Abstract
Humans have marvelled at the fit of form and function, the way organisms' traits seem remarkably suited to their lifestyles and ecologies. While natural selection provides the scientific basis for the fit of form and function, Darwin found certain adaptations vexing or particularly intriguing: sex ratios, sexual selection and altruism. The logic behind these adaptations resides in frequency-dependent selection where the value of a given heritable phenotype (i.e. strategy) to an individual depends upon the strategies of others. Game theory is a branch of mathematics that is uniquely suited to solving such puzzles. While game theoretic thinking enters into Darwin's arguments and those of evolutionists through much of the twentieth century, the tools of evolutionary game theory were not available to Darwin or most evolutionists until the 1970s, and its full scope has only unfolded in the last three decades. As a consequence, game theory is applied and appreciated rather spottily. Game theory not only applies to matrix games and social games, it also applies to speciation, macroevolution and perhaps even to cancer. I assert that life and natural selection are a game, and that game theory is the appropriate logic for framing and understanding adaptations. Its scope can include behaviours within species, state-dependent strategies (such as male, female and so much more), speciation and coevolution, and expands beyond microevolution to macroevolution. Game theory clarifies aspects of ecological and evolutionary stability in ways useful to understanding eco-evolutionary dynamics, niche construction and ecosystem engineering. In short, I would like to think that Darwin would have found game theory uniquely useful for his theory of natural selection. Let us see why this is so.
Keywords: evolutionary game theory, adaptation, fitness, natural selection, evolutionarily stable strategy, cancer
1. Introduction
Prior Darwin Reports provide excellent syntheses on evolutionary medicine, Red Queen evolution and the modern synthesis [1–3]. Only the first mentions game theory and none mentions evolutionarily stable strategies (ESS). The omission of evolutionary game theory from discussions of evolution may have several sources. Is there a lack of interest or training? Is it seen as irrelevant or inapplicable? Many may be unaware of its full scope. Here I shall take the perspective that life and natural selection are a game; and that game theory is uniquely suited to provide the conceptual framework for understanding the adaptations produced by natural selection. Had he known of it, I think Darwin would have embraced game theory.
Darwin's theory of evolution by natural selection requires three ingredients. First, there must be heritable variation. Like begets like, but with ‘mistakes’. Second, there must be a struggle for existence [4]. Populations have the capacity to grow exponentially under ideal conditions, yet limits to growth ensure they do not [5]. Third, heritable variation influences the struggle. Some heritable phenotypes (i.e. strategies) beat others in the struggle [6].
Here, I shall advocate game theory for understanding and modelling natural selection. All that follows can and should be considered as arguable. My goal is to be informative, and sometimes provocative. Mostly my goal is to stimulate enthusiasm, discourse and research towards better understanding of natural selection. I am an evolutionary ecologist interested in studying the interactions of organisms with their environment through their adaptations. I am naturally inclined towards eco-evolutionary dynamics [7], where one simultaneously considers strategy dynamics, population dynamics and environmental feedbacks [8]. This essay shall unfold through the following sections: (i) the imperative for viewing natural selection as a game, (ii) solution concepts, (iii) evolution of anisogamy, sexes and sex ratios, (iv) sexual selection, (v) ecosystem engineering and niche construction, (vi) macroevolution, and (vii) cancer.
2. The imperative for viewing natural selection as a game
Natural selection comes in three flavours: density-independent, density-dependent and frequency-dependent. The last is the most exciting, perhaps most important and certainly the most perplexing to Darwin and students of natural selection. From population and quantitative genetic models of selection, we know that density-independent selection favours the strategy that maximizes population growth rate, density-dependent selection favours the strategy that maximizes the equilibrium population size [9], and frequency-dependent selection maximizes—well it does not seem to maximize much of anything!
Game theory is well suited for frequency-dependent selection. Drawing from Darwin's postulates, individuals have expected fitnesses (per capita growth rates), which we can denote as G(v, u, x). This expected fitness is a function of the focal individual's strategy, v, the strategies of others in the population, u = (u1, … , un), and the population sizes (or densities) of the different extant strategies, x = (x1, … , xn). The strategies of u are drawn from some set of evolutionarily feasible strategies. A matrix game occurs when the strategy set is finite and discrete; a continuous trait game has a strategy set that may be continuous and even multi-dimensional for vector-valued traits. When a game occurs within species or populations, the extant strategies, u, can represent available behavioural choices to the individuals or heritable polymorphisms. As a game of coevolution and speciation, the strategies, u, represent different species. In all cases, the individual's fitness is a function of its strategy, v, the strategies of others, u, and their population sizes, x. It is a game because the best strategy for an individual probably depends on the strategies of others.
The fitness generating function, G, is so called because it becomes the fitness function for individuals using strategy ui when ui is substituted for the focal individual's strategy v. The fitness generating function imagines n ≥ 1 different strategies present in the population. Depending on the context, the different ui values represent polymorphisms within a species or they represent different species. Students of game theory will recognize G as invader fitness from adaptive dynamics—an important and widely used subset of evolutionary game theory from which many of the most significant results have emanated.
The fitness generating function models both the ecological dynamics of changes in population size (dxi/dt = xiG(v,u,x)v=ui) and the evolutionary dynamics of changes in strategy value (dui/dt = k(dG/dv)v=ui) [10]. The fitness gradient, dG/dv, evaluated at v = ui, determines whether an individual using strategy ui can improve its fitness by unilaterally increasing or decreasing its strategy. By altering assumptions regarding sources of heritable variation, the relative rates of ecological and evolutionary dynamics, and continuous versus a finite number of strategies, one can connect this evolutionary dynamic to extensions of Fisher's fundamental theorem of natural selection [11,12], Breeder's equation [13], the canonical equation of adaptive dynamics [14–18] and replicator dynamics [19–21].
Natural selection is density-independent if the strategies of others and their population sizes do not influence fitness: dG/dui = 0 and dG/dxi = 0. The fitness of an individual is based solely on its strategy, v. More broadly, natural selection can be seen as density-independent if the selection gradient, dG/dv, is independent of the strategies of others and their population sizes: d2G/dvdui = 0 and d2G/dvdxi = 0. Others' strategies and their population sizes may influence fitness, but they neither influence the evolutionary dynamics nor the adaptation that results from natural selection. Density-independent selection results in a single best strategy; a strategy, v = u*, that maximizes fitness, G, independent of u and x.
Density-dependent selection occurs when both fitness and the fitness gradient are functions of xi, d2G/dvdxi is non-zero. Yet the influence of population size on fitness and on the fitness gradient is independent of the strategies in the population: d2G/dvdui = 0 and d2G/duidxi = 0. Under this formulation, there is a single strategy that will be the adaptation, and this strategy, v = u*, will maximize the equilibrium population size, x* [22].
Fitness is frequency-dependent when changing strategy values or strategy frequencies changes fitness (dG/dui and dG/dpi are non-zero), and strongly frequency-dependent when changing the strategies of others changes the fitness gradient and hence what is adaptive to the individual: d2G/dvdui is non-zero. Such frequency dependence makes evolution a true game. Adaptations no longer must maximize anything at the population level. What is adaptive may now permit the coexistence of strategies within a species or the coexistence of species within a community. Adaptations are strategies that maximize fitness given the circumstances, where the circumstances include the strategies of others. Frequency dependence lets natural selection become the source and driver of diversity. In the last 40 years, theorists have identified the intertwined solution concepts of evolutionary game theory and, by extension, those of natural selection. Through game theory, Darwin would probably have seen the logic behind essentially all adaptations in nature!
3. Solution concepts
Levins's [23] concept of the adaptive function and fitness sets anticipates game theory as he explored conditions favouring a single generalist or two specialist species. Then came the ESS [24]. It began with matrix games [25] and grew to embrace games with continuously varying strategies, such as body size [26–29]. The 1980s saw the development of the G-function [22,30,31]. The 1990s saw adaptive dynamics [15], with exceptionally clear insights into the multiple facets of evolutionary stability [32]. This century has seen extensions into the coevolution of ecological communities [33,34], structured populations [35], games on networks and graphs [36,37], and other forms of agent-based modelling [38]. While these developments have spawned a Babel of terms and minutiae [39], they reveal distinct facets of evolutionary stability, including ESS, convergence stability and neighbourhood invasion strategy.
(a). Evolutionarily stable strategies
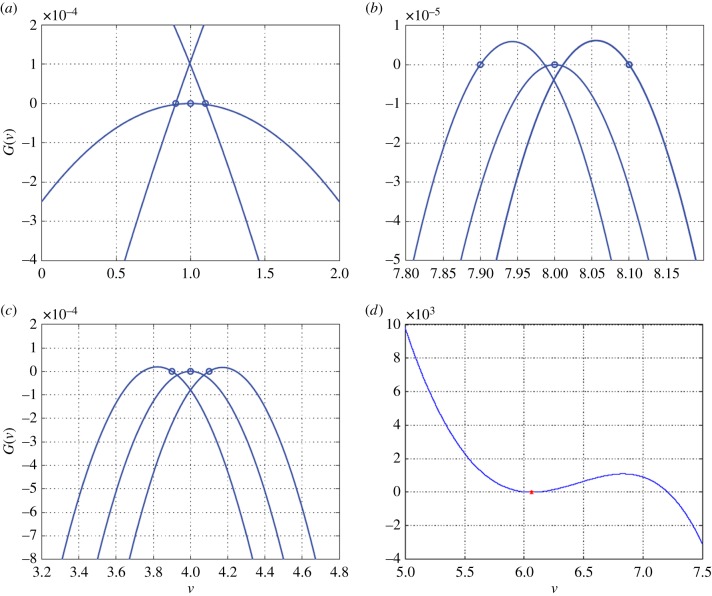
An ESS is a strategy (or set of strategies) that cannot be invaded by rare alternative strategies [40]. It views adaptations as the best trait values given the circumstances. At the ESS, no individual can gain by unilaterally changing its strategy. Thus, an ESS must be a no-regret Nash solution [41,42]. Understanding the Nash properties of adaptations would have been useful to Darwin when treating evolutionary conundrums fraught with frequency-dependence. Few textbooks or reviews of adaptations [43] and natural selection [44] make the connections between adaptations, Nash and ESS. In terms of the adaptive landscape (a plot of G versus v) [45], the ESS appear as peaks when the population is at its ESS (figure 1a–c). This is the ESS maximum principle [22,46]. Strategies that satisfy G = 0 (ecological equilibrium) and dG/dv = 0 (evolutionary equilibrium) when v = ui can be minima or maxima of the adaptive landscape. Does natural selection actually drive populations to such strategies?
Figure 1.
Three aspects of evolutionary stability. All panels show configurations of the adaptive landscape (fitness, G, plotted against the strategy of the focal individual, v, for some population value of the strategy, u, shown as the red dot on a given landscape). On each landscape, the population's strategy yields a fitness of G = 0, indicating that the population is at its equilibrium population size, x*. Panels (a–c) all show a case where a strategy is an ESS and a peak of the adaptive landscape. In (a), the ESS is both convergent stable and NIS; in (b) it is convergent stable but not NIS; and in (c), the ESS is not convergent stable or NIS. Panel (d) shows a strategy that has evolved to a minimum of the adaptive landscape that is convergent stable and NIS.
(b). Convergence stability
A peak (or valley!) of the adaptive landscape is convergent stable if populations with strategies near the peak will evolve towards the peak (figure 1a,b). The fact that an ESS might not be convergent stable was noted very early via replicator dynamics operating in a rock–scissors–paper game [40]. Peaks on the adaptive landscape may not be convergent stable, and hence may be unattainable by natural selection (figure 1c). Curiously, minima of the adaptive landscape can be convergent stable [47] and have been termed evolutionarily stable minima [48], or evolutionary branching points [49].
Darwin lacked a mechanism for speciation driven strictly by natural selection. Game theory shows us how a single species can evolve up its adaptive landscape until it resides at a convergent stable minimum (figure 1d). As a point of disruptive selection, an asexual or a sexual species (with a dose of assortative mating!) might diverge into two daughter species evolving away from this minima [50–52]. Cool! This mechanism, adaptive speciation or competitive speciation [53], is just entering our lexicon, textbooks and empirical investigations. Yet such speciation or branching from successive minima can, in theory, give rise to whole phylogenies in a manner wholly consistent with adaptive radiations [34,54]. Empirical possibilities have emerged in work on sticklebacks [55] and other examples in nature and the laboratory [56,57].
(c). Neighbourhood invader stability
A strategy has neighbourhood invader stability (NIS) if the strategy can invade any nearby resident strategy [58]. NIS means that when a population's strategy is near a peak or valley of the adaptive landscape, the strategy of the peak can successfully invade the population. Who cares?! Imagine a convergent stable ESS that is not NIS (figure 1c). Because the ESS cannot invade the population's strategy, Darwinian dynamics will evolve the population's strategy closer and closer to the peak, but it can never truly get there. Evolution towards the ESS will be slow. Alternatively, given NIS, the strategy of the convergent stable point can successfully invade the resident strategy. Natural selection to the ESS (or convergent stable minimum) can be rapid and near complete. The property of NIS might resolve a conundrum for Darwin. He saw natural selection as gradual and time consuming. Yet much empirical evidence shows rapid evolutionary change, particularly in the context of human-dominated landscapes [59,60].
Equally intriguing is how one last solution concept of mutual invasibility influences non-ESS communities [32]. If an ESS satisfies mutual invasibility, then two different strategies may be able to coexist ecologically if their strategies lie on either side of an unoccupied peak. With evolution, the two species with these different strategies will evolve towards the same peak, resulting in the extinction of one or the other. Regardless, the diversity of species that can coexist in a non-ESS community will always be the same or higher than that of an ESS community [61]. With human disturbance and global climate change many ecological communities may not be ESS. Such communities will be susceptible to invasive species and possibly result in these ‘over-saturated’ non-ESS communities [62].
4. Anisogamy, evolution of separate sexes and sex ratios
Call it the cost of males [63] or the curse of anisogamy [64], a population of hermaphrodites (or asexuals) ought to produce twice as many offspring as one where half the population is male. Darwin noted the curiosity of 50 : 50 sex ratios without offering an adaptive explanation. Game theoretic thinking explains anisogamy (extreme size differences in ‘male’ and ‘female’ gametes), the evolution of males and females from hermaphrodites, the 50 : 50 sex ratio, and much more. Such adaptations need only be the best given the circumstances. They need not serve the interests of the population or species.
Anisogamy may have resulted from a trade-off between dispersal (small gamete) and provisioning (large gamete) [64,65] and/or optimal resource allocation to gametes [66]. Bulmer & Parker [67] take this idea further, suggesting a tradeoff between gamete ‘fitness’ and zygote ‘fitness’ that becomes extreme in multicellular organisms. The production of a very small gamete needed to fertilize a very large gamete can be ESS.
Anisogamy creates a free-rider problem [68]. An individual that dispenses with the production of large, expensive gametes may be able to flood the market with small gametes. Two properties of sexual reproduction encourage free-riding. First, the fitness payoff to an individual is the same whether through a successful small or a successful large gamete. Second, at the population level, total payoffs via small gametes will equal that via large gametes. Thus, if a ‘male’ can more than double its success via small gametes by dispensing with large gametes, then it can invade a population of hermaphrodites. As males increase in frequency the payoff to hermaphrodites via their male function may drop to insignificance. The male-hermaphrodite state may be invadable by females. At this new ESS, females and males are not unlike producer–scrounger games [69].
Because the collective payoff to males equals that to females, the Fisherian [11] sex ratio becomes the expected ESS of outbreeding populations. A mother should invest equally in female and male offspring. If the cost of offspring are equal, then the ESS has a 50 : 50 sex ratio. If females cost twice as much, then a 33 : 66 female : male ratio becomes the ESS. Charnov [70] provides superb treatments on the evolution of sex ratios and sexes. Mysteries (and many would have seemed so to Darwin) become solved as adaptations in response to frequency-dependent selection. A solved mystery includes the highly skewed sex ratios of fig wasps [71–73], parasitoid wasps [74] and sawflies [75].
5. Sexual selection
Separate sexes ensure the evolution of sex-specific behavioural, morphological and physiological traits. The respective aims of quantity of matings versus quality of matings become an inevitable consequence of males, females and anisogamy. Yet ecological circumstance, along with vector-valued strategies of mate choice, mate competition, parental care and resource acquisition, has nature exhibiting a panoply of mating strategies such as run-away selection for male (and sometimes female) adornments, and lekking behaviours that allow males to cater to female choice. Females may seek resources of food, safety and parental care from males. Males and females may compete in intersexual or intrasexual contests. Different mating strategies may diversify within males and females as a within and between gender ESS. The more frequently seen roles of males providing little or less parental investment becomes inverted in many species. It seems that just about anything goes at the ESS, depending on the circumstances. Yet game theory would posit that in each case the potentially outlandish and counter-productive traits seen in mating games serve to maximize fitness given the circumstances. The mating strategies of others may be the primary circumstance!
Darwin [76] posed the problem of sexual selection as perhaps distinct form natural selection, yet the distinction disappears when its solution lies in game theory [77]. While not always explicitly game theoretic, models of Fisherian run-away selection [78,79], advantages to females of selecting gaudy males and the handicap principle [80] all aim to explain the adaptiveness of otherwise counterintuitive traits. What is clear is that sexual selection is not separate from but rather a subset of natural selection. Sexual selection studies mating behaviours as adaptations. The emerging field of social selection [81], despite protestations, is not a substitute for sexual selection, but rather a valuable expansion that studies how sexuality and mating behaviours take on expanded roles beyond simply procreation [64].
Is there an exceptionalism to sexual selection? Yes, but in underappreciated ways. There are two features that render it a highly constrained and fascinating evolutionary game. First, gender-specific traits and gender itself are conditional strategies. The actual strategy is ‘if female then _____ and if male then _____’. The strategy itself is quite androgynous. Female- and male-specific strategies are actually a subset of state dependencies. State dependencies can include the age, stage or energy state of a forager. For example, a hungry animal has less to lose from risking predation than a well-fed individual [82]. Hence it may adopt riskier feeding behaviours [83]. In state-dependent games, the solution for one group is often making the best of a bad or a good situation. This is not the immediate case for sexual selection where the combined payoff to males must equal the combined payoff to females. The sex-specific strategies of females and males can increase or decrease the size of the total pie (collective payoffs), but the split remains 50 : 50. In a sense, intrasexual ‘competition’ must always be more intense as each male or female strives for a larger portion of its gender's share.
For sexual selection, the G-function takes the form of G(vf, vm, uf, um, xf, xm) where the subscripts refer to sex-specific strategies and population sizes. Sexual selection will seem quite sensible and even concordant with the ‘rest’ of natural selection when the selection gradients favour an increase in some female or male trait, dG/dvf > 0 and dG/dvm > 0, and the increase of this trait in the whole population also increases fitness, dG/duf > 0 and dG/dum > 0. Overall, the ESS allows for a more successful population that attains a higher population density of both males and females. This might correspond to female birds adjusting clutch size optimally and males providing parental care.
Conversely, imagine the case of water striders [84,85]. Males aggressively guard their mates in a manner that risks injury and predation (dG/dvm > 0; but dG/dum < 0). Females may exacerbate the nuisance behaviour of males by foraging in riskier habitats or by adopting expensive evasive tactics (dG/dvf > 0; but dG/duf < 0). And the situation can even worsen if female behaviour simply encourages more extreme male harassment behaviours (d2G/dvfdum > 0; and dG/dvmduf > 0). The actual biology and traits may vary greatly in their appearance (witness the time and effort devoted by male and female bower birds in constructing, maintaining and scoping out displays). Yet, any time the individual male or female is selected upon to exaggerate a trait that when adopted by the group impairs fitness, the traits will seem maladaptive. While appearing to be quite useless for the more practical aspects of the struggle for existence, their roles in allowing males and females to succeed in their respective contributions to fitness makes the products of sexual selection very much ESS and the best given the circumstances.
6. Niche construction and ecological engineering
In search of termites and ants, aardvarks can dot the African savannahs with large holes. These become a public good as diverse mammals use them as dens. As ecological engineers, organisms modify the environment in ways that alter the fitness of the same or different species [86]. This environmental feedback creates new state variables, y, whose dynamics, dy/dt, are influenced by u and x. The values for y then influence fitness and the G-function becomes: G(v, u, x, y). This new dynamic component to the game, y, may be a resource or prey species, or it may be the predator of the species playing the evolutionary game. These y values can be vector-valued. In some consumer–resource games, the resource, y, may be the feedback by which others influence the fitness of the focal individual. In exploitation competition, the resource dynamic is influenced by the strategies and population sizes of others (dy/dt is a function of u, x) while the fitness of the focal individual is only influenced by its strategy and resource abundance: G(v, y). Additionally, organisms (such as beavers building lodges and dams) may intentionally engage in niche construction as an adaptation [87]. In this case, dy/dt is a function of v, the strategy of the focal individual, and natural selection may favour the evolution of strategies that intentionally manage and modulate y. Evolutionary game theory is well suited for modelling the feedbacks on the individual from its strategy, the strategies of others, their population sizes and other environmental properties.
7. Macroevolution and the existence of evolutionary technologies
This speculative section asks: to what extent can evolutionary game theory be useful for understanding macroevolution? A G-function is not unlike the German notion of a bauplan or body plan [88,89]. Organisms at higher taxonomic levels (e.g. phylum) might share a distinctive set of design rules. By its very definition, a G-function represents all individuals that share the same set of evolutionarily feasible strategies. If two individuals from the same G-function possess the same strategy in the same environment then they have the same expected fitness. In this sense, all within a G-function are evolutionarily identical [31] even if the existing populations (or species) possess very different strategies and ecologies. In time, all within a G-function have evolutionary access to their shared strategy set through recurrent mutations and/or selection. So what constitutes a different G-function?
If evolutionary constraints are indeed hierarchical, as suggested by many aspects of phylogenetics and morphometrics [90], then G-functions too will form hierarchies. Different G-functions represent the deeper branch points in phylogenies where a trait or suite of traits arises that is rather unique or relatively irreversible. G-functions, their associated strategy sets and their ecological potentials can be thought of as an evolutionary technology.
In this extension of game theory, microevolution is the repeatable and reversible evolutionary possibilities within a G-function. Different G-functions represent relatively non-repeatable and irreversible evolutionary changes. It seems quite reasonable to see the cat (Felidae) and dog (Canidae) families as different G-functions. Things like body size, limb length and the vast majority of readily changeable traits do not define these two families as separate—such traits for them and for mammals as a whole could be thought of as microevolutionary traits subject to rapid evolution in response to selection. Rather, just a handful of traits, unique and universal to each family, renders them separate G-functions. Felids have 28–30 teeth, canids 42. Cats have a clavicle (allowing for bopping and lateral arm movement); dogs do not. Perhaps those cat's eyes also qualify (with the caveat that there is still an extant cat species with the ancestral eye). For these two families, this trio of macroevolutionary traits does not seem to evolve easily or repeatably, even under strong selection. Perhaps it would simply take more time to evolve from a felid to a canid than from one felid to another? It has been some 45 Myr since their last common ancestor. Perhaps the depth of valleys separating extant species from the cat family is simply much shallower and narrower than the valley of some adaptive landscape that separates cats from dogs? We may profitably define macroevolution as the evolution of traits that are relatively irreversible and non-repeatable—such evolution results in new G-functions.
If there is value to extending game theory this far, then several research horizons emerge. What scale of taxonomy constitutes a G-function? As a crude first cut it may be at the family level. It seems that most species within a genus could fairly rapidly evolve from one into the other. At the level of orders and classes, it seems certain we are dealing with quite different evolutionary technologies. It is difficult to imagine rapid evolution from one order to another, or one class to another, and back again, via just natural selection and recurrent mutation.
Being of the same or of a different G-function influences how natural selection proceeds. The species associated with a G-function reside on the same adaptive landscape. Those of different G-functions reside on different landscapes. Species diversity can emerge both within and between evolutionary technologies. Ripa et al. [34] provide an example of a predator–prey game where the strategies of the predators (one distinct G-function) can induce disruptive selection and speciation on the prey (a separate G-function) and vice versa. Starting with a single prey and predator species, the eco-evolutionary dynamics that generate the ESS communities produce phylogenies that show patterns of speciation and diversification.
Two different G-functions may produce species that compete. If a novel G-function is wholly superior to an older one, then we might see species replacements as the superior replaces the inferior one [62]. The presence of species from the original G-function may slow or even prevent the invasion, speciation and niche filling by species of the new G-function—a phenomenon termed incumbent replacement [91]. Pit vipers (e.g. rattlesnakes, sub-family Crotalinae) found in the New World and Eastern Asia may be in the process of replacing the non-pit vipers (sub-family Viperinae) that range through much of Asia, Europe and Africa. Pit vipers possess heat sensory pits that provide infrared night vision goggles. They acquired this constraint-breaking adaptation when they diverged from a non-pit viper around 18 Ma in far eastern Asia. If the replacement process follows the model of incumbent replacement, then along their zone of contact, as non-pit viper species go extinct, they will tend to be replaced by pit viper species, but not vice versa.
If two competing G-functions are simply different, the presence of the two may reduce the number of species within each while increasing the total number of species. In examining competition between different taxa this might occur in the deserts of the world with respect to seed-eating ants, birds and rodents. An intriguing and untested pattern of diversity occurs with the families Sciuridae (mammals in the squirrel family) and Corvidae (birds of the family with crows, jays, magpies, etc.). The Great Lakes region of North America has eight species of sciurids and just two species of corvids. The UK has eight corvids and just one native sciurid, and now the introduced eastern grey squirrel. Sciurids beat corvids to North America [92] and corvids beat sciurids to Europe [93]. Might evolutionary game theory be useful or perhaps necessary to understand the diversification and coexistence patterns both within and between these two evolutionary technologies [94]?
This game theoretic approach to the history of life opens several key questions. First, are most species most of the time at or near convergent stable ESSs? If so, then one can imagine punctuated equilibria [95] as the outcome of micro- and macroevolutionary processes. At the microevolutionary scale, natural selection may quickly fill niches associated with convergent stable ESSs. Such evolution should appear fast and directed. At longer time scales, adaptive breakthroughs, constraint-breaking adaptations and other macroevolutionary traits may usher in new G-functions. Such events may be accidental and impossible to anticipate. But such events should substantially rearrange species diversity as older G-functions experience the extinction (or speciation) of many of their constituent species. Not just species but whole G-functions may exhibit fundamental and realized niche spaces. Macroevolution may be a Red Queen-like progression of new evolutionary technologies as G-functions replace or more likely add to the extant G-functions. This accumulation and replacement of evolutionary technologies gives rise to ‘progress’ in the history of life.
While the above conjectures are as yet unfounded, they do display the full scope that game theory could have provided Darwin as he developed his theory of natural selection. It also provides some insights into several trends in ecology. One of these, in conservation biology, emphasizes the need to prioritize the protection of phylogenetically distinct clades [96]. This may amount to preserving G-functions as well as individual species. As the last species of a particular G-function is lost, so is its entire evolutionary technology. By contrast, the loss of a species within a species-rich G-function does not cancel any of nature's evolutionary options. This accords with a tradition of distinguishing between the coexistence of closely related species and the study of coexistence between distantly related taxa such as desert ants and rodents [90]. This distinction finds relevance within the context of ESSs within and between G-functions.
8. Cancer as an evolutionary game
Cancer has been defined as a disease of the genes, or as a disease of unregulated proliferation. Cancer initiation requires a sequence of unfortunate mutations. Also, cancer cells are notable for their ability to proliferate. These two observations suggest heritable variation and a struggle for existence among the cancer cells. It is a small step to imagine that heritable variation influences the success of a cancer cell within its tumour. Hence, natural selection may be a prime driver of cancer progression and metastasis. The idea that cancer progresses as an evolutionary process has deep roots [97–99]; and therapies fail to cure cancer because cancer cells evolve resistance.
Cancer may be an evolutionary game [100,101]; and game theory can be used to define, understand, model and hopefully treat cancer. Most cancers represent a speciation event. A host cell becomes, essentially, a new asexual, single-celled protist. As yet it is difficult to pinpoint exactly when this cancer cell lineage truly becomes its own unit of selection. Presumably, it transitions from normal to abnormal, and eventually to being a novel G-function within the host. This transition requires unfortunate mutations, giving credence to ‘a disease of the genes’. But by the time the cancer is clinically diagnosed, the speciation event is complete, the G-function exists, and the cancer cells are playing out a Darwinian game within their tumour ecosystem.
The hallmarks of cancer [102] provide a checklist of cancer's properties. They fall nicely within a Darwinian paradigm of eco-evolutionary dynamics. Self-sufficiency in growth signals, insensitivity to anti-growth signals, evading programmed cell death (apoptosis), and limitless replicative potential simply describe the prerequisites for the cancer cell to be the unit of selection. The next two hallmarks, sustained angiogenesis (recruiting blood vasculature) and tissue invasion and metastasis, indicate limits to growth. These adaptations increase resource availability through angiogenesis (niche construction or ecological engineering), range expansion into adjacent tissue space, or invasion into another organ of the host. Additional hallmarks support a Darwinian view of cancer progression. Cancer cells exhibit ‘abnormal’ metabolisms (adaptations for swift or efficient resource acquisition for proliferation and survival?), traits to evade the immune system (anti-predator adaptations?), unstable DNA (adaptation for elevated mutation rates and evolutionary potential?) and inflammation (niche construction for increased resources and safety from the immune system?).
Cancer as an evolutionary game sees the tumour cells as the players, their survival and proliferation rates are their payoffs, and the tumour environment sets the rules. The game is played primarily between the cancer cells. It is less a game between tumour cells and the host. Some aspects of the host's immune response to the cancer cells constitute a predator–prey game—the immune cells can evolve their strategies in response to the cancer cells. Yet the other normal cells of the body are not players in the Darwinian game. They are highly dynamic and interactive components (y environmental variables) that the cancer cells ignore, evade, dupe, tolerate or exploit. These normal cells do not have a G-function (they are part of a whole organism G-function), and they do not evolve on an adaptive landscape. The tumour cells do!
The cancer patient is not a host in the traditional sense. To the cancer cells, the human is a novel world in which eco-evolutionary dynamics begin anew. The cancer cells are not part of a susceptible–infectious–resistant game between host and pathogen so typical of diseases and parasites. The patient is the entire ‘globe’ for the newly evolving and diversifying cancer G-function. The amazing and rather terrifying evolution of communicable cancers in the Tasmanian devil [103] and in domestic dogs provide notable exceptions [104]. As the cancer cells compete among themselves to secure resources, safety and space they can and do destroy their world and themselves. This just dramatizes how natural selection does not work for the good of the species or the system. Rather, natural selection promotes adaptations that diversify from valleys and reside on peaks of the adaptive landscape without regard for unintended consequences, no matter how catastrophic.
Key emerging questions for evolutionary game theory and cancer include: are clinical cancers best defined and understood as evolutionary games? Should cancer therapy be modelled and designed as a predator–prey game between the therapist and the cancer cells? Do cancer cells evolve adaptations, and if so, are most cancer cells most of the time at an ESS, or do constantly changing circumstances and lack of time prevent the traits of cancer cells from converging on peaks of their adaptive landscape? Tumours and cancer cells represent a lot of ecological heterogeneity and genetic variation. But is this heritable variation primarily driven by the accumulation of random mutations or do cancer cells diversify, speciate and evolve to occupy distinct niches within the tumour ecosystem [105,106]?
Finally, can game theory inform therapy? Adaptive therapy [107] aims to use eco-evolutionary dynamics and Darwinian principles to create therapies that anticipate the evolutionary responses of the tumour cells. Such therapies might create evolutionary double-binds [108] where different therapies elicit resistance strategies that drive the cancer cells into the arms of the other therapy [109] and that treat the tumour as a community of coexisting cancer species rather than as a single entity. When ‘treating to kill’ cannot work, it may be possible to use adaptive therapies to ‘treat to contain’. By understanding the diversity, ecologies and evolutionary potentials of the cancer cells, we may be able to design therapies that permit patients to live healthy lives with the cancer—not unlike ideas from integrated pest management.
9. Concluding thoughts
Evolutionary game theory is ready to contribute to all aspects of evolution from individual behaviours up to the history of life. Yet these developments have come lately. They tend to show up piecemeal and not as a coherent whole in textbooks on evolution, ecology and animal behaviour. Morris & Lundberg [8] provide a notable exception. Evolutionary game theory is essential for understanding adaptations and for applying the pleasing but rather odd set of evolutionary stabilities. As our understanding of molecular genetics, neurobiology and phylogenetics progresses, game theory may be essential for understanding genetics itself as an adaptation, for understanding the fit of form and function seen in neural architectures, and for understanding the ‘tempo and mode’ of evolution seen in phylogenies. We shall see. I do not desire that you, the reader, agree with all (or even much!) that is written herein. I do hope it sparks interest for how game theory can explain natural selection at all scales. Wouldn't it be great to know what Darwin would have made of evolutionary game theory?
Acknowledgements
Douglas Kelt suggested the title at the International Mammal Congress, 2009. Many thanks to Chris Whelan, Jean Powlesland, William Vickery and an anonymous reviewer for valuable discussion and edits. I owe a huge debt of gratitude to the friends, mentors and colleagues who have taught me game theory; and to a multitude of scientists (some cited herein and many not) that have advanced evolutionary game theory, adaptive dynamics, G-functions, and other means for modelling and testing for the fit of form and function.
Competing interests
I declare I have no competing interests.
Funding
Funding was provided by the European Commission, Marie Sklodowska-Curie Actions Research and Innovation Staff Exchange (RISE) SEP-210251279; and the US National Science Foundation: CNS-1248080 and IIS-1324977.
References
- 1.Stearns SC. 2012. Evolutionary medicine: its scope, interest and potential. Proc. R. Soc. B 279, 4305–4321. ( 10.1098/rspb.2012.1326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laland KN, Uller T, Feldman MW, Sterelny K, Müller GB, Moczek A, Jablonka E, Odling-Smee J. 2015. The extended evolutionary synthesis: its structure, assumptions and predictions. Proc. R. Soc. B 282, 20151019 ( 10.1098/rspb.2015.1019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brockhurst MA, Chapman T, King KC, Mank JE, Paterson S, Hurst GD. 2014. Running with the Red Queen: the role of biotic conflicts in evolution. Proc. R. Soc. B 281, 20141382 ( 10.1098/rspb.2014.1382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gause GF. 1934. The struggle for existence. Baltimore, MD: Williams and Wilkins. [Google Scholar]
- 5.Turchin P. 2001. Does population ecology have general laws? Oikos 94, 17–26. ( 10.1034/j.1600-0706.2001.11310.x) [DOI] [PubMed] [Google Scholar]
- 6.Cox CF. 1909. Charles Darwin and the mutation theory. Am. Nat. 43, 65–91. ( 10.1086/279022) [DOI] [Google Scholar]
- 7.Morris DW. 2011. Adaptation and habitat selection in the eco-evolutionary process. Proc. R. Soc. B 278, 2401–2411. ( 10.1098/rspb.2011.0604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris DW, Lundberg P. 2011. Pillars of evolution: fundamental principles of the eco-evolutionary process. Oxford, UK: Oxford University Press. [Google Scholar]
- 9.Clarke B. 1972. Density-dependent selection. Am. Nat. 106, 1–13. ( 10.1086/282747) [DOI] [Google Scholar]
- 10.Vincent TL, Cohen Y, Brown JS. 1993. Evolution via strategy dynamics. Theor. Popul. Biol. 44, 149–176. ( 10.1006/tpbi.1993.1023) [DOI] [Google Scholar]
- 11.Fisher RA. 1930. The genetical theory of natural selection: a complete variorum edition. Oxford, UK: Oxford University Press. [Google Scholar]
- 12.Charlesworth B. 1990. Optimization models, quantitative genetics, and mutation. Evolution 44, 520–538. ( 10.2307/2409433) [DOI] [PubMed] [Google Scholar]
- 13.Lande R. 1979. Quantitative genetic analysis of multivariate evolution, applied to brain: body size allometry. Evolution 33, 402–416. ( 10.2307/2407630) [DOI] [PubMed] [Google Scholar]
- 14.Dieckmann U, Law R. 1996. The dynamical theory of coevolution: a derivation from stochastic ecological processes. J. Math. Biol. 34, 579–612. ( 10.1007/BF02409751) [DOI] [PubMed] [Google Scholar]
- 15.Metz JAJ, Geritz SAH, Meszéna G, Jacobs FJA, van Heerwaarden JS. 1996. Adaptive dynamics, a geometrical study of the consequences of nearly faithful reproduction. In Stochastic and spatial structures of dynamical systems (eds van Strien SJ, Verduyn Lunel SM), pp. 183–231. Amsterdam, The Netherlands: North Holland. [Google Scholar]
- 16.Champagnat N, Ferričre R, Ben Arous4 G. 2002. The canonical equation of adaptive dynamics: a mathematical view. Selection 2, 73–83. ( 10.1556/Select.2.2001.1-2.6) [DOI] [Google Scholar]
- 17.Leimar O. 2009. Multidimensional convergence stability. Evol. Ecol. Res. 11, 191–208. [Google Scholar]
- 18.Durinx M, Metz JH, Meszéna G. 2008. Adaptive dynamics for physiologically structured population models. J. Math. Biol. 56, 673–742. ( 10.1007/s00285-007-0134-2) [DOI] [PubMed] [Google Scholar]
- 19.Zeeman EC. 1981. Dynamics of the evolution of animal conflicts. J. Theor. Biol. 89, 249–270. ( 10.1016/0022-5193(81)90311-8) [DOI] [Google Scholar]
- 20.Taylor PD, Jonker LB. 1978. Evolutionary stable strategies and game dynamics. Math. Biosci. 40, 145–156. ( 10.1016/0025-5564(78)90077-9) [DOI] [Google Scholar]
- 21.Hofbauer J, Sigmund K. 1998. Evolutionary games and population dynamics. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 22.Vincent TL, Brown JS. 2005. Evolutionary game theory, natural selection, and Darwinian dynamics. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 23.Levins R. 1962. Theory of fitness in a heterogeneous environment. I. The fitness set and adaptive function. Am. Nat. 96, 361–373. ( 10.1086/282245) [DOI] [Google Scholar]
- 24.Maynard Smith J, Price GR. 1973. The logic of animal conflict. Nature 246, 15–18. ( 10.1038/246015a0) [DOI] [Google Scholar]
- 25.Hines WGS. 1987. Evolutionary stable strategies: a review of basic theory. Theor. Popul. Biol. 31, 195–272. ( 10.1016/0040-5809(87)90029-3) [DOI] [PubMed] [Google Scholar]
- 26.Lawlor LR, Maynard Smith J. 1976. The coevolution and stability of competing species. Am. Nat. 110, 79–99. ( 10.1086/283049) [DOI] [Google Scholar]
- 27.Auslander D, Guckenheimer J, Oster G. 1978. Random evolutionarily stable strategies. Theor. Popul. Biol. 13, 276–293. ( 10.1016/0040-5809(78)90047-3) [DOI] [PubMed] [Google Scholar]
- 28.Mirmirani M, Oster G. 1978. Competition, kin selection, and evolutionary stable strategies. Theor. Popul. Biol. 13, 304–339. ( 10.1016/0040-5809(78)90049-7) [DOI] [PubMed] [Google Scholar]
- 29.McGill BJ, Brown JS. 2007. Evolutionary game theory and adaptive dynamics of continuous traits. Annu. Rev. Ecol. Evol. Syst. 38, 403–435. ( 10.1146/annurev.ecolsys.36.091704.175517) [DOI] [Google Scholar]
- 30.Vincent TL, Brown JS. 1984. Stability in an evolutionary game. Theor. Popul. Biol. 26, 408–427. ( 10.1016/0040-5809(84)90043-1) [DOI] [Google Scholar]
- 31.Brown JS, Vincent TL. 1987. A theory for the evolutionary game. Theor. Popul. Biol. 31, 140–166. ( 10.1016/0040-5809(87)90026-8) [DOI] [Google Scholar]
- 32.Geritz SA, Meszena G, Metz JA. 1998. Evolutionarily singular strategies and the adaptive growth and branching of the evolutionary tree. Evol. Ecol. 12, 35–57. ( 10.1023/A:1006554906681) [DOI] [Google Scholar]
- 33.Johansson J, Dieckmann U. 2009. Evolutionary responses of communities to extinctions. Evol. Ecol. Res. 11, 561–588. [Google Scholar]
- 34.Ripa J, Storlind L, Lundberg P, Brown JS. 2009. Niche co-evolution in consumer–resource communities. Evol. Ecol. Res. 11, 305–323. [Google Scholar]
- 35.Parvinen K, Metz JAJ. 2008. A novel fitness proxy in structured locally finite metapopulations with diploid genetics, with an application to dispersal evolution. Theor. Popul. Biol. 73, 517–528. ( 10.1016/j.tpb.2008.01.002) [DOI] [PubMed] [Google Scholar]
- 36.Lieberman E, Hauert C, Nowak MA. 2005. Evolutionary dynamics on graphs. Nature 433, 312–316. ( 10.1038/nature03204) [DOI] [PubMed] [Google Scholar]
- 37.Broom M, Rychtar J. 2013. Game-theoretical models in biology. Boca Raton, FL: CRC Press. [Google Scholar]
- 38.Nowak MA. 2006. Evolutionary dynamics. Cambridge, MA: Harvard University Press. [Google Scholar]
- 39.Apaloo J, Brown JS, Vincent TL. 2009. Evolutionary game theory: ESS, convergence stability, and NIS. Evol. Ecol. Res. 11, 489–515. [Google Scholar]
- 40.Maynard Smith J. 1982. Evolution and the theory of games. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 41.Nash J. 1951. Non-cooperative games. Annu. Math. 54, 286–295. ( 10.2307/1969529) [DOI] [Google Scholar]
- 42.Apaloo J, Brown JS, McNickle GG, Vincent TLS, Vincent TL. 2014. ESS versus Nash: solving evolutionary games. Evol. Ecol. Res. 16, 293–314. [Google Scholar]
- 43.Rose MR, Lauder GV (eds). 1996. Adaptation. New York, NY: Associated Press. [Google Scholar]
- 44.Shanahan T. 2004. The evolution of Darwinism: selection, adaptation and progress in evolutionary biology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 45.Gavrilets S. 2004. Fitness landscapes and the origin of species (MPB-41). Princeton, NJ: Princeton University Press. [Google Scholar]
- 46.Parvinen K, Heino M, Dieckmann U. 2013. Function-valued adaptive dynamics and optimal control theory. J. Math. Biol. 67, 509–533. ( 10.1007/s00285-012-0549-2) [DOI] [PubMed] [Google Scholar]
- 47.Brown JS, Pavlovic NB. 1992. Evolution in heterogeneous environments: effects of migration on habitat specialization. Evol. Ecol. 6, 360–382. ( 10.1007/BF02270698) [DOI] [Google Scholar]
- 48.Abrams PA, Matsuda H, Harada Y. 1993. Evolutionarily unstable fitness maxima and stable fitness minima of continuous traits. Evol. Ecol. 7, 465–487. ( 10.1007/BF01237642) [DOI] [Google Scholar]
- 49.Geritz SA, Metz JA, Kisdi É, Meszéna G. 1997. Dynamics of adaptation and evolutionary branching. Phys. Rev. Lett. 78, 2024–2027. ( 10.1103/PhysRevLett.78.2024) [DOI] [Google Scholar]
- 50.Cohen Y, Vincent TL, Brown JS. 1999. A G-function approach to fitness minima, fitness maxima, evolutionarily stable strategies and adaptive landscapes. Evol. Ecol. Res. 1, 923–942. [Google Scholar]
- 51.Dieckmann U, Doebeli M. 1999. On the origin of species by sympatric speciation. Nature 400, 354–357. ( 10.1038/22521) [DOI] [PubMed] [Google Scholar]
- 52.Ripa J. 2009. When is sympatric speciation truly adaptive? An analysis of the joint evolution of resource utilization and assortative mating. Evol. Ecol. 23, 31–52. ( 10.1007/s10682-008-9267-z) [DOI] [Google Scholar]
- 53.Rosenzweig ML. 1978. Competitive speciation. Biol. J. Linn. Soc. 10, 275–289. ( 10.1111/j.1095-8312.1978.tb00016.x) [DOI] [Google Scholar]
- 54.Kisdi E. 2016. Dispersal polymorphism in stable habitats. J. Theor. Biol, 392, 69–82. ( 10.1016/j.jtbi.2015.12.006) [DOI] [PubMed] [Google Scholar]
- 55.Hatfield T, Schluter D. 1999. Ecological speciation in sticklebacks: environment-dependent hybrid fitness. Evolution 53, 866–873. ( 10.2307/2640726) [DOI] [PubMed] [Google Scholar]
- 56.Rice WR, Hostert EE. 1993. Laboratory experiments on speciation: what have we learned in 40 years? Evolution 47, 1637–1653. ( 10.2307/2410209) [DOI] [PubMed] [Google Scholar]
- 57.Via S. 2001. Sympatric speciation in animals: the ugly duckling grows up. Trends Ecol. Evol. 16, 381–390. ( 10.1016/S0169-5347(01)02188-7) [DOI] [PubMed] [Google Scholar]
- 58.Apaloo J. 1997. Revisiting strategic models of evolution: the concept of neighborhood invader strategies. Theor. Popul. Biol. 52, 71–77. ( 10.1006/tpbi.1997.1318) [DOI] [PubMed] [Google Scholar]
- 59.Ashley MV, Willson MF, Pergams OR, O'Dowd DJ, Gende SM, Brown JS. 2003. Evolutionarily enlightened management. Biol. Conserv. 111, 115–123. ( 10.1016/S0006-3207(02)00279-3) [DOI] [Google Scholar]
- 60.Alberti M. 2015. Eco-evolutionary dynamics in an urbanizing planet. Trends Ecol. Evol. 30, 114–126. ( 10.1016/j.tree.2014.11.007) [DOI] [PubMed] [Google Scholar]
- 61.Geritz SA, Metz JA, Rueffler C. 2016. Mutual invadability near evolutionarily singular strategies for multivariate traits, with special reference to the strongly convergence stable case. J. Math. Biol. 72, 1081–1099. ( 10.1007/s00285-015-0944-6) [DOI] [PubMed] [Google Scholar]
- 62.Pintor LM, Brown JS, Vincent TL. 2011. Evolutionary game theory as a framework for studying biological invasions. Am. Nat. 177, 410–423. ( 10.1086/658149) [DOI] [PubMed] [Google Scholar]
- 63.Lively CM, Lloyd DG. 1990. The cost of biparental sex under individual selection. Am. Nat. 135, 489–500. ( 10.1086/285058) [DOI] [Google Scholar]
- 64.Grande T, Brown JS. 2010. The evolution of sex. In God, science, sex, gender: an interdisciplinary approach to Christian ethics (eds Jung PB, Vigen AM), ch. 7, pp. 105–122. Urbana, IL: University of Illinois Press. [Google Scholar]
- 65.Hoekstra RF. 1980. Why do organisms produce gametes of only two different sizes? Some theoretical aspects of the evolution of anisogamy. J. Theor. Biol. 87, 785–793. ( 10.1016/0022-5193(80)90117-4) [DOI] [PubMed] [Google Scholar]
- 66.Maire N, Ackermann M, Doebeli M. 2001. Evolutionary branching and the evolution of anisogamy. Selection 2, 119–132. ( 10.1556/Select.2.2001.1-2.9) [DOI] [Google Scholar]
- 67.Bulmer MG, Parker GA. 2002. The evolution of anisogamy: a game-theoretic approach. Proc. R. Soc. Lond. B 269, 2381–2388. ( 10.1098/rspb.2002.2161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baumol WJ. 1967. Welfare economics and the theory of the state. Cambridge, MA: Harvard University Press. [Google Scholar]
- 69.Giraldeau LA, Caraco T. 2000. Social foraging theory. Princeton, NJ: Princeton University Press. [Google Scholar]
- 70.Charnov EL. 1982. The theory of sex allocation. Princeton, NJ: Princeton University Press. [Google Scholar]
- 71.Hamilton WD. 1967. Extraordinary sex ratios. Science 156, 477–488. ( 10.1126/science.156.3774.477) [DOI] [PubMed] [Google Scholar]
- 72.West SA, Herre EA. 1998. Stabilizing selection and variance in fig wasp sex ratios. Evolution 52, 475–485. ( 10.2307/2411083) [DOI] [PubMed] [Google Scholar]
- 73.Greeff JM. 2002. Mating system and sex ratios of a pollinating fig wasp with dispersing males. Proc. R. Soc. Lond. B 269, 2317–2323. ( 10.1098/rspb.2002.2160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fox LR, Letourneau DK, Eisenbach J, Van Nouhuys S. 1990. Parasitism rates and sex ratios of a parasitoid wasp: effects of herbivore and plant quality. Oecologia 83, 414–419. ( 10.1007/BF00317569) [DOI] [PubMed] [Google Scholar]
- 75.Mopper S, Whitham TG. 1992. The plant stress paradox: effects on pinyon sawfly sex ratios and fecundity. Ecology 73, 515–525. ( 10.2307/1940757) [DOI] [Google Scholar]
- 76.Darwin C. 1871. Sexual selection and the descent of man. London, UK: Murray. [Google Scholar]
- 77.Mota PG. 2009/2010 Darwin's sexual selection theory: a forgotten idea. Antropologia Portuguesa 26/27, 149–161. [Google Scholar]
- 78.Fisher RA. 1915. The evolution of sexual preference. Eugenics Rev. 7, 184–192. [PMC free article] [PubMed] [Google Scholar]
- 79.Andersson MB. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 80.Zahavi A, Zahavi A. 1999. The handicap principle: a missing piece of Darwin's puzzle. Oxford, UK: Oxford University Press. [Google Scholar]
- 81.Roughgarden J. 2012. The social selection alternative to sexual selection. Phil. Trans. R. Soc. B 367, 2294–2303. ( 10.1098/rstb.2011.0282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brown JS. 1992. Patch use under predation risk: I. Models and predictions. Ann. Zool. Fenn. 29, 301–309. [Google Scholar]
- 83.Clark CW. 1994. Antipredator behavior and the asset-protection principle. Behav. Ecol. 5, 159–170. ( 10.1093/beheco/5.2.159) [DOI] [Google Scholar]
- 84.Rowe L, Arnqvist G, Sih A, Krupa JJ. 1994. Sexual conflict and the evolutionary ecology of mating patterns: water striders as a model system. Trends Ecol. Evol. 9, 289–293. ( 10.1016/0169-5347(94)90032-9) [DOI] [PubMed] [Google Scholar]
- 85.Wey TW, Chang AT, Fogarty S, Sih A. 2015. Personalities and presence of hyperaggressive males influence male mating exclusivity and effective mating in stream water striders. Behav. Ecol. Sociobiol. 69, 27–37. ( 10.1007/s00265-014-1814-8) [DOI] [Google Scholar]
- 86.Jones CG, Lawton JH, Shachak M. 1997. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 78, 1946–1957. ( 10.1890/0012-9658(1997)078%5B1946:PANEOO%5D2.0.CO;2) [DOI] [Google Scholar]
- 87.Odling-Smee FJ, Laland KN, Feldman MW. 2003. Niche construction: the neglected process in evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 88.Arthur W. 1997. The origins of animal body plans. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 89.Davidson EH, Erwin DH. 2006. Gene regulatory networks and the evolution of animal body plans. Science 311, 796–800. ( 10.1126/science.1113832) [DOI] [PubMed] [Google Scholar]
- 90.Levin M, et al. 2016. The mid-developmental transition and the evolution of animal body plans. Nature 531, 637–641. ( 10.1038/nature16994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rosenzweig ML, McCord RD. 1991. Incumbent replacement: evidence for long-term evolutionary progress. Paleobiology 17, 202–213. ( 10.1017/S0094837300010563) [DOI] [Google Scholar]
- 92.Steppan S, Hamm S. 2006. Sciuridae. The Tree of Life web project. See http://tolweb.org/Sciuridae.
- 93.Ericson PGP, Jansén A, Johansson US, Ekman J. 2005. Inter-generic relationships of the crows, jays, magpies and allied groups (Aves: Corvidae) based on nucleotide sequence data. J. Avian Biol. 36, 222–234. ( 10.1111/j.0908-8857.2001.03409.x) [DOI] [Google Scholar]
- 94.Hochberg ME, Lawton JH. 1990. Competition between kingdoms. Trends Ecol. Evol. 5, 367–371. ( 10.1016/0169-5347(90)90097-W) [DOI] [PubMed] [Google Scholar]
- 95.Gould SJ, Eldredge N. 1977. Punctuated equilibria: the tempo and mode of evolution reconsidered. Paleobiology 3, 115–151. ( 10.1017/S0094837300005224) [DOI] [Google Scholar]
- 96.Winter M, Devictor V, Schweiger O. 2013. Phylogenetic diversity and nature conservation: where are we? Trends Ecol. Evol. 28, 199–204. ( 10.1016/j.tree.2012.10.015) [DOI] [PubMed] [Google Scholar]
- 97.Cairns J. 1975. Mutation selection and the natural history of cancer. Nature 255, 197–200. ( 10.1038/255197a0) [DOI] [PubMed] [Google Scholar]
- 98.Nagy JD. 2005. The ecology and evolutionary biology of cancer: a review of mathematical models of necrosis and tumor cell diversity. Math. Biosci. Eng. 2, 381–418. ( 10.3934/mbe.2005.2.381) [DOI] [PubMed] [Google Scholar]
- 99.Thomas F, et al. 2013. Applying ecological and evolutionary theory to cancer: a long and winding road. Evol. Appl. 6, 1–10. ( 10.1111/eva.12021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tomlinson IP. 1997. Game-theory models of interactions between tumour cells. Eur. J. Cancer 33, 1495–1500. ( 10.1016/S0959-8049(97)00170-6) [DOI] [PubMed] [Google Scholar]
- 101.Gatenby RA, Vincent TL. 2003. An evolutionary model of carcinogenesis. Cancer Res. 63, 6212–6220. [PubMed] [Google Scholar]
- 102.Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. ( 10.1016/j.cell.2011.02.013) [DOI] [PubMed] [Google Scholar]
- 103.Pearse A, Swift K. 2006. Allograft theory: transmission of devil facial-tumour disease. Nature 439, 549 ( 10.1038/439549a) [DOI] [PubMed] [Google Scholar]
- 104.Murgia C, Pritchard JK, Kim SY, Fassati A, Weiss RA. 2006. Clonal origin and evolution of a transmissible cancer. Cell 126, 477–487. ( 10.1016/j.cell.2006.05.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lloyd MC, Alfarouk KO, Verduzco D, Bui MM, Gillies RJ, Ibrahim ME, Brown JS, Gatenby RA. 2014. Vascular measurements correlate with estrogen receptor status. BMC Cancer 14, 279–286. ( 10.1186/1471-2407-14-279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lloyd MC, Cunningham JA, Bui MM, Gillies RJ, Brown JS, Gatenby RA. 2016. Darwinian dynamics of intratumoral heterogeneity: not solely random mutations but also variable environmental selection forces. Cancer Res. 76, 3136–3144. ( 10.1158/0008-5472.CAN-15-2962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gatenby RA, Silva AS, Gillies RJ, Frieden BR. 2009. Adaptive therapy. Cancer Res. 69, 4894–4903. ( 10.1158/0008-5472.CAN-08-3658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gatenby RA, Brown JS, Vincent TL. 2009. Lessons from applied ecology: cancer control using an evolutionary double bind. Cancer Res. 69, 7499–7502. ( 10.1158/0008-5472.CAN-09-1354) [DOI] [PubMed] [Google Scholar]
- 109.Cunningham JJ, Gatenby RA, Brown JS. 2011. Evolutionary dynamics in cancer therapy. Mol. Pharm. 8, 2094–2100. ( 10.1021/mp2002279) [DOI] [PMC free article] [PubMed] [Google Scholar]