Abstract
We studied acute and joint toxicity of three different agrochemicals (chlorantraniliprole, flubendiamide-abamectin and penoxsulam) to Chinese tiger frog (Hoplobatrachus chinensis) tadpoles with the method of stability water tests. Results showed that the three agrochemicals increased tadpole mortality. For acute toxicity, the LC50 values after 24, 48 and 72 h of chlorantraniliprole, flubendiamide-abamectin and penoxsulam exposure were 5.37, 4.90 and 4.68 mg/L; 0.035, 0.025 and 0.021 mg/L; 1.74, 1.45 and 1.29 mg/L, respectively. The safety concentrations (SC) of chlorantraniliprole, flubendiamide-abamectin and penoxsulam to the tadpoles were 1.23, 0.30 and 0.003 mg/L, respectively. Based on these findings, chlorantraniliprole and penoxsulam were moderately toxic, while flubendiamide-abamectin was highly toxic. All pairwise joint toxicity tests showed moderate toxicity. The LC50 values after 24, 48 and 72 h of exposure were 7.08, 6.61 and 6.03 mg/L for chlorantraniliprole+penoxsulam, with corresponding values of 2.455, 2.328 and 2.183 mg/L for chlorantraniliprole+flubendiamide-abamectin, and 1.132, 1.084 and 1.050 mg/L for penoxsulam+flubendiamide-abamectin, with safe concentrations of 1.73, 0.63 and 0.30 mg/L, respectively. For toxic evaluations of pairwise combinations of the three agrochemicals, only the joint toxicity of chlorantraniliprole and flubendiamide-abamectin after 24 h was found to be synergistic, whereas all other tests were antagonistic. Our findings provide valuable information on the toxic effects of agrochemicals on amphibians and how various types of agrochemicals can be reasonably used in agricultural areas.
Keywords: Agrochemical, Acute toxicity, Joint toxicity, LC50, Safe concentration, Hoplobatrachus chinensis
Environmental degradation is one of the major causes of worldwide amphibian decline (Shuhaimi-Othman et al, 2012; Sparling & Fellers, 2009; Stuart et al, 2004; Vertucci & Corn, 1996) but is especially prominent in China, due to intensive agriculture. The influences of agriculture on the natural environment can manifest through land conservation (Hecnar, 1995), habitat fragmentation (Hayes et al, 2002) and agrochemical pollution (Blaustein et al, 2003; Davidson et al, 2002; Lavorato et al, 2013; Sparling et al, 2001). Thankfully, the wide use of agricultural chemicals and their toxicities to non-target organisms has gained increasing attention. In agricultural regions that use agrochemicals, both the redundancy and diversity of amphibians have decreased compared with those in adjacent non-agricultural regions, and even in certain areas, some species have become extinct (Bonin et al, 1997). Although agrochemicals are now generally applied within restricted areas, their application is still a global problem, and scientists have found significant and increasing agrochemical residues in frogs since the 1990s (Datta et al, 1998; Russell et al, 1997).
Many amphibian species inhabit ponds, brooks and temporary water bodies that lay eggs within agricultural regions. Likewise, their breeding and development in late spring and early summer coincides with the agrochemical application period of highest frequency and dosage (Hayes et al, 2003; Sayim, 2008; Vertucci & Corn, 1996). However, information about the effects of agrochemicals on amphibians is still limited. Some studies have demonstrated that in agrochemical polluted habitats, amphibians are characterized with high mortality, high abnormality, low hatching success and small size at metamorphosis, which have induced physical problems in individual survival, e.g. liver and kidney function attenuation, deformation, and paralysis (Harfenist et al, 1989; Kamrin, 1997; Mandrillon & Saglio, 2009; Ouellet et al, 1997; Relyea, 2005). Moreover, agrochemicals have also affected the growth, development, immunoreaction and behavior of tadpoles (Bridges, 2000; Broomhall, 2005; Brunelli et al, 2009; Christin et al, 2003; Lou et al, 2013; Zhao et al, 2013).
As the most important component of vertebrate biomass, the effects of pollution have the potential to damage the balance of entire ecosystems (Sayim, 2008). Due to their easy exposure to agrochemical contaminated water and the permeability of their skins, tadpoles are considered to be a good bio-indicator of pollution (Venturmo et al, 2003).
The tiger frog (Hoplobatrachus chinensis) is an anuran species of the Ranidae family, inhabiting farmland and surrounding ditches (Fei et al, 2009). Due to the extensive expansion of farmland, agrochemical abuse and over hunting, the wild resources of this species have declined dramatically over the past few decades (Lin & Ji, 2005; Wang et al, 2008), and it is now classified as a Class Ⅱ National Protected Species.
To detect the toxicological effects of farmland and surrounding water bodies on the tiger frog, the acute and joint toxicities of two kinds of pesticide (chlorantraniliprole and flubendiamide-abamectin) and a weedicide (penoxsulam) were analyzed. This study will provide information for the rational use of agrochemicals and the protections of amphibian species.
MATERIALS AND METHODS
Experimental agrochemicals
The following were used in this study: Chlorantraniliprole (chlorantraniliprole suspension, 200 g/L) produced by DuPont, based in the United States; Flubendiamide-abamectin (AVI-fluoroamide suspension with 10% active ingredients (6.7% flubendiamide, 3.3% abamectin)) produced by Bayer Crop Science (China), Ltd.; Penoxsulam (penoxsulam oil suspension, 25 g/L) produced by Dow Agrosciences (Indonesia) Ltd.; dechlorinated tap water (at least 48 hours), pH 6.8.
Experimental animals
Experimental tiger frog tadpoles were obtained from the breeding ponds of the Laboratory of Herpetology, Lishui University. Studied individuals were all in good health (swimming freely, with good reflexes), with homogenized size (average weight=0.03 g; average length=17.6 mm), and at G34-36 (Gosner, 1960).
Acute toxicity experiment
A few wide concentration ranges were used in the preexperiment. By observing tadpole reactions and mortalities 24 h and 48 h after the agrochemical treatments, the lethal concentration with no mortality (LC0) and with maximum mortality (LC100) were calculated and were then used as the concentration ranges in the following experiments (Xue et al, 2005).
Based on the preexperiment results, "static water changing" was used in the toxicity experiment (Zhou & Zhang, 1989). For each agrochemical, chlorantraniliprole, flubendiamide-abamectin and penoxsulam, six different concentration trials with logarithmic spacing (each trial was performed in triplicate) and one control trial were established. Totally, 10 experimental tadpoles were housed in a round 1 L plastic container with 800 mL agrochemical solution. The experimental conditions were air temperature of 29.0-29.8 ℃ and water temperature of 27.8-28.5 ℃. During the experiment, the tadpoles were fasted and the agrochemical solutions were changed completely every 24 h, with mortalities recorded at 24, 48 and 72 h intervals. If a tadpole did not respond to pressure applied on its tail by a pair of forceps, it was considered dead (Xue et al, 2005).
Joint toxicity experiment
Based on the acute toxicity experiment results, the three agrochemicals were grouped into three pairs. For each pair, one control trial and six different concentration trials in arithmetic progression were established according to their common logarithm of concentration (each trial was conducted in triplicate) (Xue et al, 2005). Specifically, chlorantraniliprole/penoxsulam: 5.62 (4.01/ 1.61), 6.17 (4.41/1.76), 6.76 (4.83/1.93), 7.41 (5.29/2.12), 8.13 (5.81/2.32), 8.91 (6.36/2.55) mg/L; chlorantraniliprole/flubendiamide-abamectin: 2.04 (2.027/0.013), 2.19 (2.176/0.014), 2.34 (2.325/0.015), 2.51 (2.494/ 0.016), 2.69 (2.672/0.018), 2.88 (2.860/0.019) mg/L; and penoxsulam/flubendiamide-abamectin: 0.95 (0.935/0.015), 1.02 (1.004/0.016), 1.09 (1.073/0.017), 1.17 (1.151/ 0.019), 1.25 (1.230/0.020), 1.34 (1.319/0.021) mg/L. All other experimental settings, animals and procedures were the same as those in the acute toxicity experiment.
Statistical analysis
Mortalities of tadpoles under different agrochemical concentrations
All statistical analyses were conducted using SPSS 16.0 (SPSS inc., Chicago, IL, USA). Prior to statistical analysis, all variables were tested for normality and homogeneity. One-way ANOVA and Tukey's post hoc multiple comparisons test were used to evaluate the effects of each agrochemical (single and joint) on the mortalities of tadpoles under different concentration and different exposure time. Repeated measures ANOVA was used to evaluate the correlated effects of concentration and exposure time of each agrochemical on the mortalities of tadpoles. All results were expressed as mean±SD, with α=0.05 taken as statistically significant.
Half lethal concentration (LC50) and safe concentration (SC)
The Karber method was used to determine the LC50 after 24, 48, and 72 h exposure of the individual and paired agrochemicals, respectively.
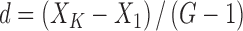 |
(1) |
Where, d is the difference of the logarithms of the two adjacent concentration trials; XK is the logarithm of the higher concentration; X1 is the logarithm of the lower concentration; and G is the concentration trials.
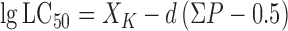 |
(2) |
Where XK is the logarithm of the concentration with 100% mortality; d is the logarithmic interval; and ΣP is the sum of the mortalities of all the trials (Xue et al, 2005).
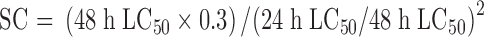 |
(3) |
According to the Environmental Safety Assessment of Agrochemical Test Guidelines provided by the Ministry of Environmental Protection of China (1989), the toxicities of agrochemicals to tadpoles were divided into low toxicity (LC50 > 10.0 mg/L), moderate toxicity (LC50=10.0-1.0 mg/L) and high toxicity (LC50 < 1.0 mg/L) (Zhang et al, 2010).
Joint toxicity evaluation
Marking's addictive index of the coeffects for aquatic toxicology was used to evaluate joint toxicities (Xiu et al, 1994).
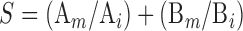 |
(4) |
Where, S is the joint toxicity of the paired agrochemicals; A and B are the experimental agrochemicals; Ai is the LC50 of agrochemical A when used alone; Am is the LC50 of agrochemical A when used jointly; Bi is the LC50 of agrochemical B when used alone; and Bm is the LC50 of agrochemical B when used jointly.
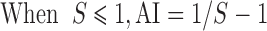 |
(5) |
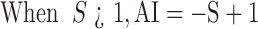 |
(6) |
When AI < 0, the coeffects were taken as antagonistic, when AI > 0, the coeffects were taken as synergistic, when AI=0, the coeffects were taken as adding effects (Zhang et al, 2011).
RESULTS
Acute toxicities of chlorantraniliprole, flubendiamide-abamectin and penoxsulam on tadpoles when used alone
When the agrochemicals were used individually, at the same exposure time point (24, 48 and 72 h), the mortalities of tadpoles increased with increasing concentrations and significant differences were found among the trials (one-way ANOVA, all P < 0.001, Table 1). The LC50 of chlorantraniliprole, flubendiamide-abamectin and penoxsulam all decreased with increasing exposure time (24, 48 and 72 h), and their SCs were 1.23, 0.003, and 0.30 mg/L, respectively. Therefore, chlorantraniliprole showed moderate toxicity, and flubendiamide-abamectin and penoxsulam showed high toxicity (Table 2).
Table 1.
Acute toxicity for three agrochemicals on H. chinensis tadpoles (n=10)
| Concentration (mg/L) | 24 h mortality (%) | 48 h mortality (%) | 72 h mortality (%) |
| Chlorantraniliprole | |||
| 0.0 | 0.0±0.0d | 0.0±0.0e | 0.0±0.0b |
| 3.3 | 0.0±0.0d | 0.0±0.0e | 10.0±10.0b |
| 4.0 | 10.0±10.0cd | 23.3±5.8d | 23.3±5.8b |
| 4.8 | 23.3±15.3c | 36.7±5.8c | 53.3±20.8a |
| 5.8 | 60.0±10.0b | 76.7±5.8b | 76.7±5.8a |
| 6.9 | 93.3±5.8a | 100.0±0.0a | |
| 8.3 | 100.0±0.0a | ||
| One-way ANOVA | F6, 14=84.119 | F5, 12=303.533 | F4, 10=25.194 |
| Flubendiamide-abamectin | |||
| 0.000 | 0.0±0.0d | 0.0±0.0d | 0.0±0.0b |
| 0.013 | 0.0±0.0d | 0.0±0.0d | 6.7±5.8b |
| 0.019 | 23.3±5.8c | 36.7±5.8c | 60.0±20.0a |
| 0.029 | 36.7±5.8c | 53.3±5.8b | 70.0±17.3a |
| 0.044 | 56.7±15.3b | 90.0±10.0a | 95.0±7.1a |
| 0.066 | 83.3±5.8a | 100.0±0.0a | |
| 0.100 | 100.0±0.0a | ||
| One-way ANOVA | F6, 14=96.467 | F5, 12=199.200 | F4, 9=26.657 |
| Penoxsulam | |||
| 0.0 | 0.0±0.0c | 0.0±0.0d | 0.0±0.0d |
| 0.8 | 0.0±0.0c | 0.0±0.0d | 0.0±0.0d |
| 1.0 | 13.3±5.8bc | 23.3±5.8c | 23.3±5.8c |
| 1.3 | 23.3±5.8bc | 46.7±15.3b | 60.0±0.0b |
| 1.6 | 30.0±0.0b | 50.0±0.0ab | 67.7±5.8b |
| 2.0 | 46.7±32.1b | 70.0±10.0a | 93.3±5.8a |
| 2.5 | 100.0±0.0a | ||
| One-way ANOVA | F6, 14=23.222 | F5, 12=40.309 | F5, 12=241.333 |
*P < 0.001; Types with different superscripts differ significantly (Tukey's test, α=0.05, a > b > c > d > e); NS: not significant.
Table 2.
Half lethal concentration (LC50) and safe concentration (SC) of the three agrochemicals administered individually and in conjunction
| Pesticide | 24 h | 48 h | 72 h | SC | Toxicity rank |
| Chlorantraniliprole | 5.37 | 4.90 | 4.68 | 1.23 | Moderately toxic |
| Flubendiamide-abamectin | 0.035 | 0.025 | 0.021 | 0.003 | Highly toxic |
| Penoxsulam | 1.74 | 1.45 | 1.29 | 0.30 | Moderately toxic |
| Chlorantraniliprole/Penoxsulam | 7.08 | 6.61 | 6.03 | 1.73 | Moderately toxic |
| Chlorantraniliprole/Flubendiamide-abamectin | 2.455 | 2.328 | 2.183 | 0.63 | Moderately toxic |
| Penoxsulam/Flubendiamide-abamectin | 1.132 | 1.084 | 1.050 | 0.30 | Moderately toxic |
Joint toxicities of chlorantraniliprole, flubendiamide-abamectin and penoxsulam on tadpoles
When the agrochemicals were used in pairs, at the same exposure time point (24, 48 and 72 h), significant differences in the mortalities of tadpoles were also found among the trials (One-way ANOVA, all P < 0.001, Table 3). The LC50 of the agrochemicals in each pair decreased with increasing exposure time. All the three joint pairs, chlorantraniliprole/penoxsulam, chlorantraniliprole/flubendiamide-abamectin and penoxsulam/flubendiamide-abamectin exhibited moderate toxicity and their LC50 at 24, 48 and 72 h were 7.08, 6.61 and 6.03 mg/L; 2.455, 2.328 and 2.183 mg/L; and 1.132, 1.084 and 1.050 mg/L, respectively, whereas their corresponding SCs were 1.73, 0.63 and 0.30 mg/L, respectively (Table 2). Compared with the acute toxicity experiment, all paired LC50 values decreased, except for that of penoxsulam in the chlorantraniliprole/penoxsulam pair. According to Marking's addictive index, all agrochemical pairwise were antagonistic, except for that of chlorantraniliprole/ flubendiamide-abamectin, which was synergistic at 24 h (Table 4).
Table 3.
Toxicity of paired agrochemicals on H. chinensis tadpoles (n=10)
| Concentration (mg/L) | 24 h mortality (%) | 48 h mortality (%) | 72 h mortality (%) |
| Chlorantraniliprole/Penoxsulam | |||
| 0 (0/0) | 0.0±0.0d | 0.0±0.0d | 0.0±0.0d |
| 5.62 (4.01/1.61) | 0.0±0.0d | 0.0±0.0d | 23.3±5.8c |
| 6.17 (4.41/1.76) | 13.3±5.8c | 30.0±0.0c | 63.3±5.8b |
| 6.76 (4.83/1.93) | 20.0±0.0c | 46.7±5.8b | 86.7±5.8a |
| 7.41 (5.29/2.12) | 83.3±5.8b | 90.0±10.0a | 100.0±0.0a |
| 8.13 (5.81/2.32) | 93.3±5.8ab | 100.0±0.0a | |
| 8.91 (6.36/2.55) | 100.0±0.0a | ||
| One-way ANOVA | F6, 14=437.667 | F5, 11=203.856 | F4, 9=215.679 |
| Chlorantraniliprole/Flubendiamide-abamectin | |||
| 0 (0/0) | 0.0±0.0d | 0.0±0.0d | 0.0±0.0b |
| 2.04 (2.027/0.013) | 0.0±0.0d | 0.0±0.0d | 23.3±25.2b |
| 2.19 (2.176/0.014) | 0.0±0.0d | 23.3±5.8cd | 60.0±0.0a |
| 2.34 (2.325/0.015) | 36.7±30.6cd | 56.7±25.2bc | 80.0±10.0a |
| 2.51 (2.494/0.016) | 60.0±17.3bc | 80.0±20.0ab | 90.0±0.0a |
| 2.69 (2.672/0.018) | 86.7±5.8ab | 100.0±0.0a | |
| 2.88 (2.860/0.019) | 100.0±0.0a | ||
| One-way ANOVA | F6, 14=28.360 | F5, 12=30.000 | F4, 9=24.191 |
| Penoxsulam/Flubendiamide-abamectin | |||
| 0 (0/0) | 0.0±0.0e | 0.0±0.0d | 0.0±0.0e |
| 0.95 (0.935/0.015) | 0.0±0.0e | 0.0±0.0d | 13.3±5.8de |
| 1.02 (1.004/0.016) | 10.0±10.0de | 26.7±25.2cd | 36.7±23.1cd |
| 1.09 (1.073/0.017) | 36.7±15.3cd | 56.7±5.8bc | 63.3±5.8bc |
| 1.17 (1.151/0.019) | 63.3±25.2bc | 83.3±5.8ab | 90.0±0.0ab |
| 1.25 (1.230/0.020) | 83.3±5.8ab | 90.0±0.0a | 100.0±0.0a |
| 1.34( 1.319/0.021) | 100.0±0.0a | ||
| One-way ANOVA | F6, 14=35.378 | F5, 12=41.076 | F5, 12=49.789 |
*P < 0.001; Types with different superscripts differ significantly (Tukey's test, α=0.05, a > b > c > d > e); NS: not significant.
Table 4.
Evaluations of paired agrochemical toxicity
| Pesticides | Exposure time (h) | LC50 | S | AI | Toxicity evaluation |
| Chlorantraniliprole/Penoxsulam | 24 | 7.08 | 2.105 | -1.105 | Antagonistic |
| 48 | 6.61 | 2.267 | -1.267 | Antagonistic | |
| 72 | 6.03 | 2.261 | -1.261 | Antagonistic | |
| Chlorantraniliprole/Flubendiamide-abamectin | 24 | 2.455 | 0.942 | 0.062 | Synergistic |
| 48 | 2.328 | 1.142 | -0.142 | Antagonistic | |
| 72 | 2.183 | 1.282 | -0.282 | Antagonistic | |
| Penoxsulam/ Flubendiamide-abamectin | 24 | 1.132 | 1.174 | -0.174 | Antagonistic |
| 48 | 1.084 | 1.464 | -0.464 | Antagonistic | |
| 72 | 1.050 | 1.644 | -0.644 | Antagonistic |
Effects of agrochemical concentration, exposure time and their correlated effects on tadpole mortalities
According to the repeated measures ANOVA, both agrochemical concentration and exposure time significantly affected the mortalities of tadpoles. All trials showed significantly correlated effects on the mortalities of tadpoles, except for the flubendiamide-abamectin/penoxsulam pair (Table 5).
Table 5.
Effects of agrochemical concentration, exposure time and their interactions on mortality of H. chinensis tadpoles
| Pesticide | Concentration | Exposure time | Concentration×Exposure time |
| Chlorantraniliprole |
F5, 12=103.159** Ⅰe, Ⅱde, Ⅲc, Ⅳc, Ⅴb, Ⅵa |
F2, 24=31.462** 24hc, 48hb, 72ha |
F10, 24=4.323 |
| Flubendiamide-abamectin |
F5, 12=133.302** Ⅰe, Ⅱde, Ⅲc, Ⅳc, Ⅴb, Ⅵa |
F2, 24=38.603** 24hc, 48hb, 72ha |
F10, 24=4.508 |
| Penoxsulam |
F5, 12=76.592** Ⅰe, Ⅱde, Ⅲc, Ⅳc, Ⅴb, Ⅵa |
F2, 24=40.831** 24hc, 48hb, 72ha |
F10, 24=4.720 |
| Chlorantraniliprole/Penoxsulam |
F5, 12=645.760** Ⅰe, Ⅱde, Ⅲc, Ⅳc, Ⅴb, Ⅵa |
F2, 24=195.470** 24hc, 48hb, 72ha |
F10, 24=30.510** |
| Chlorantraniliprole/Flubendiamide-abamectin |
F5, 12=43.905** Ⅰe, Ⅱde, Ⅲc, Ⅳc, Ⅴb, Ⅵa |
F2, 24=28.015** 24hc, 48hb, 72ha |
F10, 24=2.686 |
| Penoxsulam/Flubendiamide-abamectin |
F5, 12=53.116** Ⅰe, Ⅱde, Ⅲc, Ⅳc, Ⅴb, Ⅵa |
F2, 24=22.548** 24hc, 48hb, 72ha |
F10, 24=1.671NS |
*: P < 0.05; **: P < 0.001; Ⅰ, Ⅱ, Ⅲ, Ⅳ, Ⅴ and Ⅵ: six different concentrations of each agrochemical (see details in materials and methods); Types with different superscripts differ significantly (Tukey's test, α=0.05, a > b > c > d > e); NS: not significant.
DISCUSSION
This study evaluated the acute and joint toxicities of chlorantraniliprole, flubendiamide-abamectin and penoxsulam to tiger frog tadpoles. Chlorantraniliprole has been used for rice pest control in China since 2008. The LC50 of chlorantraniliprole in our study differed from previous findings. Huang et al (2011) found that chlorantraniliprole exhibited low toxicity in controlling Chilo suppressalis at different developmental stages and its LC50 was between 2.00 and 18.70 ng. Barbee et al (2010) also found low toxicity (LC50=951 µg/L at 96 h) in an acute toxicity experiment on Procambarus clarkii, and no effects from chlorantraniliprole on either the mortalities or behaviors of P. clarkii were found. In our study, the LC50 of chlorantraniliprole on tiger frog tadpoles at 24, 48, and 72 h were 7.08, 6.61, and 6.03 mg/L, respectively, which were much higher than those on C. suppressalis and P. clarkii. According to the Environmental Safety Assessment of Agrochemical Test Guidelines, chlorantraniliprole showed moderate toxicity to tiger frog tadpoles.
Flubendiamide-abamectin has been available since 2010 for C. suppressalis control. Lin (2012) reported it as highly toxic, with 99.64% mortality on C. suppressalis (10%, 30 mL/667 m2). Our study also showed that it was highly toxic, with LC50 values at 24, 48 and 72 h of 0.035, 0.025 and 0.021 mg/L, respectively.
Penoxsulam is a broad-spectrum weedicide, used to control species such as Myriophyllum heterophyllum (Glomski & Netherland, 2008), but its toxicity on animals is little understood. Our study found that penoxsulam exhibited moderate toxicity, with LC50 values at 24, 48 and 72 h of 1.74, 1.45 and 1.29 mg/L, respectively.
Our results showed that the toxicities of the three agrochemicals, from high to low, were flubendiamide-abamectin, penoxsulam and chlorantraniliprole, with SCs of 0.003, 0.30, and 1.23 mg/L, respectively (Table 2). Concentration, exposure time, and their interaction effects had significant influences on the mortalities of tiger frog tadpoles, except for the flubendiamide-abamectin/penoxsulam pair (Table 5).
Previous studies demonstrated that the joint toxicities of different agrochemicals and heavy metals on amphibians, such as Bufo gargarizans (Zhang et al, 2010) and Rana limnocharis (Guo et al, 2005; Nian et al, 2009), are usually synergistic. In our joint toxicity experiment, at same exposure time, all concentration trials of each pair had significant effects on the mortalities of tadpoles (Table 3). The LC50 of the chlorantraniliprole /penoxsulam pair after 24, 48, and 72 h were 7.08, 6.61, and 6.03 mg/L, respectively, whereas, their individual LC50 when used jointly were 5.01/2.04, 4.68/1.91, and 4.27/1.74 mg/L, respectively. The LC50 of chlorantraniliprole in this pair was lower than that in the acute experiment; however, the situation was opposite for penoxsulam (Table 2, 4). This pair was antagonistic in toxicity evaluation (Table 4).
The LC50 of the chlorantraniliprole/flubendiamide-abamectin pair after 24, 48 and 72 h were 2.455, 2.328 and 2.183 mg/L, respectively, whereas, their individual LC50 when used jointly were 2.45/0.017, 2.34/0.0166, and 2.32/0.0165 mg/L, respectively. The LC50 of both agrochemicals were lower than those in the acute experiment (Table 2, 4). This pair was synergistic at 24 h, but antagonistic at 48 and 72 h in toxicity evaluation (Table 4).
The LC50 of the penoxsulam/flubendiamide-abamectin pair after 24, 48 and 72 h were 1.132, 1.084 and 1.050 mg/L, respectively, whereas, their individual LC50 when used jointly were 1.114/0.0187, 1.067/0.0182, and 1.033/0.0177 mg/L, respectively. The LC50 of both agrochemicals were lower than those in the acute experiment (Table 2, 4). This pair was antagonistic in toxicity evaluation (Table 4). As a result, the response of tiger frog tadpoles to these agrochemicals (individually or jointly) is complicated as these antagonistic phenomena are correlated with animal developmental stage, as well as exposure time and concentration of chemicals (Berrill et al, 1993).
Amphibians play important roles in pest control and in the aquatic and terrestrial food webs. However, due to environmental and habitat degeneration, amphibian resources have declined dramatically in recent years. Agrochemicals can change both the quantity and quality of amphibian habitats and food resources (Berrill et al, 1993), and induce mutations during development, such as retardation and deformation, as well as abnormal behaviors (Pawar et al, 1983; Sayim, 2008). Lethal and or sub-lethal toxicities of agrochemicals might affect the natural adjustment procedures of amphibian species (especially newborn or juvenile groups), and may help explain population decline. The rational application of agrochemicals is, therefore, critical for environmental and amphibian protection.
Acknowledgements
We would like to thank Fang LIU and Dan WU for their assistance with the experiments.
Funding Statement
This work was supported by the National Natural Science Foundation of China (31270443) and the Natural Science Foundation of Zhejiang Province (LY13C030004)
REFERENCES
- 1. Barbee GC, McClain WR, Lanka SK, Stout MJ. 2010. Acute toxicity of chlorantraniliprole to non-target crayfish (Procambarus clarkii) associated with rice-crayfish cropping systems. Pest Management Science, 66 (9): 996- 1001. [DOI] [PubMed] [Google Scholar]
- 2. Berrill M, Bertham S, Wilson A, Louis S, Brigham D, Stromberg C. 1993. Lethal and sublethal impacts of insecticides on amphibian embryos and tadpoles. Environmental Toxicology and Chemistry, 12 (3): 525- 539. [Google Scholar]
- 3. Blaustein AR, Romansic JM, Kiesecker JM, Hatch AC. 2003. Ultraviolet radiation, toxic chemicals and amphibian population declines. Diversity and Distributions, 9 (2): 123- 140. [Google Scholar]
- 4. Bonin J, DesGrange JL, Rodrigued M, Quellet M. 1997. Anuran species rochiness in agricultural land scapes of Quebec: foreseeing long-term results of road call surveys. Amphibians in decline: Canadian studies of a global problem. St, Louis, Missouri: Society for the Study of Amphibians and Reptiles, 141- 149. [Google Scholar]
- 5. Bridges CM. 2000. Long-term effects of pesticides epoxure at various life stages of the southern leopard frog (Rana sphenocephala). Archives of Environmental Contamination and Toxicology, 39 (1): 91- 96. [DOI] [PubMed] [Google Scholar]
- 6. Broomhall SD. 2005. Measuring chemical impacts on amphibians: Ecotoxicology and behavioural data in governmental regulation. Applied Herpetology, 2 (3): 259- 285. [Google Scholar]
- 7. Brunelli E, Bernabò I, Berg C, Lundstedt-Enkel K, Bonacci A, Tripepi S. 2009. Environmentally relevant concentrations of endosulfan impair development, metamorphosis and behaviour in Bufo bufo tadpoles. Aquatic Toxicology, 91 (2): 135- 142. [DOI] [PubMed] [Google Scholar]
- 8. Christin MS, Gendron AD, Brousseau P, Menard L, Marcogliese DJ, Cyr D, Ruby S, Fourner M. 2003. Effects of agricultural pesticides on the immune system of Rana pipiens and on its resistance of parasitic infection. Environmental Toxicology and Chemistry, 22 (5): 1127- 1133. [PubMed] [Google Scholar]
- 9. Datta S, Hansen L, McConnell L, Baker J, Le Nior J, Seiber JN. 1998. Pesticides and PCB contaminants in fish and tadpoles from the Kaweah River basin California. Environmental Contamination and Toxicology, 60 (6): 829- 836. [DOI] [PubMed] [Google Scholar]
- 10. Davidson C, Shaffer HB, Jennings MR. 2002. Spatial tests of the pesticides droft habitat destruction, UV-B, and climate-change hypothesis for California amphibian declinces. Conservation Biology, 16 (6): 1588- 1601. [Google Scholar]
- 11. Fei L, Hu SQ, Ye CY, Huang YZ. 2009. Fauna Sinica Amphibia, Vol. 2. Anura. Beijing: Science Press, [Google Scholar]
- 12. Glomski LM, Netherland MD. 2008. Efficacy of fluridone, penoxsulam, and bispyribac-sodium on variable-leaf milfoil. Journal of Aquatic Plant Management, 46 (1): 193- 196. [Google Scholar]
- 13. Gosner KL. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica, 16 (3): 183- 190. [Google Scholar]
- 14. Guo ZY, He W, He ZB. 2005. Jonit-toxicity of clomazone and cadmiun on Rana limnocharis. Wuyi Science Journal, 21 (1): 113- 116. [Google Scholar]
- 15. Harfenist A, Power T, Clark KL, Peakall DB. 1989. A review and evaluation of the amphibian toxicological literature. Technical Report Series No.61. Ottawa: Canadian Wildlife Service., [Google Scholar]
- 16. Hayes T, Haston K, Tsui M, Hoang A, Haefelle C, Vonk A. 2003. Atrazine induced hermorphropdism at 0. 1 ppb in American leopard frogs (Rana pipiens): Laboratory and field evidence. Environmental Health Perspectives, 111 (4): 568- 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayes TB, Collins A, Lee M, Mendoza M, Noriega N, Stuart AA, Vonk A. 2002. Hermaphroditic, demasculinized forgs after exposure to the herbicide atrazine at low ecologically relevant doses. Proceedings of the National Academy of Sciences, USA, 99 (8): 5476- 6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hecnar SD. 1995. Acute and chronic toxicity of ammonium nitrate fertilizer to amphibians form southern Onataro. Environmental Toxicology and Chemistry, 14 (12): 2131- 2137. [Google Scholar]
- 19. Huang J, Wu SF, Ye GY. 2011. Evaluation of lethal effects of chlorantraniliprole on Chilo suppressalis and its larval parasitoid, Cotesia chilonis. Agricultural Sciences in China, 10 (7): 1134- 1138. [Google Scholar]
- 20. Kamrin MA. 1997. Pesticides profiles toxicity, environmental impact, and fate. New York: Lewes, [Google Scholar]
- 21. Lavorato M, Bernabò H, Crescente A, Denoël M, Tripepi S, Brunelli E. 2013. Endosulfan effects on Rana dalmatina tadpoles: Quantitative developmental and behavioural analysis. Archives of Environmental Contamination and Toxicology, 64 (2): 253- 262. [DOI] [PubMed] [Google Scholar]
- 22. Lin ZF. 2012. Study on efficacy trials to control rice stem borer by pesticide. North Rice, 42 (1): 38- 39. [Google Scholar]
- 23. Lin ZH, Ji X. 2005. Sexual dimorphism in morphological traits and food habits in tiger frogs, Hoplobatrachus rugulosus in Lishui, Zhejiang. Zoological Research, 26 (3): 255- 262. [Google Scholar]
- 24. Lou QQ, Zhang YF, Zhou Z, Shi YL, Ge YN, Ren DK, Xu HM, Zhao YX, Wei WJ, Qin ZF. 2013. Effects of perfluorooctanesulfonate and perfluorobutanesulfonate on the growth and sexual development of Xenopus laevis. Ecotoxicology, 22 (7): 1133- 1144. [DOI] [PubMed] [Google Scholar]
- 25. Mandrillon AL, Saglio P. 2009. Effects of single and combined Embryonic exposures to herbicide and conspecific chemical alarm cues on hatching and larval traits in the common frog (Rana temporaria). Archives of Environmental Contamination and Toxicology, 56 (3): 566- 576. [DOI] [PubMed] [Google Scholar]
- 26. Nian Y, Yang ZF, Wei QQ. 2009. Study on toxicity of triazophos, trichlorphon and imidacloprid on Rana limnocharis tadpole. Journal of Anhui Agriculture Science, 37 (18): 8538- 8540. [Google Scholar]
- 27. Ouellet M, Bonin J, Rodrigue J, DesGranges J, Lair S. 1997. Hind limb deformities (ectromelia, ectrodactyly) in free living anurans from agricultural habitats. Journal of Wildlife Diseases, 33 (1): 95- 104. [DOI] [PubMed] [Google Scholar]
- 28. Pawar KR, Ghate HV, Katdare M. 1983. Effect of malathion on embryonic development of the frog Microhyla ornate. Bulletin of Environmental Contamination and Toxicology, 31 (2): 170- 176. [DOI] [PubMed] [Google Scholar]
- 29. Relyea RA. 2005. The lethal impacts of Roundup and predatory stress on six species of North American tadpoles. Archives of Environmental Contamination and Toxicology, 48 (3): 351- 357. [DOI] [PubMed] [Google Scholar]
- 30. Russell RW, Gillan KA, Hafner GD. 1997. Polychlorinated biphenyls and chlorinated pesticides in sourthern Ontario, Canada, green frogs. Environmental Toxicology and Chemistry, 16 (11): 2258- 2263. [Google Scholar]
- 31. Sayim F. 2008. Acute toxic effects of malathion on the 21st stage larvae of the marsh frog. Turkish Journal of Zoology, 32 (1): 99- 106. [Google Scholar]
- 32. Shuhaimi-Othman M, Nadzifah Y, Umirah NS, Ahmad AK. 2012. Toxicity of metals to tadpoles of the common Sunda toad, Duttaphrynus melanostictus. Toxicological and Environmental Chemistry, 94 (2): 364- 376. [Google Scholar]
- 33. Sparling DW, Fellers GM. 2009. Toxicity of two insecticides to California, USA, anurans and its relevance to declining amphibian populations. Environmental Toxicology and Chemistry, 28 (8): 1696- 1703. [DOI] [PubMed] [Google Scholar]
- 34. Sparling DW, Fellers GM, McConnell LL. 2001. Pesticides and amphibian population declines in California, USA. Environmental Toxicology and Chemistry, 20 (7): 1591- 1595. [DOI] [PubMed] [Google Scholar]
- 35. Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues AS, Fischman DL, Waller RW. 2004. Status and trends of amphibian declines and extinctions worldwide. Science, 306 (5702): 1783- 1786. [DOI] [PubMed] [Google Scholar]
- 36. Venturmo A, Rosenbaum E, De Casho AC, Anguiano OL, Gana l, De Schroder TF, De Castro AC, Anguiano OL, Gauna L, De Schrodeder TF, De Angelo AMP. 2003. Biomarkers of effect in toads and frogs. Biomarkers, 8 (3-4): 167- 186. [DOI] [PubMed] [Google Scholar]
- 37. Vertucci FA, Corn PS. 1996. Evaluation of episodol acidification and amphibian declines in the rocky mountains. Ecological Applications, 6 (2): 449- 457. [Google Scholar]
- 38. Wang Y, Shao C, Wang XT. 2008. A study on relative fatness of tiger frog (Hoplobatrachus rugulosus) in Zhejiang province. Ecological Science, 27 (6): 490- 493. [Google Scholar]
- 39. Xiu RQ, Xu YX, Fu YC, Gao SR, Ren GY, J Z. 1994. Additive index of coeffects for aquatic toxicology. Environmental Chemistry, 13 (3): 269- 271. [Google Scholar]
- 40. Xue QQ, Yao D, Huang ZY, Ke Q, Wen X, Geng BL. 2005. Acute toxicity of pesticide dichlorvos and herbicide butachlor to Microhyla ornata tadpoles. Sichuan Journal of Zoology, 24 (2): 209- 212. [Google Scholar]
- 41. Zhang YL, Yuan J, Chen LP, Shao H. 2011. Joint toxicity experiment of three heavy metal on fry of Carassius auratus. Heibei Fisheries, 39 (2): 24- 27. [Google Scholar]
- 42. Zhang YP, Fang XW, Lu XY. 2010. Co-toxicity research of pesticides ruigao and feiwang to Bufo gargarizans tadpole. Hubei Agriculture Science, 49 (2): 354- 356. [Google Scholar]
- 43. Zhao HF, Chai LH, Wang HY. 2013. Effects of fluoride on metamorphosis, thyroid and skeletal development in Bufo gargarizans tadpoles. Ecotoxicology, 22 (7): 1123- 1132. [DOI] [PubMed] [Google Scholar]
- 44. Zhou YX, Zhang ZS. 1989. Toxicity testing methods of aquatic organism. Beijing: Chinese Agricultural Press, [Google Scholar]


