Abstract
Mesenchymal stem cells (MSCs) possess immunomodulatory properties, which confer enormous potential for clinical application. Considerable evidence revealed their efficacy on various animal models of autoimmune diseases, such as multiple sclerosis, systemic lupus erythematosus and uveitis. MSCs elicit their immunomodulatory effects by inhibiting lymphocyte activation and proliferation, forbidding the secretion of proinflammatory cytokines, limiting the function of antigen presenting cells, and inducing regulatory T (Treg) and B (Breg) cells. The induction of Treg and Breg cells is of particular interest since Treg and Breg cells have significant roles in maintaining immune tolerance. Several mechanisms have been proposed regarding to the MSCs-mediated induction of Treg and Breg cells. Accordingly, MSCs induce regulatory lymphocytes through secretion of multiple pleiotropic cytokines, cell-to-cell contact with target cells and modulation of antigen-presenting cells. Here, we summarized how MSCs induce Treg and Breg cells to provoke immunosuppression.
Keywords: Mesenchymal stem cells, Regulatory T cells, Regulatory B cells, Immunomodulation, Autoimmunity
Core tip: In this review, we summarized the mechanisms involved in regulatory T (Treg) and B (Breg) cell induction by mesenchymal stem cells (MSCs). In an inflammatory environment, MSCs secrete various anti-inflammatory cytokines, actively interact with immune cells and modulate them to acquire regulatory properties, thus, generate a tolerogenic environment. Particularly, by inducing Treg and Breg cells, the immunomodulation of MSCs is amplified. Therefore, genetic engineered MSCs to enhance their ability to induce Treg and Breg cells may increase their therapeutic efficacy.
INTRODUCTION
Mesenchymal stem cells (MSCs) are mesodermal progenitor cells that have a wide range of differentiation capacity. They can differentiate into adipocytes, osteocytes, chondrocytes, myocytes, fibroblasts and stromal cells[1]. In addition, some research studies have shown that MSCs, under certain conditions, can trans-differentiate to cells from ectodermal and endodermal lineage[2,3]. Among them, the ability of MSCs to develop into neurons is of particular interest. Considering that neural stem cells are limited in number and extremely difficult to be isolated while, comparatively, massive numbers of MSCs can be derived from numerous adult tissues, including, liver, kidney, adipose tissue, bone marrow, dental pulp, peripheral blood and umbilical cord blood. MSCs may serve as a reliable source of neural cells for potential cell replacement therapy or regenerative medicine.
Aside from its diverse differentiation capacity, their immunomodulatory properties also prompt researchers to study profoundly. MSCs are capable of regulating both innate and adaptive immunity. They secrete a large variety of soluble factors, including interleukin (IL)-6, IL-8, transforming growth factor-β1 (TGF-β1), indoleamine 2,3-dioxygenase (IDO), human leukocyte antigen-G (HLA-G) and prostaglandin E2 (PGE2)[4]. These factors allow MSCs to interact with components of the innate and adaptive immunity, subsequently modulate inflammation and immune tolerance. Monocytes, for instance, under the influence of MSCs-secreted IL-6, IDO and PGE2, tend to develop into anti-inflammatory M2 macrophages instead of proinflammatory M1 macrophages[5-9]. In addition, recent reports showed that human gingiva derived MSCs have converted M1 macrophages to M2[5]. Natural killer (NK) cells, on the other hands, express CD73 and acquires regulatory phenotype when exposed to MSCs[10,11]. Similarly, regulatory dendritic cells (DC) induced by MSCs were capable of secreting IL-10, a powerful anti-inflammatory cytokine[12-14]. Thus, MSCs are able to suppress innate immunity by skewing their differentiation into regulatory subtype (Figure 1).
Figure 1.
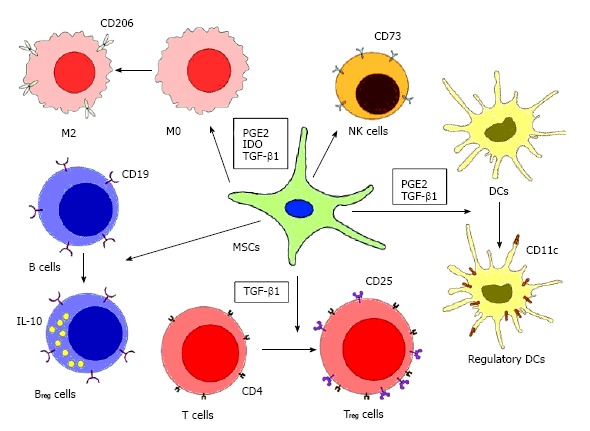
Immunosuppression by mesenchymal stem cells. MSCs suppress innate and adaptive immune responses by enhancing regulatory immune cells with tolerogenic properties. MSCs suppress macrophages by favoring monocyte polarization to anti-inflammatory M2 macrophages, increasing the production of IL-10, and decreasing the production TNF-α and IL-12. MSCs can also regulate DCs by downregulating the expression of MHC, CD40, CD80, CD83 and CD86, thus, diminishing their antigen presenting ability, while upregulating the expression of IL-10. MSCs can reduce the NK cell cytotoxicity and decrease their production of TNF-α and IFN-γ. Treg and Breg cells can be induced by MSCs, further increase the production of anti-inflammatory cytokines (IL-10 and TGF-β1). However, the mechanisms of how Breg cells are induced by MSCs are still not clear. MSCs: Mesenchymal stem cells; TNF: Tumor necrosis factor; IL: Interleukin; NK: Natural killer; DCs: Dendritic cells; IFN-γ: Interferon-γ; Treg: Regulatory T; Breg: Regulatory B; TGF: Transforming growth factor; PGE2: Prostaglandin E2; IDO: Indoleamine 2,3-dioxygenase.
MSCs can regulate adaptive immune system by suppressing the proliferation, differentiation and activation of T cell and B cell. A number of studies have demonstrated that MSCs can inhibit the proliferation of Th1 and Th17 cell, decrease the production of interferon (IFN)-γ, IL-2, IL-6 and IL-17, and downregulate the T cell activation markers, CD38 and HLA-DR[15-19]. When MSCs were co-cultured with B cell and in the presence of different B cell trophic stimuli, B cell proliferation was inhibited and they were arrested in G0/G1 phase. Moreover, B cell differentiation was prohibited as indicated by limited production of IgG, IgM and IgA[20]. In addition, the regulatory-skewing propensity of MSCs observed in innate immune system also applies to T and B lymphocyte. In fact, the ability of MSCs to expand regulatory T (Treg) cells and regulatory B (Breg) cells have been intensively studied. However, the mechanism of how Treg and Breg cells are induced by MSCs has not been fully understood. Some suggest regulatory lymphocytes induction by MSCs requires mediation of other immune cells, while others propose MSCs-released cytokines are sufficient to expand Treg and Breg cell populations, but more and more researchers have come to the consensus that MSCs can use multiple pathways to generate regulatory lymphocytes and which pathways are more favorable is determined by the microenvironment that MSCs encounter[21]. Altogether, MSCs modulate immune cells to acquire regulatory phenotype, hence, alter the inflammatory milieu into a tolerogenic one (Figure 1).
There is another advantage of using MSCs for cellular therapy. MSCs have low immunogenicity, implying that MSCs can be used for allogeneic transplantation. This property is particularly helpful to the patient whose MSCs are compromised. Thereby, MSCs possess valuable therapeutic potential to treat immune-mediated disorders[22].
Although MSCs have demonstrated as a promising immunoregulator for clinical use, the immunomodulatory and low-immunogenicity properties of MSCs are not constitutive. The function of MSCs is based on the signals from the vicinity. MSCs, in the absence of tumor necrosis factor (TNF)-α and IFN-γ may adopt pro-inflammatory phenotype, which activate T cells to response. On the contrary, when MSCs are exposed to high level of TNF-α and IFN-γ they will behave as an anti-inflammatory regulator by producing TGF-β1, IDO, and PGE2[23]. Likewise, depending on the level of IL-6, MSCs can convert monocyte into M1 or M2 macrophages[22,24-26]. Thus, before any clinical application, the plasticity of MSCs should be carefully considered. In this review, we summarized current understandings on how MSCs interact with regulatory lymphocytes, Treg and Breg cells particularly, to attenuate autoimmunity, and how this knowledge can contribute to therapeutic development.
Treg LYMPHOCYTE
The notion of “suppressive” T cells has long been proposed in 1970s. Due to technical limitation, their identities and phenotypic characteristics cannot be described until 1995, Sakagucho et al[27] isolated a unique CD4+ CD25+ T cells that can suppress immune responses and maintain immunologic self-tolerance[28]. Later, this subpopulation of T cells was named as Treg cells. For those Treg cells that undergo maturation in thymus, are referred to as thymus-dervied Treg (tTreg) cells. Three days post-maturation, tTreg cells will relocate from thymus to periphery[29]. Surprisingly, tTreg cells only comprise 5%-10% of peripheral T cells, but they are the critical regulator of autoimmunity. This is evidenced in mice lacking peripheral Treg cells. They were lethal due to various autoimmunity enhancements[29,30].
Apart from tTreg cells, Treg cells can also be generated in periphery[31,32]. Periphery-derived Treg (pTreg) cells are converted from naïve T cells (CD4+CD25-Foxp3-CD45RBhi). Upon activation of naive T cells and in the presence of particular cytokines, two main types of Treg cells can be differentiated in the periphery and in vitro, namely, T helper 3 (Th3) cells and type 1 regulatory T (Tr1) cells. Th3 cell and Tr1 cell differentiation are promoted by TGF-β and IL-10, respectively[33-35]. Both Th3 and Tr1 cells are suppressive to effector and memory T cells, and they are able to secrete cytokine for self-activation. However, one distinct phenotypical difference is Th3 cells are Foxp3+ whereas Tr1 cells are Foxp3-.
Forkhead box P3 (Foxp3) is a transcription factor that constitutively express in tTreg cells and some types of pTreg cells. It has been recognized as the master regulator of Treg cells. Scurfy, a Foxp3 gene mutated mouse, is lethal by one month after birth, displays hyperactivation of CD4+ T cells and overproduction of proinflammatory cytokines[36]. In human, immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is X-linked recessive disorder caused by mutation in Foxp3 gene[37]. Treg cells from the patients with IPEX are either dysfunction or completely vanished. As a result, IPEX patients are afflicted with various autoimmune diseases, allergy and/or inflammatory bowel disease[38]. The provoked inflammation on IPEX patients indicates the failure of immune tolerance. Foxp3 promotes its regulatory effect by enhancing the expression of IL-2 receptor (CD25), cytotoxic T cell-associated antigen-4 (CTLA-4), and glucocorticoid-induced TNF receptor family-related protein (GITR), meanwhile suppressing the production IL-2, IL-4 and IFN-γ[39]. Treg cells monitor the inflammatory status by the exogenous level of IL-2. Binding of IL-2 to CD25 would enhance the expression of Treg-cell associated genes and regulate the inflammation by suppressing effector T cell proliferation or by altering the function of antigen presenting cells[40]. Retroviral transfer of Foxp3 to naïve T cells (CD4+CD25-Foxp3-) can upregulate the expression of some Treg cell-associated genes, including CD25, CTLA-4, GITR and CD103, and the Foxp3-transduced T cells were shown to be suppressive[41]. Altogether, Foxp3 is critical to the function and the development of Treg cells and to a greater extent, the maintenance of immune homeostasis[42,43].
Treg LYMPHOCTYE INDUCTION BY MSCs
MSCs are able to induce Foxp3+ Treg cell population in vitro and in vivo. So far, several mechanisms have been proposed, including: (1) secretion of soluble mediators; (2) cell-cell interaction; and (3) modulation of antigen presenting cells (Figure 2).
Figure 2.
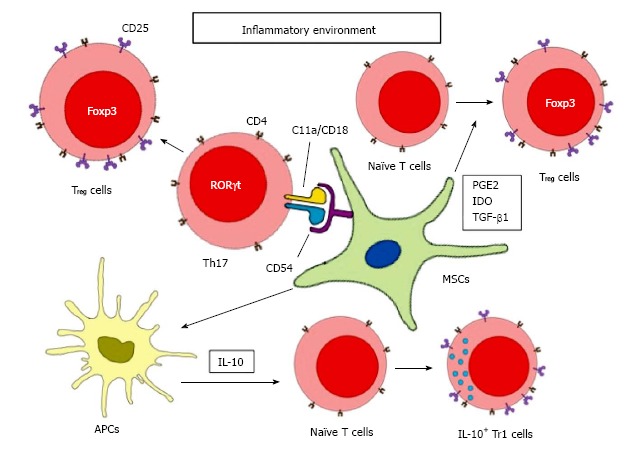
Mesenchymal stem cells-mediated regulatory T cell induction. MSCs induce Treg cells through soluble mediators stimulation, cell-cell interaction, and modulation of antigen-presenting cells. Under inflammatory environment, MSCs secretes TGF-β1, PGE2 and IDO to facilitate the differentiation of naïve T cells to Foxp3+Treg cells. MSCs can also interact with Th17 cells by direct contact via CD54 and C11a/CD18. With the presence of PGE2, differentiated Th17 cells can be converted to functional Foxp3+Treg cells. MSCs can increase the secretion of IL-10 by antigen presenting cells, which will then induce Tr1 cells differentiation. MSCs: Mesenchymal stem cells; IL: Interleukin; Treg: Regulatory T; TGF: Transforming growth factor; PGE2: Prostaglandin E2; IDO: Indoleamine 2,3-dioxygenase.
Secretion of soluble mediators
TGF-β1: MSCs can secrete TGF-β1 to promote Treg cell differentiation, especially when MSCs are placed in an inflammatory environment[21]. TGF-β1 is a potent immunosuppressor secreted by every leukocyte lineages, including macrophages, DCs, NK cells, T cells and B cells. Both TGF-β1 knockout mice and T-cell specific TGF-β receptor II knockout mice develop severe autoimmunity, leading to multiple organs failure and death, suggesting the importance of TGF-β1 in regulating peripheral tolerance[44,45]. Generally, TGF-β1 can suppress the proliferation of T cells, the activation of B cells, the maturation and antigen presentation of DCs, the cytotoxicity of NK cells, and phagocytic effect of macrophages[46]. Moreover, as mentioned earlier, TGF-β1 is able to convert naïve T cells to Foxp3+ Th3 cells, although such conversion seems to be concentration-dependent. High concentrations of TGF-β1 suppresses the expression of IL-23R and shifts the conversion to Foxp3+ Th3 cells, whereas at lower concentrations and in the presence of IL-6 and IL-21, the expression of IL-23R is enhanced and results in RORγt+ Th17 differentiation[47]. In addition, neutralizing TGF-β1 reduced mRNA and protein level of Foxp3 and CD25, further confirms its essential role in promoting Treg cell differentiation[48]. In conclusion, MSCs-secreted TGF-β1 not only acts as a suppressor of innate and adaptive immune response, it can also induce development of Treg cells from naive T cells, which further enhance the regulatory effects.
PGE2: MSCs can also secrete PGE2 to induce Treg cells. PGE2 plays a major role in suppressing chronic inflammation. PGE2 can reduce IFN-γ production of NK cells, limit the phagocytic ability of macrophages and interfere early activation of B cells[49-52]. Although PEG2 can suppress early development of DCs, it is surprising that PGE2 also stabilize matured DCs and enhance its antigen presenting capacity[53-55]. Moreover, despite PGE2 is able to shift the differentiation of naïve T cells from Th1 to Th2 cells, PGE2 also promote proinflammatory Th17 cell development by elevating IL-23 production[56]. Thereby, PEG2 is not exclusively anti-inflammatory. It also possesses the ability to provoke inflammation. Nevertheless, like TGF-β1, PGE2 can induce Foxp3+Treg cell differentiation and it is one of many soluble mediators that produce by MSCs. Diminishing PGE2 signaling when co-culture CD4+ T cells with MSCs by antagonist indomethacin fail to upregulate Foxp3 and CD25 expression. In fact, when inhibiting both TGF-β1 and PGE2 signaling, the expression of Foxp3 and CD25 further decreased[48]. Furthermore, after transferring adipose tissue-derived MSCs in asthmatic mice, the number of infiltrated inflammatory cells was significantly reduced and no obvious goblet cell hyperplasia was found in the lung. Meanwhile, the number of Treg cells was elevated. When TGF-β1 neutralizing antibodies or indomethacin was added to MSCs-treated asthmatic mice, the anti-inflammatory effects promoted by MSCs as well as the Treg cell expansion. These results demonstrated the necessity of TGF-β1 and PGE2 for Treg cell induction as well as the anti-inflammatory effect of MSCs[57].
IDO: IDO is a rate-limiting enzyme that catalyzes the degradation of tryptophan via kynurenine pathway. IDO is expressed in various cell types, including macrophages, DC and MSCs. Interestingly, IDO expression can be induced by IFN-γ and other proinflammatory cytokines. Munn et al[58] treated pregnant mice carrying allogeneic or syngeneic fetus with 1-methyltryptophan, an IDO inhibitor. As a result, allogeneic, but not syngeneic, fetuses provoked severe immune rejection[58]. Also, some studies suggested the association of tryptophan catabolism with inhibition of T cell proliferation, emphasizing its tolerogenic potential[59,60]. In addition, kynurenines, a tryptophan catabolite, can promote Treg cell induction[61]. Infusion of MSCs to kidney allograft murine model prevented graft rejection, and the Treg cell population was elevated. In contrast, allograft tolerance and Treg cell expansion diminished when the recipients were treated with IDO-deficient MSCs. These results demonstrated the importance of IDO in MSCs-mediated Treg cell induction and graft tolerance[62]. Other soluble factors, like human leukocyte antigen-G5 and haem oxygenase 1, are also shown to be involved in MSCs-mediated Treg cell induction[63,64]. However, the underlying mechanisms are not clear. More studies need to be done in order to further increase the efficacy of MSCs-based therapy and to reveal the potential risk that could cause to the patients.
Cell-cell interaction
Apart from soluble mediators, cell-cell interaction is also important to the modulatory function of MSCs and Treg cell induction. MSCs are known to express adhesion molecules on their surface, although only low level of expression can be detected in normal condition. However, after placing MSCs in inflammatory conditions, adhesion molecules, ICAM-1 and VCAM-1, chemokine ligands of CCR5 and CXCR3 are upregulated. Through these molecules, T cells are attracted and anchored to MSCs. With close proximity, adhesion molecules co-operate with IDO and NO, suppress T cell activity by inducing their apoptosis or cell arrest[65-68]. It is also worth to note that MSCs can inhibit the expression of ICAM-1, CXCR3 and α-integrin on CD3+ T cell, reduced the interaction between T cells and endothelial cells, thus, disrupted T cells from infiltrating into CNS[69]. On the other hand, MSCs can attach to Th17 cells via CCR6 and CD11a/CD18 and facilitate Th17 to adopt regulatory phenotype[70]. Moreover, when co-culture MSCs with CD4+ T cells in transwell system; Treg cells cannot be induced, even in the presence of PGE2 and TGF-β[48]. These results further confirmed cell-cell interaction is essential to the overall suppressive effect of MSCs. However, Treg cell induction ability was recovered if MSCs were co-cultured with peripheral blood mononuclear cells instead of isolated CD4+ T cells, suggesting there is an alternative pathway that does not require cell-cell contact, and it is likely, through soluble mediators in peripheral blood mononuclear cells[48].
Modulation of antigen presenting cells
Increasing evidence has indicated MSCs are able to shift macrophages, DCs and NK cells to a regulatory phenotype and alter their cytokines production. For example, MSCs skew monocyte toward M2 macrophage differentiation. Subsequently, M2 macrophages secrete CCL18 and IL-10 to exert suppressive response and induce Treg cell differentiation[26]. As discussed above, IL-10 is able to induce naïve T cell to Foxp3- Tr1 cell, which secrete high level of IL-10 and TGF-β to modulate the inflammatory microenvironment. Interestingly, although MSCs express neither IL-10 nor its receptor, MSCs are able to induce NK cells, DCs, macrophages, T cells and B cells to produce IL-10[5,10-12,17]. In addition, IL-10 is a powerful anti-inflammatory cytokine that suppresses antigen-specific immune responses, reduces pathological immune responses and promotes allograft tolerance.
In conclusion, the mechanisms underlying MSCs-mediated Treg cell development are complicated, which involve synthesis and secretion of multiple mediators, direct interaction with target cells and modulation of certain antigen-presenting cells. Apparently, there is no single pathway that governs the whole induction process, indicating that MSCs possess certain degree of plasticity. Regardless of how Treg cells are enhanced by MSCs, MSCs-activated Treg cells play a significant role on immunoregulation and affect a wide spectrum of immune responses[43,71,72]. Certainly, Treg cells can massively amplify the immunomodulatory effect of MSCs. However, the mechanism in regard to Treg cell induction is far from elaborate and additional researches are required.
Breg LYMPHOCYTE
In recent decade, Breg cells were being intensively investigated due to its immunosuppressive effect on excessive inflammation. Like Treg cells, Breg cells can produce anti-inflammatory cytokines, like TGF-β and IL-10. Among these, IL-10 is strongly associated with Breg cells since depleting IL-10-producing B cells result in chronic inflammation, outgrowth of proinflammatory T cell after autoimmune induction[73-75]. But unlike Treg cells, there is no “master regulator” being identified in Breg cells, which complicated the process of Breg cell classification. So far, there are several B cell subsets have been identified as Breg cells in mice. They are CD5+CD1dhi B (B10) cells and Tim1+ B cells[76-78]. In human, there is CD19+CD24hiCD38+CD1dhi B cells and CD19+CD24hiCD27+ B cells[79,80]. Breg cells control inflammation by suppressing IL-12 secretion from DCs, thus inhibiting Th1 and Th17 differentiation[81]. Through the secretion of TGF-β, Breg cells can induce CD4+ T cell apoptosis and anergy in CD8+ cytotoxic T cells[82,83]. Recent studies indicated that Breg cells play a role in Treg cell development and function. As Breg cells are one of the major sources of IL-10, which drive Tr1 differentiation, it is not surprising that Breg cells can expand Treg cell population during inflammation. Additionally, when B cell specific IL-10 defective mice (DBA/1IL-10 KO-/- mice) were induced with arthritis, the percentage of Tr1 was significantly decreased, indicating effects of IL-10+Breg cells on Treg cell formation[75]. Besides TGF-β and IL-10, recent studies reported that IL-35 is another pleiotropic cytokine that regulate overwhelming inflammation and autoimmunity[84,85]. Antigen-driven proliferation assay revealed that IL-35 was able to suppress CD4+ T cell proliferation[86]. Treatment with IL-35 ameliorated disease severity and reduced Th1 and Th17 cells in mice with experimental autoimmune uveoretinitis (EAU)[85]. More importantly, IL-35 can increase Treg and Breg cell populations. Similar to IL-10, IL-35-induced Treg (iTr35) cells are Foxp3-. However, adoptive transfer of iTr35 cells to various autoimmune disease animal models has sufficiently alleviated their clinical severity, and the effect was comparable to tTreg cells-treated mice[35]. On the other hand, when recombinant IL-35 was injected into the EAU mice, the frequency of B220+ IL-10+Breg cells, IL-35+Breg cells and B10 cells were upregulated in the spleen and draining lymph nodes[85]. Collectively, Breg cells exhibit anti-inflammatory and immunoregulatory effects, at least in part, by secreting multiple anti-inflammatory cytokines (TGF-β, IL-10 and IL-35), promoting differentiation of other regulatory cells, and inhibiting the proliferation and function of effector T cells.
Breg LYMPHOCYTE INDUCTION BY MSCs
Although MSCs do not constitutively express IL-10, and currently there is no evidence to indicate that MSCs produce IL-35, several studies have reported that MSCs induce IL-10+Breg cell differentiation in mouse model[87-89]. Our group studied the effects of human bone marrow-derived MSCs in experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis, and observed attenuation of clinical severity and neuroinflammation; and excitingly, these were associated with expansion of CD1dhi CD5+ Breg cells after MSCs administration[87]. Subsequently, another study demonstrated intravenous infusion of adipose tissue-derived MSCs to Roquinsan/san mice, an animal model of systemic lupus erythmatosus (SLE), lead to increased numbers of B10, B10pro and naïve Treg cells[89]. Moreover, the MSCs-mediated Breg cell induction is not restricted to murine models. Administrating MSCs into refractory chronic graft vs host disease (cGvHD) patients have improved patients’ overall clinical conditions. Consistent with murine models, MSCs increased the frequency and the function of CD5+ IL-10+Breg cells by enhancing their proliferation and survival[88]. Momentarily, we are still not clear about the mechanism regarding to MSCs-mediated Breg cell induction. It is worthwhile to ask whether the induction is IL-35 or IL-10-dependent since MSCs can induce IL-10 production by Treg cells, DCs, and M2 macrophages, implying the possibility of creating a positive feedback loop for Breg cell generation. Further understanding the mechanisms of how MSCs induce Treg and Breg cells can definitely contribute to the therapeutic development of MSCs and further improve their potential therapeutic efficacy.
THERAPEUTIC POTENTIAL OF GENETIC ENGINEERED MSCs
MSCs contain multiple properties that are suitable for therapeutical use. Wide-spectrum of differentiation capacity made it a perfect candidate for regenerative medicine. MSCs have been used to generate cartilage, bone, liver, intervertebral disc, and cardiac tissue[90]. Recent reports have suggested using MSCs for neural cell replacement. However, rather than direct neural differentiation, MSCs tend to recruit neural progenitor cells (NPCs) to the injury sites and support NPCs proliferation and differentiation[91]; Immunomodulatory properties of MSCs are potentially useful for the treatment of autoimmune diseases and GvHD. Transplanted MSCs suppressed the proliferation and activation of T cells and NK cells in type 1 diabetes animal model. Also, the level of IFN-γ and TNF-α were reduced. When MSCs were co-transplanted with pancreatic islets, MSCs protected grafted islets from immunorejection and secreted various trophic factors to promote graft vascular network[92,93]. Another intriguing advantage of using MSCs to treat immune diseases is that, unlike traditional immunotherapy in which a certain modulator act on a particular pathway, MSCs elicit their suppression on multiple immune cell types via various mechanisms. Although the immunosuppressive effects of MSCs appear very promising, further investigations are required to elucidate the underlying mechanisms, so as to prevent complications and maximize the therapeutic efficacy.
One current issue on immunotherapy is that a particular modulator or antibody may be seemingly effective, however, the therapeutic efficacy is limited since such modulator may also compromise certain cells or mediators beneficial to the disease recovery. Rituximab, for example, is a CD20 neutralizing antibody and it is believed to be an effective treatment for B and T-cell-mediated diseases, such as rheumatoid arthritis, multiple sclerosis and systemic lupus erythematosus[94-96]. Rituximab-induced B-cell depletion depends on the expression of CD20 on the cell surface, but the expression of CD20 gradually disappeared upon plasma cell differentiation[97,98]. Moreover, Breg cells were also depleted, thus, exacerbates the disease symptoms[73]. In EAE, B10 cells play an important regulatory role during the initiative phase whereas they are less involved at the late phase of the disease[99,100]. Therefore, depleting B cells by rituximab at the early phase have a potential risk of worsening the clinical conditions. As a consequence, it is necessary to develop an alternative strategy.
The immunosuppressive properties of MSCs on different murine autoimmune disease animal models support its potential clinical application. However, the immunomodulatory secretome of MSCs vary and greatly rely on the host inflammatory environment[21]. To minimize this uncertainty, a novel therapeutic strategy, in which MSCs are genetically engineered with defined immunoregulatory cytokines, has been developed. Transplantation of IL-10-engineered adipose-derived MSCs attenuated EAE by reducing the number of immune cell infiltration to the CNS, decreasing the secretion level of IL-17A, TNF-α and IL-2, and inhibiting antigen-presenting function of DC[101]. Since the immunosuppressive effect of MSCs is enhanced if they are placed proximal to the inflammatory area, Liao et al[102] engineered MSCs with CNS homing ligand genes, P-selectin glycoprotein (PSGL-1) and Sialyl-Lewisx (SLeX), along with IL-10 to EAE model. Consequently, EAE was attenuated, CNS homing ability was enhanced and their therapeutic efficacy was increased[102]. Genetic engineering of MSCs has been well studied in regenerative medicine. Different combination of treatments is documented and aims to redirect the MSCs differentiation propensity. Comparatively, genetic modification of MSCs for the treatment of autoimmune diseases is currently under development. Considering that the effect of MSCs may vary between patients with different severity of neuroinflammation, information on the clinical condition and pathology of the individual patient will probably help to predict treatment efficacy. Moreover, questions like in what phase of a particular disease introducing MSCs can improve the clinical outcome, or to what extent MSCs can elicit their suppressive effect and meanwhile, does not compromise the immunity in response to pathogens or infectious agents, are worthwhile to explore in order to safely use in human patients.
SAFETY AND CONCERNS OF MSCs AS CELLULAR THERAPIES IN PATIENTS
To date, there are nearly 500 ongoing MSC-based clinical trials. They aim to investigate the effectiveness of MSCs on treating different diseases, including GvHD, diabetes, cardiovascular diseases, hematological diseases and neurological diseases[103]. Although most of these clinical trials reported the patients were well tolerated to the MSC infusion and administration, there are some safety concerns requiring caution[104]. During in vitro expansion, MSCs can give rise to replicative senescence, which may affect the activity of surrounding healthy cells and therefore, reduce the clinical efficacy[105]. Moreover, although MSCs have low immunogenicity due to the reduced expression of co-stimulatory receptors and major histocompatibility complex (MHC) class II antigens, in vitro stimulation of pro-inflammatory cytokines on MSCs can upregulate MHC class I and MHC class II expression, compromising the hypo-immunogenicity property of MSCs.
CONCLUSION
The immunomodulatory properties of MSCs have been massively studied due to its intriguing suppressive effects on various immunological diseases. Broad-range of immune cells can be regulated by MSCs through a series of soluble mediators stimulation, chemokine attraction, and cell-to-cell interaction. MSCs-induced Treg and Breg cells enhance the immunosuppressive capacity and generate a tolerogenic microenvironment against overwhelmed inflammation. This hypothesis supports the observation that infused MSCs can only survive in the recipient for a short period of time, however, the regulatory effects of MSCs are long lasting, suggesting MSCs may act as an activator or a switcher that initiate certain cells, possibly Treg and Breg cells, to react to the inflammation and at the same time, alter the microenvironment for those cells to sustain their immunosuppressive effects. Although MSCs appear very promising as treatment in experimental models of autoimmune diseases, there are still many challenges need to overcome before MSCs can be widely use in clinical medicine.
ACKNOWLEDGMENTS
The authors would like to thank Dr. R C L Ng for help in editing the manuscript and Ms Joanne Hui for secretarial assistance.
Footnotes
Supported by Matching Fund from Stanley Ho Alumni Challenge for Translational Research in Neuroinflammation, No. 20830036.
Conflict-of-interest statement: The author, Oscar Ka-Fai Ma, certify that they have no affiliations with or involvement in any organization or entity with any financial interest. Another author, Dr. Koon Ho Chan, has received research-funding support from Merck Pharmaceutical Ltd, Novartis Pharmaceutical Ltd, Bayer HealthCare Ltd, and has received honorarium for invited lectures from Biogen Idec and UCH Pharma Ltd.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: April 29, 2016
First decision: June 16, 2016
Article in press: July 31, 2016
P- Reviewer: Jun Y, Liu L, Phinney DG, Yankee T, Zaminy A S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201. doi: 10.3389/fimmu.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 3.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.de Witte SF, Franquesa M, Baan CC, Hoogduijn MJ. Toward Development of iMesenchymal Stem Cells for Immunomodulatory Therapy. Front Immunol. 2015;6:648. doi: 10.3389/fimmu.2015.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, Nguyen AL, Kwon CW, Le AD. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46:e70. doi: 10.1038/emm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 9.Zheng G, Ge M, Qiu G, Shu Q, Xu J. Mesenchymal Stromal Cells Affect Disease Outcomes via Macrophage Polarization. Stem Cells Int. 2015;2015:989473. doi: 10.1155/2015/989473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee D, Tufa DM, Baehre H, Hass R, Schmidt RE, Jacobs R. Natural killer cells acquire CD73 expression upon exposure to mesenchymal stem cells. Blood. 2014;123:594–595. doi: 10.1182/blood-2013-09-524827. [DOI] [PubMed] [Google Scholar]
- 11.El Omar R, Xiong Y, Dostert G, Louis H, Gentils M, Menu P, Stoltz JF, Velot É, Decot V. Immunomodulation of endothelial differentiated mesenchymal stromal cells: impact on T and NK cells. Immunol Cell Biol. 2016;94:342–356. doi: 10.1038/icb.2015.94. [DOI] [PubMed] [Google Scholar]
- 12.Zhao ZG, Xu W, Sun L, You Y, Li F, Li QB, Zou P. Immunomodulatory function of regulatory dendritic cells induced by mesenchymal stem cells. Immunol Invest. 2012;41:183–198. doi: 10.3109/08820139.2011.607877. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Cai W, Huang Q, Gu Y, Shi Y, Huang J, Zhao F, Liu Q, Wei X, Jin M, et al. Mesenchymal stem cells alleviate bacteria-induced liver injury in mice by inducing regulatory dendritic cells. Hepatology. 2014;59:671–682. doi: 10.1002/hep.26670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li G, Yuan L, Ren X, Nian H, Zhang L, Han ZC, Li X, Zhang X. The effect of mesenchymal stem cells on dynamic changes of T cell subsets in experimental autoimmune uveoretinitis. Clin Exp Immunol. 2013;173:28–37. doi: 10.1111/cei.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrión F, Nova E, Luz P, Apablaza F, Figueroa F. Opposing effect of mesenchymal stem cells on Th1 and Th17 cell polarization according to the state of CD4+ T cell activation. Immunol Lett. 2011;135:10–16. doi: 10.1016/j.imlet.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Luz-Crawford P, Kurte M, Bravo-Alegría J, Contreras R, Nova-Lamperti E, Tejedor G, Noël D, Jorgensen C, Figueroa F, Djouad F, et al. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4:65. doi: 10.1186/scrt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang H, Sun J, Li Y, Duan WM, Bi J, Qu T. Human umbilical cord-derived mesenchymal stem cells suppress proliferation of PHA-activated lymphocytes in vitro by inducing CD4(+)CD25(high)CD45RA(+) regulatory T cell production and modulating cytokine secretion. Cell Immunol. 2016;302:26–31. doi: 10.1016/j.cellimm.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Alikarami F, Yari F, Amirizadeh N, Nikougoftar M, Jalili MA. The Immunosuppressive Activity of Amniotic Membrane Mesenchymal Stem Cells on T Lymphocytes. Avicenna J Med Biotechnol. 2015;7:90–96. [PMC free article] [PubMed] [Google Scholar]
- 20.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 21.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Ren G, Huang Y, Su J, Han Y, Li J, Chen X, Cao K, Chen Q, Shou P, et al. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ. 2012;19:1505–1513. doi: 10.1038/cdd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 25.Melief SM, Geutskens SB, Fibbe WE, Roelofs H. Multipotent stromal cells skew monocytes towards an anti-inflammatory interleukin-10-producing phenotype by production of interleukin-6. Haematologica. 2013;98:888–895. doi: 10.3324/haematol.2012.078055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melief SM, Schrama E, Brugman MH, Tiemessen MM, Hoogduijn MJ, Fibbe WE, Roelofs H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013;31:1980–1991. doi: 10.1002/stem.1432. [DOI] [PubMed] [Google Scholar]
- 27.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 28.Gershon RK, Cohen P, Hencin R, Liebhaber SA. Suppressor T cells. J Immunol. 1972;108:586–590. [PubMed] [Google Scholar]
- 29.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 31.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 32.Workman CJ, Szymczak-Workman AL, Collison LW, Pillai MR, Vignali DA. The development and function of regulatory T cells. Cell Mol Life Sci. 2009;66:2603–2622. doi: 10.1007/s00018-009-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 35.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 37.van der Vliet HJ, Nieuwenhuis EE. IPEX as a result of mutations in FOXP3. Clin Dev Immunol. 2007;2007:89017. doi: 10.1155/2007/89017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochs HD, Ziegler SF, Torgerson TR. FOXP3 acts as a rheostat of the immune response. Immunol Rev. 2005;203:156–164. doi: 10.1111/j.0105-2896.2005.00231.x. [DOI] [PubMed] [Google Scholar]
- 39.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 40.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 42.Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, Chatila TA. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- 43.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 46.Rubtsov YP, Rudensky AY. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat Rev Immunol. 2007;7:443–453. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]
- 47.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156:149–160. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joshi PC, Zhou X, Cuchens M, Jones Q. Prostaglandin E2 suppressed IL-15-mediated human NK cell function through down-regulation of common gamma-chain. J Immunol. 2001;166:885–891. doi: 10.4049/jimmunol.166.2.885. [DOI] [PubMed] [Google Scholar]
- 50.Walker W, Rotondo D. Prostaglandin E2 is a potent regulator of interleukin-12- and interleukin-18-induced natural killer cell interferon-gamma synthesis. Immunology. 2004;111:298–305. doi: 10.1111/j.1365-2567.2004.01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serezani CH, Chung J, Ballinger MN, Moore BB, Aronoff DM, Peters-Golden M. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. Am J Respir Cell Mol Biol. 2007;37:562–570. doi: 10.1165/rcmb.2007-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simkin NJ, Jelinek DF, Lipsky PE. Inhibition of human B cell responsiveness by prostaglandin E2. J Immunol. 1987;138:1074–1081. [PubMed] [Google Scholar]
- 53.Kaliński P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- 54.Jonuleit H, Kühn U, Müller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 55.Rieser C, Böck G, Klocker H, Bartsch G, Thurnher M. Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603–1608. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khayrullina T, Yen JH, Jing H, Ganea D. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol. 2008;181:721–735. doi: 10.4049/jimmunol.181.1.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho KS, Lee JH, Park MK, Park HK, Yu HS, Roh HJ. Prostaglandin E2 and Transforming Growth Factor-β Play a Critical Role in Suppression of Allergic Airway Inflammation by Adipose-Derived Stem Cells. PLoS One. 2015;10:e0131813. doi: 10.1371/journal.pone.0131813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 59.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596–3599. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 61.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 62.Cho KS, Park MK, Kang SA, Park HY, Hong SL, Park HK, Yu HS, Roh HJ. Adipose-derived stem cells ameliorate allergic airway inflammation by inducing regulatory T cells in a mouse model of asthma. Mediators Inflamm. 2014;2014:436476. doi: 10.1155/2014/436476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 64.Xia ZW, Xu LQ, Zhong WW, Wei JJ, Li NL, Shao J, Li YZ, Yu SC, Zhang ZL. Heme oxygenase-1 attenuates ovalbumin-induced airway inflammation by up-regulation of foxp3 T-regulatory cells, interleukin-10, and membrane-bound transforming growth factor- 1. Am J Pathol. 2007;171:1904–1914. doi: 10.2353/ajpath.2007.070096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 66.Ren G, Su J, Zhang L, Zhao X, Ling W, L’huillie A, Zhang J, Lu Y, Roberts AI, Ji W, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 67.Ren G, Zhao X, Zhang L, Zhang J, L’Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ren G, Roberts AI, Shi Y. Adhesion molecules: key players in Mesenchymal stem cell-mediated immunosuppression. Cell Adh Migr. 2011;5:20–22. doi: 10.4161/cam.5.1.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benvenuto F, Voci A, Carminati E, Gualandi F, Mancardi G, Uccelli A, Vergani L. Human mesenchymal stem cells target adhesion molecules and receptors involved in T cell extravasation. Stem Cell Res Ther. 2015;6:245. doi: 10.1186/s13287-015-0222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghannam S, Pène J, Moquet-Torcy G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010;185:302–312. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- 71.Huter EN, Punkosdy GA, Glass DD, Cheng LI, Ward JM, Shevach EM. TGF-beta-induced Foxp3+ regulatory T cells rescue scurfy mice. Eur J Immunol. 2008;38:1814–1821. doi: 10.1002/eji.200838346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 73.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 74.Carter NA, Vasconcellos R, Rosser EC, Tulone C, Muñoz-Suano A, Kamanaka M, Ehrenstein MR, Flavell RA, Mauri C. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol. 2011;186:5569–5579. doi: 10.4049/jimmunol.1100284. [DOI] [PubMed] [Google Scholar]
- 75.Carter NA, Rosser EC, Mauri C. Interleukin-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of T regulatory type 1 cells and reduction of collagen-induced arthritis. Arthritis Res Ther. 2012;14:R32. doi: 10.1186/ar3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 77.Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, Chalasani G, Sayegh MH, Najafian N, Rothstein DM. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121:3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gray M, Miles K, Salter D, Gray D, Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc Natl Acad Sci USA. 2007;104:14080–14085. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 80.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun JB, Flach CF, Czerkinsky C, Holmgren J. B lymphocytes promote expansion of regulatory T cells in oral tolerance: powerful induction by antigen coupled to cholera toxin B subunit. J Immunol. 2008;181:8278–8287. doi: 10.4049/jimmunol.181.12.8278. [DOI] [PubMed] [Google Scholar]
- 82.Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol. 2001;167:1081–1089. doi: 10.4049/jimmunol.167.2.1081. [DOI] [PubMed] [Google Scholar]
- 83.Parekh VV, Prasad DV, Banerjee PP, Joshi BN, Kumar A, Mishra GC. B cells activated by lipopolysaccharide, but not by anti-Ig and anti-CD40 antibody, induce anergy in CD8+ T cells: role of TGF-beta 1. J Immunol. 2003;170:5897–5911. doi: 10.4049/jimmunol.170.12.5897. [DOI] [PubMed] [Google Scholar]
- 84.Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, Ries S, Dang VD, Jaimes Y, Daridon C, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507:366–370. doi: 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, Wingfield PT, Kim SH, Egwuagu CE. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med. 2014;20:633–641. doi: 10.1038/nm.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 87.Guo Y, Chan KH, Lai WH, Siu CW, Kwan SC, Tse HF, Wing-Lok Ho P, Wing-Man Ho J. Human mesenchymal stem cells upregulate CD1dCD5(+) regulatory B cells in experimental autoimmune encephalomyelitis. Neuroimmunomodulation. 2013;20:294–303. doi: 10.1159/000351450. [DOI] [PubMed] [Google Scholar]
- 88.Peng Y, Chen X, Liu Q, Zhang X, Huang K, Liu L, Li H, Zhou M, Huang F, Fan Z, et al. Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of CD5+ regulatory B cells producing interleukin 10. Leukemia. 2015;29:636–646. doi: 10.1038/leu.2014.225. [DOI] [PubMed] [Google Scholar]
- 89.Park MJ, Kwok SK, Lee SH, Kim EK, Park SH, Cho ML. Adipose tissue-derived mesenchymal stem cells induce expansion of interleukin-10-producing regulatory B cells and ameliorate autoimmunity in a murine model of systemic lupus erythematosus. Cell Transplant. 2015;24:2367–2377. doi: 10.3727/096368914X685645. [DOI] [PubMed] [Google Scholar]
- 90.Nowakowski A, Walczak P, Janowski M, Lukomska B. Genetic Engineering of Mesenchymal Stem Cells for Regenerative Medicine. Stem Cells Dev. 2015;24:2219–2242. doi: 10.1089/scd.2015.0062. [DOI] [PubMed] [Google Scholar]
- 91.Lin YT, Chern Y, Shen CK, Wen HL, Chang YC, Li H, Cheng TH, Hsieh-Li HM. Human mesenchymal stem cells prolong survival and ameliorate motor deficit through trophic support in Huntington’s disease mouse models. PLoS One. 2011;6:e22924. doi: 10.1371/journal.pone.0022924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ito T, Itakura S, Todorov I, Rawson J, Asari S, Shintaku J, Nair I, Ferreri K, Kandeel F, Mullen Y. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation. 2010;89:1438–1445. doi: 10.1097/tp.0b013e3181db09c4. [DOI] [PubMed] [Google Scholar]
- 93.Sordi V, Melzi R, Mercalli A, Formicola R, Doglioni C, Tiboni F, Ferrari G, Nano R, Chwalek K, Lammert E, et al. Mesenchymal cells appearing in pancreatic tissue culture are bone marrow-derived stem cells with the capacity to improve transplanted islet function. Stem Cells. 2010;28:140–151. doi: 10.1002/stem.259. [DOI] [PubMed] [Google Scholar]
- 94.Edwards JC, Cambridge G. Sustained improvement in rheumatoid arthritis following a protocol designed to deplete B lymphocytes. Rheumatology (Oxford) 2001;40:205–211. doi: 10.1093/rheumatology/40.2.205. [DOI] [PubMed] [Google Scholar]
- 95.Edwards JC, Cambridge G. Prospects for B-cell-targeted therapy in autoimmune disease. Rheumatology (Oxford) 2005;44:151–156. doi: 10.1093/rheumatology/keh446. [DOI] [PubMed] [Google Scholar]
- 96.Anolik JH, Barnard J, Cappione A, Pugh-Bernard AE, Felgar RE, Looney RJ, Sanz I. Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum. 2004;50:3580–3590. doi: 10.1002/art.20592. [DOI] [PubMed] [Google Scholar]
- 97.Tedder TF, Engel P. CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol Today. 1994;15:450–454. doi: 10.1016/0167-5699(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 98.Uchida J, Lee Y, Hasegawa M, Liang Y, Bradney A, Oliver JA, Bowen K, Steeber DA, Haas KM, Poe JC, et al. Mouse CD20 expression and function. Int Immunol. 2004;16:119–129. doi: 10.1093/intimm/dxh009. [DOI] [PubMed] [Google Scholar]
- 99.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185:2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Payne NL, Sun G, McDonald C, Moussa L, Emerson-Webber A, Loisel-Meyer S, Medin JA, Siatskas C, Bernard CC. Human adipose-derived mesenchymal stem cells engineered to secrete IL-10 inhibit APC function and limit CNS autoimmunity. Brain Behav Immun. 2013;30:103–114. doi: 10.1016/j.bbi.2013.01.079. [DOI] [PubMed] [Google Scholar]
- 102.Liao W, Pham V, Liu L, Riazifar M, Pone EJ, Zhang SX, Ma F, Lu M, Walsh CM, Zhao W. Mesenchymal stem cells engineered to express selectin ligands and IL-10 exert enhanced therapeutic efficacy in murine experimental autoimmune encephalomyelitis. Biomaterials. 2016;77:87–97. doi: 10.1016/j.biomaterials.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 104.Nowakowski A, Walczak P, Lukomska B, Janowski M. Genetic Engineering of Mesenchymal Stem Cells to Induce Their Migration and Survival. Stem Cells Int. 2016;2016:4956063. doi: 10.1155/2016/4956063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hunsberger JG, Rao M, Kurtzberg J, Bulte JW, Atala A, LaFerla FM, Greely HT, Sawa A, Gandy S, Schneider LS, et al. Accelerating stem cell trials for Alzheimer’s disease. Lancet Neurol. 2015;15:219–230. doi: 10.1016/S1474-4422(15)00332-4. [DOI] [PubMed] [Google Scholar]


