Abstract
Background
The aim of this study was to evaluate the incidence and mortality of prostate cancer and their relationship with the Human Development Index (HDI) and its components in Asia in 2012.
Methods
This study was an ecological study conducted based on the GLOBOCAN project of the World Health Organization. The correlation between standardized incidence rate (SIR) and standardized mortality rate (SMR) of prostate cancer with HDI and its components was assessed using SPSS Inc Version 18.0 (Chicago).
Results
There were 1,094,916 incident cases of prostate cancer and 307,481 deaths recorded in 2012 worldwide. SIR and SMR due to HDI were 72 and 9.7 in very high human development regions, 37.5 and 12.9 in high human development regions, 7 and 3.7 in medium human development regions, and 14.9 and 12.1 in low human development regions per 100,000 people, respectively. A positive correlation of 0.475 was seen between SIR of prostate cancer and HDI (P ≤ 0.001). Also, a negative correlation of 0.160 was seen between SMR of prostate cancer and HDI (P = 0.032).
Conclusion
The incidence of prostate cancer is high in countries with higher development. A positive correlation was observed between the SIR of prostate cancer and the HDI and its components, such as life expectancy at birth, mean years of schooling, and the gross national income per capita. In addition, there was a negative correlation between SMR and HDI.
Keywords: Development, Incidence, Mortality, Prostate cancer
1. Introduction
Currently, cancer is one of the leading causes of death in many high-income countries.1 Prostate cancer is one of the most common cancers among men.2, 3, 4, 5 Deaths from prostate cancer are second highest after deaths from lung cancer.6 The incidence and prevalence of prostate cancer vary in different parts of the world, with the highest in North America and the lowest in South Asia.7 It is estimated that 1.1 million men with prostate cancer were diagnosed in 2012 and 70% of them (795,000 cases) were in developed countries.8 The highest age-specific incidence rate in New Zealand and Australia is 111.6 per 100,000 and the lowest rate is in South Asia with a level of 4.5 per 100,000.3, 7
Several studies have shown that the incidence of prostate cancer in recent years has grown substantially.9, 10, 11 The increase in the incidence of prostate cancer could be due to greater access to prostate-specific antigen (PSA) diagnostic tests by communities and other factors such as lifestyle, nutrition, physical activity, environmental factors, and smoking.12, 13, 14, 15 The PSA test that started in 1980 has caused a significant increase in the incidence of prostate cancer.16 Studies have shown that countries with higher social and economic levels have higher levels of prostate cancer; in contrast, prostate cancer is seen less often in countries with lower socioeconomic levels.17 It has been observed that incidence of this illness increases with age, and it is thought that the aging of the global population and increase in life expectancy will increase the incidence of this disease in the future.18, 19, 20
The effect of aging in low- and middle-income countries is greater than in high-income countries. It is predicted that there will be 20.3 million new cases of prostate cancer in 2030 and that 13.2 million of them will not survive. In 2008, there were 12.7 million new cases, 7.6 million of these died.21
Economic and social levels are known to affect the incidence and mortality of cancer.22 The Human Development Index (HDI) is a predictor of the economic and social level of different countries that can be associated with many diseases, including cancer.17, 23, 24, 25
Several studies have examined the role of the HDI and its relationship with the incidence and mortality of cancer.4, 17, 26 Due to the probable role of HDI in changing the incidence and prevalence of prostate cancer and the importance of knowledge about the incidence and prevalence of this disease in programming and performing etiology studies, and lack of an integrated worldwide study of HDI, this study investigated the standardized incidence rate (SIR) and standardized mortality rate (SMR) of prostate cancer and their relationship with HDI in 2012.
2. Materials and methods
2.1. Method for estimating age-specific incidence and mortality rates in the global cancer project by the international agency for research on cancer
This was a global ecologic study to assess the correlation between the age-specific incidence and mortality rate and the HDI, and its particulars, including life expectancy at birth, mean years of schooling, and gross national income per capita. Data about the age-specific incidence and mortality rates for every country in 2012 were obtained from the global cancer project (http://globocan.iarc.fr/Default.aspx),27 and the HDI from the Human Development Report 2013,28 which includes information about the HDI for every country in 2012.
2.2. Estimate of age-specific incidence rate
The methods of estimation are country specific, and the quality of the estimation depends upon the quality and amount of information available for each country. In theory, there are as many methods as countries, and because of the variety and the complexity of these methods, an overall quality score for the incidence and mortality estimates combined is almost impossible to establish. However, an alphanumeric scoring system that independently describes the availability of incidence and mortality data has been established at the country level. The combined score is presented together with the estimates for each country with the aim of providing a broad indication of the robustness of the estimation.
The methods to estimate the sex- and age-specific incidence rates of cancer for a specific country fall into one of the following broad categories, in priority order: (1) rates projected to 2012 (38 countries); (2) most recent rates applied to 2012 population (20 countries); (3) estimates from national mortality by modeling, using incidence mortality ratios derived from recorded data in country-specific cancer registries (13 countries); (4) estimates from national mortality estimates by modeling, using incidence mortality ratios derived from recorded data in local cancer registries in neighboring countries (nine European countries); (5) estimates from national mortality estimates using modeled survival (32 countries); (6) estimates as the weighted average of the local rates (16 countries); (7) one cancer registry covering a part of a country is used as representative of the country profile (11 countries); (8) age/sex specific rates for all cancers were partitioned using data on relative frequency of different cancers (by age and sex) (12 countries); and (9) rates are those of neighboring countries or registries in the same area (33 countries).27
2.3. Estimate of age-specific mortality rate
Depending on the degree of detail and accuracy of the national mortality data, six methods have been utilized in the following order of priority: (1) rates projected to 2012 (69 countries); (2) most recent rates applied to 2012 population (26 countries); (3) estimates as the weighted average of regional rates (one country); (4) estimates from national incidence estimates by modeling, using country-specific survival (two countries); (5) estimates from national incidence estimates using modeled survival (83 countries); and (6) rates are those of neighboring countries or registries in the same area (three countries).8
2.4. HDI
HDI is a composite measure of indicators along three components, including life expectancy, educational attainment, and command over the resources needed for a decent living. All groups and regions have seen notable improvement in all HDI components, with faster progress in low- and medium-HDI countries. On this basis, the world is becoming less unequal. Nevertheless, national averages hide large variations in human experience. Wide disparities remain within developing and developed countries and income inequality within and among many countries has been rising. According to the HDI, countries are divided into four categories: very high HDI (≥ 0.80), high HDI (0.80 > HDI > 0.710), medium HDI (0.710 ≥ HD ≥ 0.535), and low HDI (<0.535).28
2.5. Statistical analysis
In this study, we used the correlation bivariate method for assessment of the correlation between age-specific incidence and mortality rate with HDI and its details, which include life expectancy at birth, mean years of schooling, and gross national income per capita. Statistical significance was assumed at P < 0.05. All reported P values were two-sided. Statistical analyses were performed using SPSS Inc Version 18.0 (Chicago).
3. Results
In total, 1,094,916 cases of prostate cancer were recorded worldwide in 2012. The following 10 countries had the highest number of recent cases: United States (US) 233,159 cases; Brazil 72,536 cases; Germany 68,262 cases; France (metropolitan) 56,841 cases; Japan 55,970 cases; China 46,745 cases; United Kingdom 45,406 cases; Italy 44,525 cases; Spain 27,853 cases; and Canada 27,087. These countries account for 678,384 cases (61.95%) of the global total.
The following 10 countries had the highest SIR of prostate cancer: France 129.7 per 100,000 people; Martinique 123.1 per 100,00; Norway 227.2 per 100,000; Trinidad and Tobago 115.2 per 100,000; Barbados 123.9 per 100,000; Sweden and Ireland 114.6 per 100,000; Australia 119 per 100,000; New Caledonia 107.2 per 100,000; French Polynesia 114.9 per 100,000; and Switzerland 114.2 per 100,000. The following 10 countries had the lowest SIR of prostate cancer: Uzbekistan 1.7 per 100,000 people; Bhutan 1.2 per 100,000; Nepal 1.5 per 100,000; Uzbekistan 2.0 per 100,000; Turkmenistan 2.1 per 100,000; Tajikistan 2.3 per 100,000; Yemen with 2.7 per 100,000; Sri Lanka 3.0 per 100,000; North Korea 3.2 per 100,000; and 3.4 per 100,000.
The SIR due to HDI was 72 per 100,000 people in very high human development regions; 37.5 per 100,000 in high human development regions; 7.0 per 100,000 in medium human development regions; and 14.9 per 100,000 in low human development regions. Also, SIR was 58.5 per 100,000 people in the World Health Organization (WHO) Europe Region; 75 per 100,000 in the WHO Americas Region; 12.6 per 100,000 in the WHO Western Pacific Region; 9.7 per 100,000 in the WHO South-East Asia Region; and 26.8 per 100,000 in the WHO Africa Region. Also, it was 68 per 100,000 people in more-developed regions and 14.5 per 100,000 in less-developed areas.
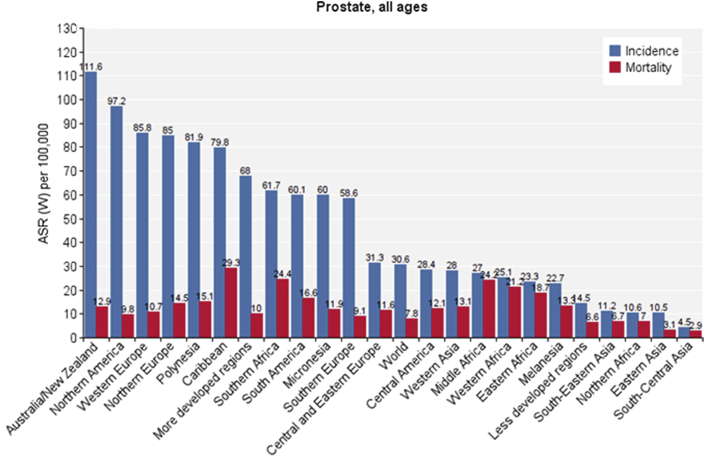
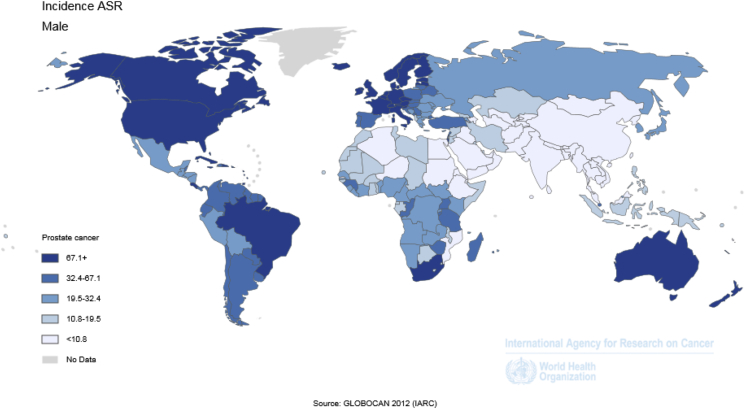
The number of cases and crude SIR of prostate cancer in different regions of the world is shown in Table 1. Different parts of the world are sorted from high to low according to SIR. The highest and lowest standardized regions can be observed for each sex (Fig. 1, Fig. 2 and Table 1).
Table 1.
Number and rate of crude and standardized incidence and mortality of prostate cancer worldwide, in 2012.
| Prostate – estimated incidence, all ages |
Prostate – estimated mortality, all ages |
||||||
|---|---|---|---|---|---|---|---|
| Population | No. | Crude rate | ASR (W) | Population | No. | Crude rate | ASR (W) |
| Australia/New Zealand | 25,296 | 185.7 | 111.6 | Caribbean | 7,970 | 38.0 | 29.3 |
| Oceania | 26,130 | 138.3 | 101.9 | Southern Africa | 3,759 | 12.9 | 24.4 |
| North America | 260,336 | 150.2 | 97.2 | Middle Africa | 5,900 | 8.9 | 24.2 |
| Western Europe | 161,881 | 174.2 | 85.8 | Western Africa | 14,277 | 8.8 | 21.2 |
| Northern Europe | 81,696 | 165.4 | 85.0 | Sub-Saharan Africa | 37,802 | 8.7 | 20.9 |
| Polynesia | 227 | 65.1 | 81.9 | WHO Africa Region | 37,486 | 8.5 | 19.9 |
| Caribbean | 18,719 | 89.3 | 79.8 | Eastern Africa | 13,866 | 7.9 | 18.7 |
| WHO Americas Region | 412,739 | 87.6 | 75.0 | Africa | 42,802 | 8.0 | 17.0 |
| Micronesia/Polynesia | 352 | 56.3 | 72.3 | Latin America and Caribbean | 51,313 | 17.2 | 16.6 |
| Very high human development | 734,128 | 129.0 | 72.0 | South America | 34,386 | 17.4 | 16.6 |
| European Union (EU-28) | 345,195 | 139.0 | 70.4 | Polynesia | 43 | 12.3 | 15.1 |
| More developed regions | 741,966 | 122.4 | 68.0 | Northern Europe | 18,099 | 36.7 | 14.5 |
| Southern Africa | 10,266 | 35.3 | 61.7 | Micronesia/Polynesia | 67 | 10.7 | 13.7 |
| Europe | 400,364 | 112.0 | 61.3 | Melanesia | 253 | 5.4 | 13.3 |
| South America | 114,701 | 58.0 | 60.1 | Western Asia | 10,422 | 8.4 | 13.1 |
| Micronesia | 125 | 45.2 | 60.0 | WHO Americas Region | 85,425 | 18.1 | 13.1 |
| Southern Europe | 91,355 | 118.0 | 58.6 | Oceania | 4,250 | 22.5 | 13.0 |
| WHO Europe Region | 419,915 | 96.1 | 58.5 | High human development | 72,623 | 14.2 | 12.9 |
| Latin America and Caribbean | 152,403 | 51.2 | 54.2 | Australia/New Zealand | 3,930 | 28.9 | 12.9 |
| IARC membership (24 countries) | 790,747 | 60.2 | 50.2 | Central America | 8,957 | 11.3 | 12.1 |
| High human development | 195,839 | 38.2 | 37.5 | Low human development | 39,096 | 6.0 | 12.1 |
| Central and Eastern Europe | 65,432 | 47.5 | 31.3 | Micronesia | 24 | 8.7 | 11.9 |
| World | 1,094,916 | 30.8 | 30.6 | Central and Eastern Europe | 25,862 | 18.8 | 11.6 |
| Central America | 18,983 | 24.0 | 28.4 | WHO Europe Region | 101,419 | 23.2 | 11.5 |
| Western Asia | 21,829 | 17.6 | 28.0 | Europe | 92,328 | 25.8 | 11.3 |
| Sub-Saharan Africa | 51,945 | 12.0 | 27.9 | European Union (EU-28) | 71,789 | 28.9 | 10.9 |
| Middle Africa | 6,892 | 10.4 | 27.0 | Western Europe | 28,138 | 30.3 | 10.7 |
| WHO Africa Region | 51,689 | 11.8 | 26.8 | Middle-East and Northern Africa | 15,422 | 6.8 | 10.2 |
| Western Africa | 17,600 | 10.9 | 25.1 | More developed regions | 142,014 | 23.4 | 10.0 |
| Eastern Africa | 17,187 | 9.8 | 23.3 | North America | 34,112 | 19.7 | 9.8 |
| Africa | 59,493 | 11.1 | 23.2 | Very high human development | 131,685 | 23.1 | 9.7 |
| Melanesia | 482 | 10.4 | 22.7 | Southern Europe | 20,229 | 26.1 | 9.1 |
| Middle-East and Northern Africa | 29,377 | 12.9 | 19.7 | IARC membership (24 countries) | 157,081 | 12.0 | 8.4 |
| Low human development | 47,809 | 7.3 | 14.9 | World | 307,481 | 8.6 | 7.8 |
| Less developed regions | 352,950 | 12.0 | 14.5 | Northern Africa | 5,000 | 4.8 | 7.0 |
| WHO Western Pacific Region | 153,167 | 16.2 | 12.6 | South-Eastern Asia | 15,841 | 5.3 | 6.7 |
| South-Eastern Asia | 26,451 | 8.8 | 11.2 | Less developed regions | 165,467 | 5.6 | 6.6 |
| Northern Africa | 7,548 | 7.3 | 10.6 | WHO East Mediterranean Region | 12,141 | 3.8 | 6.2 |
| Eastern Asia | 118,583 | 14.5 | 10.5 | Asia | 82,676 | 3.8 | 3.8 |
| WHO East Mediterranean Region | 18,585 | 5.8 | 9.7 | Medium human development | 63,739 | 3.5 | 3.8 |
| Asia | 196,190 | 9.0 | 9.4 | WHO South-East Asia Region | 24,932 | 2.6 | 3.6 |
| Medium human development | 115,942 | 6.4 | 7.0 | WHO Western Pacific Region | 45,977 | 4.9 | 3.5 |
| WHO South-East Asia Region | 38,515 | 4.1 | 5.5 | Eastern Asia | 37,553 | 4.6 | 3.1 |
| South-Central Asia | 29,327 | 3.2 | 4.5 | South-Central Asia | 18,860 | 2.0 | 2.9 |
ASR (W), age-standardized rate (world); IARC, International Agency for Research on Cancer.
Fig. 1.
Standardized incidence and mortality rate of prostate cancer worldwide in 2012. ASR (W), age-standardised rate (world).
Fig. 2.
Geographical distribution of prostate cancer incidence rate worldwide in 2012 (extracted from GLOBOCAN 2012). ASR, age-standardised rate.
In the year 2012, 307,481 deaths occurred from prostate cancer worldwide. Mortality was highest in the following 10 countries: US 30,383 cases; China 22,603 cases; Brazil 17,218 cases; Germany 12,548 cases; India 2,231 cases; Japan 1,164 cases; Russian Federation 11,480 cases, United Kingdom 10,595 cases; Nigeria 9,628 cases; and Indonesia 919 cases; giving a total of 147,521 fatal cases (47.97%).
The following 10 countries had the highest SMR for prostate cancer: Trinidad and Tobago 58.9 per 100,000 people; Guyana 48.2 per 100,000; Barbados 45.6 per 100,000; Burundi 40.2 per 100,000; Jamaica 40.2 per 100,000; Uganda 38.8 per 100,000; Bahamas 36.6 per 100,000; Dominican Republic 34.6 per 100,000; Haiti 32.3 per 100,000; and Guinea 30.2 per 100,000. The following countries had the lowest SMR for prostate cancer: Bhutan 0.7 per 100,000 people; Nepal 1.2 per 100,000; Bangladesh 1.2 per 100,000; North Korea 1.3 per 100,000; Turkmenistan 1.5 per 100,000; Uzbekistan 1.5 per 100,000; Sri Lanka 1.6 per 100,000; Tajikistan 1.9 per 100,000; and Yemen 2.3 per 100,000.
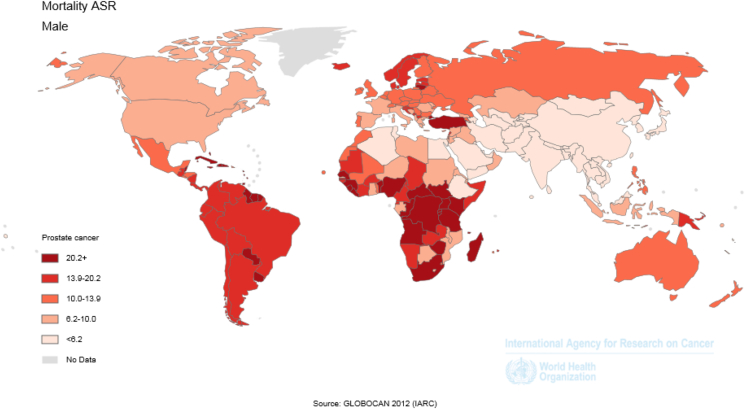
SMR based on HDI was 9.7 per 100,000 people in very high human development regions; 12.9 per 100,000 in high human development regions; 3.7 per 100,000 in medium human development regions; and 12.1 per 100,000 in low human development regions. Also, SIR was 11.5 per 100,000 people in the WHO Europe Region; 13.1 per 100,000 in the WHO Americas Region; 3.5 per 100,000 in the WHO Western Pacific Region; 3.6 per 100,000 in the WHO South-East Asia Region; 6.2 per 100,000 in the WHO East Mediterranean Region; and 19.9 per 100,000 in the WHO Africa Region. SMR was 10.0 per 100,000 people in more-developed regions and 6.6 per 100,000 in less-developed regions (Fig. 1, Fig. 3 and Table 1).
Fig. 3.
Geographical distribution of prostate cancer mortality rate worldwide in 2012 (extracted from GLOBOCAN 2012). ASR, age-standardised rate.
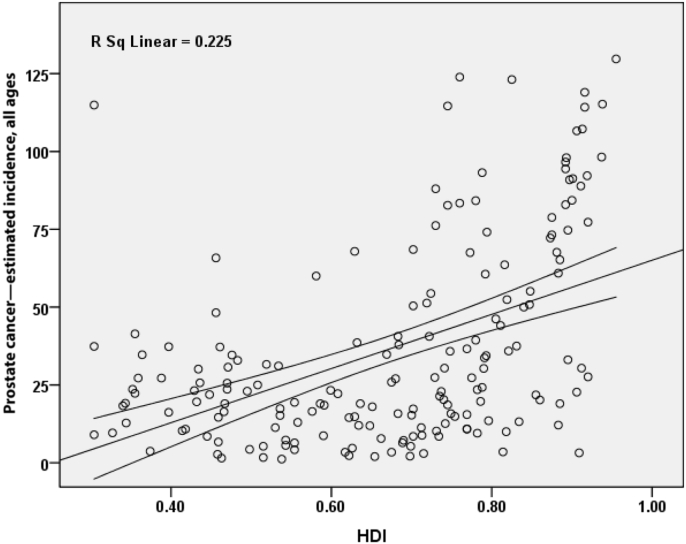
3.1. SIR and HDI
A positive correlation of 0.475 was seen between SIR of prostate cancer and HDI and this correlation was statistically significant (P ≤ 0.001) (Fig. 4). Also, there was a positive correlation between HDI components and SIR. Between SIR and life expectancy at birth, there was a positive correlation of 0.289 (P ≤ 0.001); between SIR and mean education years, there was a positive correlation of 0.422 (P ≤ 0.001); and between SIR and income level per person in the population, there was a positive correlation of 0.353 (P ≤ 0.001).
Fig. 4.
Correlation between HDI and standardized incidence rate of prostate cancer worldwide in 2012. HDI, Human Development Index.
Countries were classified according to HDI as follows: 47 in the very high human development category; 46 in the high human development category; 46 countries in the medium human development category; 44 countries in the low human development category; and 8 countries in the unknown category. Table 2 shows the numbers that were related to HDI and their components.
Table 2.
Human Development Index in 2012.
| Population | Human Development Index | Life expectancy at birth (y) | Mean years of schooling | Gross national income per capita (US$) |
|---|---|---|---|---|
| Human Development Index groups | ||||
| Very high human development | 0.905 | 80.1 | 11.5 | 33,391 |
| High human development | 0.758 | 73.4 | 8.8 | 11,501 |
| Medium human development | 0.640 | 69.9 | 6.3 | 5,428 |
| Low human development | 0.466 | 59.1 | 4.2 | 1,633 |
| Regions | ||||
| Arab States | 0.652 | 71.0 | 6.0 | 8,317 |
| East Asia and Pacific | 0.683 | 72.7 | 7.2 | 6,874 |
| Europe and Central Asia | 0.771 | 71.5 | 10.4 | 12,243 |
| Latin America and the Caribbean | 0.741 | 74.7 | 7.8 | 10,300 |
| South Asia | 0.558 | 66.2 | 4.7 | 3,343 |
| Sub-Saharan Africa | 0.475 | 54.9 | 4.7 | 2,010 |
| Least developed countries | 0.449 | 59.5 | 3.7 | 1,385 |
| Small island developing states | 0.648 | 69.8 | 7.3 | 5,397 |
| World | 0.694 | 70.1 | 7.5 | 10,184 |
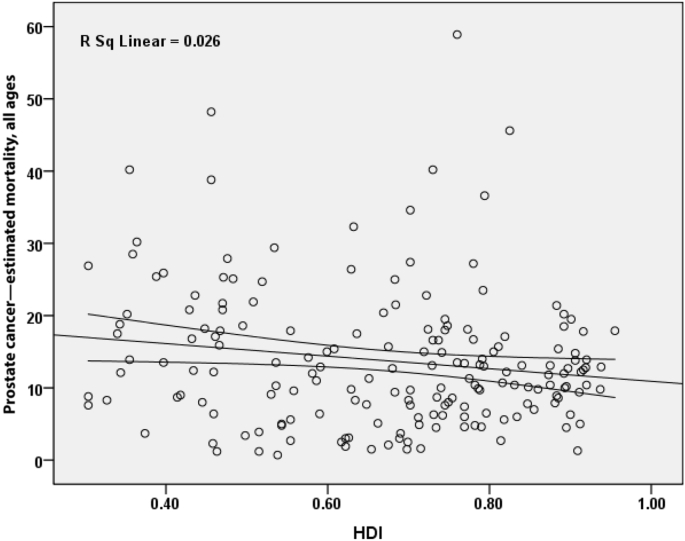
3.2. SMR and HDI
There was a negative correlation of 0.160 between SMR for prostate cancer and HDI and it was statistically significant (P = 0.032) (Fig. 5). Also, correlations were observed between HDI components and standardized incidence. Between SMR and life expectancy at birth, there was a negative correlation of 0.155 (P = 0.038); between SMR and mean education years, there was a negative correlation of 0.120 (P = 0.108); and between income level per person in the population, there was a negative correlation of 0.109 (P = 0.145).
Fig. 5.
Correlation between HDI and standardized mortality rate of prostate cancer worldwide in 2012. HDI, Human Development Index.
4. Discussion
The results of this study showed that the exclusive incidence rate of prostate cancer has a significant and direct relation with HDI worldwide. Also, all components of HDI (life expectancy at birth, education level, and income level) were related to the incidence of cancer. Countries like France and Norway that had the highest age-specific incidence and mortality rate of prostate cancer were counted as high HDI countries and countries such as Bhutan and Nepal that had the lowest incidence and mortality were counted as low HDI countries. Other studies have also shown higher incidence of prostate cancer in high HDI countries.4, 17, 29 The reasons may include diet, lifestyle, widespread clinical examinations, and most importantly, access to preventive services like PSA testing, and the presence of cancer registry systems in countries with higher HDI.
One of the human development components that had a positive and significant relation with incidence of prostate cancer in this study was life expectancy at birth. Studies have shown that prostate cancer has a close relationship with age, such that many cases occur in men aged >80 years.30 Those living in countries with a greater life expectancy relevant to the development indicators are more likely to have cancer, including prostate cancer.31, 32 This is related to a decrease in communicable diseases and increase in treatment methods of other diseases, and as a result, enhancement of life expectancy and confrontation with old-age-related diseases like prostate cancer.33
Another component of human development is education and awareness. Results of this study showed that there was a significant positive association with prostate cancer incidence. People who have a higher education level have better diets, more physical activity, and are less affected by communicable diseases, and the chance of being affected by noncommunicable disease increases.34 In a study performed in the US, the incidence of prostate cancer in educated men was greater than in the less educated population.35
The third part of HDI is sufficient income, which is related to gross domestic product. This study showed positive and significant relationships between gross domestic product and prostate cancer. As high-income countries have better access to preventive and treatment services, the diagnosis of illness is higher and they show higher incidence.36, 37 In this study, more access to screening for PSA was related to socioeconomic level and income.38, 39
The results of this study showed a significant negative correlation between SMR of prostate cancer and HDI. However, this relationship was different for various components of HDI as it was only significant for life expectancy with no significant relationship observed in the number of education years and income level.
The countries with the highest SMR for prostate cancer included five in North America and the Caribbean (Trinidad and Tobago, Barbados, Jamaica, Bahamas, Dominican Republic, and Haiti); three in Africa (Burundi, Uganda, and Guinea), and one in South America (Guyana). None of these countries are counted as very high HDI countries.17 The noticeable point about these countries is that in terms of cultural characteristics, socioeconomic status, dietary habits, tobacco use, and access to preventive and treatment services, they are at the same level as countries in similar regions, and this may explain the high mortality of prostate cancer in the geographical regions surrounding these countries.
As mentioned earlier, the only HDI component that had a significant relationship with SMR for prostate cancer was life expectancy at birth. Among the 10 countries with the highest prostate cancer mortality, the best life expectancy was in Barbados, with 78 years.17 In comparison with cancer exclusive incidence, that is, more frequently seen in high HDI countries, we can say that care, treatment, and patient survival in high HDI countries are better, cancer is diagnosed at a younger age when the disease is still in its primary stages, and prognosis is better. Conversely, in low HDI countries, the disease is diagnosed in older men and at a more advanced stage, which results in worse prognosis, less surveillance, and higher mortality.13, 40, 41
The results of the survey did not show significant correlation, except for the level of education and mortality of prostate cancer, which was in line with the findings of Pakzad et al4 in Asian countries. In a study performed in the US, people who were educated for < 12 years in comparison with those educated for > 12 years showed higher SMR.42
The present study did not show a significant relationship between level of income and SMR for prostate cancer, which was similar to other studies in this field.4 Other studies in this context did not present a linear and fixed procedure and we cannot speak with certainty about the relationship between income levels and mortality of prostate cancer.
This study had some limitations. This study was an ecological study and the limitations include ecological fallacy and lack of correlation between group and individual results.
In conclusion, the incidence of prostate cancer is higher in countries that are more developed. A positive correlation was observed between the SIR of prostate cancer and HDI and its components, such as life expectancy at birth, mean years of schooling, and income level per person of the population. In addition, there was a negative correlation between the SMR and HDI. Epidemiological transition is expected to occur in communities with increasing HDI and reduced incidence of infectious diseases and increased incidence of noncommunicable diseases such as cancer. However, although the incidence is high, the mortality rate is reduced, which represents an improvement of diagnosis and efficacy of treatment in prostate cancer patients with increasing levels of HDI.
Conflicts of interest
None declared.
References
- 1.de Souza J.A., Hunt B., Asirwa F.C., Adebamowo C., Lopes G. Global health equity: cancer care outcome disparities in high-, middle-, and low-income countries. J Clin Oncol. 2016;34:6–13. doi: 10.1200/JCO.2015.62.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center M.M., Jemal A., Lortet-Tieulent J., Ward E., Ferlay J., Brawley O. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 3.Bashir M.N. Epidemiology of prostate cancer. Asian Pac J Cancer Prev. 2015;16:5137–5141. doi: 10.7314/apjcp.2015.16.13.5137. [DOI] [PubMed] [Google Scholar]
- 4.Pakzad R., Mohammadian-Hafshejani A., Ghoncheh M., Pakzad I., Salehiniya H. The incidence and mortality of prostate cancer and its relationship with development in Asia. Prostate Int. 2015;3:135–140. doi: 10.1016/j.prnil.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pakzad R., Rafiemanesh H., Ghoncheh M., Sarmad A., Salehiniya H., Hosseini S. Prostate cancer in Iran: trends in incidence and morphological and epidemiological characteristics. Asian Pac J Cancer Prev. 2016;17:839–843. doi: 10.7314/apjcp.2016.17.2.839. [DOI] [PubMed] [Google Scholar]
- 6.Schröder F.H., Hugosson J., Roobol M.J., Tammela T.L., Ciatto S., Nelen V. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 8.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 9.Cook M.B., Rosenberg P.S., McCarty F.A., Wu M., King J., Eheman C. Racial disparities in prostate cancer incidence rates by census division in the United States, 1999–2008. Prostate. 2015;75:758–763. doi: 10.1002/pros.22958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Somdyala N.I., Parkin D.M., Sithole N., Bradshaw D. Trends in cancer incidence in rural Eastern Cape Province; South Africa, 1998–2012. Int J Cancer. 2015;136:E470–E474. doi: 10.1002/ijc.29224. [DOI] [PubMed] [Google Scholar]
- 11.Feletto E., Bang A., Cole-Clark D., Chalasani V., Rasiah K., Smith D.P. An examination of prostate cancer trends in Australia, England, Canada and USA: is the Australian death rate too high? World J Urol. 2015;33:1677–1687. doi: 10.1007/s00345-015-1514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grossfeld G.D., Carroll P.R. Prostate cancer early detection: a clinical perspective. Epidemiol Rev. 2015;23:173–180. doi: 10.1093/oxfordjournals.epirev.a000786. [DOI] [PubMed] [Google Scholar]
- 13.Jemal A., Fedewa S.A., Ma J., Siegel R., Lin C.C., Brawley O. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314:2054–2061. doi: 10.1001/jama.2015.14905. [DOI] [PubMed] [Google Scholar]
- 14.Basch E., Oliver T.K., Vickers A., Thompson I., Kantoff P., Parnes H. Screening for prostate cancer with prostate-specific antigen testing: American Society of Clinical Oncology provisional clinical opinion. J Clin Oncol. 2012;30:3020–3025. doi: 10.1200/JCO.2012.43.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hébert J.R., Hurley T.G., Harmon B.E., Heiney S., Hebert C.J., Steck S.E. A diet, physical activity, and stress reduction intervention in men with rising prostate-specific antigen after treatment for prostate cancer. Cancer Epidemiol. 2012;36:e128–e136. doi: 10.1016/j.canep.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seamonds B., Yang N., Anderson K., Whitaker B., Shaw L.M., Bollinger J.R. Evaluation of prostate-specific antigen and prostatic acid phosphatase as prostate cancer markers. Urology. 1986;28:472–479. doi: 10.1016/0090-4295(86)90146-9. [DOI] [PubMed] [Google Scholar]
- 17.Bray F., Jemal A., Grey N., Ferlay J., Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13:790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 18.Briganti A., Spahn M., Joniau S., Gontero P., Bianchi M., Kneitz B. Impact of age and comorbidities on long-term survival of patients with high-risk prostate cancer treated with radical prostatectomy: a multi-institutional competing-risks analysis. Eur Urol. 2013;63:693–701. doi: 10.1016/j.eururo.2012.08.054. [DOI] [PubMed] [Google Scholar]
- 19.Edwards B.K., Noone A.M., Mariotto A.B., Simard E.P., Boscoe F.P., Henley S.J. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–1314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daskivich T.J., Fan K.-H., Koyama T., Albertsen P.C., Goodman M., Hamilton A.S. Effect of age, tumor risk, and comorbidity on competing risks for survival in a US population-based cohort of men with prostate cancer. Ann Intern Med. 2013;158:709–717. doi: 10.7326/0003-4819-158-10-201305210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 22.Krieger N., Chen J.T., Waterman P.D., Soobader M.J., Subramanian S.V., Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156:471–482. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 23.Ghoncheh M., Mirzaei M., Salehiniya H. Incidence and mortality of breast cancer and their relationship with the human development index (HDI) in the world in 2012. Asian Pac J Cancer Prev. 2015;16:8439–8443. doi: 10.7314/apjcp.2015.16.18.8439. [DOI] [PubMed] [Google Scholar]
- 24.Pakzad R., Mohammadian-Hafshejani A., Mohammadian M., Pakzad I., Safiri S., Khazaei S. Incidence and mortality of bladder cancer and their relationship with development in Asia. Asian Pac J Cancer Prev. 2015;16:7365–7374. doi: 10.7314/apjcp.2015.16.16.7365. [DOI] [PubMed] [Google Scholar]
- 25.Mahdavifar N., Ghoncheh M., Pakzad R., Momenimovahed Z., Salehiniya H. Epidemiology, incidence and mortality of bladder cancer and their relationship with the development index in the world. Asian Pac J Cancer Prev. 2016;17:381–386. doi: 10.7314/apjcp.2016.17.1.381. [DOI] [PubMed] [Google Scholar]
- 26.Ghoncheh M., Mohammadian-Hafshejani A., Salehiniya H. Incidence and mortality of breast cancer and their relationship to development in Asia. Asian Pac J Cancer Prev. 2015;16:6081–6087. doi: 10.7314/apjcp.2015.16.14.6081. [DOI] [PubMed] [Google Scholar]
- 27.Ferlay J., Soerjomataram I., Ervik M., Dikshit R., Eser S., Mathers C. International Agency for Research on Cancer; Lyon (France): 2013. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet]http://globocan.iarc.fr [cited 2016 Feb 2]. Available from: [Google Scholar]
- 28.Malik K. 2013. Human development report 2013. The rise of the South: human progress in a diverse world (March 15, 2013) UNDP-HDRO Human Development Reports. [Google Scholar]
- 29.Soerjomataram I., Lortet-Tieulent J., Parkin D.M., Ferlay J., Mathers C., Forman D. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380:1840–1850. doi: 10.1016/S0140-6736(12)60919-2. [DOI] [PubMed] [Google Scholar]
- 30.Hsing A.W., Chokkalingam A.P. Prostate cancer epidemiology. Front Biosci. 2006;11:1388–1413. doi: 10.2741/1891. [DOI] [PubMed] [Google Scholar]
- 31.Bray F., Ren J.S., Masuyer E., Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133–1145. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 32.Walz J., Gallina A., Saad F., Montorsi F., Perrotte P., Shariat S.F. A nomogram predicting 10-year life expectancy in candidates for radical prostatectomy or radiotherapy for prostate cancer. J Clin Oncol. 2007;25:3576–3581. doi: 10.1200/JCO.2006.10.3820. [DOI] [PubMed] [Google Scholar]
- 33.Mathers C.D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winkleby M.A., Jatulis D.E., Frank E., Fortmann S.P. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am J Pub Health. 1992;82:816–820. doi: 10.2105/ajph.82.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clegg L.X., Reichman M.E., Miller B.A., Hankey B.F., Singh G.K., Lin Y.D. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20:417–435. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Etzioni R., Penson D.F., Legler J.M., di Tommaso D., Boer R., Gann P.H. Overdiagnosis due to prostate-specific antigen screening: lessons from US prostate cancer incidence trends. J Nat Cancer Inst. 2002;94:981–990. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 37.Welch H.G., Albertsen P.C. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986–2005. J Nat Cancer Inst. 2009;101:1325–1329. doi: 10.1093/jnci/djp278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guessous I., Cullati S., Fedewa S.A., Burton-Jeangros C., Courvoisier D.S., Manor O. Prostate cancer screening in Switzerland: 20-year trends and socioeconomic disparities. Prev Med. 2016;82:83–91. doi: 10.1016/j.ypmed.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Smith R.A., Brooks D., Cokkinides V., Saslow D., Brawley O.W. Cancer screening in the United States, 2013: a review of current American Cancer Society guidelines, current issues in cancer screening, and new guidance on cervical cancer screening and lung cancer screening. CA Cancer J Clin. 2013;63:88–105. doi: 10.3322/caac.21174. [DOI] [PubMed] [Google Scholar]
- 40.Qaseem A., Barry M.J., Denberg T.D., Owens D.K., Shekelle P. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2013;158:761–769. doi: 10.7326/0003-4819-158-10-201305210-00633. [DOI] [PubMed] [Google Scholar]
- 41.Heidenreich A., Bastian P.J., Bellmunt J., Bolla M., Joniau S., van der Kwast T. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent—update 2013. Eur Urol. 2014;65:124–137. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 42.Albano J.D., Ward E., Jemal A., Anderson R., Cokkinides V.E., Murray T. Cancer mortality in the United States by education level and race. J Nat Cancer Inst. 2007;99:1384–1394. doi: 10.1093/jnci/djm127. [DOI] [PubMed] [Google Scholar]