Abstract
Food restriction (FR) and refeeding (Re) have been suggested to impair body mass regulation and thereby making it easier to regain the lost weight and develop over-weight when FR ends. However, it is unclear if this is the case in small mammals showing seasonal forging behaviors. In the present study, energy budget, body fat and serum leptin level were measured in striped hamsters that were exposed to FR-Re. The effects of leptin on food intake, body fat and genes expressions of several hypothalamus neuropeptides were determined. Body mass, fat content and serum leptin level decreased during FR and then increased during Re. Leptin supplement significantly attenuated the increase in food intake during Re, decreased genes expressions of neuropepetide Y (NPY) and agouti-related protein (AgRP) of hypothalamus and leptin of white adipose tissue (WAT). Hormone-sensitive lipase (HSL) gene expression of WAT increased in leptin-treated hamsters that were fed ad libitum, but decreased in FR-Re hamsters. This indicates that the adaptive regulation of WAT HSL gene expression may be involved in the mobilization of fat storage during Re, which partly contributes to the resistance to FR-Re-induced overweight. Leptin may be involved in the down regulations of hypothalamus orexigenic peptides gene expression and consequently plays a crucial role in controlling food intake when FR ends.
Keywords: Body mass, Food restriction, Hypothalamus neuropeptides, Leptin, Striped hamster
Abbreviations: AgRP: agouti-related protein; CART: cocaine- and amphetamine-regulated transcript; DEI: digestive energy intake; FR-Re: food restriction and refeeding; GEI: gross energy intake; HSL: hormone-sensitive lipase (EC 3.1.1.3); NPY: neuropeptide Y; Ob-Rb: long form of the leptin receptor; POMC: pro-opiomelanocortin; WAT: white adipose tissue.
Leptin, the production of the ob gene, is mainly expressed in adipose tissue and plays important roles in the regulation of both energy intake and expenditure (Fernández-Galaz et al, 2002; Friedman & Halaas, 1998; Friedman, 2011; Zhang et al, 1994). Serum leptin level is found to reduce in animals under food restriction (FR) and then to increase following ad libitum refeeding (Re) (Gutman et al, 2006; Wisse et al, 1999; Zhang & Wang, 2008). In rodents and even humans, the fall in serum leptin is considered to be an important signal for the switch between fed and fasted states, allowing leptin to function both as starvation and satiety signals (Ahima et al, 1996; Ahima, 2005; Kastin & Pan, 2000). Thus, leptin may become a possible candidate that is involved in the regulation of energy budget in animals subjected to FR and Re. Leptin gene expression is proportioned to the size of adipose tissue (Frederich et al, 1995). Strong positive correlations have been observed between leptin mRNA expression and plasma leptin levels and total body fat (Ahima et al, 2000; Frederich et al, 1995; Maffei et al, 1995). Hormone-sensitive lipase (HSL; EC 3.1.1.3), an enzyme responsible for the release of free fatty acids (FFA) from stored triacylglycerols (TG) in adipose tissues (Fielding & Frayn, 1998), has been proposed to play an essential role in the regulation of body weight and fat mass (Fortier et al, 2005; Harada et al, 2003). HSL is active, and the esterification pathway is not activated in the fasted state; while in the fed state, HSL is suppressed and esterification is stimulated (Fielding & Frayn, 1998; Frayn et al, 1995). So far, it is unclear if it is involved in the TG mobilization in wild animals showing the resistance to FR-Re-induced over-weight.
Previous studies suggested that the effect of leptin on energy balance is mediated mainly by neuronal targets in the hypothalamus via its long isoform receptor (Ob-Rb) (Ahima et al, 2000). Hypothalamic neuropeptides involved in leptin action are classified into two major groups: orexigenic peptides, including neuropeptide Y (NPY) and agouti-related protein (AgRP), and anorexigenic peptides, e.g. pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) (Ahima et al, 2000). Food deprivation and/or restriction were found to increase synthesis of NPY and AgRP and to decrease production of POMC and CART in the arcuate nucleus of the hypothalamus in laboratory mice and rats (Brady et al, 1990), as well as in Siberian hamsters (Phodopus sungorus, Dailey & Bartness, 2010; Mercer et al, 2000). Re induced a decrease of NPY gene expression in hypothalamus (Sucajtys-Szulc et al, 2009). Thus, it suggests that hypothalamus orexigenic or anorexigenic peptides, or both, may be involved in the regulation of energy balance in animals experiencing FR and Re.
The striped hamster (Cricetulus barabensis) is a major rodent in northern China and is also distributed in Russia, Mongolia, and Korea (Zhang & Wang, 1998). This species feeds mainly on stems and leaves of plant during summer and on foraging crop seeds in winter (Song & Wang, 2002, 2003; Zhang & Wang, 1998). Thus, they must experience great seasonal fluctuations in food quality and availability (Zhang & Wang, 1998). Unlike other wild rodents, such as Djungarian hamsters (Phodopus sungorus) (Klingenspor et al, 2000; Mercer, 1998), Brandt's voles (Lasiopodomys brandtii, Li & Wang, 2005a) and Mongolian gerbils (Meriones unguiculatus) (Li & Wang, 2005b), striped hamsters do not show significant changes in body mass after being maintained in an outside enclosure for over a year (Zhao ZJ, unpublished data). We previously found a significant decrease in body mass in stochastic FR hamsters, which was not followed by a body mass regaining after Re (Zhao & Cao, 2009). Thus, striped hamster may become a potential model suitable for studying the resistance to FR-Re-induced overweight. In the present study, energy budget, body fat and serum leptin were measured in striped hamsters subjected to FR and Re. The effect of leptin administration on energy budget and hypothalamus NPY/AgRP, POMC/CART and Ob-Rb as well as subcutaneous white adipose tissue (WAT) leptin and HSL genes expression were examined during the period of FR-Re. We hypothesized that striped hamsters would decrease energy expenditure to cope with the lower food intake during the period of FR, but increase food intake when food was plentiful and consequently increase the accumulation of body fat, showing a significant regaining of body mass. Leptin might be involved in controlling energy intake associated with the regulation of hypothalamus genes expression when FR ends and consequently played a crucial role in the resistance to over-weight. If leptin is a hormone that prevents post-fast hyperphagia, it would be expected that plasma leptin level would increase significantly in the hamsters following ad libitum Re.
MATERIALS AND METHODS
Animals and experimental design
Striped hamsters were obtained from an out-breeding colony started with animals that were initially trapped in 2009 from farmland at the center of Hebei Province (E115℃13', S38℃12'), North China Plain. Hamsters were singly housed in plastic cages (29 cm ×18 cm ×16 cm) with fresh saw dust bedding at 21±1 ℃ with a 12 h: 12 h light: dark cycle (lights on at 08: 00). Food (standard rodent chow; Beijing KeAo Feed Co., Beijing, China) and water were provided ad libitum. Adult male hamsters, 4−6 months old, were used in the present study. Baseline measurements of body mass and food intake were carried out on a daily basis before experiment started. All animal protocols were in compliance with the Animal Care and Use Committee of College of Life and Environmental Science, Wenzhou University.
Experiment 1: To examine the effect of Re on food intake, body mass and fat mass, 24 hamsters were assigned randomly into three groups: (1) control group (n=8) that animals were fed ad libitum for 7 weeks; (2) FR-1w-Re group (n=8) that hamsters were restricted to 85% of initial food intake for 1 week and refed ad libitum for 6 weeks; and (3) FR-3w-Re group (n=8), during which hamsters were restricted to 85% of initial food intake for 3 weeks followed by ad libitum Re for 4 weeks. Based on the data collected from the hamsters subjected to different extents of food restriction from 60%, 70% to 90% (Zhao, 2012), the hamsters in the present study were restricted to 85% of ad libitum food intake in both FR-1w-Re and FR-3w-Re groups.
Experiment 2 was designed to examine the role of leptin in the regulations of body mass and energy intake (Figure 1), Twenty-four hamsters were randomly assigned into one of the four groups (n=6 in each group): Ad-PBS and Ad-leptin group in which hamsters were fed ad libitum and treated with PBS and leptin, respectively, and FR-PBS and FR-leptin group in which FR hamsters were treated with PBS and leptin, respectively. The changes in body mass and food intake were measured daily for 8 days (from day 0 to day 7), during which all hamsters in the four groups were fed ad libitum. Animals were fed ad libitum for other 10 days in two Ad groups (from day 8 to day 17). On day 8, hamsters in two FR groups were restricted to 85% of initial food intake for 8 days (from day 8 to day 14), and then refed ad libitum for other 3 days (from day 15 to day 17). On day 10, hamster was anesthetized with isoflurane and implanted subcutaneously on the dorsal side with a miniosmotic pump (Alzet model 1007D; capacity, 100 μL; release rate, 0.5 μL/h; duration, 7 days; Durect, Cupertino, CA, USA) containing either recombinant murine leptin (100 μg dissolved in 100 μL phosphate-buffered saline (PBS; Peprotech, USA) or PBS. Changes in food intake and body mass were measured daily according to the method mentioned in experiment 1.
Figure 1.
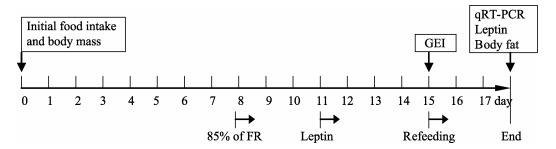
Timeline showing days of food restriction and measurements taken for striped hamsters
Food intake and body mass
Food intake was calculated as the mass of food missing from the hopper every day, subtracting orts mixed in the bedding. The initial food intake was calculated as the average of daily food intake over 8 days during the period of the baseline measurements. Hamsters in two experimental groups were provided with 85% of ad libitum food intake during the period of FR. Food intake and body mass were measured daily throughout the experiment.
Body fat content
Animals were euthanized by decapitation between 0900 and 1100 h at the end of FR-Re. Body fat content was measured as described previously (Zhao et al, 2010). Briefly, the gastrointestinal tracts and liver, heart, lung, spleen, pancreas and kidneys were separated and removed. The remaining carcass was weighed to determine wet mass, dried in an oven at 60 ℃ for 10 days to a constant mass, and then weighed again to determine dry mass. Total body fat was extracted from the dried carcass by ether extraction in a Soxhlet apparatus.
Energy budget
Gross energy intake (GEI), digestive energy intake (DEI) and digestibility were measured over the last three days (day 15 to day 17) of the experiment. In detail, food was provided quantitatively, and the spillage of food mixed with bedding (if it was observed) and feces were collected from each cage over the 3 days. The spillage of food and feces were sorted and separated manually after they were dried at 60 ℃ to constant mass. Gross energy contents of the diet and feces were determined using a Parr 1281 oxygen bomb calorimeter (Parr Instrument, Moline, IL, USA). GEI, DEI and digestibility were calculated as follows (Liu et al, 2003; Zhao et al, 2013a):
GEI (kJ/d)=food intake (g/d)×dry matter content of the diet (%)×energy content of food (kJ/g);
DEI (kJ/d)=GEI−(dry mass of feces (g/d)×energy content of feces (kJ/g));
Digestibility (%)=DEI/GEI×100%.
Serum leptin level and body fat content
Animals were euthanized by decapitation at the end of the experiment. Hypothalamus and WAT were collected quickly and stored at liquid nitrogen for Real-time RT-QPCR analysis. Trunk blood was collected for serum leptin measurements. Serum leptin level was quantified by radio-immunoassay (RIA) using the Linco 125I Multi-species Kit (Cat. No. XL-85K, Linco Research Inc.), following the standard kit instructions. The lower and upper limits of the assay kit were 1 and 50 ng/mL, and the inter- and intra-assay variations were <3.6% and 8.7%, respectively. Body fat content of dry carcass was measured as described in experiment 1.
Real-time RT-QPCR analysis
Total RNA was isolated from frozen hypothalamus and WAT using the TRIzol method (TaKaRa, China) according to the manufacturer's protocol. RNA concentration and purity were determined by A260 and A280 optical density (OD) measurements and A260/ A280 ratio determination using a Spectrophotometer. First-strand cDNA synthesis was performed on 2 μg of total RNA with AMV Reverse Transcriptase (TAKARA, China) using random primer Oligo (dT)18. The reverse transcription reaction (2 μL) was used as a template for the subsequent PCR reaction using gene-specific primers (Table 1). Primers were designed using laboratory mouse and rat NCBI RefSeq sequence entry data that was submitted to the Primer 5 software. The qPCR reaction setup and plate preparation were standardized and carried out according to standard operating protocols in our lab. The reaction consisted of SYBR Premix EX Tag TM (2×), 12.5 μL; 3' primers (10 μmol/L), 0.5 μL; 5' primers (10 μmol/L), 0.5 μL; ROX Reference Dye II (50×), 5 μL and template cDNA, 2 μL. qPCR was performed using SYBR Green Master Mix in Mx3000P Real-Time QPCR system (Stratagene, La Jolla, CA, USA) under user-defined thermal cycling conditions (95 ℃, 30 s; and 40 cycles at 95 ℃ for 5 s, 55 ℃ for 30 s and 72 ℃ for 30 s), and the reaction finished by the built in melt curve. All samples were quantified for relative quantity of gene expression by using actin expression as an internal standard.
Table 1.
Gene-specific primer sequences used for Real-time RT-QPCR analysis
| Gene | Primers (5'-3') | Size (bp) |
| NPY (forward) | ACCCTCGCTCTGTCCCTG | 186 |
| NPY (reverse) | AATCAGTGTCTCAGGGCTA | |
| AgRP (forward) | TGTTCCCAGAGTTCCCAGGTC | 227 |
| AgRP (reverse) | ATTGAAGAAGCGGCAGTAGCAC | |
| POMC (forward) | GGTGGGCAAGAAGCGACG | 205 |
| POMC (reverse) | CTTGTCCTTGGGCGGGCT | |
| CART (forward) | TACCTTTGCTGGGTGCCG | 260 |
| CART (reverse) | AAGTTCCTCGGGGACAGT | |
| Ob-Rb (forward) | CAGTGTCGATACAGCTTGGA | 200 |
| Ob-Rb (reverse) | TTGCATATTTAACTGAGGGT | |
| Leptin (forward) | AACCCTCATCAAGACCATT | 240 |
| Leptin (reverse) | GCCAGCAGATGGAGAAGG | |
| HSL (forward) | CACTACAAACGCAACGAG | 224 |
| HSL (reverse) | CTGAGCAGGCGGCTTACC | |
| Actin (forward) | AAAGACCTCTATGCCAACA | 196 |
| Actin (reverse) | ACATCTGCTGGAAGGTGG |
NPY: neuropeptide; Y; AgRP, agouti-related protein; POMC: pro-opiomelanocortin; CART; cocaine- and amphetamine-regulated transcript; Ob-Rb: long form of the leptin receptor; HSL: hormone-sensitive lipase (EC 3.1.1.3).
Statistics
Data were analyzed using SPSS 13.0 statistic software. Experiment 1, the differences in body mass, food intake, carcass mass and body fat mass and content between Control, FR-1w-Re and FR-3w-Re groups were analyzed using one-way ANOVA. Experiment 2, body mass and food intake throughout the experiment were examined using RM-ANOVA. Differences in body mass, food intake and genes expression of hypothalamus neuropeptides, and leptin and HSL of WAT were examined using two-way ANOVA (FR×leptin), followed by Tukey's HSD post-hoc tests where required. Data are presented as means±SE, with significance set at P < 0.05.
RESULTS
Experiment 1: Body mass change and food intake
There were no differences in body mass and food intake between Control, FR-1w-Re and FR-3w-Re groups prior to the experiment (body mass: F2, 21=0.01, P > 0.05; food intake: F2, 21=0.10, P > 0.05, Figure 2A). Body mass decreased significantly during FR periods compared with that of baseline measurements on day 0 to day 7 (post hoc, P < 0.05) and increased during the period of Re (post hoc, P < 0.05). Both FR-1w and FR-3w hamsters showed lower body masses than controls (day 7: F2, 21=49.07, P < 0.01; day 21: F2, 21=34.79, P < 0.01) and then returned to the levels of controls shortly after they were refed (day 29: F2, 21=3.11, P > 0.05, day 49: F2, 21=0.35, P > 0.05). Food intake was lower in FR hamsters than that of controls (day 2: F2, 21=7.27, P < 0.05, Figure 2B), and was increased shortly in Re hamsters (day 8: F2, 21=11.78, P < 0.05, day 22: F2, 21=4.30, P < 0.05). No difference in food intake was observed between the three groups on day 23 and thereafter (day 23: F2, 21=1.24, P > 0.05; day 49: F2, 21=0.92, P > 0.05, Figure 2B).
Figure 2.
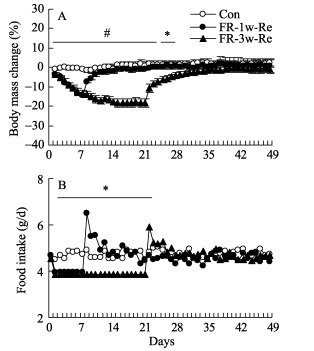
Body mass change (A) and food intake (B) in striped hamsters restricted to 85% of initial food intake for 1 and 3 weeks and then refed ad libitum (FR-1w-Re and FR-3w-Re) *
Carcass mass and fat content
Wet and dry masses of carcass were not different between Control, FR-1w-Re and FR-3w-Re groups (Table 2). FR-1w-Re and FR-3w-Re hamsters had similar body fat mass to control hamsters (Table 2). Fat content was not affected by FR-Re, and no difference was observed between the three groups (Table 2).
Table 2.
Carcass mass and fat content in striped hamsters subjected to food restriction and refeeding (FR-Re)
| Control | FR-1w-Re | FR-3w-Re | F | P | |
| 8 | 8 | 8 | |||
| Body mass (g) | 34.7±1.6 | 34.0±1.2 | 33.9±1.3 | 0.12 | NS |
| Carcass | |||||
| Wet mass (g) | 26.5±1.4 | 25.5±1.0 | 25.8±1.1 | 0.19 | NS |
| Dry mass (g) | 9.2±0.6 | 9.1±0.6 | 9.2±0.5 | 0.01 | NS |
| Fat mass (g) | 2.9±0.4 | 3.0±0.3 | 2.9±0.3 | 0.01 | NS |
| Fat content (%) | 30.9±2.5 | 32.0±1.9 | 31.3±1.9 | 0.06 | NS |
NS: non-significant between the three groups (P > 0.05).
Experiment 2: Body mass
There was no difference in body mass between the four groups prior to the experiment and during the period of baseline measurements (day 7: FR-Re, F1, 20=3.52, P > 0.05; leptin: F1, 20=0.14, P > 0.05, Figure 3A). FR resulted in a significant reduction in body mass in FR hamsters compared with Ad hamsters (day 10, FR-Re: F1, 20=50.62, P < 0.01). Leptin supplement had no impact on body mass changes in either FR or Ad hamsters during the period of FR (day 11: F1, 20=1.90, P > 0.05, day 14: F1, 20=1.99, P > 0.05). After hamsters were refed ad libitum, body mass significantly increased in FR-Re-PBS group (day 14-17: F3, 15=4.83, P < 0.05), and failed to increase in FR-Re-leptin group (day 14-17: F3, 15= 0.74, P > 0.05). On day 17, body mass changes were -1.5±0.4%, -7.0±0.7%, -4.3±0.9% and -8.9±1.7% in Ad-PBS, Ad-leptin, FR-Re-PBS and FR-Re-leptin groups (FR-Re: F1, 20=5.11, P < 0.01; leptin: F1, 20=22.80, P < 0.01, Figure 3A).
Figure 3.
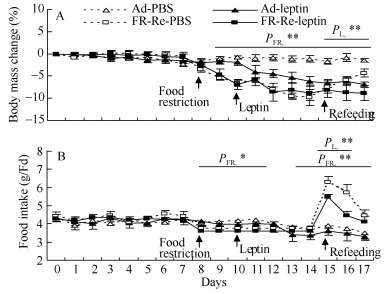
Effects of leptin administration on body mass change (A) and food intake (B) in striped hamsters subjected to FR-Re
Food intake, DEI and digestibility
Food intake averaged 4.4±0.2, 4.3±0.2, 4.4±0.2 and 4.3±0.2 g/d in Ad-PBS, Ad-leptin, FR-Re-PBS and FR-Re-leptin groups at the end of baseline measurements (day 7, FR-Re: F1, 20=0.21, P > 0.05; leptin: F1, 20=0.15, P > 0.05, Figure 3B), respectively. Ad hamsters had significantly higher food intake than FR hamster during the FR period (day 8: F1, 20=5.68, P < 0.05), while it was not affected by leptin supplement (day 11: F1, 20=0.51, P > 0.05). After being refed ad libitum, hamsters in FR-Re-PBS and FR-Re-leptin groups consumed 6.3±0.3 and 5.5±0.1 g/d, respectively, which were higher by 60.8% and 36.6% than Ad hamsters (day 15: F1, 20=90.36, P < 0.01). Leptin supplement imposed a significant effect on food intake during Re period (day 15: F1, 20=6.48, P < 0.05, day 16: F1, 20=5.58, P < 0.05), by which food intake is lower in FR-Re-leptin group than that in FR-Re-PBS group (day 15: post hoc, P < 0.05; day 16: post hoc, P < 0.05, Figure 3B). During the period of Re, GEI and DEI were increased by 67% and 65% in FR-Re-PBS group, and 35% and 36% in FR-Re-leptin group, respectively, compared with that in Ad controls (Table 3). There was no effect of FR-Re and leptin supplement on digestibility (Table 3).
Table 3.
Effects of leptin administration on gross energy intake (GEI), digestibility, fat content and serum leptin level in striped hamsters subjected to FR-Re
| Ad-PBS | Ad-leptin | FR-Re-PBS | FR-Re-leptin | PFR | PLeptin | |
| GEI (kJ/d) | 60.4±3.0c | 61.2±5.3c | 101.0±5.4a | 81.6±1.1b | ** | * |
| DEI (kJ/d) | 49.7±2.6c | 50.1±4.2c | 82.2±5.0a | 67.6±1.4b | ** | NS |
| Digestibility (%) | 82.3±0.5 | 82.1±2.6 | 81.3±1.8 | 82.8±0.6 | NS | NS |
| Body mass (g) | 35.1±1.9 | 33.3±1.6 | 35.4±1.6 | 32.9±1.5 | NS | NS |
| Carcass | ||||||
| Wet mass (g) | 26.2±1.4 | 24.6±1.3 | 25.3±1.6 | 24.2±1.3 | NS | NS |
| Dry mass (g) | 9.7±0.8 | 8.1±0.5 | 8.9±0.7 | 8.1±0.9 | NS | NS |
| Fat content (%) | 35.7±4.3 | 21.8±2.0 | 29.3±2.6 | 21.9±7.3 | NS | * |
| Serum leptin (ng/mL) | 3.48±0.80c | 5.63±1.01b | 3.00±0.10c | 12.43±1.99a | * | ** |
DEI: digestive energy intake; Ad-PBS or Ad-leptin: hamsters that were fed ad libitum and treated with PBS or leptin; FR-Re-PBS or FR-Re-leptin: food-restricted hamsters that were treated with PBS or leptin and refed ad libitum; PFR *: significant effect of FR; PFR **: P < 0.01; PLeptin *: significant effect of leptin manipulation (P < 0.05); PLeptin **: P < 0.01; NS: nonsignificance. Different letters (a, b or c) on the same row indicate significant difference (P < 0.05).
Body fat content and serum leptin level
Body fat content was significantly affected by leptin supplement, by which leptin administration to Ad hamsters decreased by 39% in fat content (Table 3). Fat content was lower by 25% in FR-Re-leptin group than that in FR-Re-PBS group. The effect of FR-Re on body fat content was not significant (Table 3). Serum leptin level was significantly affected by both FR-Re and leptin supplement, by which leptin administration to either Ad or FR-Re hamsters induced significant increase in leptin level (Table 3).
Hypothalamus NPY/AgRP, POMC/CART and Ob-Rb genes expression
Hypothalamus NPY and AgRP genes expression were significantly affected by FR-Re, by which the hamsters in FR-Re-PBS group showed 48% higher NPY and 50% higher AgRP genes expression compared with those in Ad-PBS group (NPY: F1, 28=10.59, P < 0.01; AgRP: F1, 28=12.37, P < 0.01, Figure 4A, B). Leptin supplement led to 28% and 40% lower NPY and AgRP genes expression in Ad hamsters, and 22% and 21% lower NPY and AgRP for FR-Re hamsters, respectively, whereas the effect of leptin supplement was not statistically significant (NPY: F1, 28=1.81, P=0.19; AgRP: F1, 28=2.44, P=0.13). POMC, CART and Ob-Rb genes expression were not affected by either FR-Re (POMC: F1, 28=3.22, P > 0.05; CART: F1, 28=2.48, P > 0.05; Ob-Rb: F1, 28=0.65, P > 0.05) or leptin supplement (POMC: F1, 28=0.50, P > 0.05, post hoc, P > 0.05, Figure 4C; CART: F1, 28=0.39, P > 0.05, post hoc, P > 0.05, Figure 4D; Ob-Rb: F1, 28=1.44, P > 0.05, post hoc, P > 0.05, Figure 4E).
Figure 4.
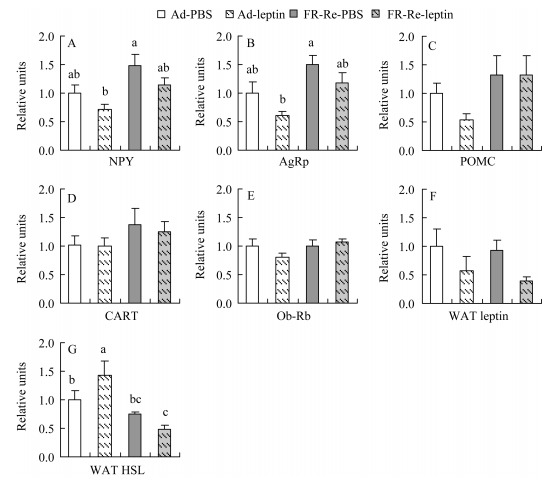
Effects of leptin administration on hypothalamus genes expression of NPY (A), AgRP (B), POMC (C), CART (D), Ob-Rb (E) and WAT leptin (F) and HSL (G) in striped hamsters subjected to FR-Re
WAT leptin and HSL genes expression
Leptin supplement led to a significant decrease in WAT leptin gene expression; it decreased by 42% in Ad hamsters and by 57% in FR-Re hamsters compared with their counterparts that treated with PBS (F1, 28=4.75, P < 0.05). No effect of FR-Re on WAT leptin gene expression was observed (F1, 28=0.33, P > 0.05, Figure 4F). WAT HSL gene expression was affected by FR-Re (F1, 28= 14.69, P < 0.01), but not impacted by leptin supplement (F1, 28=0.23, P > 0.05). HSL gene expression in ad-leptin group was significantly higher than that in other three groups (post hoc, P < 0.05, Figure 4G).
DISCUSSION
In the present study, striped hamsters showed weight losses during food restriction and regaining of the lost weight during ad libitum Re. This is inconsistent with the previous studies that have been performed on many other rodents under food restriction and/or Re (Ferguson et al, 2007; Gutman et al, 2006; Marinković et al, 2007; Rozen et al, 1994; Zhang & Wang, 2008; Zhao & Cao, 2009; Zhao et al, 2013b). Rozen et al (1994) claimed that the discrepancies were independent of age, strain, or sex, but perhaps a matter of diet, as well as the energy budget of the animals and the duration of Re period.
Animals usually show hyperphagia when food restriction ends (Brownlow et al, 1993; Cameron & Speakman, 2011; Clinthorne et al, 2010; Harris et al, 1986; Speakman & Mitchell, 2011; Zhao & Cao, 2009). In the present study, energy intake was also significantly increased in striped hamsters during Re. This can explain that the rapid regaining of the lost weight is likely due to notable elevation in energy intake. This is consistent with the study performed on the same strain of hamsters under stochastic FR-Re (Zhao & Cao, 2009). In addition, we found that the increase in food intake was short, which occurred only on the first several days during Re and then fell rapidly to the levels of ad libitum controls. This suggests that the short elevation of food intake contributes to the rapid regaining of the lost weight only, resulting in a failure of accumulating more fat deposits than controls during long term of Re. Inconsistently, hyperphagia continued throughout Re period in Siberian hamsters, resulting in a seasonally appropriate body mass (Archer et al, 2007). This result suggests that the control of food intake may play a crucial role in the resistance to overweight or obesity in striped hamsters.
We observed that leptin supplement had a significant effect on food intake during the period of Re, by which energy intake was lowered by 19% (P < 0.05) in hamsters treated with leptin than controls treated with PBS (101.0 kJ/d and 81.6 kJ/d, respectively). Similarly, Wittert et al (2004) found that an increase in body mass was attenuated in leptin-treated rats compared with PBS-treated counterparts. Leptin administration to food-restricted rats reduced food intake and prevented regaining of the lost body mass (Fernández-Galaz et al, 2002). These results indicate that leptin is likely involved in controlling energy intake when food restriction ends and consequently plays a crucial role in the resistance to obesity induced by FR-Re.
In the present study, changes in serum leptin level paralleled changes in fat content, implying that the mobilization of fat storage is likely changed in striped hamsters in response to FR-Re. We observed that WAT leptin and HSL genes expression were unchanged in hamsters following ad libitum Re. In the same strain of hamster, food deprivation resulted in down-regulation of leptin gene expression and up-regulation of HSL gene expression, which both returned back to the levels of controls after Re (Zhao, unpublished data). In laboratory rodents, fasting decreased leptin gene expression, whereas subsequent Re increased leptin gene expression (Saladin et al, 1995). These results suggest that both leptin and HSL are likely involved in the regulation of body weight and fat mass in response to changes in food availability (Fortier et al, 2005; Harada et al, 2003). WAT HSL gene expression is probably being stimulated in fasted state, and then the mobilization of fat storage is being enhanced, consequently providing energy to animals in negative energy balance. When food is plentiful, HSL gene expression may be suppressed in the fed state and esterification may be stimulated, resulting in the accumulation of fat, which would increase the capacity to cope with the next periods of food shortage (Fielding & Frayn, 1998; Frayn et al, 1995). Consequently, we could conclude that the adaptive regulation of WAT HSL gene expression may be involved in the mobilization of fat storage in wild animals showing resistance to FR-Re-induced overweight.
In the present study, we observed that hypothalamus NPY and AgRP genes expression were increased by 48% and 50% in FR-Re hamsters, respectively, compared with their counterparts fed ad libitum. It has been previously observed that the expression levels of NPY and AgRP increase in the arcuate nucleus in food-restricted or -deprived mice (Hahn et al, 1998; Mizuno & Mobbs, 1999) and rats (Bi et al, 2003; Brady et al, 1990; Rijke et al, 2005; Sucajtys-Szulc et al, 2008, 2009). For striped hamsters, food deprivation also induced significant increases in hypothalamus NPY and AgRP genes expression (Zhao, unpublished data). Here, both orexigenic peptides genes expression were up-regulated in FR hamsters. However, the genes expression of hypothalamus anorexigenic peptides, e.g. POMC and CART, were unchanged in FR-Re striped hamster. This may indicate that the effect of food availability on POMC and CART expression is less consistent with that in other studies, within which those genes expressions are unchanged or decreased in food restricted or -deprived laboratory rodents (Bi et al, 2003; Brady et al, 1990; Rijke et al, 2005). These results suggest that hypothalamus NPY and AgRP genes expression, rather than POMC and CART, may be more likely involved in the energy balance and body weight regulations in animals experiencing the changes in food availability.
Leptin supplement led to 28% and 40% of down-regulations of hypothalamus NPY and AgRP genes expression in hamsters fed ad libitum. In addition, no effects of leptin supplement on POMC, CART and Ob-Rb genes expression were observed. Inconsistently, leptin administration to laboratory rodents resulted in significant down-regulations of hypothalamus NPY and AgRP genes expression and/or up-regulations of POMC and CART expression (Ahima et al, 2000; Friedman & Halaas, 1998; Kristensen et al, 1998; Stephens et al, 1995). The reasons for these inconsistencies are unclear, which may reflect a species-specific response to leptin supplement. Additionally, we observed that leptin supplement decreased WAT leptin gene expression by 42% in hamsters fed ad libitum and by 57% in FR-Re animals compared with their counterparts treated with PBS. Leptin administration to the hamsters fed ad libitum induced a significant up-regulation of WAT HSL gene expression. Similarly, leptin administration increased HSL mRNA expression but decreased leptin gene expression in laboratory mice (Zhang et al, 2008) and rats (Scarpace et al, 1998). HSL is classically considered to be the enzyme catalyzing the rate-limiting step of the mobilization of stored triacylglycerol (lipolysis) (Lucas et al, 2003). It has been previously proposed that in rats the reduction in fat mass is most likely due to the increased HSL mRNA (Kristensen et al, 1998). In the striped hamsters in the current study, we did not found any changes in energy intake following leptin treatment, but observed a significant decrease in body fat content, which was paralleled with the up-regulation of HSL gene expression, indicating that the mobilization of fat was enhanced in leptin-treated hamsters. This suggests that effects of leptin may be partly mediated via WAT HSL gene expression and consequently play an essential role in the regulations of body weight and fat mass.
The resistance to overweight of body mass may suggest a special strategy in adaption to ambient environment. In general, rodents with large size and big fat are easier to be killed by the predators than the small and lean rodents. Data from the present study may indicate that striped hamsters showing resistance to overweight decrease their predation risk and consequently increase survival rate. However, more evidence is necessary to support this assumption.
In summary, body mass and fat content were decreased in striped hamsters during FR and then increased during Re. FR-Re induced a regaining of lost weight, but did not result in overweight relative to ad libitum controls. During Re, striped hamsters showed a short stage of hyperphagia, which likely contributed to the regaining of body mass. Serum leptin level was significantly decreased during FR and increased during Re. Leptin supplement attenuated the increase in food intake during Re. Hypothalamus NPY and AgRP genes expression, rather than POMC and CART, may be more likely involved in the regulations of energy balance and body weight in animals experiencing the changes in food availability. Leptin may be involved in the down-regulations of hypothalamus orexigenic peptides genes expression and consequently plays a crucial role in controlling food intake when FR ends.
Funding Statement
This work was supported by the National Natural Science Foundation of China (31270458, 31070367) and partly supported by the grant from Zhejiang Province (pd2013374)
REFERENCES
- 1. Ahima RS. 2005. Central actions of adipocyte hormones. TRENDS in Endocrinology and Metabolism, 16 (7): 307- 313. [DOI] [PubMed] [Google Scholar]
- 2. Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. 1996. Role of leptin in the neuroendocrine response to fasting. Nature, 382 (6588): 250- 252. [DOI] [PubMed] [Google Scholar]
- 3. Ahima RS, Saper CB, Flier JS, Elmquist JK. 2000. Leptin regulation of neuroendocrine systems. Frontiers in Neuroendocrinology, 21 (2): 263- 307. [DOI] [PubMed] [Google Scholar]
- 4. Archer ZA, Moar KM, Logie TJ, Reilly L, Stevens V, Morgan PJ, Mercer JG. 2007. Hypothalamic neuropeptide gene expression during recovery from food restriction superimposed on short-day photoperiod-induced weight loss in the Siberian hamster. American Journal of Physiology-Regulatory Integrative and Comparative Physiology, 293 (3): 1094- 1101. [DOI] [PubMed] [Google Scholar]
- 5. Bi S, Robinson BM, Moran TH. 2003. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. American Journal of Physiology-Regulatory Integrative and Comparative Physiology, 285 (5): 1030- 1036. [DOI] [PubMed] [Google Scholar]
- 6. Brady LS, Smith MA, Gold PW, Herkenham M. 1990. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology, 52 (5): 441- 447. [DOI] [PubMed] [Google Scholar]
- 7. Brownlow BS, Park CR, Schwartz RS, Woods SC. 1993. Effect of meal pattern during food restriction on body weight loss and recovery after refeeding. Physiology & Behavior, 53 (3): 421- 424. [DOI] [PubMed] [Google Scholar]
- 8. Cameron KM, Speakman JR. 2011. Reduction of dietary energy density reduces body mass regain following energy restriction in female mice. Journal of Nutrition, 141 (2): 182- 188. [DOI] [PubMed] [Google Scholar]
- 9. Clinthorne JF, Adams DJ, Fenton JI, Ritz BW, Gardner EM. 2010. Short-term re-feeding of previously energy-restricted C57BL/6 male mice restores body weight and body fat and attenuates the decline in natural killer cell function after primary influenza infection. Journal of Nutrition, 140 (8): 1495- 1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dailey MJ, Bartness TJ. 2010. Arcuate nucleus destruction does not block food deprivation induced increases in food foraging and hoarding. Brain Research, 1323 94- 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferguson M, Sohal BH, Forster MJ, Sohal RS. 2007. Effect of long-term caloric restriction on oxygen consumption and body temperature in two different strains of mice. Mechanisms of Ageing and Development, 128 (10): 539- 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernández-Galaz C, Fernández-Agulló T, Pérez C, Peralta S, Arribas C, Andrés A, Carrascosa JM, Ros M. 2002. Long-term food restriction prevents ageing-associated central leptin resistance in wistar rats. Diabetologia, 45 (7): 997- 1003. [DOI] [PubMed] [Google Scholar]
- 13. Fielding BA, Frayn KN. 1998. Lipoprotein lipase and the disposition of dietary fatty acids. British Journal of Nutrition, 80 (6): 495- 502. [DOI] [PubMed] [Google Scholar]
- 14. Fortier M, Soni K, Laurin N, Wang SP, Mauriège P, Jirik FR, Mitchell GA. 2005. Human hormone-sensitive lipase (HSL): expression in white fat corrects the white adipose phenotype of HSL-deficient mice. Journal of Lipid Research, 46 (9): 1860- 1867. [DOI] [PubMed] [Google Scholar]
- 15. Frayn KN, Coppack SW, Fielding BA, Humphreys SM. 1995. Coordinated regulation of hormone-sensitive lipase and lipoprotein lipase in human adipose tissue in vivo: implications for the control of fat storage and fat mobilization. Advances in Enzyme Regulation, 35 163- 178. [DOI] [PubMed] [Google Scholar]
- 16. Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. 1995. Leptin levels reflect body lipid content in mice: Evidence for diet-induced resistance to leptin action. Nature Medicine, 1 (12): 1311- 1314. [DOI] [PubMed] [Google Scholar]
- 17. Friedman JM. 2011. Leptin and the regulation of body weight. Keio Journal of Medicine, 60 (1): 1- 9. [DOI] [PubMed] [Google Scholar]
- 18. Friedman JM, Halaas JL. 1998. Leptin and the regulation of body weight in mammals. Nature, 395 (6704): 763- 770. [DOI] [PubMed] [Google Scholar]
- 19. Gutman R, Choshniak I, Kronfeld-Schor N. 2006. Defending body mass during food restriction in Acomys russatus: a desert rodent that does not store food. American Journal of Physiology-Regulatory Integrative and Comparative Physiology, 290 (4): 881- 891. [DOI] [PubMed] [Google Scholar]
- 20. Hahn TM, Breininger JF, Baskin DG, Schwartz MW. 1998. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nature Neuroscience, 1 (4): 271- 272. [DOI] [PubMed] [Google Scholar]
- 21. Harada K, Shen WJ, Patel S, Natu V, Wang J, Osuga J, Ishibashi S, Kraemer FB. 2003. Resistance to high-fat diet-induced obesity and altered expression of adipose-specific genes in HSL-deficient mice. American Journal of Physiology-Endocrinology and Metabolism, 285 (6): 1182- 1195. [DOI] [PubMed] [Google Scholar]
- 22. Harris RBS, Kasser TR, Martin RJ. 1986. Dynamics of recovery of body composition after overfeeding, food restriction or starvation of mature female rats. Journal of Nutrition, 116 (12): 2536- 2546. [DOI] [PubMed] [Google Scholar]
- 23. Kastin AJ, Pan W. 2000. Dynamic regulation of leptin entry into blood-brain barrier. Regulatory Peptides, 92 (1-3): 37- 43. [DOI] [PubMed] [Google Scholar]
- 24. Klingenspor M, Niggemann H, Heldmaier G. 2000. Modulation of leptin sensitivity by short photoperiod acclimation in the Djungaran hamster, Phodopus sungorus. Journal of Comparative Physiology B-Biochemical Systemic and Environmental, 170 (1): 37- 43. [DOI] [PubMed] [Google Scholar]
- 25. Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S. 1998. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature, 393 (6680): 72- 76. [DOI] [PubMed] [Google Scholar]
- 26. Li XS, Wang DH. 2005. a. Regulation of body weight and thermogenesis in seasonally acclimatized Brandt's voles (Microtus brandti). Hormones and Behavior, 48 (3): 321- 328. [DOI] [PubMed] [Google Scholar]
- 27. Li XS, Wang DH. 2005. b. Seasonal adjustments in body mass and thermogenesis in Mongolian gerbils (Meriones unguiculatus): the roles of short photoperiod and cold. Journal of Comparative Physiology B-Biochemical Systemic and Environmental, 175 (8): B593- B600. [DOI] [PubMed] [Google Scholar]
- 28. Liu H, Wang DH, Wang ZW. 2003. Energy requirements during reproduction in female Brandt's voles (Microtus brandti). Journal of Mammalogy, 84 1410- 1416. [Google Scholar]
- 29. Lucas S, Tavernier G, Tiraby C, Mairal A, Langin D. 2003. Expression of human hormone-sensitive lipase in white adipose tissue of transgenic mice increases lipase activity but does not enhance in vitro lipolysis. Journal of Lipid Research, 44 (1): 154- 163. [DOI] [PubMed] [Google Scholar]
- 30. Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM. 1995. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nature Medicine, 1 (11): 1155- 1161. [DOI] [PubMed] [Google Scholar]
- 31. Marinković P, Pesić V, Loncarević N, Smiljanić K, Kanazir S, Ruzdijić S. 2007. Behavioral and biochemical effects of various food-restriction regimens in the rats. Physiology & Behavior, 92 (3): 492- 499. [DOI] [PubMed] [Google Scholar]
- 32. Mercer JG. 1998. Regulation of appetite and body weight in seasonal mammals. Comparative Biochemistry and Physiology C-Toxicology & Pharmacology, 119 (3): C295- 303. [DOI] [PubMed] [Google Scholar]
- 33. Mercer JG, Moar KM, Ross AW, Hoggard N, Morgan PJ. 2000. Photoperiod regulates arcuate nucleus POMC, AGRP, and leptin receptor mRNA in Siberian hamster hypothalamus. American Journal of Physiology-Regulatory Integrative and Comparative Physiology, 278 (1): 271- 281. [DOI] [PubMed] [Google Scholar]
- 34. Mizuno TM, Mobbs CV. 1999. Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology, 140 (2): 814- 817. [DOI] [PubMed] [Google Scholar]
- 35. Rijke CE de, Hillebrand JJG, Verhagen LAW, Roeling TAP, Adan RAH. 2005. Hypothalamic neuropeptide expression following chronic food restriction in sedentary and wheel-running rats. Journal of Molecular Endocrinology, 35 (2): 381- 390. [DOI] [PubMed] [Google Scholar]
- 36. Rozen R, Brigant L, Apfelbaum M. 1994. Effects of cycles of food restriction followed by ad libitum refeeding on body composition and energy expenditure in obsess rats. American Journal of Clinical Nutrition, 59 (3): 560- 565. [DOI] [PubMed] [Google Scholar]
- 37. Saladin R, De Vos P, Guerre-Millo M, Leturque A, Girard J, Staels B, Auwerz J. 1995. Transient increase in obese gene expression after food intake or insulin administration. Nature, 377 (6549): 527- 529. [DOI] [PubMed] [Google Scholar]
- 38. Scarpace PJ, Nicolson M, Matheny M. 1998. UCP2, UCP3 and leptin gene expression: modulation by food restriction and leptin. Journal of Endocrinology, 159 (2): 349- 357. [DOI] [PubMed] [Google Scholar]
- 39. Song ZG, Wang DH. 2002. The maximum metabolizable energy intake and the relationship with basal metabolic rate in the striped hamster Cricetulus barabensis. Acta Theriologica, 47 417- 423. [Google Scholar]
- 40. Song ZG, Wang DH. 2003. Metabolism and thermoregulation in the striped hamster Cricetulus barabensis. Journal of Thermal Biology, 28 (6-7): 509- 514. [Google Scholar]
- 41. Speakman JR, Mitchell SE. 2011. Caloric restriction. Molecular Aspects of Medicine, 32 (3): 159- 221. [DOI] [PubMed] [Google Scholar]
- 42. Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A, Mackellar W, Rosteck PR Jr, Schoner B, Smith D, Tinsley FC, Zhang XY, Heiman M. 1995. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature, 377 (6549): 530- 532. [DOI] [PubMed] [Google Scholar]
- 43. Sucajtys-Szulc E, Goyke E, Korczynska J, Stelmanska E, Rutkowski B, Swierczynski J. 2008. Chronic food restriction differentially affects NPY mRNA level in neurons of the hypothalamus and in neurons that innervate liver. Neuroscience Letters, 433 (3): 174- 177. [DOI] [PubMed] [Google Scholar]
- 44. Sucajtys-Szulc E, Goyke E, Korczynska J, Stelmanska E, Rutkowski B, Swierczynski J. 2009. Refeeding after prolonged food restriction differentially affects hypothalamic and adipose tissue leptin gene expression. Neuropeptides, 43 (4): 321- 325. [DOI] [PubMed] [Google Scholar]
- 45. Wisse BE, Campfield LA, Marliss EB, Morais JA, Tenenbaum R, Gougeon R. 1999. Effect of prolonged moderate and severe energy restriction and refeeding on plasma leptin concentrations in obese women. American Journal of Clinical Nutrition, 70 (3): 321- 330. [DOI] [PubMed] [Google Scholar]
- 46. Wittert GA, Turnbull H, Hope P, Morley JE, Horowitz M. 2004. Leptin prevents obesity induced by a high-fat diet after diet-induced weight loss in the marsupial Scrassicaudata. American Journal of Physiology -Regulatory Integrative and Comparative Physiology, 286 (4): 734- 739. [DOI] [PubMed] [Google Scholar]
- 47. Zhang LN, Wang DH. 2008. Effects of food restriction and refeeding on energy balance regulation in Mongolian gerbils Meriones unguiculatus. BFDG Abstracts/Appetite, 51 (3): 758- 758. [Google Scholar]
- 48. Zhang W, Della-Fera MA, Hartzell DL, Hausman D, Baile CA. 2008. Adipose tissue gene expression profiles in ob/ob mice treated with leptin. Life Sciences, 83 (1-2): 35- 42. [DOI] [PubMed] [Google Scholar]
- 49. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature, 372 (6505): 425- 432. [DOI] [PubMed] [Google Scholar]
- 50. Zhang ZB, Wang ZW. 1998. Ecology and Management of Rodent Pests in Agriculture. Ocean Publishing House, [Google Scholar]
- 51. Zhao ZJ. 2012. Effect of food restriction on energy metabolism and thermogenesis in striped hamster. Acta Theriologica Sinica, 32 (4): 297- 305. [Google Scholar]
- 52. Zhao ZJ, Cao J. 2009. Plasticity in energy budget and behavior in Swiss mice and striped hamsters under stochastic food deprivation and refeeding. Comparative Biochemistry and Physiology A-Molecular and Integrative Physiology, 154 (1): 84- 91. [DOI] [PubMed] [Google Scholar]
- 53. Zhao ZJ, Chen JF, Wang DH. 2010. Diet-induced obesity in the short-day-lean Brandt's vole. Physiology & Behavior, 99 (1): 47- 53. [DOI] [PubMed] [Google Scholar]
- 54. Zhao ZJ, Zhu QX, Chen KX, Wang YK, Cao J. 2013. a. Energy budget, behavior and leptin in striped hamsters subjected to food restriction and refeeding. PLoS One, 8 (1): e54244- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhao ZJ, Wei WT, Li MZ, Cao J. 2013. b. Body mass, energy budget and leptin of mice under stochastic food restriction and refeeding. Zoological Research, 34 (6): 574- [PubMed] [Google Scholar]


