Abstract
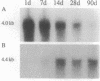
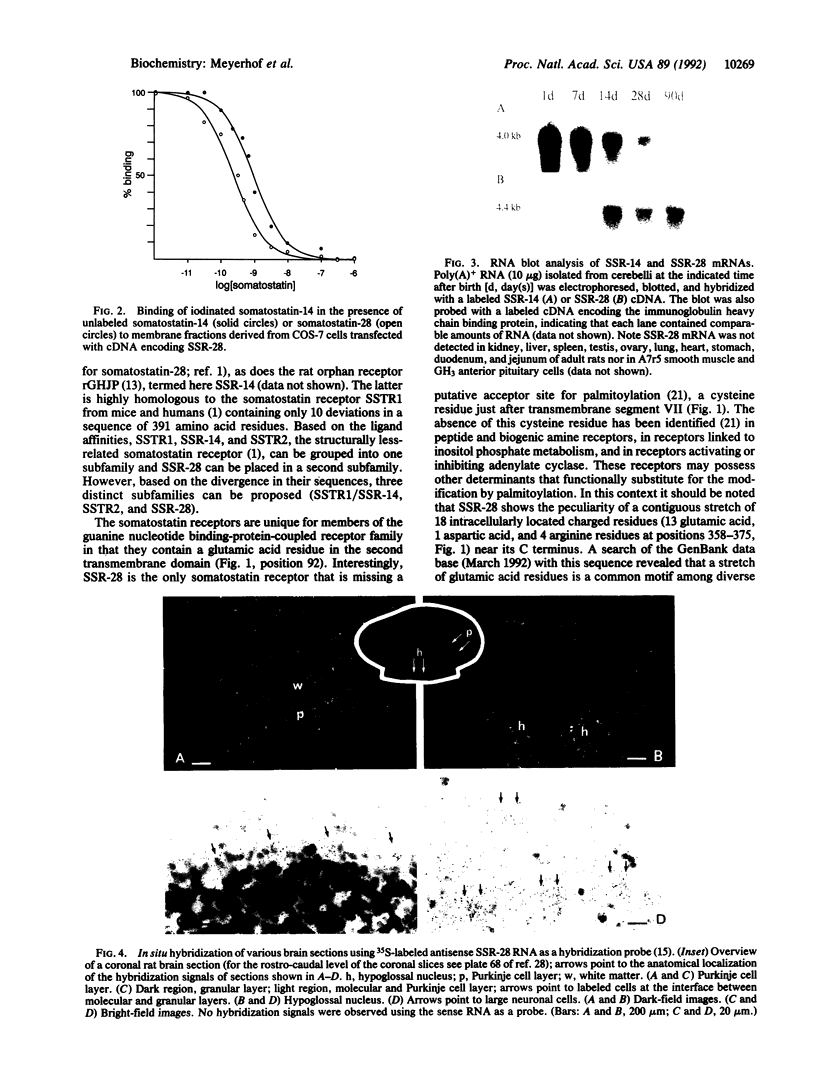
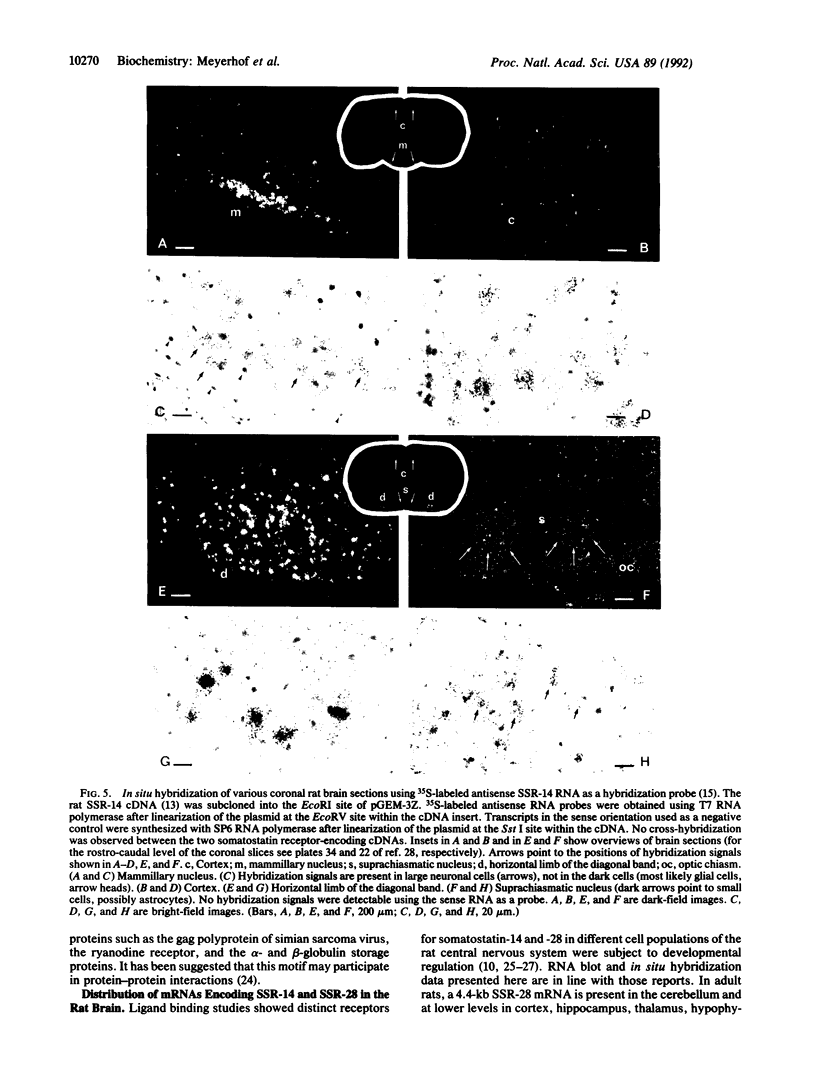
The tetradecapeptide somatotropin-release inhibiting factor somatostatin-14 regulates the release of peptide hormones and also functions as neurotransmitter. The octacosapeptide somatostatin-28, the N-terminally extended form of somatostatin-14, shows similar biological activities yet with different potencies. Both peptides most likely function through distinct receptors. Here we report on the molecular and functional characterization of a somatostatin-28 receptor (SSR-28) cloned from a rat brain cDNA library. The nucleotide sequence contains an open reading frame for a protein of 428 amino acid residues with a predicted molecular mass of 47 kDa. Binding assays using radiolabeled somatostatin-14 and membranes from COS cells transfected with the cloned cDNA show that this receptor, SSR-28, has a higher binding affinity for somatostatin-28 (IC50 = 0.24 nM) than for somatostatin-14 (IC50 = 0.89 nM). RNA blot analysis reveals a 4.4-kilobase mRNA in rat cerebellum and at significantly lower abundance in other brain regions. In situ hybridization indicates that SSR-28 mRNA is present in the granular and Purkinje cell layers of the cerebellum and in the large cells of the hypoglossal nucleus of the brain stem. Signals for SSR-28 mRNA do not overlap with those of a previously cloned rat receptor that preferentially binds somatostatin-14 (SSR-14). SSR-14 mRNA is found in the medial cerebellar nucleus, horizontal limb of the diagonal band, various hypothalamic nuclei, and in layers IV and V of the cortex. In the rat cerebellum, SSR-14 and SSR-28 mRNAs are developmentally regulated; the levels of the former are highest around birth and levels of the latter are highest at the adult stage.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Dodd J., Kelly S. Is somatostatin an excitatory transmitter in the hippocampus? Nature. 1978 Jun 22;273(5664):674–675. doi: 10.1038/273674a0. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Fehr S., Ivell R., Koll R., Schams D., Fields M., Richter D. Expression of the oxytocin gene in the large cells of the bovine corpus luteum. FEBS Lett. 1987 Jan 1;210(1):45–50. doi: 10.1016/0014-5793(87)81295-4. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez B. J., Leroux P., Laquerrière A., Coy D. H., Bodenant C., Vaudry H. Transient expression of somatostatin receptors in the rat cerebellum during development. Brain Res. 1988 May 1;468(1):154–157. doi: 10.1016/0165-3806(88)90018-1. [DOI] [PubMed] [Google Scholar]
- Heiman M. L., Murphy W. A., Coy D. H. Differential binding of somatostatin agonists to somatostatin receptors in brain and adenohypophysis. Neuroendocrinology. 1987 Jun;45(6):429–436. doi: 10.1159/000124788. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Iversen S. D., Bloom F., Douglas C., Brown M., Vale W. Calcium-dependent release of somatostatin and neurotensin from rat brain in vitro. Nature. 1978 May 11;273(5658):161–163. doi: 10.1038/273161a0. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Protein kinase recognition sequence motifs. Trends Biochem Sci. 1990 Sep;15(9):342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Krisch B., Buchholz C., Mentlein R. Somatostatin binding sites on rat diencephalic astrocytes. Light-microscopic study in vitro and in vivo. Cell Tissue Res. 1991 Feb;263(2):253–263. doi: 10.1007/BF00318767. [DOI] [PubMed] [Google Scholar]
- Kuno T., Andresen J. W., Kamisaki Y., Waldman S. A., Chang L. Y., Saheki S., Leitman D. C., Nakane M., Murad F. Co-purification of an atrial natriuretic factor receptor and particulate guanylate cyclase from rat lung. J Biol Chem. 1986 May 5;261(13):5817–5823. [PubMed] [Google Scholar]
- Leroux P., Quirion R., Pelletier G. Localization and characterization of brain somatostatin receptors as studied with somatostatin-14 and somatostatin-28 receptor radioautography. Brain Res. 1985 Nov 11;347(1):74–84. doi: 10.1016/0006-8993(85)90890-x. [DOI] [PubMed] [Google Scholar]
- Libert F., Parmentier M., Lefort A., Dinsart C., Van Sande J., Maenhaut C., Simons M. J., Dumont J. E., Vassart G. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science. 1989 May 5;244(4904):569–572. doi: 10.1126/science.2541503. [DOI] [PubMed] [Google Scholar]
- Lin H. C., Lei S. P., Wilcox G. An improved DNA sequencing strategy. Anal Biochem. 1985 May 15;147(1):114–119. doi: 10.1016/0003-2697(85)90016-8. [DOI] [PubMed] [Google Scholar]
- Meyerhof W., Paust H. J., Schönrock C., Richter D. Cloning of a cDNA encoding a novel putative G-protein-coupled receptor expressed in specific rat brain regions. DNA Cell Biol. 1991 Nov;10(9):689–694. doi: 10.1089/dna.1991.10.689. [DOI] [PubMed] [Google Scholar]
- Pittman Q. J., Siggins G. R. Somatostatin hyperpolarizes hippocampal pyramidal cells in vitro. Brain Res. 1981 Sep 28;221(2):402–408. doi: 10.1016/0006-8993(81)90791-5. [DOI] [PubMed] [Google Scholar]
- Raynor K., Wang H. L., Dichter M., Reisine T. Subtypes of brain somatostatin receptors couple to multiple cellular effector systems. Mol Pharmacol. 1991 Aug;40(2):248–253. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikant C. B., Patel Y. C. Receptor binding of somatostatin-28 is tissue specific. Nature. 1981 Nov 19;294(5838):259–260. doi: 10.1038/294259a0. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y., Yatsunami K., Tsujimoto M., Khorana H. G., Ichikawa A. The amino acid sequence of a glutamic acid-rich protein from bovine retina as deduced from the cDNA sequence. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3116–3119. doi: 10.1073/pnas.88.8.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W., Rivier C., Brazeau P., Guillemin R. Effects of somatostatin on the secretion of thyrotropin and prolactin. Endocrinology. 1974 Oct;95(4):968–977. doi: 10.1210/endo-95-4-968. [DOI] [PubMed] [Google Scholar]
- Vale W., Rivier C., Brown M. Regulatory peptides of the hypothalamus. Annu Rev Physiol. 1977;39:473–527. doi: 10.1146/annurev.ph.39.030177.002353. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Post S. R., Wang K., Tager H. S., Bell G. I., Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):251–255. doi: 10.1073/pnas.89.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]