Abstract
CD4 expression is rare in diffuse large B-cell lymphoma (DLBCL), with 4 previously reported cases. Its significance is uncertain. We report five patients with CD4+ DLBCL and one CD4+ primary mediastinal large B-cell lymphoma. Cases were identified by searching the electronic database of the department; each was reviewed. Average age was 56 years. Neoplastic cells expressed CD20 (5/6 tested cases). BCL2/BCL6 expression were seen in 3/3 tested cases, suggesting a germinal center origin. Additionally, expression of T-cell antigens CD2 and CD5 was noted in 2/2 and CD7 in 1/1 tested case. CD3 was negative in all. Lymph nodes were commonly involved (67%). Patients received chemotherapy +/- radiation (6/6) and bone marrow transplant (2/6). Average survival was 44.2 mo. CD4 expression in DLBCL raises questions of lineage commitment. CD4+ DLBCL is rare; care should be exercised not to diagnose these as T-cell lymphomas. A subset behaves aggressively.
Keywords: CD4+, Diffuse large B-cell lymphoma, T-cell lymphoma, Lineage infidelity
Core tip: Aberrant expression of T-cell antigens including CD4 in ALK-negative diffuse large B-cell lymphoma (DLBCL) is a rare phenomenon that raises interesting biological and diagnostic considerations. With regards to our series of DLBCLs expressing CD4, it appears that at least a subset may behave aggressively based on our data.
INTRODUCTION
Most cases of diffuse large B-cell lymphoma (DLBCL) show expression of B-cell antigens CD19, CD20, CD22, and CD79a. Variable expression of CD10, BCL-6, IRF4/MUM1, FOXP1, and less commonly CD5, is also observed. Surface/cytoplasmic light chain expression is usually present[1].
The expression of T-cell associated antigens is not seen in benign lymphoid proliferations and is uncommon in B-cell non-Hodgkin lymphoma (B-NHL), most frequently occurring in the setting of chronic lymphocytic leukemia/small lymphocytic lymphoma[2]. In a series of 101 B-NHL, CD2, CD5, and CD7 expression was seen in only one quarter of cases[3]. CD8 (2%-3% of cases) expression has also been documented[4]. CD3 and CD4 expression, however, are exceedingly rare in DLBCL with only 4 cases of the latter reported in the world literature to the best of our knowledge[5-7]. Herein, we report a series of six cases of CD4+ large B-cell lymphoma (LBCL) identified from our institution and review the pertinent literature to determine the incidence and possible mechanisms of aberrant T-cell antigen expression in B-NHL.
LITERATURE
Permission for the study was granted by the Washington University (WU) Human Studies Committee. Consecutive cases diagnosed within a 21-year period in patients 0-99 years old were identified by searching the Copath database of the Division of Anatomic Pathology at Barnes Jewish Hospital/WU (WU/BJC) using the terms “large B-cell lymphoma” and “CD4” in any field. Each hit was then individually reviewed to identify cases of DLBCL with concomitant CD4 expression or aberrant expression of other T-cell antigens by immunohistochemistry or flow cytometry.
Clinical data were acquired from WU/BJC Clinical Desktop, CoPath, and Touchwork databases, and by clinician interview. Mortality data were also procured from the Social Security Death Index.
RESULTS
Histologic and clinical features for all six cases are detailed in Table 1. Patients ranged in age from 22-79 years (average 54 years), and included 3 men and 3 women. Due to the retrospective nature of the study information about extent of disease at presentation was limited to three patients, and ranged from relatively limited disease in patients 1 and 2 to more extensive involvement in patient 5. Lymph node biopsies were obtained from the cervical or supraclavicular regions in 4 patients. In patient 1 tissue from the left maxillary sinus was biopsied, and in patient 6 the skin of the right leg was sampled.
Table 1.
Summary of histologic and clinical features of ALK-negative, CD4-positive diffuse large B-cell lymphomas
| Case no. | Age/sex | Diagnosis | Immunophenotype | Molecular findings | Gross description | Treatment | Clinical outcome | Site | Stage |
| 1 | 73/M | DLBCL | CD20, BCL-2, CD10, CD5, CD43, BCL-6, and CD4 | Soft, gelatinous appearing mass | R-CHOP × 4 | Complete remission at 4 mo follow-up; free of disease at 6 mo follow-up | Left maxillary sinus | I-E | |
| 2 | 22/F | PMLBCL | CD45, CD20, CD79a, with variable expression of CD2, CD4, CD30, CD23, BCL-6 and BCL-2 | 12 cycles of R-VACOP-B; 2 cycles of R-ESHAP mediastinal radiation (40-50 Gy); matched, unrelated donor stem cell transplant with Bu/Cy conditioning; salvage chemotherapy with GND; SGN-35 | Dead; 14 mo survival from disease discovery; Progressive disease | Supraclavicular lymph node | IIE-X-B | ||
| 3 | 79/F | DLBCL and FL(3a) | CD45 (focal), CD20, CD3, CD21 (focal), Bcl-2, Pax-5, subset expression of CD2, CD4, CD5, CD7, CD8 Bcl-6, MUM1, and CD10 | R-CHOP | Dead; 6 mo survival from disease diagnosis | Left neck lymph node | |||
| 4 | 67/M | CLL/SLL with transformation to LBCL | CD45, CD30, EMA, CD4 (subset), and CD43, rare weak CD2 | 46, XY[18]; clonal IGH rearranged; IGVH unmutated; TCR gamma rearrangement negative | Fludarabine and cytoxan × 3; fludarabine, Rituxan, and mitoxantrone × 2; R-CHOP × 4; R-CHOP; BEAM and auto transplant | Dead; 15 mo survival; Progressive disease | Left subclavicular lymph node | ||
| 5 | 26/M | B-cell large cell lymphoma | CD20, CD30 (weak), CD4 (subset) | R-CHOP × 5, field radiotherapy | No evidence of relapse at 72 mo; lost to follow up | Left posterior cervical lymph node | IV-A-E | ||
| 6 | 55/F | Malignant lymphoma, diffuse cleaved large cell type, with B-cell differentiation | CD20, MB-2, CD4, BCL2, and CD43 PCNA | Lost to follow-up | Right leg skin |
CLL/SLL: Chronic lymphocytic leukemia/small lymphocytic lymphoma; CD: Cluster of differentiation; DLBCL: Diffuse large B-cell lymphoma; F: Female; M: Male; PMLBCL: Primary mediastinal large B-cell lymphoma; CHOP: Rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate; R-ESHAP: Rituximab, etoposide, methylprednisolone, high-dose cytarabine, and cisplatin; VACOP: Vinblastine, adriamycin, cyclophosphamide, etoposide, prednisone, bleomycin; GND: Gemcitabine, vinorelbine, and doxorubicin; SGN-35: Brentuximab vedotin; BEAM: Carmustine, etoposide, cytarabine, and melphalan; FL: Follicular lymphoma.
Grossly, the tumors were noted to have a soft, grey/gelatinous appearance in two cases, and in one of these cases it was mistaken as a myxoma initially. In terms of immunophenotype, the neoplastic cells usually expressed CD45 (4/4 tested cases), CD20 (5/6 tested cases), CD19, and PAX5. BCL2, BCL6, and CD10 expression were also seen implying a germinal center origin (Figure 1, Figure 2, Figure 3). BCL2 and BCL6 expression were seen in 3/3 tested cases. CD4 was expressed in the vast majority of cells in 5/6 cases, and was positive in approximately 1/2 of the large cells in the biopsy from patient 4 (Figure 3). All cases were tested for CD3 expression by immunohistochemistry and were negative. With the exception of patient 4, the malignant cells in all patients were positive for CD20 immunohistochemistry. A monoclonal rearrangement of IgH by polymerase chain reaction (PCR) established the malignant lineage of the cells from patient 4. In addition to CD4, aberrant expression of T-cell antigens CD2 and CD5 was noted in two cases each and CD7 in one case. No TCR rearrangements were found in one tested case.
Figure 1.
Diffuse effacement of underlying tissue architecture by neoplastic cells in left maxillary sinus biopsy, patient 1 (hematoxylin and eosin, original magnification × 400).
Figure 2.
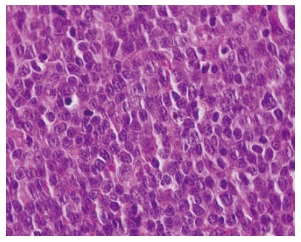
Large malignant cells with increased nuclear:cytoplasmic ratio, multiple nucleoli, irregular and nuclear contours arranged in sheets, case 1 (hematoxylin and eosin, original magnification × 1000).
Figure 3.
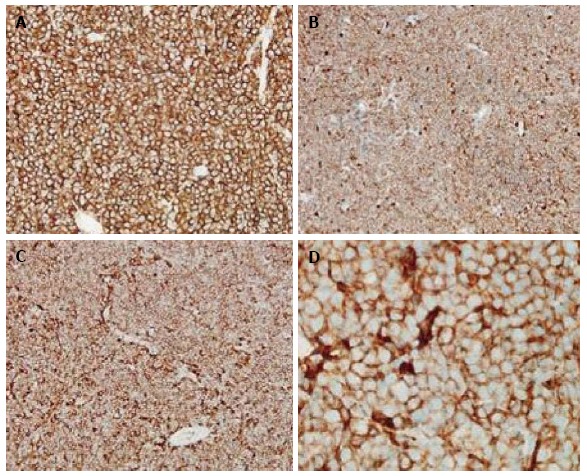
Immunohistochemistry results (clockwise). A: CD20 (original magnification × 200); B: CD5 (original magnification × 200); C: CD4 (original magnification × 200); D: CD4 (original magnification × 1000).
Five patients were diagnosed with DLBCL, including one (patient 4) in which the DLBCL represented a Richter transformation of a prior chronic lymphocytic leukemia/ small lymphocytic lymphoma and one (patient 3) with DLBCL and associated grade 3a follicular lymphoma. Patient 4 presented with primary mediastinal large B-cell lymphoma.
Clinical follow-up was available for 5/6 patients, who were all treated with multiagent chemotherapy. Two patients received stem cell transplants. Two patients had an initial response until lost to further follow-up at 6 and 72 mo, respectively, and 3 died of progressive disease between 6-15 mo after original diagnosis. Average survival was 44.2 mo overall, and 11.7 mo for those who died.
CONCLUSION
Herein, we report the largest series of ALK-negative CD4-positive DLBCL. Five cases were DLBCL, NOS and one was an example of PMLBCL. In our cohort (n = 6), the average patient age was 56 years (range: 22-79). There was no sex predilection (3 males, 3 females). Lymph nodes were the most common site of involvement (67%). All patients received multi-agent chemotherapy, and two patients underwent stem cell transplantation. Average overall survival (n = 6) was 44.2 mo. For those who died (n = 3), average survival was only 11.7 mo.
The aberrant expression of T-cell associated antigens is not seen in benign lymphoid proliferations and is rather uncommon in B-NHL[7,8]. Nonetheless, aberrant expression of CD5, CD2, CD3, CD4, CD7, CD8, and CD45RO (UCHL-1) have all been reported in B-cell lymphomas. Most commonly, aberrant expression of T-cell antigens is seen in CLL and mantle cell lymphoma. Overall, the reported frequency of T-cell antigen expression in B-NHL ranges from 19% to 25%[8]. CD4 expression is unusual in B-NHL. It has been reported in plasmablastic lymphoma (3 cases), DLBCL associated with chronic inflammation/pyothorax-associated lymphoma (1 case), and CLL (1 case)[9]. Interestingly, CD4 positivity is frequently seen in ALK+ DLBCL with one series citing a frequency of 64% (14/22 cases) in these rather uncommon neoplasms[10]. In DLBCL, NOS, CD4 expression is particularly rare with only 4 cases reported in the world literature to the best of our knowledge[6,8,11].
Review of the literature shows four prior reports or ALK-negative, CD4-positive DLBCL (Table 2). The earliest case was identified by Olack et al[8] who described a case of DLBCL that was positive for CD19, surface kappa light chain, CD4, and CD7 by flow cytometry. The patient was an 81-year-old man with moderate lymphadenopathy but no organomegaly or bone marrow involvement. He had stage III disease and was lost to follow up after 13 mo of stable disease.
Table 2.
Summary of ALK-negative, CD4-positive diffuse large B-cell lymphomas reported in the literature
| Case no. | Age/sex | Diagnosis | Immunophenotype | Molecular findings | Gross description | Treatment | Clinical outcome | Site | Stage | Ref. |
| 1 | 81/F | DLBCL | CD19, CD4, CD7 | Lost to follow-up after 13 mo of stable disease | N/A | III | [2] | |||
| 2 | 82/M | DLBCL | CD4+, CD5+, CD19+, CD20+, CD23+, CD25+, kappa+ | R-CHOP × 5 | Complete remission after chemotherapy | Left cervical and left inguinal lymph node | [11] | |||
| 3 | 55/F | DLBCL | CD20 (weak), BCL2, PAX5, surface kappa, MUM1, and CD4 | Clonal IgH gene rearrangement and no BCL2 gene rearrangement | Uniformly soft, greyish tissue | Mega Chop; R- Mega CHOP × 5 | Complete remission after chemotherapy | Ileum | [6] | |
| 4 | 73/M | DLBCL | CD45 (dim), CD19, PAX5, CD20, CD10, BCL6, BCL2, surface lambda light chain, and CD4 | Clonal IgH gene rearrangement and a BCL2 gene rearrangement | Adjuvant chemotherapy | Ileum | [6] |
CD: Cluster of differentiation; DLBCL: Diffuse large B-cell lymphoma; F: Female; M: Male; N/A: Not available; CHOP: Rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, mega.
The next patient was an 82-year-old man who was found to have left cervical and left inguinal lymph node swelling. Biopsy of an involved lymph node showed DLBCL. Dual staining confirmed that the cells of interest were positive for both CD4 and CD19. Complete remission was achieved after five cycles of rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate (R-CHOP)[11].
2010, Arrondini et al[6] reported 2 cases of CD4+ DLBCL occurring in the small bowel. The first of these was a 55-year-old woman who presented with lymphoma entrapping the last part of the ileum, pancreas, and omentum. Molecular studies showed a clonal IgH rearrangement and no BCL2 rearrangement. The patient received dose-escalated (Mega) CHOP but two weeks later required a laparotomy for resection of 60 cm of ulcerated and perforated small bowel. The patient received 5 more cycles of R-MegaCHOP and achieved complete remission.
The second case reported by Arrondini et al[6] involved a 73-year-old man who presented with DLBCL involving the ileum. A staging bone marrow biopsy was negative. The patient was treated with adjuvant chemotherapy. Two years later, he presented with an enlargement of the pancreatic head and a mass involving the right adrenal gland and superior pole of the right kidney. Retroperitoneal lymph-node fine needle aspiration showed numerous large lymphoid cells with centrally-located nucleoli and vesicular chromatin.
Aberrant expression of CD4 in DLBCL is a rare but intriguing finding that raises questions of lineage fidelity and the biology underlying such aberrant expression. Under physiological conditions, lineage commitment and differentiation are considered unidirectional and irreversible processes[12]. That is to say, a lymphocyte committed to the B-cell program is destined to become a B-cell, one that we do not normally expect to dedifferentiate to an immature form. So how then does one explain aberrant T-cell antigen expression in B-cell lymphomas? Various hypotheses, some of which challenge this paradigm, have been advanced to account for this unusual phenomenon.
Some believe that aberrant expression is the result of neoplastic transformation at stem cell level before commitment to either B or T-cell differentiation[13,14]. Others postulate expansion of a normal subpopulation expressing T cell antigens [CD5+ B1 cells, CD2(+) B cells, CD7(+) B cells][8,15]. It is also possible that deregulated control of gene expression in malignant B cells leads to the activation of some otherwise silent or repressed genes of T-cell differentiation[8,13,15-18]. For example, deregulated/damaged PAX5 might downregulate B-cell antigens and lead to aberrant expression of a T-cell antigen such as CD4. This notion is corroborated by murine models in which conditional deletion of PAX5 resulted in dedifferentiation of mature peripheral B cells into early uncommitted progenitors that were able to rescue T lymphopoiesis in the thymus of T-cell-deficient mice[12].
The import of T-cell antigen expression in B-NHL lies beyond its identification as a biological curiosity or its role in helping us understand lymphopoiesis, and in turn lymphomagenesis. There are practical implications as well. Particularly, recognition of T-cell antigen expression in B-NHL is important to avoid erroneous diagnostic consideration of a T-cell malignancy, a potential pitfall that is potentiated by sometimes weak expression of B-cell defining markers. Given that T-cell markers are not routinely performed in cases of DLBCL, the actual incidence of CD4 positivity may actually be higher than cited in the literature. However, results from sizeable series looking at T-cell antigen expression in B-NHL would argue against this possibility and suggest that CD4 expression is in fact rare[3].
We must also consider whether there are any prognostic implications. The data are not entirely clear in this regard. There are isolated reports showing worse outcomes for T-Ag (+) B-NHLs. Some report increased extranodal involvement and a higher International Prognostic Index (high and high intermediate) score in such cases[3]. On the other hand, Olack et al[8] did not find any difference when T-Ag (+) B-NHLs were compared to their normal B-NHL counterparts. With regards to our series of DLBCLs expressing CD4, it appears that at least a subset may behave aggressively based on our data. In summary, aberrant CD4 expression in ALK-negative DLBCL is a rare, but documented, phenomenon that raises interesting biological and diagnostic considerations.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Manuscript source: Invited manuscript
Specialty type: Clinical medicine
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: April 13, 2016
First decision: May 19, 2016
Article in press: July 31, 2016
P- Reviewer: Alshehabi Z, Kupeli S, Mehdi I S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
References
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. [Google Scholar]
- 2.Kaleem Z, White G, Zutter MM. Aberrant expression of T-cell-associated antigens on B-cell non-Hodgkin lymphomas. Am J Clin Pathol. 2001;115:396–403. doi: 10.1309/v8yg-8pp4-b4te-9x6j. [DOI] [PubMed] [Google Scholar]
- 3.Inaba T, Shimazaki C, Sumikuma T, Okano A, Hatsuse M, Okamoto A, Takahashi R, Ashihara E, Hibi S, Sudo Y, et al. Expression of T-cell-associated antigens in B-cell non-Hodgkin’s lymphoma. Br J Haematol. 2000;109:592–599. doi: 10.1046/j.1365-2141.2000.02006.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Chen C, Lau S, Raghavan RI, Rowsell EH, Said J, Weiss LM, Huang Q. CD3-positive large B-cell lymphoma. Am J Surg Pathol. 2009;33:505–512. doi: 10.1097/PAS.0b013e318185d231. [DOI] [PubMed] [Google Scholar]
- 5.Carulli G, Stacchini A, Marini A, Ciriello MM, Zucca A, Cannizzo E, Aliberti S, Demurtas A, Novero D, Calcagno L, et al. Aberrant expression of CD8 in B-cell non-Hodgkin lymphoma: a multicenter study of 951 bone marrow samples with lymphomatous infiltration. Am J Clin Pathol. 2009;132:186–190; quiz 306. doi: 10.1309/AJCPNCOHS92ARWRQ. [DOI] [PubMed] [Google Scholar]
- 6.Arrondini M, Barreca A, Aliberti S, Demurtas A, Tondat F, Novero D, Stacchini A. CD4-positive diffuse large B cell lymphoma identified by flow cytometry: two case reports. Int J Hematol. 2010;92:198–203. doi: 10.1007/s12185-010-0631-8. [DOI] [PubMed] [Google Scholar]
- 7.Wallentine JC, Perkins SL, Tripp SR, Bruggman RD, Bayerl MG. Diffuse large B-cell lymphoma with coexpression of CD3 in a pediatric patient: a case report, review of the literature, and tissue microarray study. J Pediatr Hematol Oncol. 2009;31:124–127. doi: 10.1097/MPH.0b013e31818b354a. [DOI] [PubMed] [Google Scholar]
- 8.Olack BJ, Jaramillo A, Zhang L, Swanson C, Rayan K, Goodnight DM, Kaleem Z, Howard T, Mohanakumar T. The role of indirect antigen recognition in islet xenograft rejection. Transplant Proc. 2001;33:784–785. doi: 10.1016/s0041-1345(00)02254-5. [DOI] [PubMed] [Google Scholar]
- 9.Jani P, Qi XY, Chang H. Aberrant expression of T-cell-associated markers CD4 and CD7 on B-cell chronic lymphocytic leukemia. Am J Hematol. 2007;82:73–76. doi: 10.1002/ajh.20747. [DOI] [PubMed] [Google Scholar]
- 10.Reichard KK, McKenna RW, Kroft SH. ALK-positive diffuse large B-cell lymphoma: report of four cases and review of the literature. Mod Pathol. 2007;20:310–319. doi: 10.1038/modpathol.3800742. [DOI] [PubMed] [Google Scholar]
- 11.Nakazato T, Suzuki K, Mihara A, Sanada Y, Kakimoto T, Yoshida S. [CD4-positive diffuse large B-cell lymphoma] Rinsho Ketsueki. 2009;50:568–573. [PubMed] [Google Scholar]
- 12.Cobaleda C, Jochum W, Busslinger M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature. 2007;449:473–477. doi: 10.1038/nature06159. [DOI] [PubMed] [Google Scholar]
- 13.Burns GF, Nash AA, Worman CP, Barker CR, Hayhoe FG, Cawley JC. A human leukaemic cell expressing hybrid membrane phenotypes. Nature. 1977;268:243–245. doi: 10.1038/268243a0. [DOI] [PubMed] [Google Scholar]
- 14.Aisenberg AC, Bloch KJ, Wilkes BM. Malignant lymphoma with dual B and T cell markers. Analysis of the neoplastic cells with monoclonal antibodies directed against T cell subsets. J Exp Med. 1981;154:1709–1714. doi: 10.1084/jem.154.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foon KA, Billing RJ, Terasaki PI. Dual B and T markers in acute and chronic lymphocytic leukemia. Blood. 1980;55:16–20. [PubMed] [Google Scholar]
- 16.Pridans C, Holmes ML, Polli M, Wettenhall JM, Dakic A, Corcoran LM, Smyth GK, Nutt SL. Identification of Pax5 target genes in early B cell differentiation. J Immunol. 2008;180:1719–1728. doi: 10.4049/jimmunol.180.3.1719. [DOI] [PubMed] [Google Scholar]
- 17.Cobaleda C, Schebesta A, Delogu A, Busslinger M. Pax5: the guardian of B cell identity and function. Nat Immunol. 2007;8:463–470. doi: 10.1038/ni1454. [DOI] [PubMed] [Google Scholar]
- 18.Nutt SL, Heavey B, Rolink AG, Busslinger M. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 1999;401:556–562. doi: 10.1038/44076. [DOI] [PubMed] [Google Scholar]


