Abstract
Background
Prostate cancer (PCa) is the most common cancer in men in Western countries. In-vitro and in-vivo studies suggest that oxidative stress (OS) and antioxidants play a key role in the pathogenesis of chronic diseases including PCa, which is promoted by the production of reactive oxygen species and impaired antioxidant defense mechanisms. This study evaluates the association between OS and men with PCa.
Methods
A literature search was carried out on Medline, PubMed, and ScienceDirect databases, as well as manual searches from inception up to August 2015 using the keywords “Oxidative stress” or “Reactive oxygen species” or “Lipid peroxidation” AND “Prostate cancer.” All studies including data on the measurement of OS biomarkers in PCa were included.
Results
Twenty-three case control studies were retrieved with sample sizes ranging from 15 to 3,613 (6,439 participants in total). Markers of OS were significantly higher in patients with PCa compared with control groups in 21 studies. Two self-controlled case studies comparing OS between PCa cells and non-PCa cells in tissue biopsies found OS to be statistically higher in PCa cancer cells. Results on markers of antioxidant capacity (superoxide dismutase, catalase, glutathione, glutathione reductase, glutathione peroxidase, uric acid, lutein, lycopene, beta carotein, vitamin A, vitamin C, vitamin E, and total antioxidants) were not completely consistent in their association with PCa.
Conclusions
Upregulated OS profiles and impairment of antioxidant defense systems may play a role in men with PCa. To confirm these findings, robust clinical trials utilizing a personalized approach which monitors both OS and antioxidant markers during therapy are warranted.
Keywords: Antioxidant, Oxidative stress, Prostate cancer, Peroxidation, Reactive oxygen species
1. Introduction
Prostate cancer (PCa) is the most common malignancy and the second leading cause of cancer-related mortality in men in the Western world including Australia and the USA.1 PCa is a multifocal neoplasm, which forms solid tumors of glandular origin. The risk of PCa increases with age but the etiology and pathogenesis are poorly understood.2 Clinically localized PCa is managed with observation, surgery, or radiation treatment; the latter may be combined with androgen-deprivation therapy (ADT). Men with metastatic disease3 are managed almost exclusively with ADT and chemotherapy. The natural history of untreated PCa is still poorly understood, with a wide variation in outcomes in patients with apparently similar cancers based on standard staging and pathological grading.4 Being able to risk stratify individuals with regard to clinical risk is therefore necessary in order to personalize therapeutic strategies, with the aim of minimizing harm to those at low risk, and maximizing the therapeutic armament to those at highest risk. The most widely used biomarker to detect and monitor PCa is prostate-specific antigen. However, strong clinical and preclinical evidence for the role of elevated cellular reactive oxygen species (ROS) and impaired protective mechanisms as a driver of PCa susceptibility4, 5 points to the potential clinical utility of these markers. The term oxidative stress has been used to refer to the imbalance between levels of ROS and the protective “antioxidant” mechanisms, resulting in an accumulation of molecular damage in DNA, proteins, and lipids.5
Several clinical studies have demonstrated that increased OS is related to PCa and that some antioxidants have the potential to protect men from PCa.6, 7, 8 Multiple in-vitro and in-vivo studies have attempted to elucidate the mechanisms of initiation and progression of PCa in relation to OS.9 Oxidative free radicals caused by multiple factors such as modulation of androgens, inflammation, vitamin D, tumor suppressor protein (p53), antioxidants, and age-related OS may initiate PCa.10 More specifically, in men with PCa it has been suggested that serum androgens promote ROS production and accumulation in PCa cells.10 Androgen-associated redox homeostasis is involved in the signal transduction network of multimeric redox-sensitive transcription factors, enzymes, and epigenetic modifications. Androgens have been shown to promote cancer by increasing reactive oxygen derivatives in tissue.11 Androgen-induced ROS levels in prostate epithelial cells play a critical role in PCa development, progression, and recurrence.12 Further, studies demonstrate that castration or estrogen therapy can lead to the regression of cancer in patients with metastatic PCa.13 Hence, it may be proposed that ADT combined with antioxidant agents may inhibit the progression of PCa.14
To date, no robust randomized controlled trials have been conducted to determine the impact of OS on the risk of developing PCa. A number of studies have attempted to examine the effect of exogenous antioxidants in preventing cancer recurrence and reducing the risk of developing cancer. These studies include lung,15 breast,16, 17 colorectal,18 gastrointestinal,19 head and neck,20 leukemia,21 bladder cancer,22 and PCa,23, 24 and findings have been inconsistent. Further, there have been reported risks of antioxidants instead of their protective effects. The major limitation of these studies is the lack of consideration of the redox imbalance between oxidation and antioxidants, and the double-edged effect of exogenous antioxidants. Supplementation of exogenous antioxidants in the long-term without monitoring the redox balance can result in beneficial as well as harmful effects depending on the concentration of ROS and the required amounts to maintain or re-establish redox homeostasis in each individual patient.25 Studies suggest that high doses of exogenous antioxidants could paradoxically act as a pro-oxidant by disrupting the redox balance. Thus, the balance between oxidation and antioxidants is a critical issue to consider in an individual patient when assessing the anticancer effect of antioxidants. To our knowledge, there have been no literature reviews examining the association between OS and men with PCa. Furthermore, none of the aforementioned studies have examined the effect of the redox balance in men with PCa. Thus, we conducted a systemic review to clarify the association between OS and men with PCa.
2. Materials and methods
2.1. Search strategy
A literature search was carried out on Medline, PubMed, and ScienceDirect databases from inception up to August 2015 using the keywords “Oxidative stress” or “Reactive oxygen species” or “Free radical” or “Lipid peroxidation” AND “Prostate cancer.” A manual search was also conducted from the retrieved articles. Inclusion criteria were articles that presented data on OS biomarkers, with the full article published in English. Acceptable study designs were case control, nested case control, prospective cohort, or randomized control trials. We excluded articles based on animal and cell models.
2.2. Quality assessment
The quality of each article included in this review was assessed using the Newcastle–Ottawa Scale following the Cochrane Collaboration recommendation.26, 27 The Newcastle–Ottawa Scale was developed jointly by the University of Newcastle (Australia) and the University of Ottawa (Canada) to assess the quality of nonrandomized studies to be included in systematic reviews.27 It has been widely used since at least 2004,28 and the results from several validation studies have been published.29, 30 The total score ranges from 0 to 9, with a higher score indicating greater quality.
2.3. Data extraction
A review template was developed specifying the key information about each study (Table 1, Table 2). Two reviewers (B.O. and S.L.) independently applied the inclusion and quality assessment criteria. The two reviewers compared results and resolved any discrepancies in the published articles.
Table 1.
The characteristics of the studies involving oxidative stress and antioxidants.
| Author, y, country | Study population | Study design | Sample size Mean age |
Control group | Sample collection | Outcome measurement & method | Clinical variables | Results & conclusion |
|---|---|---|---|---|---|---|---|---|
| Klotz et al33 1988 Germany |
PCa and benign BPH | Case control study 2 arms |
Total N = 26 Case (n = 16) 66 y Control BPH (n = 10) |
BPH | Tissue specimens | Nitric oxide: iNOS iNOS immunostaining |
Age, clinical diagnosis |
|
| Baltaci et al34 2001 Turkey |
High-grade prostatic intraepithelial neoplasia (PIN) |
Case control study 4 arms |
Total N = 80 BPH (n = 20) Low-grade PIN: (n = 20) High-grade PIN (n = 20) Primary prostatic adenocarcinomas (n = 20) |
Tissue samples | Nitric oxide: iNOS iNOS immunostaining |
|
||
| Camphausen et al31 2004 Sweden |
PCa | Case control study 2 arms |
Total N = 15 Case: (n = 15) Control: Historical data |
Historical data | Urine sample | Oxidative stress: 8-iso-PGF2 and 15-keto-dihydro-PGF2 Student t test |
ND |
|
| Iynem et al35 2004 Turkey |
Metastatic PCa patients |
Prospective case control study 2 arms |
Total N = 41 Case: Metastatic PCa (n = 21) 69 y Control (n = 20) 64 y |
Healthy volunteer nonsmokers |
Blood samples | Oxidative stress: MDA Antioxidant status: GSH, GSH-Px, GR, GST, and Vit E |
ND |
|
| Yilmaz et al36 2004 Turkey |
Newly diagnosed PCa and Treated benign prostatic nodular hyperplasia (BPNH) patients |
Case control study with 3 arms |
Total N = 121 Case PCa (n = 21) 66 y BPNH (n = 50) 64 y Control (n = 50) 66 y |
Age, sex, BMI matched healthy participants | Blood samples | Oxidative stress: MDA Antioxidant status: Cu, Zn, Cu-Zn SOD, and GSH-Px Data analysis: The Mann–Whitney U-test |
PSA level, transrectal ultrasonography, and biopsy Gleason sum. |
|
| Miyake H37 2004 Japan |
PCa limited to prostate stage (T1-T4) | Prospective case control study 2 arms |
Total N = 115 Case (n = 82) Control (n = 33) |
Age-matched healthy participants | Urine samples | Oxidative Stress: DNA damage: Urinary 8-OHdG and creatinine (Cr) Serum prostate Specific antigen (PSA) Data analysis: The Mann–Whitney U-test |
Serum PSA, clinical stage, metastasis, biopsy, Gleason score |
|
| Srivastava38 2005 India |
PCa and benign BPH | Case control study 3 arms |
Total N = 107 Case (n = 47) 62 y BPH (n = 55) 60 y Control (n = 25) 61 y |
Healthy men | Blood samples | Oxidative stress: MDA Antioxidant status: GST from serum, GSH-Px and GSH Data analysis: One-way ANOVA and Linear regression model |
ND |
|
| Almushatat et al39 2006 UK |
BPH, localized and metastatic PCa |
Case control study 4 arms |
Total N = 112 Localized PCa (n = 40) 70 y Metastatic PCa (n = 38) 74 y BPH (n = 20) 67 y Control (n = 14) 64 y |
Healthy subjects | Blood samples | Oxidative stress: MDA Antioxidant status: Plasma retinol, a-tocopherol, lutein, lycopene, α-carotene and β-carotene Inflammation biomarker: C-reactive protein (CRP) Data analysis: ANOVA (Kruskal–Wallis and Mann–Whitney U-test |
Age, PSA, Gleason score, cholesterol |
|
| Aydin et al40 2006 Turkey |
Newly diagnosed PCa patients and benign prostatic hyperplasia (BPH) patients | Case control study 3 arms |
Total N = 85 Case Nonmetastatic PCa (n = 25) 68 y BPH (n = 36) 64 y Control (n = 24) 65 y |
Sex-matched healthy volunteers | Blood samples | Oxidative stress: MDA Antioxidant status: SOD, GSH-Px, CAT, Cu-Zn SOD Data analysis: ANOVA tests followed by Tukey–Kramer's multiple comparisons test a posteriori |
ND |
|
| Ozmen et al41 2006 Turkey |
PCa | Case control study 2 arms |
Total N = 41 Case (n = 20) 72 y Control (n = 21) 66 y |
Healthy men | Blood samples | Oxidative stress: MDA Antioxidant status: Vitamin A,C,E and selenium in serum Fe and trace element (Ni, Zn, Co, and Cu) Data analysis: Student t test |
ND |
|
| Surapanenet al42 2006 India |
PCa | Case control study 2 arms |
Total N = 60 Case (n = 30) Control (n = 30) |
Healthy men | Blood samples | Oxidative stress: MDA Antioxidant status: GSH, SOD, and GST Data analysis: unpaired t-test |
ND |
|
| Yossepowitch43 2007 Israel |
PCa completed either radical prostatectomy or receiving androgen deprivation therapy |
Case control study 4 arms |
Total N = 104 Case: (n = 79) Localized undergoing radical prostatectomy (N = 42) 63 y Metastatic disease receiving androgen deprivation therapy (n = 37) HSPC (n = 15) HRPC (n = 22) 63 y Control (n = 25) 72 y |
Age matched healthy men | 12-h fasting blood sample | Oxidative stress: MDA Antioxidant status: Uric acid, vitamin E (α-tocopherol), copper induced peroxidation (CucCL2), nd Vmax (OD246) OD max. Data analysis: ANOVA on log transformed data. Univariate binary logistic regression analyses Ordinal logistic regression model |
PSA, biopsy, Gleason score, age, smoking history, vitamin supplements, and lipid profiles |
|
| Kotrikadze et al44 2008 Georgea |
PCa and BPH | Case control study 3 arms |
Total N = 30 Case (n = 15) BPH (n = 15) Control (n = 15) |
Healthy males of similar age | Blood sample | Oxidative stress: MDA Antioxidant status: SOD, CAT, Ceruloplasmin (Cp), GSH, GSH-Px, and glutathione-reductase (GR) Data analysis: Means of standard variation statistics MINITAB (basic statistic), |
|
|
| Akinloye et al45 2009 Nigeria |
PCa | Case control study 4 arms |
Total N = 170 Case (n = 120) Low-grade PSA (5–10 ng/mL) (n = 33), Medium-grade PSA (11–20 ng/mL) (n = 45) High-grade PSA (> 20 ng/mL) (n = 42) Control (n = 20) |
Healthy volunteers PSA concentration < 3.0 ng/mL |
Blood samples | Oxidative stress: Microsomal membrane Antioxidant status: SOD, CAT, reduced GSH, Uric acid and Vitamin C and E. Data was analysis: One-way ANOVA followed by the post-hoc Duncan multiple range test for analysis of biochemical data |
PSA, ALT, AST, Total bilirubin |
|
| Arsova-Sarafinovsk et al46 2009 Turkey |
PCa and BPH | Case control study 3 arms |
Total N = 312 Cases Case (n = 107) BPH (n = 167) Control (n = 38) |
Healthy volunteers | Blood sample | Oxidative stress: MDA and 8-OHdG NO2−/NO3− and cGMP Antioxidant status: CuZn-SOD, GSH-Px, and CAT Data analysis: ANOVA and Tukey–Kramer multiple comparisons test a posteriori or Kruskal–Wallis nonparametric test, Dunn's multiple comparisons test |
Smoking, family history of cancer, Gleason score and PSA |
|
| Hoque et al32 2010 USA |
PCa | Nested case-control design 2 arms |
Total n = 3,613 Case (n = 1,808) 64 years Control (n = 1,805) 64 y |
Biopsy negative | Biopsy and blood samples | Oxidative stress: Carbonyl Data analysis χ2 test for categorical variables and t test for continuous variables and multiple logistic regression analysis |
Age, race, education, physical activity, smoking, fruit intake, vegetable intake, and family history of PCa, and BMI |
|
| Battisit et al47 2011 Brazil |
PCa | Case control study 3 arms |
Total N = 110 Case (n = 55) Metastatic (n = 23) Non-metastatic (n = 32) Control (n = 55) |
Age matched-healthy men |
Blood sample | Oxidative stress: MDA and carbonylation Antioxidant status: CAT,SOD, Vitamin C and vitamin E Data analysis: One-way ANOVA followed by the Duncan's multiple test. |
Metastasis, standard treatment, Gleason score, family history, smoking and alcohol intake |
|
| Barocas et al48 2011 USA |
Patients with high grade prostatic intraepithelial neoplasia (HGPIN) and PCa |
Case control study 3 arms |
Total N = 500 HGPIN (n = 140) 66 y PCa (n = 200) 68 y Control (n = 160) 67 y |
Confirmed biopsy negative | Urine and biopsy | Oxidative stress: F2IP Data analysis: Multivariable linear and logistic regression was used |
BMI race, health history, family history and other risk factors (smoke), biopsy, DRE results, current use of NSAIDs and statins, and transrectal ultrasound prostate volume. |
|
| Wozniak et al49 2012 Poland |
PCa limited to prostate gland (T1ABCN0M0, T2ABCN0M0Gx, and T1ABCN0M0Gx) |
Prospective case control study 2 arms |
Total N = 90 Case (n = 60) 67 y Control (n = 30) 62 y |
Healthy men. | Blood sample | Oxidative stress: TBARS Antioxidant status: GSH-Px, CAT, and SOD Data analysis: ANOVA |
Age, PSA, Gleason score, TNM, hemoglobin |
|
| Brys et al50 2013 Poland |
PCa | Case control study 2 arms |
Total N = 537 Case (n = 304) 61 y Control (n = 233) 65 y |
Healthy individuals | Blood and urine samples. | Oxidative stress: 8-isoPGF2a Antioxidant status: Uric acid and glucose Data analysis: Q-Dixon test, Mann–Whitney and Cox regression analysis |
Age, PSA, Gleason score, TNM, hemoglobin, Prostate volume |
|
| Pande et al51 2013 India |
Newly diagnosed PCa patients Stage (1-4) Metastatic (n = 16), non-metastatic (n = 24) |
Case control study 2 arms |
Total N = 80 Case (n = 40) 64 y Control (n = 40) 68 y |
Age-matched healthy individuals |
Venous blood collection, and the serum was used for various biochemical and hematologic investigations |
Oxidative stress: 8-OHdG, carbonyls and MDA Antioxidant status: Total antioxidant status Angiogenesis: VEGF levels Data analysis: Student t test, One-way ANOVA, Pearson's correlation coefficient |
PSA level at diagnosis, transrectal ultrasound, and biopsy Gleason score |
|
| Kosova et al52 2014 Turkey |
PCa and BPH | Prospective case control study 2 arms |
Total N = 40 Case (n = 20) Control (n = 20) |
Age matched BPH | Blood sample | Oxidative stress: 8-OHdG and MDA Caspase-3 Data analysis Mann–Whitney U test, Wilcoxon test |
Age, sex, weight, and height |
|
| Yang et al53 2015 USA |
PCa | Prospective nested case control study 2 arms |
Total N = 48 Case (n = 24) 60 y Control (n = 24) 60 y |
Age-matched healthy subjects |
Blood and urine samples |
Oxidative stress; Urine F2-isoprostanes FlOPs Carboxymethyl-lysine (CML) Data analysis: Conditional logistic regression model. transformed the variables into a logarithmic scale |
Age, BMI, smoking status (current and past smoker, or never smoked), family history of PCa, history of benign prostatic hyperplasia, history of hypertension, history of diabetes, and other demographic characteristics |
|
ABTS, 2,2′-azino-di-(3-ethylbenzthiazoline sulfonate); AGE, advanced glycation end products; BMI, body mass index; BPH, benign prostate hyperplasia; CAT, catalase; cGMP, cyclic guanosine monophosphate; CI, confidence interval; CML, carboxymethyl-lysine; DNPH, 2,4-dinitrophenyl hydrazine; DRE, Digital rectal examination; FAD, flavin adenine dinucleotide; FIGO, International Federation of Gynecology and Obstetrics; FlOPs, fluorescent oxidation products (lipid, protein and DNA); F2IP, F2-isoprostane; FRAP, ferric reducing antioxidant power; GSH, glutathione; GSH-Px, glutathione peroxidase; GR, glutathione reductase; GST, glutathione S-transferase; HNSCC, head and neck squamous cell carcinoma; HRPC, hormone refractory prostate cancer; HSPC, hormone sensitive prostate; MDA, malondialdehyde; NADPH, reduced nicotine amide adenine dinucleotide; ND, not described; NSAID, nonsteroidal anti-inflammatory drug; OSCC, oral squamous cell carcinoma; SOD, superoxide dismutase; TBARS, thiobarbituric acid; 8-isoPGF2, 4-HNE, 4-hydroxy-2-nonenal.
Table 2.
Oxidative stress and antioxidant profiles in prostate cancer patients.
| Oxidative biomarkers |
Antioxidant indicators |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipid peroxidation |
Protein peroxidation |
Endogenous antioxidant |
Exogenous antioxidant |
|||||||||||||||||
| MDA | Microsomal membrane | Isoprostanes | NO2 −/NO3− /iNOS/ cGMP |
DNA damage 8-OHdg |
Carbonylation | Glycation (CML) | CAT | GSH | GR | GSH-Px | GST | SOD | Bilirubin | Uric acid |
Lutein, Lycopen, β-carotein |
Vit A |
Vit C |
Vit E |
Total antioxidant (TAS) | |
| Klotz et al 1988 |
+ | |||||||||||||||||||
| Baltaci et al 2001 | + | |||||||||||||||||||
| Camphausen et al 2004 | NS | |||||||||||||||||||
| Iynem et al 2004 | + | − | − | − | + | − | ||||||||||||||
| Yilmaz et al 2004 |
+ | − | − | |||||||||||||||||
| Miyake H 2004 |
+ | |||||||||||||||||||
| Srivastava et al 2005 | + | − | − | + | ||||||||||||||||
| Almushatat et al 2006 | + | − | ||||||||||||||||||
| Aydin A et al 2006 | + | NS | − | − | ||||||||||||||||
| Ozmen et al 2006 | + | − | − | − | ||||||||||||||||
| Surapaneni et al 2006 | + | − | NS | + | ||||||||||||||||
| Yossepowitch et al 2007 | + | − | + | |||||||||||||||||
| Kotrikadzet al 2008 | + | − | + | + | − | − | ||||||||||||||
| Akinloye et al 2009 | + | − | − | − | + | − | − | − | ||||||||||||
| Arsova-Sarafinovsk et al 2009 | + | + | NS | − | − | − | ||||||||||||||
| Hoque et al 2010 | NS | |||||||||||||||||||
| Battisit et al 2011 | + | + | − | + | + | − | − | |||||||||||||
| Barocas DA et al 2011 | + | |||||||||||||||||||
| Wozniak et al 2012 | + | NS | − | NS | ||||||||||||||||
| Brys et al 2013 |
+ | + | ||||||||||||||||||
| Pande et al 2013 | + | + | + | − | ||||||||||||||||
| Kosova et al 2014 |
+ | + | ||||||||||||||||||
| Yang et al 2015 | NS | + | ||||||||||||||||||
+, significant increase in prostate cancer; −, significant decrease in prostate cancer; CAT, catalase; cGMP, cyclic guanosine monophosphate; CML, carboxymethyl-lysine; GR, glutathione reductase; GSH, glutathione; GSH-Px, glutathione peroxidase; GST, glutathione S-transferase; iNOS, inducible nitric oxide synthase; MDA, malondialdehyde; NS, not significant; SOD, superoxide dismutase; Vit, vitamin; 8-isoPGF2, 4-HNE, 4-hydroxy-2-nonenal.
2.4. Statistical analysis for meta-analysis
The meta-analysis was conducted using a random-effects model, which assumes that the effect size has a distribution rather than a fixed value, i.e., the effect size varies within the population. The summary statistic of interest is the standardized mean difference; specifically, the difference in mean for PCa patients compared with healthy controls, divided by the standard deviation pooled across these groups. For each effect size we calculated a 95% confidence interval (CI). Restricted maximum likelihood estimation was used to estimate the model. Analyses were conducted using the metafor package in R 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria). The relevant studies covered a range of oxidation and antioxidants, but these were dominated by malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px), so to allow for comparisons of sufficient size and homogeneity, and to avoid the complication of including multiple outcomes per study, we chose to analyze only MDA, SOD, and GSH-Px. The results presented are an overall effect size and CI, as well as a forest plot. Heterogeneity of effects for each outcome was reported using I2, which represents the percentage of variability due to heterogeneity rather than chance.
3. Results
3.1. Study characteristics
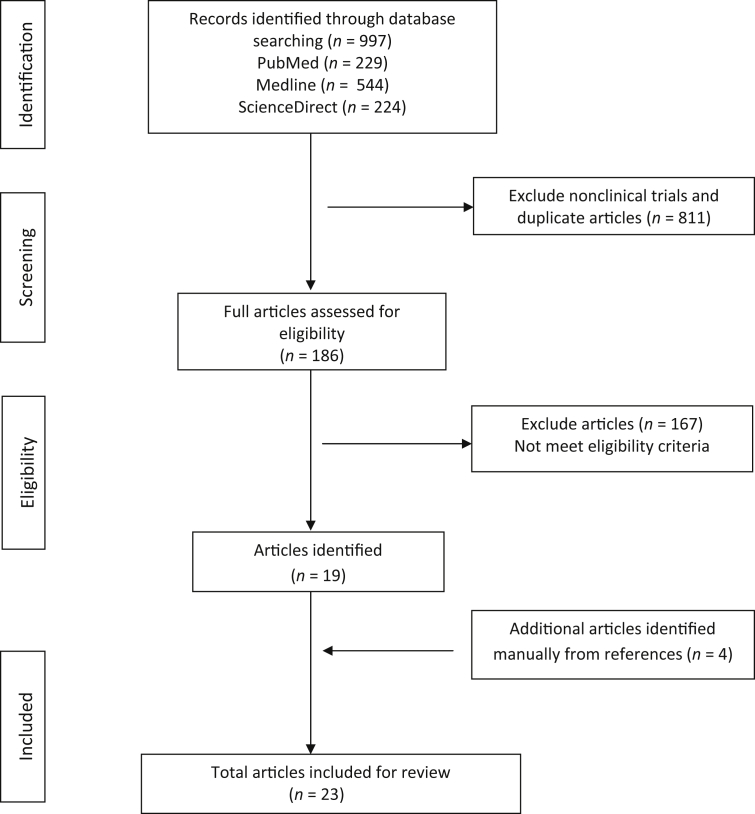
Twenty-three case control studies were identified (Fig. 1). In 23 studies, the total number of participants was 6,377 of which 3,558 were PCa cases. Seven studies were undertaken in Turkey; three studies each in India and USA; two studies in Poland; one study each in Brazil, Georgia, Germany, Japan, Nigeria, Sweden, and the UK. All study participants were recruited from urology clinics. Sample sizes ranged from 15 to 3,163.31, 32 The mean age of participants ranged from 60 years to 74 years. Twenty-three studies were conducted using a case control study design, 19 were case control studies, two were prospective, one was nested, and one was a subset of the Nashville Men's Health study.
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses.
Comparative and control groups varied. Two self-controlled case studies compared oxidation status with the biopsy specimens of PCa cells and non-PCa cells33, 34. Twenty-one case control studies compared oxidation status between patients with PCa and healthy volunteers. Studies included two arms (n = 11), three arms (n = 7), or four arms (n = 3). One study used historical data as the control group, two studies used men with benign prostate hyperplasia as the comparator, two studies used a negative biopsy, and 18 studies used age-matched healthy men as the control group.
3.2. Oxidative stress and antioxidant status in PCa
Of 23 studies, 21 studies reported at least one of the markers of OS to be significantly higher in patients compared with controls while two studies reported no significant difference. OS was analyzed on biopsy (n = 2), urine (n = 2), blood (n = 16), and both urine and blood (n = 3) samples of participants (Table 1, Table 2). Fifteen studies measured both OS and antioxidant values whilst eight studies measured OS only. OS value was measured based on lipid peroxidation (n = 19), protein oxidation (n = 9), or both lipid and protein peroxidation (n = 5). Eight OS biomarkers were identified among 23 studies. Most studies (n = 14) measured MDA while nine studies used different biomarkers including 8-hydroxy-2' -deoxyguanosine (8-OHdg; n = 4), isoprostanes (n = 4), inducible nitric oxide synthase (n = 3), carbonylation (n = 2), glycation (n = 2), microsomal membrane (n = 1), and cyclic guanosine monophosphate (n = 1).
Markers of antioxidant capacity assessed in the studies (Table 2) can be categorized as endogenous [catalase (CAT; n = 6); glutathione (GSH; n = 6); glutathione reductase (GR; n = 2); GSH-Px; n = 7; glutathione S-transferase (GST; n = 3); SOD; n = 8; uric acid (n = 3); bilirubin (n = 1)]; and exogenous [i.e., markers reflecting dietary intake such as (lutein (n = 1); lycopene (n = 1); β-carotein (n = 1); vitamin A (n = 1); vitamin C (n = 3); vitamin E (n = 4); and total antioxidants (n = 1)]. Twelve studies measured antioxidant values based on more than two indicators. Three studies measured antioxidant values based on only one indicator. Seven studies reported GSH-Px values to be low in patients with PCa. The values of six other antioxidant indicators (CAT, GSH, GR, SOD, uric acid, and vitamin E) were more variable, but tended to be lower in patients compared with the control group. Conversely, two antioxidant indicator values (GST and bilirubin) were higher in patients.
3.3. Result of meta-analysis
Studies that reported data of sample size, mean value of OS, mean value of antioxidant, and standard deviation were included in the meta-analysis (Fig. 2, Fig. 3, Fig. 4). In studies included in this review, MDA, SOD, and GSX-Px were the most commonly used indicator for OS and antioxidants, respectively.
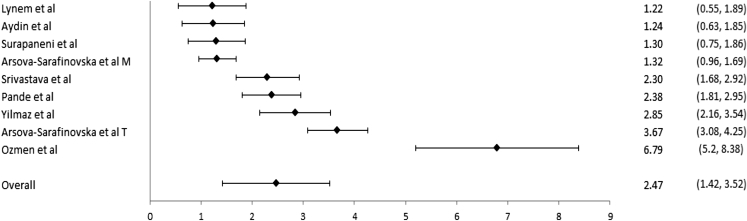
Fig. 2.
Forest plot for malondialdehyde. For each study, the marker indicates the effect size and the error bars are 95% confidence intervals. For Arsova-Sarafinovska et al, “M” indicates Macedonian patients and “T” indicates Turkish patients.
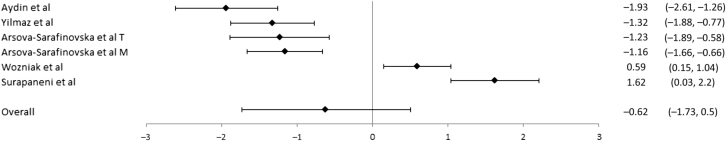
Fig. 3.
Forest plot for superoxide dismutase. For each study, the marker indicates the effect size and the error bars are 95% confidence intervals. For Arsova-Sarafinovska et al, “M” indicates Macedonian patients and “T” indicates Turkish patients.
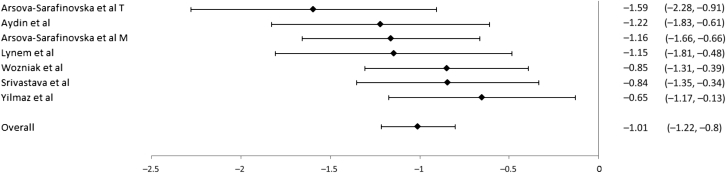
Fig. 4.
Forest plot for glutathione peroxidase. For each study, the marker indicates the effect size and the error bars are 95% confidence intervals. For Arsova-Sarafinovska et al, “M” indicates Macedonian patients and “T” indicates Turkish patients.
For the comparison of MDA (n = 9), the mean effect size was 2.47 [95% CI (1.42, 3.52)] indicating that MDA levels were significantly higher for PCa patients than controls. Heterogeneity for this outcome was high (I2 = 96%). For the comparison of SOD (n = 13), the mean effect size was −0.62 [95%, CI (−1.73, 0.50)], indicating no differences between PCa patients and controls. Heterogeneity for this outcome was high (I2 = 96%). For GSX-Px (n = 8), the mean effect size was −1.01 [95%, CI (−1.22, –0.80)], indicating GSX-Px levels were lower for PCa patients than controls. Heterogeneity for this outcome, as measured by I2, was 0%.
3.4. Study quality
The overall quality of the studies included in this review, assessed using the Newcastle–Ottawa Scale, was moderate with an average score of 6.83 (standard deviation = 1.19, range, 4–8) on a nine-point scale. The main areas where quality was lacking were comparability of cases and controls on the basis of the design and analysis. Nonreporting of the nonresponse rates was a major factor for low scores in the provision of well-defined outcome measures.
4. Discussion
Over the last decades, epidemiological, experimental, and clinical studies have demonstrated that markers of OS are associated with the development and progression of cancer.9 The present study seeks to evaluate the association between PCa and OS and antioxidants. Overall, results of our review confirm that markers of OS are increased in PCa patients compared with healthy controls, with the strongest and most consistent circulating biomarker being MDA. To our knowledge, this is the first systemic review conducted to examine the association between OS and antioxidants in men with PCa.
The present study found that most OS biomarkers were significantly higher in patients with PCa than the control group. Of 23 studies, 21 studies reported at least one marker of OS to be higher in men with PCa, whereas two studies did not detect any significant differences between the two groups. These results are consistent with recent studies examining the correlation of OS and the risk of cancer in various tumor groups which reported significantly increased lipid peroxidation and DNA damage in breast,54, 55 brain,56 colorectal,57 lung,58 liver,59 head and neck60 cancers, and oral squamous cell61 carcinoma.
The oxidation of lipid or lipid peroxidation is one of the most commonly reported indices of OS which is recognized as a pathological factor contributing to chronic disease including cancer and aging.62, 63 The most frequently studied markers of lipid peroxidation are MDA and isoprostanes.64 Of 23 studies, 14 studies measured OS with MDA and reported OS to be associated with PCa. In those studies, high levels of OS in PCa were consistent, although, MDA was measured using different methods such as thiobarbituric acid test, thiobarbituric acid-reactive substances test, and chromatographic assays (high performance liquid chromatography-diode array detection-fluoro and Liquid chromatography–mass spectrometry- diode array detection). One study reported that MDA levels correlated with the Gleason score and progression of disease.36 However, another study43 observed an association between OS and advanced PCa but not localized PCa. To confirm this result will require further studies with an adequate sample size.
Four studies measured lipid peroxidation with isoprostanes, which are prostaglandin-like compounds formed from the free radical catalyzed peroxidation of arachidonic acid. Two studies48, 50 reported urine F2-isoprostane generated by lipid peroxidation to be associated with PCa whilst two other studies did not.31, 53 These two studies31, 53 were likely hampered by the limitation of small sample sizes. Further, differences in study populations may also be a cause of divergent findings. One study53 was conducted in diabetic and hypoglycemia patients while the former two studies were conducted in nondiabetic patients.
To measure outcomes of protein oxidation, DNA damage with 8-OHdG and with NO2– or NO3– are commonly used in cancer research.64 Three studies reported a difference of 8-OHdG between two groups while one study did not observe a difference. The latter study suggested that there was a need to improve the validated analytical procedure of measuring 8-OHdG using plasma or serum samples. The results of these three studies are similar to previous studies which demonstrated that higher DNA damage correlated with the risk of PCa.65, 66
Three studies which measured protein oxidation with NO2– or NO3– showed OS to be consistently higher in PCa. These findings are in agreement with previous studies which suggested that ROS, including oxygen and nitrogen-free radicals, may cause specific oxidative DNA damage and play a leading role in initiation and promotion of carcinogenesis.67
CAT, SOD, and GSH related enzymes are considered primary endogenous antioxidants while vitamins C, E, and A (converted from beta-carotene) are considered exogenous antioxidants as they are directly involved in elimination of ROS.46, 68 Both endogenous and exogenous antioxidants protect cells against ROS induced during metabolism in living organisms.46 For example, GSH-Px removes both H2O2 and lipid peroxides using GSH. SOD metabolizes and protects the cells against O2–, mediated by lipid peroxidation, and CAT acts on H2O2 and decomposes it to H2O and OH–. The exogenous antioxidants (vitamins A, C, and E) at the molecular and cellular level are also considered to be effective in eliminating free radicals and prevent chronic diseases including cancer.68 Thus, our review further evaluated the relationship between antioxidants and men with PCa, in addition to OS.
Fifteen studies measured antioxidant indicators. SOD was measured in eight studies with erythrocytes or whole blood. Five studies reported low SOD levels in patients while two studies42, 47 reported contrasting results, and one study49 found no differences. CAT was measured on erythrocytes and whole blood samples in six studies. Four studies44, 45, 46, 47 reported lower CAT levels in patients and two studies40, 49 found no differences. The main reason for the inconsistency in SOD and CAT values could be attributed to the progression of disease.49 This hypothesis is consistent with Battisit et al47 who observed that an alteration in CAT and SOD values existed between patients with localized disease and those with bone metastases.
Further, four studies reported lower GSH levels in patients whereas two studies reported conflicting results.44, 47 All seven studies that measured GSH-Px showed GSH-Px values to be lower in patients. In contrast, GST values were higher in men with PCa. Overall, the GSH-dependent enzyme levels were either decreased or increased or unchanged. The reason for the inconsistency of GSH-dependent enzyme activities could be influenced by the prostate-specific antigen values, as suggested by several studies.45, 51 Furthermore, GSH and GSH-dependent enzymes have been known to be of central importance in the detoxification of peroxides, hydroperoxides, xenobiotics, and drugs.69 Hence, modification of GSH-dependent enzyme activities can be explained by the interdependence and dynamics of the GSH enzyme family pathway. GSH is turned to gluthathione disulfide by GSH-Px. Gluthathione disulfide is reduced again by GR using nicotinamide adenine dinucleotide phosphate as a cofactor. GSH has the ability to directly scavenge cellular ROS nonenzymatically as well as serving as a cofactor for GSH-Px in the reduction of H2O2 and other peroxide species.70 GSH-Px is also responsible for detoxifying other lipid peroxides to the corresponding alcohol.70 GST catalyzes the conjugation of GSH to a wide variety of endogenous and exogenous electrophilic compounds. GSH conjugation is the first step in the mercapturic acids pathway that leads to the elimination of toxic compounds.71
Six studies measured exogenous antioxidant substances.35, 39, 41, 43, 45, 47, 51 Both vitamin A (including beta-carotene, lycopen, leutin) and vitamin C were found to be lower in patients with PCa. With regard to vitamin E, four studies found lower levels in patients whereas one study43 reported contrasting results. The authors43 attributed this difference to different study populations. Patients participating in this study received ADT whilst most other studies included patients who did not receive anticancer treatment. ADT may alter vitamin E levels. Future studies are needed to confirm this.
A major limitation of this review is the inability to control for potential confounding factors. It is possible that the risk of PCa is influenced by multiple factors such as radiation, pollution, alcohol, diet, smoking, anxiety and stress, inflammation, drugs, and chronic diseases, which can all modulate OS levels. Most papers included in this review did not report these variables. Despite this limitation, our results suggest that redox imbalance is more common in men with PCa which may be useful for designing future randomized controlled trials.
In conclusion, the results of our review suggest that dysregulation of redox balance occurs in patients with PCa. OS biomarkers MDA and 8OH-dg as well as antioxidant parameters SOD, CAT, GSH enzyme family, and vitamins C and E may be potentially predictive biomarkers of PCa. Robust studies are required to elucidate whether reduced antioxidant enzyme levels are caused by the counteraction to OS or enhanced oxidation, which occurs as a result of depleted antioxidants over a prolonged period of time.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Secondary Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012.
- 2.Grönberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–864. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 3.Loblaw D.A., Virgo K.S., Nam R., Somerfield M.R., Ben-Josef E., Mendelson D.S. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2007 Update of an American Society of Clinical Oncology Practice Guideline. J Clin Oncol. 2007;25:1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 4.Lund L., Svolgaard N., Poulsen M.H. Prostate cancer: a review of active surveillance. Res Rep Urol. 2014;6:107–112. doi: 10.2147/RRU.S41653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalyanaraman B. Teaching the basics of redox biology to medical and graduate students: oxidants, antioxidants and disease mechanisms. Redox Biol. 2013;1:244–257. doi: 10.1016/j.redox.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khandrika L., Kumar B., Koul S., Maroni P., Koul H.K. Oxidative stress in prostate cancer. Cancer Lett. 2009;282:125–136. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L., Stacewicz-Sapuntzakis M., Duncan C., Sharifi R., Ghosh L., van Breemen R. Oxidative DNA damage in prostate cancer patients consuming tomato sauce-based entrees as a whole-food intervention. J Natl Cancer Inst. 2001;93:1872–1879. doi: 10.1093/jnci/93.24.1872. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y., Cui R., Xiao Y., Fang J., Xu Q. Effect of carotene and lycopene on the risk of prostate cancer: a systematic review and dose-response meta-analysis of observational studies. PLoS One. 2015;10:e0137427. doi: 10.1371/journal.pone.0140415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radical Bio Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minelli A., Bellezza I., Conte C., Culig Z. Oxidative stress-related aging: a role for prostate cancer? Biochim Biophys Acta. 2009;1795:83–91. doi: 10.1016/j.bbcan.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Ripple M.O., Wilding G., Henry W.F., Wilding G. Prooxidant-antioxidant shift induced by androgen treatment of human prostate carcinoma cells. J Natl Cancer Inst. 1997;89:40–48. doi: 10.1093/jnci/89.1.40. [DOI] [PubMed] [Google Scholar]
- 12.Mehraein-Ghomi F., Lee E., Church D.R., Thompson T.A., Basu H.S., Wilding G. JunD mediates androgen-induced oxidative stress in androgen dependent LNCaP human prostate cancer cells. Prostate. 2008;68:924–934. doi: 10.1002/pros.20737. [DOI] [PubMed] [Google Scholar]
- 13.Huggins C., Hodges C.V. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232–240. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 14.Miyake H., Hara I., Gleave M.E., Eto H. Protection of androgen-dependent human prostate cancer cells from oxidative stress-induced DNA damage by overexpression of clusterin and its modulation by androgen. Prostate. 2004;61:318–323. doi: 10.1002/pros.20087. [DOI] [PubMed] [Google Scholar]
- 15.The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 16.Greenlee H., Hershman D., Jacobson J. Use of antioxidant supplements during breast cancer treatment: a comprehensive review. Breast Cancer Res Treat. 2009;115:437–452. doi: 10.1007/s10549-008-0193-0. [DOI] [PubMed] [Google Scholar]
- 17.Nechuta S., Lu W., Chen Z., Zheng Y., Gu K., Cai H. Vitamin supplement use during breast cancer treatment and survival: a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2011;20:262–271. doi: 10.1158/1055-9965.EPI-10-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albanes D., Malila N., Taylor P.R., Huttunen J.K., Virtamo J., Edwards B.K. Effects of supplemental alpha-tocopherol and beta-carotene on colorectal cancer: results from a controlled trial (Finland) Cancer Causes Control. 2000;11:197–205. doi: 10.1023/a:1008936214087. [DOI] [PubMed] [Google Scholar]
- 19.Wang G.Q., Dawsey S.M., Li J.Y., Taylor P.R., Li B., Blot W.J. Effects of vitamin/mineral supplementation on the prevalence of histological dysplasia and early cancer of the esophagus and stomach: results from the General Population Trial in Linxian, China. Cancer Epidemiol Biomarkers Prev. 1994;3:161–166. [PubMed] [Google Scholar]
- 20.Bairati I., Meyer F., Gélinas M., Fortin A., Nabid A., Brochet F. Randomized trial of antioxidant vitamins to prevent acute adverse effects of radiation therapy in head and neck cancer patients. J Clin Oncol. 2005;23:5805–5813. doi: 10.1200/JCO.2005.05.514. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy D.D., Tucker K.L., Ladas E.D., Rheingold S.R., Blumberg J.B., Kelly K.M. Low antioxidant vitamin intakes are associated with increases in adverse effects of chemotherapy in children with acute lymphoblastic leukemia. Am J Clin Nutr. 2004;79:1029–1036. doi: 10.1093/ajcn/79.6.1029. [DOI] [PubMed] [Google Scholar]
- 22.Lotan Y., Goodman P.J., Youssef R.F., Svatek R.S., Shariat S.F., Tangen C.M. Evaluation of Vitamin E and Selenium Supplementation for the Prevention of Bladder Cancer in SWOG Coordinated SELECT. J Urol. 2012;187:2005–2010. doi: 10.1016/j.juro.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lippman S.M., Klein E.A., Goodman P.J., Lucia M.S., Thompson I.M., Ford L.G. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the selenium and vitamin e cancer prevention trial (select) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaziano J., Glynn R.J., Christen W.G., Kurth T., Belanger C., MacFadyen J. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouayed J., Bohn T. Exogenous antioxidants—Double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev. 2010;3:228–237. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins J.P.T., Green S., Higgins J.P.T., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. The Cochrane Collaboration; London: 2011. [Google Scholar]
- 27.Wells G., Shea B., O'Connell D., Peterson J., Welch V., Losos M. 2013. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet] [cited 2015 Dec 15]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 28.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 29.Oremus M., Oremus C., Hall G.B.C., McKinnon M.C., ECT & Cognition Systematic Review Team Inter-rater and test–retest reliability of quality assessments by novice student raters using the Jadad and Newcastle–Ottawa Scales. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartling L., Milne A., Hamm M.P., Vandermeer B., Ansari M., Tsertsvadze A. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J Clin Epidemiol. 2013;66:982–993. doi: 10.1016/j.jclinepi.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Camphausen K., Ménard C., Sproull M., Goley E., Basu S., Coleman C.N. Isoprostane levels in the urine of patients with prostate cancer receiving radiotherapy are not elevated. Int J Radiat Oncol. 2004;58:1536–1539. doi: 10.1016/j.ijrobp.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Hoque A., Ambrosone C.B., Till C., Goodman P.J., Tangen C., Kristal A. Serum oxidized protein and prostate cancer risk within the prostate cancer prevention trial. Cancer Prev Res. 2010;3:478–483. doi: 10.1158/1940-6207.CAPR-09-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klotz T., Bloch W., Volberg C., Engelmann U., Addicks K. Selective expression of inducible nitric oxide synthase in human prostate carcinoma. Cancer. 1998;82:1897–1903. [PubMed] [Google Scholar]
- 34.Baltaci S., Orhan D., Gögüs Ç., Türkölmez K., Tulunay O., Gögüs O. Inducible nitric oxide synthase expression in benign prostatic hyperplasia, low- and high-grade prostatic intraepithelial neoplasia and prostatic carcinoma. BJU Int. 2001;88:100–103. doi: 10.1046/j.1464-410x.2001.02231.x. [DOI] [PubMed] [Google Scholar]
- 35.Iynem A.H., Alademir A.Z., Obek C., Kural A.R., Konukoğlu D., Akçay T. The effect of prostate cancer and antiandrogenic therapy on lipid peroxidation and antioxidant systems. Int Urol Nephrol. 2004;36:57–62. doi: 10.1023/b:urol.0000032676.31470.b2. [DOI] [PubMed] [Google Scholar]
- 36.Yilmaz M.I., Saglam K., Sonmez A., Gok D.E., Basal S., Kilic S. Antioxidant system activation in prostate cancer. Biol Trace Elem Res. 2004;98:13–19. doi: 10.1385/BTER:98:1:13. [DOI] [PubMed] [Google Scholar]
- 37.Miyake H., Hara I., Kamidono S., Eto H. Oxidative DNA damage in patietns with prostate cancer and its response to treatment. J Urol. 2004;171:1533–1536. doi: 10.1097/01.ju.0000116617.32728.ca. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava D.S.L., Mittal R.D. Free radical injury and antioxidant status in patients with benign prostate hyperplasia and prostate cancer. Indian J Clin Biochem. 2005;20:162–165. doi: 10.1007/BF02867419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Almushatat A.S., Talwar D., McArdle P.A., Williamson C., Sattar N., O'Reilly D.S. Vitamin antioxidants, lipid peroxidation and the systemic inflammatory response in patients with prostate cancer. Int J Cancer. 2006;118:1051–1053. doi: 10.1002/ijc.21451. [DOI] [PubMed] [Google Scholar]
- 40.Aydin A., Arsova-Sarafinovska Z., Sayal A., Eken A., Erdem O., Erten K. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clin Biochem. 2006;39:176–179. doi: 10.1016/j.clinbiochem.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 41.Ozmen H., Erulas F.A., Karatas F., Cukurovali A., Yalcin O. Comparison of the concentration of trace metals (Ni, Zn, Co, Cu and Se), Fe, vitamins A, C and E, and lipid peroxidation in patients with prostate cancer. Clin Chem Lab Med. 2006;44:175–179. doi: 10.1515/CCLM.2006.032. [DOI] [PubMed] [Google Scholar]
- 42.Surapaneni K.M., Venkata G.R. Lipid peroxidation and antioxidant status in patients with carcinoma of prostate. Indian J Physiol Pharmacol. 2006;50:350–354. [PubMed] [Google Scholar]
- 43.Yossepowitch O., Pinchuk I., Gur U., Neumann A., Lichtenberg D., Baniel J. Advanced but not localized prostate cancer is associated with increased oxidative stress. J Urol. 2007;178:1238–1244. doi: 10.1016/j.juro.2007.05.145. [DOI] [PubMed] [Google Scholar]
- 44.Kotrikadze N., Alibegashvili M., Zibzibadze M., Abashidze N., Chigogidze T., Managadze L. Activity and content of antioxidant enzymes in prostate tumors. Exo Oncol. 2008;30:244–247. [PubMed] [Google Scholar]
- 45.Akinloye O., Adaramoye O., Kareem O. Changes in antioxidant status and lipid peroxidation in Nigerian patients with prostate carcinoma. Pol Arch Med Wewn. 2009;119:526–532. [PubMed] [Google Scholar]
- 46.Arsova-Sarafinovska Z., Eken A., Matevska N., Erdem O., Sayal A., Savaser A. Increased oxidative/nitrosative stress and decreased antioxidant enzyme activities in prostate cancer. Clinc Biochem. 2009;42:1228–1235. doi: 10.1016/j.clinbiochem.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Battisti V., Maders L.D.K., Bagatini M.D., Reetz L.G., Chiesa J., Battisti I.E. Oxidative stress and antioxidant status in prostate cancer patients: relation to Gleason score, treatment and bone metastasis. Biomed Pharmacother. 2011;65:516–524. doi: 10.1016/j.biopha.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Barocas D.A., Motley S., Cookson M.S., Chang S.S., Penson D.F., Dai Q. Oxidative stress measured by urine F2-isoprostane level is associated with prostate cancer. J Urol. 2011;185:2102–2107. doi: 10.1016/j.juro.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wozniak A., Masiak R., Szpinda M., ila-Kierzenkowska C., Wozniak B., Makarewicz R. Oxidative stress markers in prostate cancer patients after HDR brachytherapy combined with external beam radiation. Oxid Med Cell Longev. 2012;2012:789870. doi: 10.1155/2012/789870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brys M., Morel A., Forma E., Krzeslak A., Wilkosz J., Rozanski W. Relationship of urinary isoprostanes to prostate cancer occurrence. Mol Cell Biochem. 2013;372:149–153. doi: 10.1007/s11010-012-1455-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pande D., Negi R., Karki K., Dwivedi U.S., Khanna R.S., Khanna H.D. Simultaneous progression of oxidative stress, angiogenesis, and cell proliferation in prostate carcinoma. Urol Oncol. 2013;31:1561–1566. doi: 10.1016/j.urolonc.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 52.Kosova F., Temeltaş G., Arı Z., Lekili M. Possible relations between oxidative damage and apoptosis in benign prostate hyperplasia and prostate cancer patients. Tumor Biol. 2014;35:4295–4299. doi: 10.1007/s13277-013-1560-y. [DOI] [PubMed] [Google Scholar]
- 53.Yang S., Pinney S.M., Mallick P., Ho S.M., Bracken B., Wu T. Impact of oxidative stress biomarkers and carboxymethyllysine (an advanced glycation end product) on prostate cancer: a prospective study. Clin Genitourin Cancer. 2015;13:e347–e351. doi: 10.1016/j.clgc.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rajneesh C.P., Manimaran A., Sasikala K.R., Adaikappan P. Lipid peroxidation and antioxidant status in patients with breast cancer. Singapore Med J. 2008;49:640–643. [PubMed] [Google Scholar]
- 55.Sener D.E., Gonenc A., Akinci M., Torun M. Lipid peroxidation and total antioxidant status in patients with breast cancer. Cell Biochem Funct. 2007;25:377–382. doi: 10.1002/cbf.1308. [DOI] [PubMed] [Google Scholar]
- 56.YİLmaz N., Dulger H., KİYmaz N., Yilmaz C., Bayram I., Ragip B. Lipid peroxidation in patients with brain tumor. Int J Neurosci. 2006;116:937–943. doi: 10.1080/00207450600553141. [DOI] [PubMed] [Google Scholar]
- 57.Jiao L., Taylor P.R., Weinstein S.J., Graubard B.I., Virtamo J., Albanes D. Advanced glycation end-products, soluble receptor for advanced glycation end-products and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:1430–1438. doi: 10.1158/1055-9965.EPI-11-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaynar H., Meral M., Turhan H., Keles M., Celik G., Akcay F. Glutathione peroxidase, glutathione-S-transferase, catalase, xanthine oxidase, Cu–Zn superoxide dismutase activities, total glutathione, nitric oxide, and malondialdehyde levels in erythrocytes of patients with small cell and non-small cell lung cancer. Cancer Lett. 2005;227:133–139. doi: 10.1016/j.canlet.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 59.Suman J., Renu S., Suman S., Varadkar A.M. Activities of some antioxidant enzymes and lipid peroxidation in liver cancer patients. Int J Curr Res Rev. 2012;4:59–63. [Google Scholar]
- 60.Dahiya K., Dhankhar R., Madaan H., Singh V., Arora K. Nitric oxide and antioxidant status in head and neck carcinoma before and after radiotherapy. Ann Clin Lab Sci. 2012;42:94–97. [PubMed] [Google Scholar]
- 61.Rasool M., Khan S.R., Malik A., Khan K.M., Zahid S., Manan A. Comparative studies of salivary and blood sialic acid, lipid peroxidation and antioxidative status in oral squamous cell carcinoma (OSCC) Pak J Med Sci. 2014;30:466–471. doi: 10.12669/pjms.303.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pratt D.A., Tallman K.A., Porter N.A. Free radical oxidation of polyunsaturated lipids: new mechanistic insights and the development of peroxyl radical clocks. Acc Chem Res. 2011;44:458–467. doi: 10.1021/ar200024c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moselhy H.F., Reid R.G., Yousef S., Boyle S.F. A specific, accurate, and sensitive measure of total plasma malondialdehyde by HPLC. J Lipid Res. 2013;54:852–858. doi: 10.1194/jlr.D032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karimi Galougahi K., Antoniades C., Nicholls S.J., Cahnnon K.M., Figtree G.A. Redox biomarkers in cardiovascular medicine. Eur Heart J. 2015;36:1576–1582. doi: 10.1093/eurheartj/ehv126. [DOI] [PubMed] [Google Scholar]
- 65.Lockett K.L., Hall M.C., Clark P.E., Chuang S.C., Robinson B., Lin H.Y. DNA damage levels in prostate cancer cases and controls. Carcinogenesis. 2006;27:1187–1193. doi: 10.1093/carcin/bgi288. [DOI] [PubMed] [Google Scholar]
- 66.Malins D.C., Johnson P.M., Wheeler T.M., Barker E.A., Polissar N.L., Vinson M.A. Age-related radical-induced DNA damage is linked to prostate cancer. Cancer Res. 2001;61:6025–6028. [PubMed] [Google Scholar]
- 67.Valavanidis A., Vlachogianni T., Fiotakis C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Exotoxicol Rev. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 68.Valko M., Izakovic M., Mazur M., Rhodes C.J., Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 69.Saydam N., Kirb A., Demir Ö., Hazan E., Oto O., Saydam O. Determination of glutathione, glutathione reductase, glutathione peroxidase and glutathione S-transferase levels in human lung cancer tissues. Cancer Lett. 1997;119:13–19. doi: 10.1016/s0304-3835(97)00245-0. [DOI] [PubMed] [Google Scholar]
- 70.Backos D.S., Franklin C.C., Reigan P. The role of glutathione in brain tumor drug resistance. Biomed Pharmacoher. 2012;83:1005–1012. doi: 10.1016/j.bcp.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 71.Townsend D.M., Tew K.D. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene. 2003;22:7369–7375. doi: 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]