Abstract
Cestode larvae spend one phase of their two-phase life cycle in the viscera of rodents, but cases of cestodes infecting subterranean rodents have only been rarely observed. To experimentally gain some insight into this phenomenon, we captured approximately 300 plateau zokors (Eospalax baileyi), a typical subterranean rodent inhabiting the Qinghai-Tibet Plateau, and examined their livers for the presence of cysts. Totally, we collected five cysts, and using a mitochondrial gene (cox1) and two nuclear genes (pepck and pold) as genetic markers, we were able to analyze the taxonomy of the cysts. Both the maximum likelihood and Bayesian methods showed that the cysts share a monophyly with Taenia mustelae, while Kimura 2-parameter distances and number of different sites between our sequences and T. mustelae were far less than those found between the examined sequences and other Taeniidae species. These results, alongside supporting paraffin section histology, imply that the cysts found in plateau zokors can be regarded as larvae of T. mustelae, illustrating that zokors are a newly discovered intermediate host record of this parasite.
Keywords: Endoparasites, New host record, Phylogenetic relationships, Subterranean rodent
Larval taeniid cestodes (Taeniidae, Cyclophyllidea, Cestoda) are known to require either human or other herbivorous mammals—generally rodents—to serve as intermediate hosts during their larval stage (Knapp et al, 2011). While there are many parasites within humans and rodents, larval cestodes are a special epidemiological focus, because they can cause serious pathological changes in viscera and tissues, and even death of the host (Eckert et al, 2001; Hoberg, 2002). While humans and other herbivorous mammals are often used as hosts, subterranean rodents are generally not thought to be viable host options. As a widely distributed group of species that live primarily underground and are highly adapted to that environment (Lacey et al, 2000; Nevo, 1999), most major activities—foraging, mating, and breeding, etc. —take place underground. Consequently, these rodents have rare contact with predators (Begall et al, 2007), but more importantly, since subterranean rodents generally forage underground parts of plants, they have a markedly smaller probability of encountering food contaminated by cestode eggs. As a result, it is commonly believed that these animals have a comparatively rare chance of becoming infected by cestodes, but that preconception may be, at best, flawed, or even incorrect, because these animals are typically hidden, the presence of cestodiasis is not well empirically studied.
Boev et al (1971) first identified E. multilocularis from cysts isolated from a zokor species (Myospalax sp.) in Kazakhstan. Li et al (1985) and Hong & Lin (1987) reported the same identification of cysts from the viscera of Chinese zokors (M. fontanieri) in Ningxia, China. Because of the paucity of adult cestode phenotypic characteristics and the great plasticity of larvae, identifying the Taeniidae species using traditional histologic examination of paraffin slices is prone to errors (Nakao et al, 2010). Thankfully, modern molecular technologies provide far more accurate methods of identification. In this study, we sought to use both approaches to gain a more complete picture of cestode infections. To that end, we collected several cysts from the livers of captured plateau zokors (Eospalax baileyi) (Figure 1A) and used molecular phylogenetic methods to determine the phylogeny of the larval cysts.
Figure 1.
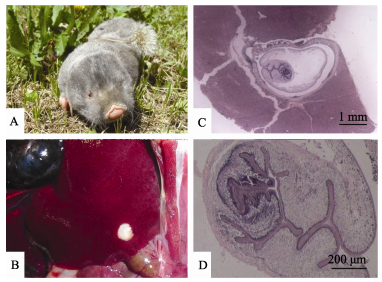
Photographs of Samples
MATERIALS AND METHODS
Totally, 300 plateau zokors were captured for study from Datong County (N37°7.5′, E101°48.7′), in the east of Qinghai Province, China. After euthanizing the specimens and dissecting them, liver tissues were extract and tissues that appeared to be infected (Figure 1B) were fixed in 4% formalin and embedded in paraffin wax, with 3-5 µm sections prepared for histopathological observation. The samples were stained overnight with Delafield’s haematoxylin, destained with 70% ethanol containing 1% hydrochloric acid, dehydrated in ethanol, and then cleared with xylene and mounted in Canada balsam. The found cysts that were to be used for molecular analyses were fixed in 95% ethanol before total genomic DNA was extracted using a spin column kit (DNeasy tissue kit, Qiagen, Germany) and then used as a template for PCR. Here, two cysts were randomly selected for molecular analyses. Partial fragments of the mitochondrial gene for cytochrome c oxidase subunit 1 gene (cox1) (approximately 880 bp) were amplified using previously published primers cox1/F and cox1/R by Nakao et al (2000). We also selected genes for phosphoenolpyruvate carboxykinase (pepck) (approximately 1 650 bp) and DNA polymerase delta (pold) (approximately 2 000 bp) to serve as targets for nuclear markers in the cyst DNA (Knapp et al, 2011).
PCR was performed using a 40 µL final reaction volume, with 40 to 60 ng of genomic DNA, 0.6 mmol/L dNTPs, 0.2 µmol/L of each primer, 1U Taq polymerase and the manufacturer-supplied reaction buffer. Thermocycling was conducted in a T-Gradient Thermoblock PCR machine (Biometra, Gottingen, Germany). After initial denaturation at 94 ℃ for 7 min, the reaction proceeded for 35 cycles as follows: 30 s at 94 ℃, 30 s at 54 ℃ to 56 ℃ and 90 s at 72 ℃ and terminated with a final extension step of 72 ℃ for 5 min. Resulting PCR products were purified using a CASpure PCR Purification Kit following the manufacturer’s recommended protocols (Casarray, Shanghai, China), and directly sequenced using the same primers that were previously used for amplifying the sequences mentioned above. Sequencing reactions were conducted in a Biometra thermocycler using a DYEnamic ET Terminator Cycle Sequencing Kit (Amersham Biosciences, UK) following the manufacturer’s protocols. Sequencing products were later separated and analyzed on an ABI 3730 DNA Analysis System (Applied Biosystems, USA). Putative exon regions for both the pepck and pold genes were extracted from each of the respective sequence alignments under the guidance of previously published exon–intron maps for E. multilocularis (Knapp et al, 2011).
We obtained the sequences of cox1, pepck and pold genes from the available species in the family (9 Echinococcus and 15 Taenia taxa) from GenBank to serve as a basis for identifying taxonomic status of the cystic larvae using phylogenetic methods. The additional taxa Hymenolepis diminuta for cox1 and Dipylidium caninum for the two nuclear genes (pepck and pold) were included as outgroups for reconstructing maximum likelihood (ML) and Bayesian trees (accession numbers of these sequences are in Table 1). The coding sequences for each gene were aligned using ClustalW in MEGA 5.0 (Tamura et al, 2011), cut to the length of the shortest sequence. Maximum likelihood trees were generated in PAUP 4b10 (Swofford, 2002) and the Bayesian trees in MrBayes 3.2 (Ronquist et al, 2012), each with 1 000 bootstrap replicates. Nucleotide substitution models were selected using the Akaike information criterion (AIC) within Modeltest 3.7 (Posada & Crandall, 1998) running on PAUP 4b10 and in MrModeltest 2.3 (Posada & Crandall, 2001) running on MrBayes3.2. The Kimura 2-parameter pairwise divergence (K2P) and the number of different nucleotide substitutions (N) among sequences was calculated using MEGA 5.0.
Table 1.
GenBank accession numbers for all reference sequences (9 Echinococcus and 15 Taenia taxa and 2 outgroups Dipylidium caninum and Hymenolepis diminuta) for each gene used in this study
RESULTS
Totally, 300 plateau zokors were captured and then examined for the presence of cestode parasites. Among these five separate cysts were detected in five of the examined zokors, each ranging from 3-5 mm in diameter (Figure 1B). The cysts were found deeply embedded, and partly or fully covered by liver tissue. Physically, they are thin-walled and either transparent to translucent. Slices of the tissues (depicted in Figure 1C, D) showed that only one larva attached to the inner face of the cyst. The larva was about 3.5 mm in major diameter with no sign of scolex formation. Together, these physical characteristics suggest a close relationship of the observed cysts with Taenia mustelae Gmelin 1790 (Freeman, 1956).
Three genes each from two cysts were sequenced and the resulting sequences submitted to GenBank (accession numbers: KC898934-KC898939). The cox1, pepck, and pold (partial sequences) were 820 bp, 1 041 bp, and 1 873 bp in length, respectively. The putative exon regions for pepck and pold genes were likewise 921 bp and 867 bp, respectively. Finally, three alignments in lengths of 384 bp, 921 bp, and 867 bp respectively for the cox1, pepck, and pold genes were used for molecular analyses. Testing showed that the three genes differed at only one or two sites between the DNA sequences from the larvae from the two cysts.
Further phylogenetic analysis yielded both ML and Bayesian trees, (Figure 2) both of which clearly demonstrated that the sequences of the cysts and T. mustelae were closely related. Further analysis between the sequences of the cysts and other sequences yielded K2P and N values (Table 2), and the values for both measurements between the studied sequences and earlier reported sequences of T. mustelae were far less than those between our sequences and other Taeniidae species.
Figure 2.
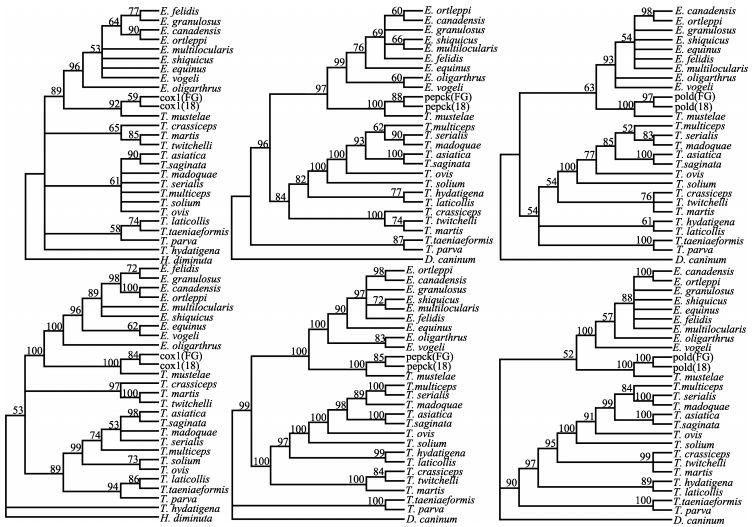
Phylogenetic trees of cyst tissues found in Plateau Zokors using the cox1 and exon data sets of pepck and pold
Table 2.
Mean values of different sites (N) and Kimura 2-parameter distances (K2P) between the sampled sequences and earlier published sequences for cox1, pepck, and pold genes
| cox1 (384 bp) | pepck (921 bp) | pold (867 bp) | ||||
| N | K2P | N | K2P | N | K2P | |
| E. granulosus | 52.0 | 0.1500 | 83.5 | 0.0980 | 92.0 | 0.1170 |
| E. multilocularis | 50.0 | 0.1430 | 81.5 | 0.0955 | 87.0 | 0.1100 |
| E. shiquicus | 48.0 | 0.1370 | 83.5 | 0.0980 | 91.0 | 0.1150 |
| E. felidis | 48.0 | 0.1370 | 82.5 | 0.0965 | 88.0 | 0.1110 |
| E. oligarthrus | 43.0 | 0.1220 | 80.5 | 0.0945 | 90.0 | 0.1140 |
| E. ortleppi | 48.0 | 0.1370 | 83.5 | 0.0980 | 90.0 | 0.1140 |
| E. equinus | 46.0 | 0.1310 | 82.5 | 0.0965 | 85.0 | 0.1070 |
| E. vogeli | 48.0 | 0.1370 | 78.5 | 0.0915 | 85.0 | 0.1070 |
| E. canadensis (G7) | 51.0 | 0.1470 | 82.5 | 0.0965 | 90.0 | 0.1140 |
| T. asiatica | 56.0 | 0.1630 | 104.5 | 0.1255 | 104.0 | 0.1335 |
| T. crassiceps | 50.0 | 0.1430 | 99.5 | 0.1190 | 122.0 | 0.1610 |
| T. hydatigena | 56.0 | 0.1630 | 99.5 | 0.1185 | 96.5 | 0.1230 |
| T. multiceps | 55.0 | 0.1590 | 110.5 | 0.1335 | 103.0 | 0.1325 |
| T. saginata | 54.0 | 0.1560 | 104.5 | 0.1255 | 103.0 | 0.1325 |
| T. solium | 55.0 | 0.1590 | 90.5 | 0.1070 | 105.0 | 0.1355 |
| T. laticollis | 54.0 | 0.1560 | 111.5 | 0.1345 | 129.0 | 0.1710 |
| T. madoquae | 53.0 | 0.1530 | 102.5 | 0.1230 | 97.0 | 0.1235 |
| T. martis | 55.0 | 0.1600 | 99.5 | 0.1190 | 110.0 | 0.1435 |
| T. ovis | 56.0 | 0.1630 | 101.5 | 0.1215 | 100.0 | 0.1275 |
| T. parva | 54.0 | 0.1560 | 122.5 | 0.1505 | 139.0 | 0.1855 |
| T. serialis | 57.0 | 0.1660 | 101.5 | 0.1215 | 96.0 | 0.1225 |
| T. taeniaeformis | 59.0 | 0.1720 | 117.5 | 0.1425 | 143.0 | 0.1910 |
| T. twitchelli | 48.0 | 0.1370 | 99.5 | 0.1195 | 118.0 | 0.1555 |
| T. mustelae | 10.0 | 0.0270 | 3.5 | 0.0035 | 4.0 | 0.0045 |
DISCUSSION
Both the histopathological and molecular analyses we conducted indicated that the cysts can be regarded as larvae of T. mustelae, meaning that the zokors are a newly identified host for T. mustelae infection. A recent DNA barcoding of taeniids using the same cox1 gene segment as we used in this study found that the optimum threshold for distinguishing a Taenia species is 3.6% of K2P distance (Galimberti et al, 2012), which is higher than the value (2.7%) found between our samples and T. mustelae. Given this threshold, we can conclude that our speculation on the nature of the cysts as being T. mustelae larvae is likely accurate. Unfortunately, the “gold standard” of determining a taeniid species relies on using both molecular data of adult specimens as well as morphological observations. Though our present findings are intriguing, further studies, such as survey of potential hosts for adult taeniids, are still necessary to make a definitive taxonomic review of the cysts.
Combined with former reports, our results make it clear that that zokors can harbor two Taeniidae species with two different genuses. Taeniid parasites require two mammalian hosts to perpetuate their life cycles. Terrestrial carnivorous predators act as hosts for the adult worms, while their prey, such as the zokors and other rodents, act as intermediate hosts for the cystic larvae. Zokors are commonly thought to have only rare contact with predators because of their absolute underground habitat. An earlier study noted that the numbers of plateau zokors in the pellets and food remains of Buteo hemilasius and Bubo bubo only consisted~5% of total prey individuals (Cui et al, 2003). Likewise, the average feeding intensity by predators of the zokor, such as the red fox (Vulpes vulpes), polecat (Mustela eversmanni) and weasel (M. altaica) of plateau zokor was much lower than that of the sympatric distributed plateau pika (Ochotona curzoniae) (Yang et al, 2007). Moreover, as a typical subterranean rodent, plateau zokors mainly feed on underground roots and shoots of plants (Zhang, 1999), both of which are less likely to be contaminated by cestode eggs contained in the feces of carnivorous hosts. Collectively, these characteristics make the zokors somewhat unlikely infectious targets for taeniid cestodes. The reality that zokors can indeed be infected by both Taenia and Echinococcus larvae shows that an underground habitat alone cannot prevent infection by such parasites, which may then hold some interesting implications for studies of similar parasitic organisms
One possibility that may explain the infection is that when zokors collect food items from underground, they may also pull down the aboveground parts of plants. In fact, recent studies on winter caches of plateau zokors showed that they collected considerable amount of aboveground plant parts, some of which are even positively selected as food sources (Xie et al, 2013). This novel observation gives some credence to our observed infection of zokors by parasites, because if zokors actually harvest aboveground sources of food, they may have more chances to become infected by cestodes as expected. Another possibility is that while zokors constitute only a small part of food resources of predators, some carnivores, such as the polecat, frequently invade to their burrows in search of food (Zheng et al, 1983) Even when unsuccessful, such forays into the underground zokor burrows may increase the probability of contamination of cestodes eggs (from feces) in the zokor habitats and consequently increase their infection rate. Since there are many predators and other herbivorous mammals such as plateau pika, root vole (Microtus oeconomus), hamster (Cricetulus longicaudatus), and marmot (Marmota himalayana) that are sympatrically distributed with plateau zokors, the taeniid parasites that infect zokors may complete their life cycles locally, placing many of these wild mammals of the plateau at risk for cestodiasis, though this clearly needs further detailed follow-up. Accordingly, we suggest that the health challenges of endoparasites from zokors (and probably other subterranean rodent species) should not be neglected and indeed warrant greater attention and observation.
Funding Statement
This work was supported by the West Light Foundation of the Chinese Academy of Sciences and the Chinese Academy of Sciences President Scholarship (to G. Lin)
REFERENCES
- 1. Begall S, Burda H, Schleich CE. 2007. Subterranean Rodents: News from Underground. Berlin: Springer. [Google Scholar]
- 2. Boev SN, Bondareva VI, Tazieva ZKh. 1971. Vospriimchivost' nekotorykh vidov gryzunov K al' veokokky. Voprosy Prirodnoi Ochagovosti Boleznei, (4): 152- 159. [Google Scholar]
- 3. Cui QH, Lian XM, Zhang TZ, Su JP. 2003. Food habits comparison between Buteo hemilasius and Bubo bubo. Chinese Journal of Zoology, 38 (6): 57- 63. [Google Scholar]
- 4. Eckert J, Gemmell MA, Meslin FX, Pawlowski ZS. 2001. WHO/OIE Manual on Echinococcosis in Humans and Animals: a Public Health Problem of Global Concern. Paris: World Organization for Animal Health and World Health Organization. [Google Scholar]
- 5. Freeman RS. 1956. Life history studies on Taenia mustelae Gmelin, 1790 and the taxonomy of certain taenioid cestodes from Mustelidae. Canadian Journal of Zoology, 34 (4): 219- 242. [Google Scholar]
- 6. Galimberti A, Romano DF, Genchi M, Paoloni D, Vercillo F, Bizzarri L, Sassera D, Bandi C, Genchi C, Ragni B, Casiraghi M. 2012. Integrative taxonomy at work: DNA barcoding of taeniids harboured by wild and domestic cats. Molecular Ecology Resources, 12 (3): 403- 413. [DOI] [PubMed] [Google Scholar]
- 7. Hoberg EP. 2002. Taenia tapeworms: their biology, evolution and socioeconomic significance. Microbes and Infection, 4 (8): 859- 866. [DOI] [PubMed] [Google Scholar]
- 8. Hong L, Lin Y. 1987. Investigation of growth and pathological of E. multilocularis in the human and animals. Endemic Diseases Bulletin, 2 (2): 51- 61. [Google Scholar]
- 9. Knapp J, Nakao M, Yanagida T, Okamoto M, Saarma U, Lavikainen A, Ito A. 2011. Phylogenetic relationships within Echinococcus and Taenia tapeworms (Cestoda: Taeniidae): An inference from nuclear protein-coding genes. Molecular Phylogenetics and Evolution, 61 (3): 628- 638. [DOI] [PubMed] [Google Scholar]
- 10. Lacey EA, Patton J, Cameron G. 2000. Life Underground: the Biology of Subterranean Rodents. Chicago: University of Chicago Press. [Google Scholar]
- 11. Li W, Zhang G, Huang G, Wang J, Li Z, Li M. 1985. Investigation on infection of the genera Echinococcus in Ningxia. Endemic Diseases Bulletin, 1 (2): 131- 133. [Google Scholar]
- 12. Nakao M, Sako Y, Yokoyama N, Fukunaga M, Ito A. 2000. Mitochondrial genetic code in cestodes. Molecular and Biochemical Parasitology, 111 (2): 415- 424. [DOI] [PubMed] [Google Scholar]
- 13. Nakao M, Yanagida T, Okamoto M, Knapp J, Nkouawa A, Sako Y, Ito A. 2010. State-of-the-art Echinococcus and Taenia: Phylogenetic taxonomy of human-pathogenic tapeworms and its application to molecular diagnosis. Infection Genetics and Evolution, 10 (4): 444- 452. [DOI] [PubMed] [Google Scholar]
- 14. Nevo E. 1999. Mosaic Evolution of Subterranean Mammals: Regression, Progression, and Global Convergence. New York: Oxford University Press. [Google Scholar]
- 15. Posada D, Crandall KA. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics, 14 (9): 817- 818. [DOI] [PubMed] [Google Scholar]
- 16. Posada D, Crandall KA. 2001. Selecting the best-fit model of nucleotide substitution. Systematic Biology, 50 (4): 580- 601. [PubMed] [Google Scholar]
- 17. Ronquist F, Teslenko M, van Mark PD, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3. 2: Efficient bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61 (3): 539- 542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Swofford DL. 2002. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods) 4.0 Beta. Massachusetts: Sinauer Associates. [Google Scholar]
- 19. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28 (10): 2731- 2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xie JX, Lin GH, Liu CX, Yang CH, Deng XG, Cui XF, Li B, Zhang TZ, Su JP. 2014. Diet selection in overwinter caches of plateau zokor (Eospalax baileyi). Acta Theriologica, 59 (2): 337- 345. [Google Scholar]
- 21. Yang SM, Wei WH, Yin BF, Fan NC, Zhou WY. 2007. Predation risk and survival game of plateau pika and plateau zokor in alpine meadow ecosystem. Acta Ecologica Sinica, 27 (12): 4972- 4978. [Google Scholar]
- 22. Zhang YM. 1999. Effect of plateau zokor on characters and succession of plant communities in alpine meadow. Zoological Research, 20 (6): 435- 440. [Google Scholar]
- 23. Zheng SW, Zeng JX, Cui RX. 1983. On ecology and energy dynamics of masked polecat (Mustela eversmanni) in Haibei Qinghai Province. Acta Theriologica Sinica, 3 (1): 35- 46. [Google Scholar]


