Abstract
Both dust and silica phytoliths have been shown to contribute to reducing tooth volume during chewing. However, the way and the extent to which they individually contribute to tooth wear in natural conditions is unknown. There is still debate as to whether dental microwear represents a dietary or an environmental signal, with far-reaching implications on evolutionary mechanisms that promote dental phenotypes, such as molar hypsodonty in ruminants, molar lengthening in suids or enamel thickening in human ancestors. By combining controlled-food trials simulating natural conditions and dental microwear textural analysis on sheep, we show that the presence of dust on food items does not overwhelm the dietary signal. Our dataset explores variations in dental microwear textures between ewes fed on dust-free and dust-laden grass or browse fodders. Browsing diets with a dust supplement simulating Harmattan windswept environments contain more silica than dust-free grazing diets. Yet browsers given a dust supplement differ from dust-free grazers. Regardless of the presence or the absence of dust, sheep with different diets yield significantly different dental microwear textures. Dust appears a less significant determinant of dental microwear signatures than the intrinsic properties of ingested foods, implying that diet plays a critical role in driving the natural selection of dental innovations.
Keywords: controlled-food trials, dental microwear texture analysis, diet, dust, grit, tooth wear
1. Introduction
Today, global warming and habitat fragmentation, together with deforestation in inter-tropical latitudes, favour the spread of arid water-depleted environments frequently swept by windblown dust [1]. In arid and peripheral biomes, winds may amass tonnes per square kilometre a year of dust grains far smaller than a millimetre [2,3]. The expectation is that animals living in such environments contend with intensified, abrasive diets [3]. Over the Cenozoic Era, several episodes of climate change have favoured the expansion of such open and arid habitats with the development of grasslands [4,5]. These changing conditions played a key role in the selection of dental innovations to counterbalance intensive tooth wear among many clades of mammals [6,7]. In North American and Eastern Mediterranean biomes, dental phenotypes such as high crowned molars (hypsodonty) of extinct horses and antelopes, respectively, were more favourably selected as they were better suited to the spread of grasslands [8,9]. Conversely, exogenous abrasive particles on vegetation could have driven convergent evolution towards hypsodonty in South America [10,11]. Such examples feed the debate about whether dental microwear textures and tooth wear as a whole carry a dietary or an environmental signal [12–14].
During mastication, repeated tooth-on-tooth contact (attrition) yields dental facets and tooth-on-food contact (abrasion) tends to wear them [15]. Tooth wear varies with the way upper and lower teeth occlude and reduce foods depending on the mechanical properties of the dietary bolus [16,17], and thus has been used to infer diets of extinct mammals [18,19]. A recent study [14] confirms that isolated silica phytoliths can produce striations on the enamel surface during mastication [20,21]. These results place the plant defences hypothesis [22–26] at the forefront of the debate as selective pressure towards dental innovations such as enamel thickening, tooth lengthening or hypsodonty [27–29]. Apart from food mechanical properties and bio-silica in plant tissues [30], soil and windblown mineral particles deposited on food found in arid habitats can also contribute to tooth wear [12,31]. Dental microwear may combine both biotic and abiotic signals that palaeobiologists have to untangle. The debate is deepened by the paucity of experimental studies on large mammals [32,33] to characterize the factors at the root of tooth wear aetiology on one hand and by the lack of data regarding the amount of dust deposited on vegetation on the other [34]. By reducing the dietary breadth, controlled-food trials allow for a direct quantification of the effect of each item type separately (i.e. browse versus graze) supplemented or not with a load of exogenous particles.
Here, large-scale controlled-food trials build a unique model with which to untangle environmental from dietary signals. We simulate the effects of differences in diet and the presence of dust (simulating the Harmattan windblown dust) in food on tooth enamel after a 70-day controlled-food trial conducted on 40 ewes raised in a covered sheepfold. Differences in dental abrasion were evaluated through dental microwear textural analysis [16,35]. This is a suitable method for a month-scale study, as opposed to molar mesowear [15] or occlusal wear gradient [36], which reflect tooth wear at a longer time scale.
2. Material and methods
The controlled-food trials were carried out at the Mourier farm (Haute-Vienne, France; agreement number B-87-176-01), under the supervision of the Centre Interrégional d'Information et de Recherche en Production Ovine (CIIRPO) and the Institut de l'Elevage (Idele). G.M. and D.G., who have official approval to carry out such procedures, designed these trials [37]. They were performed on domestic sheep (Ovis aries), using only ewes (females) from the Vendéen breed. All experiments were conducted on cull ewes (meaning sheep no longer suitable for breeding) and were sold for meat. None of the ewes were put down solely for the purpose of the controlled-food trials. None of the trials required the sheep to be handled, pricked or anaesthetized. Sheep had full access to foods with which they were familiar.
All of these ewes spent three months together in the very same grass-dominated pasture before the experiment started. Given that dental microwear is known to reflect the last few days or weeks of the dietary habits [38,39], it was assumed that their dental microwear signatures prior to beginning the controlled-food trials reflected a homogeneous grazing signal. The ewes were kept inside a covered sheepfold and fed from 15 July to 2 October 2014. The trial started with a 5-day period of adaptation to the diet: over the 5 days, the proportion of clover (or grasses) was gradually increased. The sheep were not kept on hay, which they would have eaten, but on dust-free wood shavings. Feeding troughs were covered with a plastic film and cleaned out daily to avoid contamination. None of the ewes lost weight during the experiments.
Forty sheep were included in this study, divided into four groups of 10. Two groups were fed on a red clover-dominated silage and the other two groups were fed on a multi-specific assemblage highly dominated by grasses. Silica phytolith and cellulose contents expressed as percentages of dry matter weight for each fodder, as well as toughness of red clover and of a set of grasses measured on fresh plants, are given in electronic supplementary material, tables S1 and S2 (see also electronic supplementary material, figure S1). The ewes had full access to the food. Ewes were given approximately 1.650 kg (dry matter weight) of clover fodder and approximately 1.550 kg (dry matter weight) of grass fodder per day per ewe. These amounts were defined by giving large amounts of fodder and measuring how much the ewes had consumed in 24 h.
Every day, a load of dust was added to the fodder of one of the 10-ewe samples per diet category (electronic supplementary material, table S1). Fodder and dust were placed in large troughs, which were cleaned daily. For several days, the remaining dust was gathered and measured. This showed that more than 90% of the dust load was ingested by the ewes. The quantity and the properties of the dust used in the controlled-food testing follow the study of Breuning-Madsen & Awadzi [34]. To our knowledge, this is the only study in inter-tropical latitudes quantifying the dust deposits on vegetation. It was conducted in Ghana and aimed at quantifying such deposits due to the Harmattan winds blowing from the Sahara from November to March. One month of dust accumulation represents on average 3.3 g m−2, an average calculated from three consecutive years. Ten ewes forage on approximately 40 m2 a day. Consequently, the food was laden with 132 g of dust per 10-ewe sample to simulate the amount of dust deposited by the Harmattan on a meadow in 30 days. To meet the conditions met in the Harmattan windblown dust in Ghana, dust load was sieved to retain only grains below 100 µm, and was composed of 72–74% quartz grains and 18–20% Mg-feldspaths.
The dental microwear textural analyses were performed using the Scale-Sensitive Fractal Analysis with Toothfrax and Sfrax software (Surfract, www.surfract.com) following Scott et al. [35]. For each specimen, a surface of 200 × 200 µm (1550 × 1550 points; figure 1) is scanned with a Leica DCM8 (Leica Microsystems; figure 1). Abnormal peaks were automatically erased with a batch algorithm computed on ImageJ software based on mathematical morphological tools (electronic supplementary material, figure S2). Photosimulations of all of the 40 surfaces analysed in this study are shown in electronic supplementary material, figure S3, and individual textural parameters are given in electronic supplementary material, table S3. Four microwear variables are used in this study (table 1). All variables have been described in further detail by Scott et al. [35]. Complexity (area-scale fractal complexity, Asfc) is a measure of the roughness at a given scale. Anisotropy (epLsar or exact proportion of length-scale anisotropy of relief) measures the orientation concentration of surface roughness. Textural fill volume (Tfv) is the result of an algorithm that fills a surface with square cuboids of different volumes. Tfv does not depend on the surface shape but on its finer texture. Heterogeneity of complexity (heterogeneity of area-scale fractal complexity, HAsfc) quantifies the variation of complexity observed within scan. HAsfc is calculated in each surface through 81 cells.
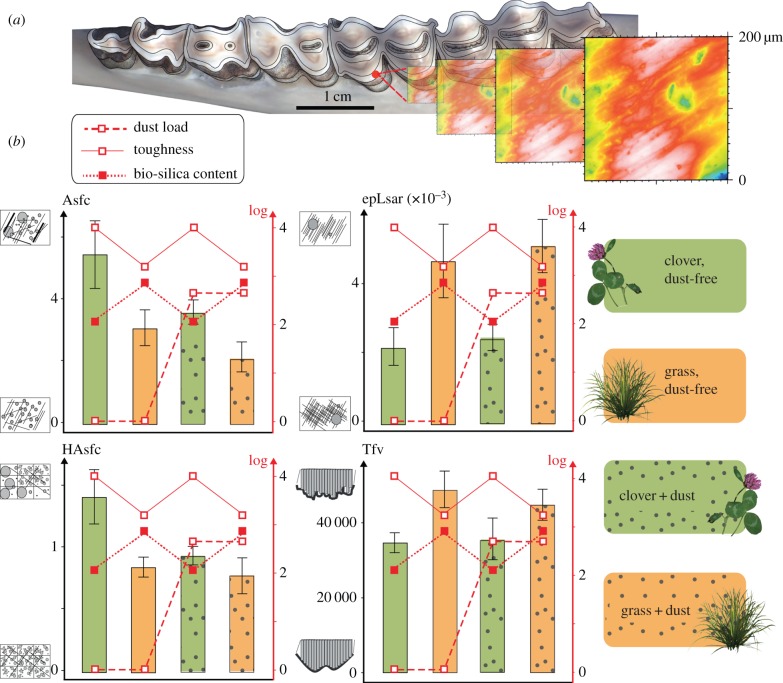
Figure 1.
From teeth to dental microwear textural parameters. (a) Mandible of a sheep with a two-dimensional image with false colours to represent the topography. Scans were made on the shearing facet of the protoconid. (b) Barplots of the mean and standard error of the mean of the four parameters. Asfc: complexity, epLsar: anisotropy, Tfv: textural fill volume, HAsfc: heterogeneity. Food and exogenous material properties (bio-silica content, toughness of clover and grass stems, and dust load; log-transformed) are drawn in red lines.
Table 1.
Summary statistics of textural parameters. Mean (m), standard deviation (s.d.) and standard error of the mean (s.e.m.) of the four dental microwear textural parameters (Asfc, epLsar, HAsfc and Tfv) and the wear textural index (WTI) for the four samples of ewes (clover-fed, grass-fed, dust-laden and dust-free groups).
| samples | clover-fed |
grass-fed |
|||||
|---|---|---|---|---|---|---|---|
| all | dust-free | dust | all | dust-free | dust | ||
| N | 20 | 10 | 10 | 20 | 10 | 10 | |
| Asfc | m | 4.499 | 5.429 | 3.569 | 2.590 | 3.064 | 2.115 |
| s.d. | 2.712 | 3.471 | 1.252 | 1.716 | 1.836 | 1.531 | |
| s.e.m. | 0.607 | 1.098 | 0.396 | 0.384 | 0.58 | 0.484 | |
| epLsar (×10−3) | m | 2.299 | 2.170 | 2.427 | 4.875 | 4.658 | 5.092 |
| s.d. | 1.466 | 1.745 | 1.207 | 2.889 | 3.392 | 2.451 | |
| s.e.m. | 0.328 | 0.552 | 0.382 | 0.646 | 1.073 | 0.775 | |
| HAsfc | m | 1.173 | 1.413 | 0.935 | 0.807 | 0.842 | 0.774 |
| s.d. | 0.562 | 0.697 | 0.235 | 0.361 | 0.253 | 0.458 | |
| s.e.m. | 0.125 | 0.22 | 0.074 | 0.081 | 0.080 | 0.145 | |
| Tfv | m | 35178.6 | 34674.9 | 35682.2 | 46856.6 | 48878.6 | 44834.6 |
| s.d. | 13404.7 | 8438.2 | 17537.7 | 14129.1 | 15503.1 | 13115.4 | |
| s.e.m. | 2997.4 | 2668.4 | 5545.9 | 3159.4 | 4902.5 | 4147.5 | |
| WTI | m | −0.802 | −1.313 | −0.293 | 0.803 | 0.650 | 0.955 |
| s.d. | 1.205 | 1.483 | 0.537 | 1.020 | 1.097 | 0.972 | |
| s.e.m. | 0.269 | 0.469 | 0.170 | 0.228 | 0.347 | 0.307 | |
As textural parameters violated conditions for parametric tests, they were rank-transformed before each analysis [40,41]. Two-way factorial ANOVAs (with diet and dust load as factors) with post hoc tests for each parameter were used to determine the sources of significant variation. Jackknife resampling techniques were also used as a further investigation into the solidity of our results. All possible ‘leave-one-out’ combinations of samples were generated and used to test null hypotheses. Combining all of the parameters into a set of linear combinations assisted dietary classification. A principal components analysis was performed on the four textural parameters and the 40 ewes without an a priori classification. The first component of the analysis carries 46.9% of the variation seen in the total sample (electronic supplementary material, table S5). One-way ANOVA highlights significant differences in coordinates only along PC1 between the different ewe samples (electronic supplementary material, table S5). Accordingly, coordinates along the first component are taken to form the wear textural index (WTI).
3. Results
Iterations of two-way ANOVAs through jackknife resampling procedures on textural parameters between the four 10-ewe samples do not detect any significant effects of dust, nor interaction between dust and diet: only diet is significant. This suggests diet is the main factor controlling dental abrasion (tables 1 and 2a, figure 1b). The ewes fed on clover fodders have higher complexity (Asfc) and heterogeneity (HAsfc) and lower anisotropy (epLsar) than the ewes fed on grass fodders (table 1 and figure 1b). This supports, as indicated by empirical data [42], the premise that grazing on monocotyledonous herbs and browsing on dicotyledonous plants, and even forbs from the herbaceous layer, generates contrasted signatures in dental abrasion, with less complex and heterogeneous and more anisotropic textures for grazers. It is worth mentioning here that the present ewe dataset shows an unexpected pattern for the textural fill volume (Tfv) when compared with earlier studies [42]. Grass-fed ewes have higher Tfv than clover-fed ones (table 1 and figure 1b). Among all textural parameters, anisotropy (epLsar) shows the strongest coupling with the amount of bio-silica in the diet. A similar coupling can be seen with Tfv (figure 1b). Complexity (Asfc) and heterogeneity (HAsfc) show a coupling with dust load for the two clover-fed ewe samples, which show less complex and less heterogeneous textures (figure 1b). The same trends, although less pronounced, are seen when the grass-fed ewes are considered. Clover- and grass-fed ewes differ from each other with different sets of parameters according to the presence or absence of dust. This unbalanced shift highlights the difficulty to extract a dietary signal when isolated textural parameters are considered.
Table 2.
Multivariate statistics and post hoc test results. Two-way ANOVAs with a Jackknife resampling procedure (a) on the four parameters and (b) on the wear textural index. (c) Post hoc tests result from the jackknife resampling procedure (electronic supplementary material, methods). Percentages represent the frequency of significant difference (p-value < 0.05). Above the diagonal: Tukey's honest significant difference test. Below the diagonal: Fisher's least significant difference test. d.f., degrees of freedom; SS, sum of squares; MS, mean of squares.
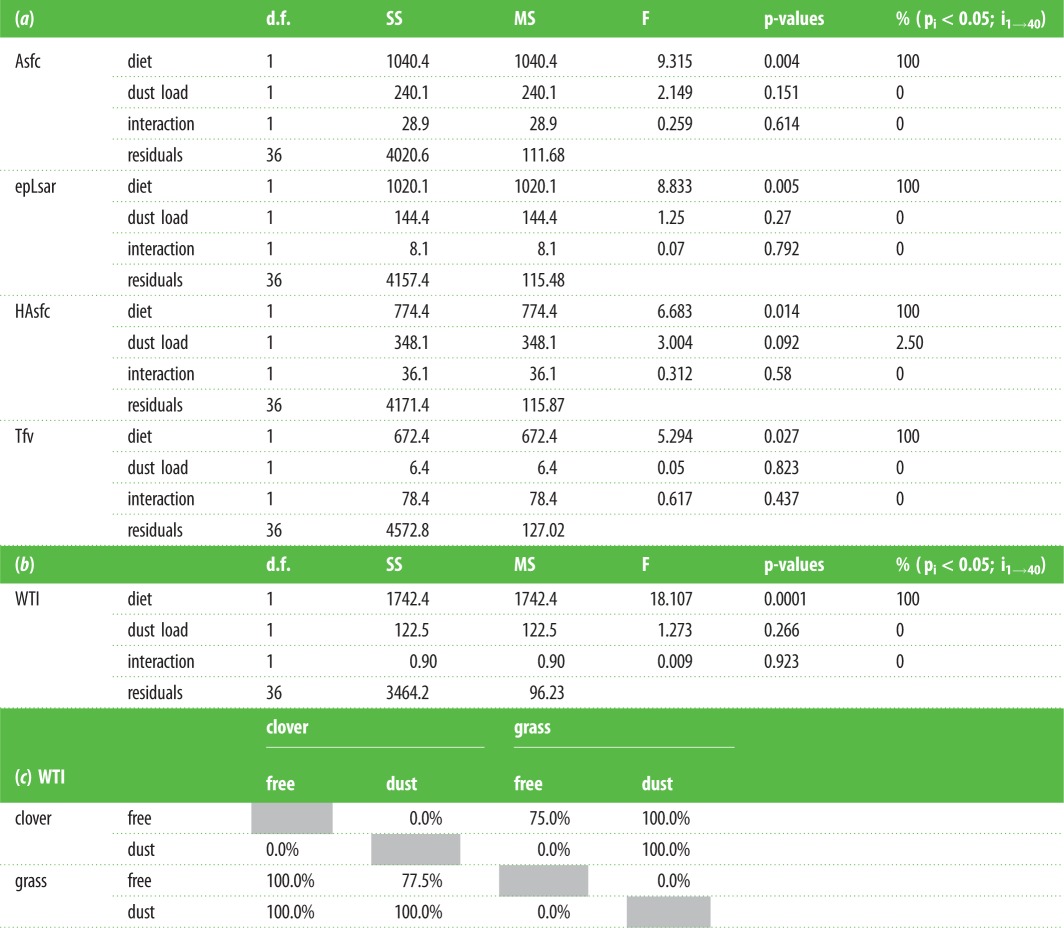 |
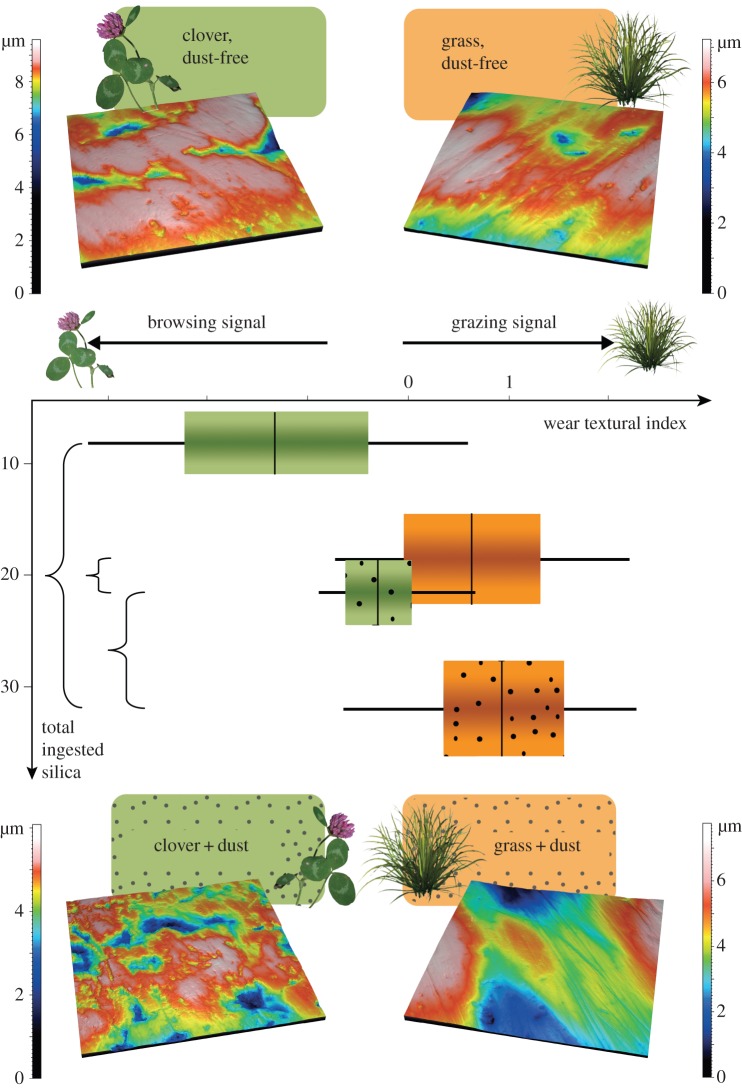
A principal components analysis combining the four textural parameters was carried out on the four ewe samples to generate a new set of variables emphasizing variance among individuals without a priori assignation. The four components, PC1 to PC4, represent 46.89, 31.68, 14.68 and 6.74% of the variance, respectively (figure 2; electronic supplementary material, methods). Iterations of two-way ANOVAs on PC1 to PC3 through jackknife resampling procedures do not detect any significant effects of dust, nor interaction between dust and diet; only diet is significant in PC1. Thus, we refer to the individual scores along the PC1 as the WTI, effectively combining the four textural parameters (table 2b and figure 2). When looking at iterations through jackknife resampling procedures on the combination of the conservative HSD test (Tukey's honest significant differences) and the less conservative LSD test (Fisher's least significant differences), both samples of ewes fed on grass fodders differ from sheep fed on dust-free clover fodder, and on dust-laden clover fodder to a lesser extent (meaning only the less conservative test is significant; table 2c and figure 2). Even though ewes fed dust-laden clover fodder ingested more silica particles than the sheep fed on dust-free grass fodder, the latter sample displays a significantly higher WTI in most of the iterations when LSD tests are considered. Besides, there is no significant difference between the two samples of ewes fed on grasses although one of them ingested an exogenous silica complement (table 2c and figure 2).
Figure 2.
Diagram combining wear textural index (WTI) and total ingested silica index (combining bio-silica content and dust load). Boxes represent the mean and the confidence interval of the mean (at 95%), and whiskers correspond to minimum and maximum WTI values. Braces pinpoint significant differences between samples. Three-dimensional surface renderings of the dental microwear textures shown here are the ones having the WTI the closest to the mean of the sample to which they belong.
4. Discussion
Although exogenous particles probably contribute to tooth reduction by loss of enamel and dentine matter, dust supplements, simulating natural conditions met in inter-tropical savannahs today, do not overwhelm the dietary signal carried by the dental microwear textures. The contrasted grazing and browsing signatures are maintained when the food is laden with dust. Consequently, this implies that differences in bio-silica contents embedded in food tissue and in food mechanical properties such as hardness and toughness, as well as the way mastication operates, produce these differences in dental abrasion. Combining isolated textural parameters into a single linear combination through multivariate analyses ensures better discrimination between browsers and grazers, and is inescapable in order to extract the biotic signal carried by the dental microwear textures. Our results based on controlled-food trials reinforce the reliability of dental abrasion as a dietary proxy. The dental microwear textural analysis tracks differences in dietary habits in environments free from dust and rich in airborne dust. However, one may argue that higher amounts of ingested exogenous particles than measured in natural conditions such as overgrazed pastures by livestock [3] might shift the browsing signals further towards the ones seen for grazing ruminants in dust-free habitats. This would not be an issue when looking at niche partitioning among mammals from the same locality, as they would be uniformly affected by similar amounts of airborne dust. Similar results have been found on wild sympatric rodents inhabiting arid windblown environments in South Africa [43]. The issue could then arise when comparing different localities affected by different amounts of airborne dust. For example, can we still discriminate between grazers from a dust-free grassland and browsers from a dust-laden bushland? Our results suggest that the persistence of the dietary signal would allow for relatively reliable dietary reconstructions. This persistent presence of the dietary signal in dental microwear textures despite simulating dust-covered vegetation supports the preponderant role in dental abrasion of (preferred or fallback) challenging foods (i.e. foods that are hard, tough and/or rich in silica phytoliths). Hence, food can be assumed to be one of the key factors promoting dental innovations such as molar hypsodonty in ruminants [6,27], molar lengthening in suids [29] or enamel thickening in hominids during the Neogene [28,44]. Among those, robust australopithecines have received a lot of attention over the last few years [13]. Initially assumed to be exclusively a ‘nut cracker’ [45,46], the differences in molar microwear textures between species and populations were later interpreted as reflecting diverse feeding habits. This makes it difficult to identify precisely which foods promote enamel thickness in Paranthropus. Nuts apart, silica-bearing and tough foods also have to be considered [45,46]. Another hypothesis explains microwear texture differences by the dust-rich environments [12] in which Paranthropus lived, and thus considers exogenous particles as driving natural selection [3]. Our controlled-food trials emphasize the prevalence of a dietary signal over an exogenous particle signal at the microscopic level, substantiating the importance of biotic factors, even at a seasonal scale, in driving the natural selection of dental innovations.
5. Conclusion
Both dust and silica phytoliths have been shown to contribute to reducing tooth volume during chewing. By combining the longest and the largest published controlled-food trials on domestic sheep to date with three-dimensional dental microwear textural analysis, we assessed the way and the extent to which dust contributes to dental microwear textures compared with differences in diet. Dental microwear textures reflect not an environmental signal but a dietary one. Indeed, we show first that differences in dental microwear textures reflect dietary differences. We also show that the presence of dust on food items simulating natural conditions met in Western Africa, where the Harmattan winds carry dust from the Sahara to regions with high primary productivity, did not significantly overwhelm the dental microwear textural signal. Our study makes it clear that the intrinsic properties of ingested foods play a more critical role than dust in driving the natural selection of dental innovations.
Supplementary Material
Acknowledgements
We thank the staff from the Mourier Station, J.-M. Grolleau (Limovin), F. Vannier (CIIRPO) and J. Merceron (Institut de l'Elevage), as well as G. Reynaud, G. Florent, J. Surault and S. Riffaut (iPHEP). We are grateful to I. Calandra, F. Guy, T. Lehmann, the three reviewers and the editors for their helpful comments.
Ethics
This study was conducted at the Ferme du Mourier (agreement number B-87-176-01). None of the controlled food trials required the sheep to be handled, pricked or anaesthetized. Sheep had full access to foods with which they were familiar. None of the cull ewes were put down solely for the purpose of the controlled food trials.
Data accessibility
The supporting individual data used in the study as well as the scan photosimulations of studied ewes are given in the electronic supplementary material, table S3 and figure S3, respectively.
Authors' contributions
G.M., C.B., J.-R.B. designed the research; G.M. and D.G. designed the animal experiments; G.M. and A.R. analysed dental microwear texture data; G.M., A.R., N.B. and A.F. explored alternative statistic tools, A.N. analysed plant residues after mineralization and acid attacks; G.M., A.R. and X.M. analysed food mechanical properties; G.M. and D.P. developed algorithms to erase abnormal peaks from raw surfaces; G.M. and A.R. wrote the manuscript with the contributions from all co-authors.
Competing interests
We declare we have no competing interests.
Funding
The Project TRIDENT was funded by the French National Research Agency, ANR (ANR-13-JSV7-0008-01; PI: G.M.).
References
- 1.Tegen I, Lacis AA, Fung I. 1996. The influence on climate forcing of mineral aerosols from disturbed soils. Nature 380, 419–422. ( 10.1038/380419a0) [DOI] [Google Scholar]
- 2.O'Hara SL, Clarke ML, Elatrash MS. 2006. Field measurements of desert dust deposition in Libya. Atmos. Environ. 40, 3881–3897. ( 10.1016/j.atmosenv.2006.02.020) [DOI] [Google Scholar]
- 3.Madden RH. 2014. Hypsodonty in mammals: evolution, geomorphology and the role of earth surface processes. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 4.Edwards EJ, et al. 2010. The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328, 587–591. ( 10.1126/science.1177216) [DOI] [PubMed] [Google Scholar]
- 5.Cerling TE, Harris JR, MacFadden BJ, Leakey MG, Quade J, Eisenmann V, Ehleringer JR. 1997. Global vegetation change through the Miocene/Pliocene boundary. Nature 389, 153–158. ( 10.1038/38229) [DOI] [Google Scholar]
- 6.Jernvall J, Fortelius M. 2002. Common mammals drive the evolutionary increase of hypsodonty in the Neogene. Nature 417, 538–540. ( 10.1038/417538a) [DOI] [PubMed] [Google Scholar]
- 7.Mendoza M, Palmqvist P. 2008. Hypsodonty in ungulates: an adaptation for grass consumption or for foraging in open habitat? J. Zool. 274, 134–142. ( 10.1111/j.1469-7998.2007.00365.x) [DOI] [Google Scholar]
- 8.Strömberg CAE. 2011. Evolution of grasses and grassland ecosystems. Annu. Rev. Earth Planet. Sci. 39, 517–544. ( 10.1146/annurev-earth-040809-152402) [DOI] [Google Scholar]
- 9.Mihlbachler MC, Rivals F, Solounias N, Semprebon GM. 2011. Dietary change and evolution of horses in North America. Science 331, 1178–1181. ( 10.1126/science.1196166) [DOI] [PubMed] [Google Scholar]
- 10.Strömberg CAE, Dunn RE, Madden RH, Kohn MJ, Carlini AA. 2013. Decoupling the spread of grasslands from the evolution of grazer-type herbivores in South America. Nat. Commun. 4, 1478 ( 10.1038/ncomms2508) [DOI] [PubMed] [Google Scholar]
- 11.Dunn RE, Strömberg CAE, Madden RH, Kohn MJ, Carlini AA. 2015. Linked canopy, climate, and faunal change in the Cenozoic of Patagonia. Science 347, 258–261. ( 10.1126/science.1260947) [DOI] [PubMed] [Google Scholar]
- 12.Lucas PW, et al. 2013. Mechanisms and causes of wear in tooth enamel: implications for hominin diets. J. R. Soc. Interface 10, 20120923 ( 10.1098/rsif.2012.0923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood B. 2013. Palaeontology: gritting their teeth. Nature 493, 486–487. ( 10.1038/493486a) [DOI] [PubMed] [Google Scholar]
- 14.Xia J, Zheng J, Huang D, Tian ZR, Chen L, Zhou Z, Ungar PS, Qian L. 2015. New model to explain tooth wear with implications for microwear formation and diet reconstruction. Proc. Natl Acad. Sci. USA 112, 10 669–10 672. ( 10.1073/pnas.1509491112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaiser TM, Müller DWH, Fortelius M, Schulz E, Codron D, Clauss M. 2013. Hypsodonty and tooth facet development in relation to diet and habitat in herbivorous ungulates: implications for understanding tooth wear. Mamm. Rev. 43, 34–46. ( 10.1111/j.1365-2907.2011.00203.x) [DOI] [Google Scholar]
- 16.Scott RS, Bergstrom TS, Brown CA, Grine FE, Teaford MF, Walker A, Ungar PS. 2005. Dental microwear texture analysis reflects diets of living primates and fossil hominins. Nature 436, 693–695. ( 10.1038/nature03822) [DOI] [PubMed] [Google Scholar]
- 17.Schulz E, Calandra I, Kaiser TM. 2013. Feeding ecology and chewing mechanics in hoofed mammals: 3D tribology of enamel wear. Wear 300, 169–179. ( 10.1016/j.wear.2013.01.115) [DOI] [Google Scholar]
- 18.Calandra I, Merceron G. 2016. Dental microwear texture analysis in mammalian ecology. Mamm. Rev. 46, 215–228. ( 10.1111/mam.12063) [DOI] [Google Scholar]
- 19.DeSantis LRG. 2016. Dental microwear textures: reconstructing diets of fossil mammals. Surf. Topogr. Metrol. Prop. 4, 23002 ( 10.1088/2051-672X/4/2/023002) [DOI] [Google Scholar]
- 20.Ciochon RL, Piperno DR, Thompson GR. 1990. Opal phytoliths found on the teeth of the extinct ape Gigantopithecus blacki: implications for paleodietary studies. Proc. Natl Acad. Sci. USA 87, 8120–8124. ( 10.1073/pnas.87.20.8120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gügel IL, Grupe G, Kunzelmann K-H. 2001. Simulation of dental microwear : characteristic traces by opals phytoliths give clues to ancient human dietary behavior. Am. J. Phys. Anthropol. 114, 124–138. ( 10.1002/1096-8644(200102)114:2%3C124::AID-AJPA1012%3E3.0.CO;2-S) [DOI] [PubMed] [Google Scholar]
- 22.Massey FP, Hartley SE. 2006. Experimental demonstration of the antiherbivore effects of silica in grasses: impacts on foliage digestibility and vole growth rates. Proc. R. Soc. B 273, 2299–2304. ( 10.1098/rspb.2006.3586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNaughton SJ, Tarrants JL. 1983. Grass leaf silicification: natural selection for an inducible defense against herbivores. Proc. Natl Acad. Sci. USA 80, 790–791. ( 10.1073/pnas.80.3.790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucas PW, Turner IM, Dominy NJ, Yamashita N. 2000. Mechanical defences to herbivory. Ann. Bot. 86, 913–920. ( 10.1006/anbo.2000.1261) [DOI] [Google Scholar]
- 25.Calandra I, Zub K, Szafrańska PA, Zalewski A, Merceron G. 2016. Silicon-based plant defences, tooth wear and voles. J. Exp. Biol. 219, 501–507. ( 10.1242/jeb.134890) [DOI] [PubMed] [Google Scholar]
- 26.Vicari M, Bazely DR. 1993. Do grasses fight back? The case for antiherbivore defences. Trends Ecol. Evol. 8, 137–141. ( 10.1016/0169-5347(93)90026-L) [DOI] [PubMed] [Google Scholar]
- 27.Damuth J, Janis C. 2011. On the relationship between hypsodonty and feeding ecology in ungulate mammals, and its utility in palaeoecology. Biol. Rev. 86, 733–758. ( 10.1111/j.1469-185X.2011.00176.x) [DOI] [PubMed] [Google Scholar]
- 28.Guy F, Lazzari V, Gilissen E, Thiery G. 2015. To what extent is primate second molar enamel occlusal morphology shaped by the enamel-dentine junction? PLoS ONE 10, e0138802 ( 10.1371/journal.pone.0138802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris JM, Cerling TE. 2006. Dietary adaptations of extant and Neogene African suids. J. Zool. 256, 45–54. ( 10.1017/S0952836902000067) [DOI] [Google Scholar]
- 30.Baker G, Jones LHP, Wardrop ID. 1959. Cause of wear in sheeps’ teeth. Nature 184, 1583–1584. ( 10.1038/1841583b0) [DOI] [PubMed] [Google Scholar]
- 31.Sanson GD, Kerr SA, Gross KA. 2007. Do silica phytoliths really wear mammalian teeth? J. Archaeol. Sci. 34, 526–531. ( 10.1016/j.jas.2006.06.009) [DOI] [Google Scholar]
- 32.Hoffman JM, Fraser D, Clementz MT. 2015. Controlled feeding trials with ungulates: a new application of in vivo dental molding to assess the abrasive factors of microwear. J. Exp. Biol. 218, 1538–1547. ( 10.1242/jeb.118406) [DOI] [PubMed] [Google Scholar]
- 33.Mainland IL. 1998. Dental microwear and diet in domestic sheep (Ovis aries) and goats (Capra hircus): distinguishing grazing and fodder-fed ovicaprids using a quantitative analytic approach. J. Archaeol. Sci. 25, 1259–1271. ( 10.1006/jasc.1998.0301) [DOI] [Google Scholar]
- 34.Breuning-Madsen H, Awadzi TW. 2005. Harmattan dust deposition and particle size in Ghana. CATENA 63, 23–38. ( 10.1016/j.catena.2005.04.001) [DOI] [Google Scholar]
- 35.Scott RS, Ungar P, Bergstrom TS, Brown CA, Childs BE, Teaford MF, Walker A. 2006. Dental microwear texture analysis: technical considerations. J. Hum. Evol. 51, 339–349. ( 10.1016/j.jhevol.2006.04.006) [DOI] [PubMed] [Google Scholar]
- 36.Galbany J, Romero A, Mayo-Alesón M, Itsoma F, Gamarra B, Pérez-Pérez A, Willaume E, Kappeler PM, Charpentier MJE. 2014. Age-related tooth wear differs between forest and savanna primates. PLoS ONE 9, e94938 ( 10.1371/journal.pone.0094938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.2012. Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 83, 301–309. ( 10.1016/j.anbehav.2011.10.031) [DOI] [PubMed] [Google Scholar]
- 38.Teaford MF, Oyen OJ. 1989. In vivo and in vitro turnover in dental microwear. Am. J. Phys. Anthropol. 80, 447–460. ( 10.1002/ajpa.1330800405) [DOI] [PubMed] [Google Scholar]
- 39.Merceron G, Escarguel G, Angibault J-M, Verheyden-Tixier H. 2010. Can dental microwear textures record dietary inter-individual dietary variations? Public Libr. Sci. ONE 5, e9542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conover WJ, Iman RL. 1981. Rank transformations as a bridge between parametric and nonparametric statistics. Am. Stat. 35, 124–129. [Google Scholar]
- 41.Sokal SR, Rohlf FJ. 1969. Biometry. New York, NY: WE Freeman and Company. [Google Scholar]
- 42.Scott JR. 2012. Dental microwear texture analysis of extant African Bovidae. Mammalia 76, 157–174. ( 10.1515/mammalia-2011-0083) [DOI] [Google Scholar]
- 43.Burgman JH, Leichliter J, Avenant NL, Ungar PS. 2016. Dental microwear of sympatric rodent species sampled across habitats in southern Africa: implications for environmental influence. Integr. Zool. 11, 111–127. ( 10.1111/1749-4877.12188) [DOI] [PubMed] [Google Scholar]
- 44.Lucas PW, Constantino P, Wood B, Lawn B. 2008. Dental enamel as a dietary indicator in mammals. Bioessays 30, 374–385. ( 10.1002/bies.20729) [DOI] [PubMed] [Google Scholar]
- 45.Ungar PS, Grine FE, Teaford MF. 2008. Dental microwear and diet of the Plio-Pleistocene hominin Paranthropus boisei. PLoS ONE 3, e2044 ( 10.1371/journal.pone.0002044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Constantino PJ, Borrero-Lopez O, Pajares A, Lawn BR. 2015. Simulation of enamel wear for reconstruction of diet and feeding behavior in fossil animals: a micromechanics approach. Bioessays 38, 89–99. ( 10.1002/bies.201500094) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The supporting individual data used in the study as well as the scan photosimulations of studied ewes are given in the electronic supplementary material, table S3 and figure S3, respectively.