Abstract
Background
Transrectal ultrasound-guided prostate biopsies (TRUSBx), in spite of being one of the most frequently performed urological office procedures, are associated with a spectrum of complications, most significantly including infection. The aim of the study is to evaluate the prevalence of fluoroquinolone-resistant bacteria in rectal swabs from our local population prior to TRUSBx and to identify risk factors among a patient population harboring fluoroquinolone-resistant organisms.
Methods
We prospectively included 541 men who were submitted for TRUSBx in our center from March 2011 to June 2015. The indications for TRUSBx were an elevated prostate-specific antigen level and/or abnormal digital rectal exam. All patients were randomly divided into two groups: Group 1 (n = 279 cases) who received standard empirical prophylactic antibiotics and Group 2 who received targeted prophylaxis based on a rectal swab culture and susceptibility result. Differences in risk factors between quinolone-resistant and nonresistant patients were compared. Univariate and multivariate analyses were performed to identify independent potential risk factors associated with fluoroquinolone-resistant rectal flora.
Results
Sixteen out of 271 men developed infectious complications after TRUSBx in the group receiving standard empirical prophylaxis (5.7%). No men in the group who received targeted prophylactic antibiotic guided by rectal swab developed infectious complications. Among the 262 patients who underwent prebiopsy rectal swab cultures, 76 men (29%) displayed fluoroquinolone-resistant rectal flora (29%). In the multivariate analysis, a history of antibiotic exposure before prostate biopsy was the only independent factor associated with an increased risk of fluoroquinolone resistance.
Conclusion
Determining the prevalence of fluoroquinolone resistance in rectal flora has important implications in the selection of targeted prophylactic antibiotic regimens. Antimicrobial profiles guided by rectal swabs may prove useful to optimize prophylaxis prior to TRUSBx; this strategy is effective at reducing the rates of infectious complications, including sepsis, especially in men at higher risk of infectious complications.
Keywords: Biopsy, Prophylactic antibiotic, Prostate biopsy, Transrectal
1. Introduction
Transrectal ultrasound-guided prostate biopsies (TRUSBx), in spite of being a frequently performed urological procedure, are associated with a spectrum of complications, most significantly including infection, which affects up to 5% of patients.1 In the most severe cases, infection leads to sepsis, which poses significant morbidity to patients with inpatient hospital stays, intensive care requirements, and even death. Escherichia coli is the pathogen most commonly associated with infections after TRUSBx.2, 3, 4
Antibiotic prophylaxis with fluoroquinolones (FQ) is currently used on a regular routine basis for preventing sepsis after TRUSBx because of its broad spectrum activity against gram-positive and gram-negative organisms and the convenience of using an oral agent with a high sustained concentration in urine and prostate tissue;5, 6, 7 however, there is growing evidence that the infection rate after TRUSBx is on the rise and it continues to be a problem, frequently caused by an increasing prevalence of FQ-resistant organisms.8, 9, 10, 11, 12
In an attempt to reduce the rate of infectious complications, there are an increasing number of publications citing different prophylaxis regimens.13, 14, 15 The aim of this study is to evaluate the prevalence of FQ-resistant bacteria in rectal swabs from men presented to the outpatient department at Alexandria University Hospital, Egypt, prior to TRUSBx and to identify the risk factors among a patient population harboring FQ-resistant organisms.
2. Materials and methods
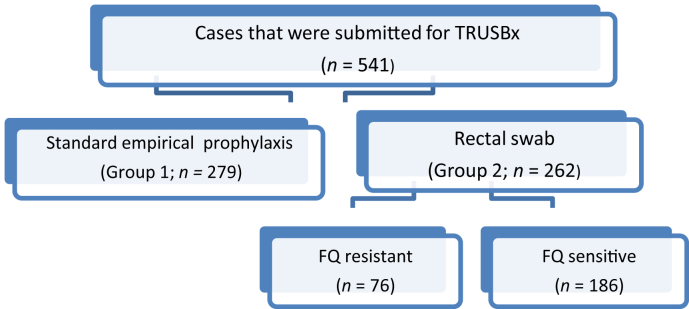
We prospectively included 541 men who were submitted for TRUSBx in our center from March 2011 to June 2015. All patients were randomly divided into two groups: Group 1 (n = 279 cases) who received standard empirical prophylactic antibiotic with oral ciprofloxacin 500 mg and metronidazole 500 mg at least 1 hour before biopsy and continued this twice daily for 3 days (a total of 6 doses of each antibiotic) and Group 2 who received targeted prophylaxis based on a rectal swab culture and susceptibility results performed 1 week before TRUSBx provided in a 3-day regimen on the day before the biopsy, the day of the biopsy, and the day after the biopsy (Fig. 1).
Fig. 1.
Study cases. FQ, fluroquinolones; TRUSBx, transrectal ultrasound-guided prostate biopsies.
Sealed opaque envelopes were used as the method of randomization which were placed into a box and mixed. Allocation concealment was achieved using an independent person (“biopsy nurse”) who selected one of the sealed opaque envelopes blindly. Thus patients were randomly allocated to Group 1 or Group 2 before the procedure. The study was approved through the Institutional Review Board.
Demographic data were obtained for all patients, as well as diabetic status, recurrent urinary tract infections (UTI), presence of a urinary catheter, prior FQ exposure within 6 months before the biopsy, and past TRUS biopsies (Table 1). The results of rectal swabs and infectious complications within 30 days of the biopsy were also recorded. The definition of post-TRUSBx infection was distinct clinical presentations, including fever > 38.5°C, UTI, pyelonephritis, bacteremia, prostatitis, systemic inflammatory response syndrome, and sepsis. The indications for TRUS biopsies were an elevated prostate-specific antigen level (PSA) and/or abnormal digital rectal exam. All TRUSBx were performed at our institution in an office-based setting.
Table 1.
Patients' demographics.
| Variables | Group 1 (n = 279) | Group 2 (n = 262) | P |
|---|---|---|---|
| Mean age (y) | 63.6 | 65.2 | 0.461 |
| Mean PSA (ng/mL) | 18 | 22 | 0.624 |
| Mean prostate volume (mL) | 64 | 59 | 0.187 |
| No. of diabetes patients | 73 (26.2) | 80 (30.5) | 0.422 |
| AAC (mean) | 0.252 | ||
|
175 | 156 | |
|
65 | 57 | |
|
32 | 38 | |
|
7 | 11 | |
| Prior UTI | 136 (48.7) | 116 (44.3) | 0.582 |
| Presence of urinary catheter | 35 (12.5) | 26 (9.9) | 0.182 |
| Prior antibiotic exposure within 6 mo | 90 (32.2) | 87 (33.2) | 0.922 |
| Prior prostate biopsy | 52 (18.6) | 54 (20.6) | 0.863 |
Data are presented as n (%).
ACC, age-adjusted Charlson comorbidity score; PSA, prostate-specific antigen; UTI, urinary tract infection.
2.1. TRUSBx technique
All patients provided informed consent before the biopsy after they had been instructed by the physician regarding all possible complications. Patients were strictly advised not to take nonsteroidal anti-inflammatories and anticoagulant medications for a week before the application.
A standard prebiopsy preparation was applied for all patients. No enema was used. Patients only fasted the night before. The biopsy procedure was carried out under local periprostatic anesthesia. TRUS-guided biopsies were achieved through transrectal ultrasonography using a 7-MHz probe attached. Biopsies were carried out with the patient in the left decubital position using an automated biopsy gun with a disposable 18-G biopsy needle.
All biopsies were carried out through a systematic approach (a standard 12-core biopsy taken from the base, mid gland, and the apex of the right and left sides of the lateral and far-lateral peripheral zone). Two transitional zone biopsies were added in cases of a previous history of negative biopsies.
Patients were advised to present to the emergency department if they developed symptoms of sepsis within 30 days of the biopsy.
All patients with sepsis, as defined as fever > 38°C in the presence of constitutional symptoms, were admitted for inpatient management. Empiric treatment with meropenem 1 g [intravenous (i.v.)] twice daily was commenced after collecting blood and urine for culture.
The main outcome criterion was the incidence of bacteriuria (defined as ≥ 103colony-forming units/mL) within 30 days of biopsy. Secondary endpoints included the incidence of clinical symptoms of fever, flushing, chills, or any weakness on physical examination and UTI, defined as the association of leukocyturia (>5 cells/high-power field) and bacteriuria, or any significant change in the biological results suggesting an infection including a blood cell count and C-reactive protein.
2.2. Statistical analysis
Differences in risk factors between quinolone-resistant and nonresistant patients were compared using a Fisher exact test with statistical significance ascribed at P < 0.05. Univariate and multivariate analyses were performed to identify independent potential risk factors associated with FQ-resistant rectal flora. Statistical analyses were performed with SPSS version 21.0 (SPSS Inc., Chicago, IL, USA) statistical software package.
3. Results
The mean age of patients was 63.6 years and 65.2 years in Group 1 and Group 2, respectively (P = 0.4).There was no difference in mean PSA value (P = 0.62) or age-adjusted Charlson comorbidity score (P = 0.25) between the two groups.
Sixteen men out of 279 men developed infectious complications after TRUSBx in the group receiving standard empirical prophylaxis (5.7%) in the form of fever and pyelonephritis including two cases of sepsis. None of the two men admitted for sepsis required intensive care treatment and all were successfully managed with i.v. fluids and i.v. meropenem (1 g twice daily). No men in the group who received targeted prophylactic antibiotic guided by rectal swab developed infectious complications. This result was statistically significant (P = 0.003). Culture and susceptibility results for the two men with sepsis demonstrated FQ resistant extended-spectrum β-lactamase-producing Escherichia coli.
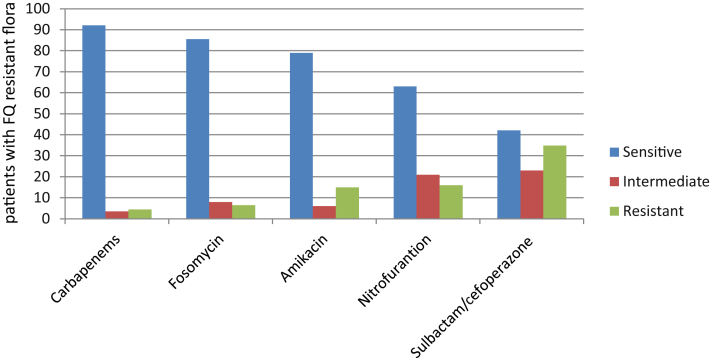
Among the 262 patients who underwent prebiopsy rectal swab cultures, 76 men (29%) displayed FQ-resistant rectal flora (29%). Of the 76 bacterial isolates, 84.2% were E. coli and 10.5% were Klebsiella pneumonia. Antimicrobial susceptibility testing to FQ-resistant strains obtained by rectal swab showed the highest resistance notably to cotrimoxazole (85%), followed by cefotaxime (63%), and the highest sensitivity shown to carbapenems (92.1%), fosomycin (85.5%), amikacin (79%), followed by nitrofurantoin (63%), and sulbactam/cefoperazone (42.1%; Fig. 2).
Fig. 2.
Antimicrobial susceptibility testing to fluoroquinolone-resistant strains.
Age, diabetic status, presence of a urinary catheter, and a history of prior prostate biopsy were not associated with FQ resistance. Prior UTI and antibiotic exposure before prostate biopsy was associated with quinolone resistance (P = 0.041 and 0.001, respectively; Table 2).
Table 2.
Risk factors and quinolone resistance status.
| Variables | FQ resistant (n = 76) | FQ sensitive (n = 186) | P |
|---|---|---|---|
| Age ≥ 65 (y) | 12 (15.7) | 30 (16.1) | 0.18 |
| Diabetes | 28 (36.8) | 52 (27.9) | 0.62 |
| Prior UTI | 39 (51.3) | 77 (41.33) | 0.041a) |
| Presence of urinary catheter | 9 (11.8) | 17 (9.1) | 0.52 |
| Prior antibiotic exposure within 6 mo | 45 (59.2) | 42 (22.5) | 0.001a) |
| Prior prostate biopsy | 18 (23.6) | 36 (19.3) | 0.24 |
Data are presented as n (%).
FQ, fluoroquinolone; UTI, urinary tract infection.
Statistically significant.
In the univariate analysis, prior UTI and history of exposure to antibiotics increased the risk of FQ resistance [odds ratio (OR), 4.45; 95% confidence interval (CI), 1.36–10.52; P = 0.02, and OR, 34.2 CI, 3.16–178.52; P = 0.001, respectively] (Table 3).
Table 3.
Univariate logistic regression analysis of factors influencing quinolone resistance.
| FQ resistance | OR (95% CI) | P |
|---|---|---|
| Age ≥ 65 y | 1.28 (0.42–4.35) | 0.453 |
| Diabetes | 3.47 (0.1–6.32) | 0.520 |
| Prior UTI | 4.45 (1.36–10.52) | 0.02a) |
| Presence of urinary catheter | 1.08 (0.22–6.43) | 0.38 |
| Prior antibiotic exposure within 6 mo | 34.2 (3.16–178.52) | 0.001a) |
| Prior prostate biopsy | 3.02 (0.82–6.88) | 0.24 |
CI, confidence interval; FQ, fluoroquinolone; OR, odds ratio; URI, urinary tract infection.
Statistically significant.
In the multivariate analysis, a history of antibiotic exposure before prostate biopsy was the only independent factor associated with increased risk of FQ resistance (OR, 41.2; 95% CI, 3.16–328.46; P = 0.008; Table 4).
Table 4.
Multivariate logistic regression analysis of factors influencing quinolone resistance.
| FQ resistance | OR (95% CI) | P |
|---|---|---|
| Prior UTI | 1.62 (0.85–11.08) | 0.659 |
| Prior antibiotic exposure within 6 mo | 41.2 (3.16–328.46) | 0.008a) |
CI, confidence interval; FQ, fluoroquinolone; OR, odds ratio; URI, urinary tract infection.
Statistically significant.
4. Discussion
The use of prophylactic antibiotics prior to TRUSBx reaches a consensus; however, data to guide the optimal choice of prophylactic antibiotic are currently lacking. No standard regimen for antibiotic prophylaxis has been formulated despite the need to optimize prophylaxis against infectious complications post-TRUSBx being recognized a long time ago.16
Multiple strategies aimed at improving the safety and acceptability of prostate biopsies are the subject of ongoing research studies. Different prophylactic regimens depending on local microbiological profiles, and alternate approaches to prostate biopsies, such as the transperineal approach, are considered depending on availability.17 Rectal disinfection and washing the biopsy needle with povidone–iodine are other approaches used with the aim of reducing infection complications post-TRUSBx in some centers.18, 19
The European Association of Urology guideline recommends the use of a FQ as a first-line agent for the prevention of infection from transrectal prostate biopsy, with ciprofloxacin being superior to ofloxacin.20 Our current clinical practice usually includes an empirical oral FQ antibiotic usage of 3 days starting the day before the procedure owing to their excellent prostatic penetration;7 these agents also provide good coverage against the key pathogens implicated in post-TRUSBx infectious complications.21 However, FQ resistance has emerged as a growing problem resulting in a significant increase in infectious complications in men undergoing TRUS biopsies.
Many reports have shown that the prevalence of FQ-resistant E. coli in Western countries is on the rise.9, 11 In the USA, FQ-resistant bacteria were identified in 22% of samples from rectal swab cultures before TRUSBx.22 Furthermore, in American men who developed acute prostatitis after TRUSBx, the FQ-resistant rate was reported to be 57.1%.23 Batura et al24 obtained rectal swabs from 445 men undergoing TRUSBx and found a 13.3% incidence of FQ-resistant coliforms. The present study looks at the local susceptibility for FQ in our patient population, with an incidence of FQ resistance as high as 29% which is relatively high and may be due to an increase in antibiotic prescriptions in the past few years. In light of the worldwide emergence of FQ resistance and its increasing implication in post-TRUSBx infectious complications including sepsis, the current recommendations for antimicrobial prophylaxis need to be reevaluated.
Our results showed that targeted antibiotic prophylaxis guided by a pre-TRUSBx rectal swab was dramatically effective in lowering postbiopsy infectious complications. No men in the group who received targeted prophylactic antibiotics guided by a rectal swab developed an infectious complication. In contrast, men treated with empirical prophylactic antibiotic regimen had a 5.7% rate of infectious complications despite receiving ciprofloxacin and metronidazole for 3 days. This rate matches the worldwide reported incidence for infectious complication post-TRUSBx of around 2–6%.17 Two cases of sepsis had cultures of ciprofloxacin-resistant E. coli, suggesting that ciprofloxacin resistance is a significant contributor to the failure of standard prophylaxis. This may be attributed to the frequent unsupervised use of these antibiotics or inadequate dosing with subsequent development of resistance in the rectal flora, creating potentially worse problems in cases of post-TRUS biopsy sepsis, where these drugs would have reduced efficacy as empirical treatment.
Several reports in literature have suggested that rectal swab cultures before a biopsy may allow for an individualized and targeted approach to providing prophylactic antibiotics and decreased overall cost of care. Duplessis et al22 showed that rectal cultures obtained before TRUSBx with the use of selective media to identify FQ-resistant Enterobacteriaceae facilitate targeted antibiotic prophylaxis and appear to be highly efficacious in reducing postprostatic biopsy infection rates. Taylor et al23 reported no infectious complications in 112 men who received targeted antimicrobial prophylaxis and this was associated with a reduced cost of care. Our data show similar results, with no cases of infectious complications in the group that received targeted antibiotic prophylaxis guided by rectal swab culture, whereas 16 cases among 279 patients receiving empirical FQ prophylaxis developed infectious complications including two cases of sepsis (P = 0.003).
Concerns regarding the practical implementation of rectal swab collection need considering. Optimal timing of swab collection, duration of targeted antibiotic regimen, target population, and cost effectiveness has to be clearly established. The feasibility, and cost effectiveness of routine rectal swabs for every patient undergoing TRUSBx must be considered, as it represents a significant burden for clinical microbiology laboratories, and would incur several additional costs (culture media, targeted antibiotics, etc.).
In a previous study comparing cost-effectiveness of targeted versus empirical prophylaxis per 100 men undergoing TRUSBx, Taylor et al23 showed that the total cost of managing infectious complications in patients in the empirical group was US $13,219 including hospital admission, outpatient and emergency room visits, prolonged antibiotic treatment, diagnostic imaging procedures, laboratory tests, and professional fees. Cost-effectiveness analysis revealed that targeted prophylaxis yielded a cost savings of US $4,499 per post-TRUSBx infectious complication averted. Currently, there is no conclusive evidence of the overall benefit of routine adoption of targeted prophylaxis and the burden of the additional costs limits the widespread replacement of such an approach.
A more practical and feasible approach to optimize the use of antimicrobial prophylaxis for TRUSBx is to consider the selective application of rectal swabs before prostate biopsy in patients with high risk factors and to use the standard protocol for low-risk patients. However, men at high risk for developing post-TRUSBx infections are not yet well defined. A number of recent studies tried to look at these risk factors for infection post-TRUSBx.
Liss et al11 obtained rectal swabs from 136 men before TRUSBx over a 3-month period. In 22% of patients, rectal cultures showed quinolone-resistant E. coli, and patients with diabetes, Asian ethnicity, and a prior history of TRUSBx were determined to be at a higher risk for colonization with resistant organisms, although these differences did not reach statistical significance. In this series, five patients (3.6%) developed post-TRUSBx fever, among which only one had a positive rectal culture. Kanafani et al25 and Lautenbach et al26 pointed out in their studies that previous use of FQ was an independent risk factor for acquiring infections with extended-spectrum β-lactamase-producing E. coli-producing organisms. Similar to those observations, prior UTI and history of exposure to antibiotics increased the risk of FQ resistance by univariate analysis in the present study. In the multivariate analysis, a history of antibiotic exposure before prostate biopsy was the only independent factor associated with increased risk of FQ resistance (P = 0.008).
As an alternative to FQ standard prophylaxis, changing to broad-spectrum antimicrobials with extremely low resistance rates in rectal flora, eliminating the need for rectal culture has also been suggested.12, 14 However, the high cost and frequent use of these antibiotics again may lead to the subsequent development of resistance in rectal microbiota, potentially limiting its use in prophylaxis prior to TRUSBx. Our patients' antimicrobial susceptibility testing showed the highest sensitivity to cabapenems, fosomycin, and amikacin.
The present study raises awareness of local susceptibility for FQ in our patient population and highlights the utility of a rectal swab as an approach to optimize antibiotic prophylaxis prior to TRUSBx in high-risk patients. Thus a benefit of screening prior to TRUSBx and targeted prophylaxis should be considered as a thoughtful, predictable alternative to routine empirical prophylaxis. Using a risk stratification approach seems to be effective at identifying those men at higher risk of infectious complications. Future studies will need to evaluate the cost effectiveness and clinical utility of a prebiopsy rectal culture in targeting antibiotic prophylaxis.
We acknowledge several limitations of the present study. Firstly, there might be a recall bias, relying on patient recall for past histories of UTI and antibiotic use. Similarly, the frequency of exposure to FQ and the duration preceding TRUSBx could not be ascertained, therefore it is possible that some patients might have forgotten these past events which may have led to an underestimation of the total number of patients with a history of antibiotic use. Secondly, a true cost-benefit analysis between routine empirical antibiotic prophylaxis and targeted antibiotic prophylaxis has not been formally undertaken in our study. Finally, lack of culture standardization in microbiological laboratories may represent a limiting factor.
In conclusion, determining the prevalence of FQ resistance in rectal flora has important implications in the selection of targeted prophylactic antibiotic regimens. Antimicrobial profiles guided by rectal swabs may prove useful to optimize prophylaxis prior to TRUSBx; this strategy is effective at reducing the rates of infectious complications, including sepsis, especially in men at higher risk for infectious complications.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Challacombe B., Dasgupta P., Patel U., Amoroso P., Kirby R. Recognizing and managing the complications of prostate biopsy. BJU Int. 2011;108:1233–1234. doi: 10.1111/j.1464-410X.2011.10621.x. [DOI] [PubMed] [Google Scholar]
- 2.Nam R.K., Saskin R., Lee Y., Liu Y., Law C., Klotz L.H. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2010;183:963–968. doi: 10.1016/j.juro.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 3.Loeb S., van den Heuvel S., Zhu X., Bangma C.H., Schröder F.H., Roobol M.J. Infectious complications and hospital admissions after prostate biopsy in a European randomized trial. Eur Urol. 2012;61:1110–1114. doi: 10.1016/j.eururo.2011.12.058. [DOI] [PubMed] [Google Scholar]
- 4.Loeb S., Carter H.B., Berndt S., Ricker W., Schaeffer E.M. Complications of prostate biopsy: Data from SEER-Medicare. J Urol. 2011;186:1830–1834. doi: 10.1016/j.juro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puig J., Darnell A., Bermúdez P., Malet A., Serrate G., Baré M. Transrectal ultrasound-guided prostate biopsy: is antibiotic prophylaxis necessary? Eur Radiol. 2006;16:939–943. doi: 10.1007/s00330-005-0076-2. [DOI] [PubMed] [Google Scholar]
- 6.Kapoor D.A., Klimberg I.W., Malek G.H., Wegenke J.D., Cox C.E., Patterson A.L. Single-dose oral ciprofloxacin versus placebo for prophylaxis during transrectal prostate biopsy. Urology. 1998;52:552–558. doi: 10.1016/s0090-4295(98)00296-9. [DOI] [PubMed] [Google Scholar]
- 7.Aron M., Rajeev T.P., Gupta N.P. Antibiotic prophylaxis for transrectal needle biopsy of the prostate: a randomized controlled study. BJU Int. 2000;85:682–685. doi: 10.1046/j.1464-410x.2000.00576.x. [DOI] [PubMed] [Google Scholar]
- 8.Ismail M., Saini A., Nigam R. Ciprofloxacin-resistant infection after transrectal ultrasonography-guided prostate biopsy: should we reassess our practice? BJU Int. 2011;108:305–306. doi: 10.1111/j.1464-410X.2011.10473.x. [DOI] [PubMed] [Google Scholar]
- 9.Zaytoun O.M., Vargo E.H., Rajan R., Berglund R., Gordon S., Jones J.S. Emergence of fluoroquinolone-resistant Escherichia coli as cause of postprostate biopsy infection: implications for prophylaxis and treatment. Urology. 2011;7:1035–1041. doi: 10.1016/j.urology.2010.12.067. [DOI] [PubMed] [Google Scholar]
- 10.Akduman B., Akduman D., Tokgöz H., Erol B., Türker T., Ayoğlu F. Long-term fluoroquinolone use before the prostate biopsy may increase the risk of sepsis caused by resistant microorganisms. Urology. 2011;78:250–255. doi: 10.1016/j.urology.2011.02.065. [DOI] [PubMed] [Google Scholar]
- 11.Liss M.A., Chang A., Santos R., Nakama-Peeples A., Peterson E.M., Osann K. Prevalence and significance of fluoroquinolone resistant Escherichia coli in patients undergoing transrectal ultrasound guided prostate needle biopsy. J Urol. 2011;185:1283–1288. doi: 10.1016/j.juro.2010.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kehinde E.O., Al-Maghrebi M., Sheikh M., Anim J.T. Combined ciprofloxacin and amikacin prophylaxis in the prevention of septicemia after transrectal ultrasound guided biopsy of the prostate. J Urol. 2013;189:911–915. doi: 10.1016/j.juro.2012.08.237. [DOI] [PubMed] [Google Scholar]
- 13.Adibi M., Hornberger B., Bhat D., Raj G., Roehrborn C.G., Lotan Y. Reduction in hospital admission rates due to post-prostate biopsy infections after augmenting standard antibiotic prophylaxis. J Urol. 2013;189:535–540. doi: 10.1016/j.juro.2012.08.194. [DOI] [PubMed] [Google Scholar]
- 14.Batura D., Rao G.G., Nielson P.B., Charlett A. Adding amikacin to fluoroquinolone-based antimicrobial prophylaxis reduces prostate biopsy infection rates. BJU Int. 2011;107:760–764. doi: 10.1111/j.1464-410X.2010.09715.x. [DOI] [PubMed] [Google Scholar]
- 15.Simsir A., Kismali E., Mammadov R., Gunaydin G., Cal C. Is it possible to predict sepsis, the most serious complication in prostate biopsy? Urol Int. 2010;84:395–399. doi: 10.1159/000296290. [DOI] [PubMed] [Google Scholar]
- 16.Aus G., Ahlgren G., Bergdahl S., Hugosson J. Infection after transrectal core biopsies of the prostate—Risk factors and antibiotic prophylaxis. Br J Urol. 1996;77:851–855. doi: 10.1046/j.1464-410x.1996.01014.x. [DOI] [PubMed] [Google Scholar]
- 17.Williamson D.A., Barrett L.K., Rogers B.A., Freeman J.T., Hadway P., Paterson D.L. Infectious complications following transrectal ultrasound (TRUS) guided prostate biopsy: new challenges in the era of multi-drug resistant Escherichia coli. Clin Infect Dis. 2013;52:267–274. doi: 10.1093/cid/cit193. [DOI] [PubMed] [Google Scholar]
- 18.Abughosh Z., Margolick J., Goldenberg S.L., Taylor S.A., Afshar K., Bell R. A prospective randomized trial of povidone-iodine prophylactic cleansing of the rectum before transrectal ultrasound guided prostate biopsy. J Urol. 2013;189:1326–1331. doi: 10.1016/j.juro.2012.09.121. [DOI] [PubMed] [Google Scholar]
- 19.Koc G., Un S., Filiz D.N., Akbay K., Yilmaz Y. Does washing the biopsy needle with povidone-iodine have an effect on infection rates after transrectal prostate needle biopsy? Urol Int. 2010;85:147–151. doi: 10.1159/000314340. [DOI] [PubMed] [Google Scholar]
- 20.Heidenreich A., Bastian P.J., Bellmunt J., Bolla M., Joniau S., Mason M.D. European Association of Urology; Arnhem (NL): 2013. Guidelines on prostate cancer [Internet]http://www.uroweb.org/gls/pdf/09_Prostate_Cancer_LR.pdf [cited 2013 May 10]. Available from: [Google Scholar]
- 21.Campeggi A., Ouzaid I., Xylinas E., Lesprit P., Hoznek A., Vordos D. Acute bacterial prostatitis after transrectal ultrasound-guided prostate biopsy: Epidemiological, bacteria and treatment patterns from a 4-year prospective study. Int J Urol. 2014;21:152–155. doi: 10.1111/iju.12207. [DOI] [PubMed] [Google Scholar]
- 22.Duplessis C.A., Bavaro M., Simons M.P., Marguet C., Santomauro M., Auge B. Rectal cultures before transrectal ultrasound-guided prostate biopsy reduce post-prostatic biopsy infection rates. Urology. 2012;79:556–561. doi: 10.1016/j.urology.2011.09.057. [DOI] [PubMed] [Google Scholar]
- 23.Taylor A.K., Zembower T.R., Nadler R.B., Scheetz M.H., Cashy J.P., Bowen D. Targeted antimicrobial prophylaxis using rectal swab cultures in men undergoing transrectal ultrasound guided prostate biopsy is associated with reduced incidence of postoperative infectious complications and cost of care. J Urol. 2012;187:1275–1279. doi: 10.1016/j.juro.2011.11.115. [DOI] [PubMed] [Google Scholar]
- 24.Batura D., Rao G.G., Nielsen P.B. Prevalence of antimicrobial resistance in intestinal flora of patients undergoing prostatic biopsy: implications for prophylaxis and treatment of infections after biopsy. BJU Int. 2010;106:1017–1020. doi: 10.1111/j.1464-410X.2010.09294.x. [DOI] [PubMed] [Google Scholar]
- 25.Kanafani Z.A., Mehio-Sibai A., Araj G.F., Kanaan M., Kanj S.S. Epidemiology and risk factors for extended-spectrum beta-lactamase-producing organisms: a case control study at a tertiary care center in Lebanon. Am J Infect Control. 2005;33:326–332. doi: 10.1016/j.ajic.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Lautenbach E., Patel J.B., Bilker W.B., Edelstein P.H., Fishman N.O. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: Risk factors for infection and impact of resistance on outcomes. Clin Infect Dis. 2001;32:1162–1171. doi: 10.1086/319757. [DOI] [PubMed] [Google Scholar]