Abstract
BACKGROUND
Tracking longitudinal measurements of growth and decline in lung function in patients with persistent childhood asthma may reveal links between asthma and subsequent chronic airflow obstruction.
METHODS
We classified children with asthma according to four characteristic patterns of lung-function growth and decline on the basis of graphs showing forced expiratory volume in 1 second (FEV1), representing spirometric measurements performed from childhood into adulthood. Risk factors associated with abnormal patterns were also examined. To define normal values, we used FEV1 values from participants in the National Health and Nutrition Examination Survey who did not have asthma.
RESULTS
Of the 684 study participants, 170 (25%) had a normal pattern of lung-function growth without early decline, and 514 (75%) had abnormal patterns: 176 (26%) had reduced growth and an early decline, 160 (23%) had reduced growth only, and 178 (26%) had normal growth and an early decline. Lower baseline values for FEV1, smaller bronchodilator response, airway hyperresponsiveness at baseline, and male sex were associated with reduced growth (P<0.001 for all comparisons). At the last spirometric measurement (mean [±SD] age, 26.0±1.8 years), 73 participants (11%) met Global Initiative for Chronic Obstructive Lung Disease spirometric criteria for lung-function impairment that was consistent with chronic obstructive pulmonary disease (COPD); these participants were more likely to have a reduced pattern of growth than a normal pattern (18% vs. 3%, P<0.001).
CONCLUSIONS
Childhood impairment of lung function and male sex were the most significant predictors of abnormal longitudinal patterns of lung-function growth and decline. Children with persistent asthma and reduced growth of lung function are at increased risk for fixed airflow obstruction and possibly COPD in early adulthood. (Funded by the Parker B. Francis Foundation and others; ClinicalTrials.gov number, NCT00000575.)
Graphical abstract
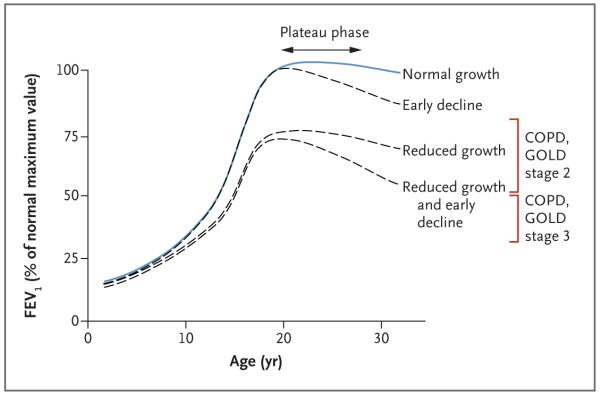
In persons without lung disease, forced expiratory volume in 1 second (FEV1) reaches its maximal level in late adolescence or early adulthood and remains stable for several years, a period known as the plateau of lung function, before gradually declining thereafter (Fig. 1).1 Under the construct described by Speizer and Tager,1 the pattern of FEV1 growth and decline in childhood and early adulthood is an important determinant of lung function in later adulthood; both reduced growth resulting in a low maximal level of lung function and early decline are associated with the subsequent development of chronic airflow obstruction.2-4
Figure 1. Longitudinal Lung-Function Trajectories.
Possible lung-function trajectories during the first three decades of life are shown; the lung function plotted for each age is the percentage of the maximum forced expiratory volume in 1 second (FEV1) in a person without lung disease; the maximum value is usually attained at the age of 18 to 30 years. A normal pattern of lung-function growth and decline is characterized by a steep increase during adolescence, a plateau in early adulthood, and a gradual decline into old age. Abnormal trajectories include reduced growth, normal growth and an early decline, and reduced growth and an early decline. The red brackets indicate FEV1 criteria according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 2 (FEV1 ≥50% and <80%) and stage 3 (FEV1 ≥30% and <50%) of chronic obstructive pulmonary disease (COPD), when accompanied by a ratio of FEV1 to forced vital capacity that is less than 0.70. The figure is adapted from Speizer and Tager.1
Determinants of abnormal patterns of FEV1 growth and decline are multifactorial and complex, and identification of factors associated with the timing of a decline from the maximal level requires longitudinal data, which are sparse, particularly for persons with asthma, a population at risk for chronic airflow obstruction.4-15 The Childhood Asthma Management Program (CAMP) cohort was followed from enrollment, at the age of 5 to 12 years, into the third decade of life, with at least annual prebronchodilator and postbronchodilator spirometry and detailed concomitant assessments. The long follow-up offers the opportunity to examine the trajectory of lung growth and the decline from maximum growth in a large cohort of persons who had persistent, mild-to-moderate asthma in childhood and to determine the demographic and clinical factors associated with abnormal patterns of lung growth and decline.
METHODS
CAMP STUDY DESIGN AND PARTICIPANTS
CAMP was a randomized, placebo-controlled trial of inhaled antiinflammatory treatments for mild-to-moderate childhood asthma followed by three phases of observational follow-up; the trial and all follow-up phases included at least annual prebronchodilator and postbronchodilator spirometry (Fig. S1 in Supplementary Appendix 1, available with the full text of this article at NEJM.org).16,17 A total of 1041 children, 5 to 12 years of age, were enrolled in the trial between December 1993 and September 1995. Inclusion was restricted to children with a history of chronic asthma who, during a 28-day run-in period, had asthma symptoms or a low morning peak flow on 8 or more days; inclusion also required a methacholine challenge with a concentration of 12.5 mg per milliliter or less that resulted in a reduction in FEV1 by at least 20% (since airway responsiveness is determined by the provocative concentration of methacholine required to reduce the FEV1 by at least 20% [methacholine PC20], with higher values indicating less airway responsiveness). Written informed consent was obtained from the parents or guardians of all the participants.
Participants were randomly assigned to receive budesonide (200 μg twice a day), nedocromil (8 mg twice a day), or placebo, all by inhalation. Treatment was continued for a mean of 4.5 years; the treatment component of the trial ended in 1999, and asthma care was transferred to each participant’s health care provider. We found that the antiinflammatory medications did not have a better long-term effect than placebo on lung-function growth.17 Observational follow-up continued for 13 years in three consecutive phases; more than 85% of the original 1041 participants participated in at least one observational follow-up phase, and 80% participated in all three phases.
Prebronchodilator FEV1 values for persons without asthma who were participants in the third National Health and Nutrition Examination Survey (NHANES III),18 adjusted for sex, self-reported race or ethnic group, age, and height at each spirometric measurement, were used as the basis of comparison for FEV1 in CAMP participants. NHANES III, conducted from 1988 through 1994, examined 7429 healthy lifelong nonsmokers and measured prebronchodilator FEV1 and forced vital capacity (FVC) according to American Thoracic Society recommendations. The cohort included whites, Mexicans, and blacks, and different equations for predicted FEV1 were established for each race or ethnic group and sex on the basis of age and height.18
CLASSIFICATION OF LUNG-FUNCTION PATTERNS IN A SUBSET OF PARTICIPANTS
In the current study, we categorized a subset of CAMP participants according to four patterns of lung-function growth and decline: normal growth with a normal plateau or maximum not yet reached, normal growth and an early decline, reduced growth and a normal plateau or maximum not yet reached, and reduced growth and an early decline. Normal growth was defined as an FEV1 growth curve that was almost always at or above the 25th percentile of values in NHANES III, and reduced growth was defined as an FEV1 growth curve that was almost always below the 25th percentile, on the basis of visual inspection. An early decline in lung function was defined as an earlier-than-expected decrease of at least two data points from the maximal level on the basis of the corresponding normal FEV1 growth curves in the NHANES III cohort. Pattern classification was subjectively assessed by experts according to the overall shape of the participants’ FEV1 curve over time. Full classification procedures are provided in Supplementary Appendix 1, Part 2A.
Graphic smoothing was performed on each CAMP participant’s available prebronchodilator FEV1 measures with the use of robust, locally weighted scatterplot smoothing regression.19 The participant’s raw measures and smoothed measures and the NHANES percentiles for a person of the same sex, race or ethnic group, age, and height as the CAMP participant at each spirometric assessment were graphed on the same plot. Two pulmonologists with asthma expertise independently classified the pattern of lung-function growth and decline on all graphs from the same randomly selected set of 110 participants according to protocol. Each pulmonologist also determined whether maximum lung function had been reached and, if so, whether the plateau phase or a decline had begun (see the full set of pattern coding sheets in Supplementary Appendix 2). A participant was considered to have no plateau and an immediate decline if the smoothed curve immediately declined from the maximum level. The pulmonologists then compared their classifications to arrive at a consensus classification for each participant. Finally, one of the pulmonologists classified the remaining 931 participants. If that pulmonologist could not classify a participant’s data, the other pulmonologist was asked to do so, and a consensus classification was determined, if possible.
STATISTICAL ANALYSIS
Our primary analyses were limited to participants who had at least one spirometric measurement at 23 years of age or older and were assigned to one of the four patterns of lung growth and decline. The homogeneity of the pattern groups with respect to demographic and clinical characteristics (age, sex, baseline lung function, timing of the FEV1 plateau, timing of the decline in FEV1, body-mass index, and status with respect to atopy and allergy) was assessed with the use of a two-sided chi-square test for nonordered categories (for categorical variables) and analysis of variance (for continuous variables). Also, the trajectory of lung-function growth was assessed according to sex, with the use of two-sided t-tests (for continuous variables) and chi-square tests (for categorical variables).
The Kaplan–Meier method for estimating survivor functions and the associated log-rank test were used to compare male and female participants with respect to median ages at the time of maximum lung function, the plateau period, and the start of the decline in function.20 Multinomial logistic regression21 was used to relate the four-category outcome variable (normal lung-function growth [reference category], normal growth and an early decline, reduced growth, and reduced growth and an early decline) to explanatory demographic, behavioral, and clinical risk factors, resulting in three sets of conditional odds ratios for each risk factor per one-unit change in that factor. Each participant’s outcome was further characterized by applying the spirometric criteria of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification22 to the final postbronchodilator spirometric assessment in early adulthood. The exact Mantel–Haenszel chi-square statistic for trend was used to test the ordered cross-classification of the GOLD spirometric category in relation to the four patterns of lung growth and decline. All statistical analyses were performed with the use of SAS, version 9.3 (SAS Institute) or STATA/IC, version 13.1 (StataCorp).
RESULTS
CLASSIFICATION OF THE STUDY PARTICIPANTS
We attempted to classify all 1041 CAMP participants; Supplementary Appendixes 1 (Part 2), 2, 3, and 4 include all 1041 plots for readers who wish to complete their own classifications. The two pulmonologists with asthma expertise had very good agreement on their classifications for the full set of 110 participants (kappa = 0.83; 95% confidence interval [CI], 0.76 to 0.91; P<0.001) and excellent agreement in their classifications for the 101 participants with identifiable patterns (kappa = 0.92; 95% CI, 0.86 to 0.98; P<0.001); additional details are provided in Table S1 in Supplementary Appendix 1.23,24 One of the expert graders then proceeded to classify the remaining 931 participants. He was able to reach a decision for 842 of these participants; joint review by the two experts was required to reach a decision for the remaining 89 participants. Thus, a total of 19% of the pattern classifications were determined by both experts. With the classification results combined for the initial set of 110 participants and the remaining set of 931, a total of 949 participants were assigned to a pattern group, and 92 had undetermined patterns (Fig. S2 in Supplementary Appendix 1). In addition, two researchers with pulmonary expertise classified a different random sample of 100 participants and also had a very high correspondence between their classifications (kappa = 0.89; 95% CI, 0.81 to 0.96; P<0.001) (Table S2 in Supplementary Appendix 1).
LUNG-FUNCTION PATTERNS IN A SUBSET OF PARTICIPANTS
Primary analysis was completed for 684 of the 949 participants (72%) who had at least one FEV1 measurement at the age of 23 to 30 years. These 684 participants had a total of 15,798 spirometric sessions (median number of sessions per participant, 24 [interquartile range, 22 to 25]; median number of sessions at the age of 23 years or older, 3 [interquartile range, 2 to 5]). The mean (±SD) age at the last spirometric measurement was 26.0±1.8 years (range, 23 to 30).
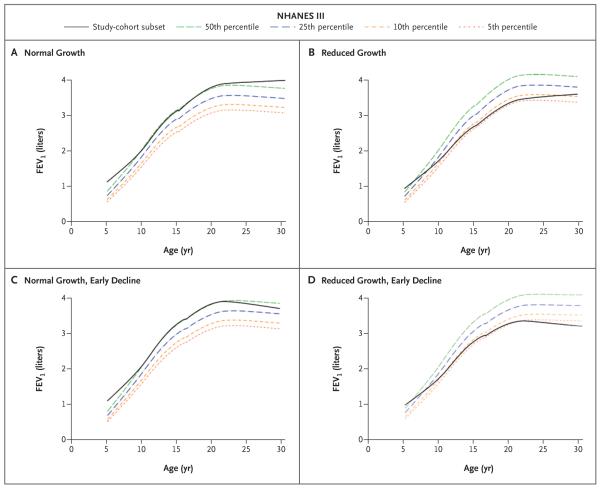
A total of 170 of these 684 participants (25%) were classified as having normal lung-function growth without an early decline. Of these 170 participants, 26% had a plateau in growth, with maximum lung-function growth at a mean age of 22.3 years, whereas 74% had not yet reached maximum lung-function growth at the time of the last assessment (Table 1). A total of 178 of the 684 participants (26%) were classified as having normal growth and an early decline; the mean age at maximum lung function was 20.6 years, and the mean age at the start of the decline was 21.1 years. A total of 160 participants (23%) were classified as having reduced growth without an early decline; 19% of these 160 participants reached a plateau, with maximum lung-function growth at a mean age of 21.9 years, whereas 81% had not yet reached maximum lung-function growth at the time of the last assessment. A total of 176 participants (26%) were classified as having reduced growth and an early decline; the mean age at maximum lung function was 20.6 years, and the mean age when lung function declined was 21.3 years. Figure 2 shows averaged prebronchodilator FEV1 trajectories for each pattern group.
Table 1.
Characteristics of the Study Participants According to the Pattern of Lung-Function Growth and Decline.*
| Characteristic | Normal Growth (N = 170) |
Normal Growth and Early Decline (N = 178) |
Reduced Growth (N = 160) |
Reduced Growth and Early Decline (N = 176) |
P Value† |
|---|---|---|---|---|---|
| Maximum lung function attained — no. (%) | 45 (26) | 178 (100) | 30 (19) | 176 (100) | <0.001 |
| Age at maximum lung function — yr | 22.3±2.2 | 20.6±2.2 | 21.9±1.7 | 20.6±1.8 | <0.001 |
| Plateau phase | <0.001 | ||||
| Plateau not attained, maximum lung function not reached — no. (%) |
125 (74) | 0 | 130 (81) | 0 | |
| No plateau, immediate decline — no. (%) | 0 | 112 (63) | 0 | 106 (60) | |
| Maximum lung function reached, plateau attained — no. (%) |
45 (26) | 66 (37) | 30 (19) | 70 (40) | |
| Age when plateau attained — yr | 22.3±2.2 | 20.6±2.1 | 21.9±1.7 | 20.5±1.6 | <0.001 |
| Plateau phase completed — no. (%) | 1 (1)‡ | 66 (37) | — | 70 (40) | 0.60 |
| Duration of plateau — yr | 2.0‡ | 1.5±0.6 | — | 1.8±0.9 | 0.03 |
| Decline phase begun — no. (%) | 1 (1)‡ | 178 (100) | 0 | 176 (100) | |
| Had an early decline — no. (%) | 0 | 178 (100) | 0 | 176 (100) | |
| Age at start of any decline — yr | 24.0‡ | 21.1±2.3 | — | 21.3±2.0 | 0.46 |
| Demographic and physical characteristics | |||||
| Male sex — no. (%) | 82 (48) | 100 (56) | 114 (71) | 109 (62) | <0.001 |
| Age at randomization — yr | 9.3±1.7 | 9.7±1.7 | 9.3±1.8 | 9.9±1.7 | 0.006 |
| Prepubertal at randomization — no. (%)§ | 119 (70) | 106 (60) | 112 (70) | 106 (60) | 0.04 |
| Body-mass index at randomization — z score | 0.50±0.97 | 0.78±0.94 | 0.18±1.04 | 0.44±1.05 | <0.001 |
| Interval between diagnosis of asthma and enrollment — no. (%) |
0.003 | ||||
| <3 yr | 50 (29) | 45 (25) | 31 (19) | 28 (16) | |
| 3–6 yr | 85 (50) | 84 (47) | 81 (51) | 79 (45) | |
| ≥7 yr | 35 (21) | 49 (28) | 48 (30) | 69 (39) | |
| Maternal cigarette smoking during gestation — no. (%) | 17 (10) | 27 (15) | 20 (12) | 27 (15) | 0.40 |
| Lung function at randomization | |||||
| Prebronchodilator FEV1 — % of predicted value | 100.5±13.4 | 99.7±12.9 | 87.5±12.6 | 83.8±12.9 | <0.001 |
| Prebronchodilator FEV1:FVC — % of predicted value | 81.9±6.9 | 81.6±7.5 | 76.5±7.9 | 76.5±8.4 | <0.001 |
| Bronchodilator response — %¶ | 8.9±7.8 | 8.2±7.8 | 12.7±9.9 | 12.4±11.3 | <0.001 |
| Airway responsiveness — log mg/ml∥ | 0.3±1.2 | 0.4±1.1 | −0.2±1.1 | −0.2±1.1 | <0.001 |
| Lifetime smoking — pack-yr** | 0.5±1.5 | 0.4±1.4 | 0.4±1.1 | 0.5±1.5 | 0.97 |
| Age and spirometry at last visit | |||||
| Age — yr | 25.7±1.7 | 26.0±1.8 | 25.8±1.9 | 26.3±1.7 | 0.01 |
| Prebronchodilator FEV1 — % of predicted value | 104.3±7.6 | 97.7±9.5 | 87.1±7.9 | 79.7±10.0 | <0.001 |
| Prebronchodilator FEV1:FVC — % of predicted value | 80.4±6.4 | 78.1±7.2 | 73.0±8.0 | 71.2±9.6 | <0.001 |
Plus–minus values are means ±SD. FEV1 denotes forced expiratory volume in 1 second, and FVC forced vital capacity.
P values (two-sided) are for the comparison across pattern groups and are based on a chi-square test for nonordered categories (categorical variables) or analysis of variance (continuous variables).
One participant with a normal-growth pattern was determined to have a decline in lung function; however, the decline was not earlier than expected.
Prepubertal was defined as no Tanner components (pubic hair and either breast stage for girls or genital stage for boys) greater than stage 1.
Bronchodilator response was calculated as [(postbronchodilator FEV1 – prebronchodilator FEV1) ÷ prebronchodilator FEV1] × 100.
Airway responsiveness was defined as the concentration of methacholine that caused a 20% decrease in FEV1.
Data on smoking exposure were restricted to cigarette smoking. Smoking exposure was determined through the end of follow-up.
Figure 2. Average Prebronchodilator FEV1 Trajectories for 684 Study Participants According to Pattern Classification, as Compared with FEV1 in Persons without Asthma.
Values for prebronchodilator FEV1 in the study participants are group averages and are based on robust, locally weighted scatterplot smoothing regression. Also shown are percentiles of FEV1 in persons without asthma who were participants in the third National Health and Nutrition Examination Survey (NHANES III)18 and were matched to our study participants for sex, race or ethnic group, age, and height at each spirometric session. Panel A shows the average FEV1 trajectory for participants classified as having normal lung-function growth without an early decline (170 participants), Panel B shows the trajectory for participants who had reduced growth without an early decline (160 participants), Panel C shows the trajectory for participants with normal growth and an early decline (178 participants), and Panel D shows the trajectory for participants who had reduced growth and an early decline (176 participants).
Of the 684 participants, 429 (63%) reached a maximum in FEV1. For 218 participants (32%), lung function began to decline immediately after the maximum level had been reached, with no observable plateau on the lung-function curve; for 211 participants (31%), lung function plateaued; and for 137 (20%), the plateau phase was completed. Among participants with a complete plateau phase, the duration of the plateau ranged from 0.8 to 5.2 years (mean, 1.6±0.8); the duration did not differ significantly according to sex but on average was 0.3 years longer in the group with reduced growth and an early decline than in the group with normal growth and an early decline (P = 0.03) (Tables S3 and S4 in Supplementary Appendix 1). Maximum lung function, the plateau phase, and decline in lung function occurred on average a year earlier in women than in men; however, the difference in the time to a plateau was not significant on the basis of a log-rank test (Fig. S3 in Supplementary Appendix 1).
CHARACTERISTICS OF THE LUNG-FUNCTION GROUPS
The four lung-function groups differed according to sex (P<0.001), age at enrollment (P = 0.006), race or ethnic group (P<0.001), interval between diagnosis of asthma and enrollment (P = 0.003), bronchodilator response at enrollment (P<0.001), airway responsiveness at enrollment (P<0.001), and lung function at enrollment (P<0.001) (Table 1, and Table S5 in Supplementary Appendix 1).
Characteristics of the 684 participants with at least one spirometric measurement at the age of 23 years or older, as compared with the remaining 357 participants, are shown in Table S6 in Supplementary Appendix 1. Participants with a spirometric measurement at the age of 23 years or older were older (P<0.001) and were more likely to be prepubertal at baseline (P = 0.008) than the remaining participants but were similar with respect to sex, race or ethnic group, asthma severity, interval between asthma diagnosis and enrollment, atopy status, and assigned treatment group.
RISK FACTORS FOR ABNORMAL LUNG-FUNCTION PATTERNS
Results of the multinomial logistic-regression analysis of risk factors for abnormal patterns of lung growth and decline (normal growth and an early decline, reduced growth, and reduced growth and an early decline) are shown in Table S7 in Supplementary Appendix 1. As compared with participants who had a normal growth pattern, those with a pattern of normal growth and an early decline had a higher body-mass index at enrollment (odds ratio, 1.39; P = 0.02), a greater likelihood of maternal cigarette smoking during gestation (odds ratio, 2.33; P = 0.04), and more episode-free days during the trial (odds ratio, 1.01 per 1% change in episode-free days; P = 0.03) but were otherwise similar.
Participants with the reduced-growth pattern, as compared with those who had normal growth, had lower FEV1 values at enrollment (odds ratio, 0.86 per 1% change in the predicted value; P<0.001), a lower bronchodilator response (odds ratio, 0.91 per 1% change; P<0.001), and greater airway hyperresponsiveness (odds ratio, 0.61 per unit change in log-transformed milligrams per milliliter; P<0.001); were more likely to be male (odds ratio, 8.18; P<0.001); were younger at enrollment (odds ratio, 0.55 per year of age; P<0.001); had a lower level of parental education (odds ratio for at least a college degree vs. a lower level, 0.33; P = 0.002); were more likely to have vitamin D insufficiency (odds ratio, 2.15; P = 0.03); and received more courses of prednisone per year during the trial (odds ratio, 4.12 for each additional course; P = 0.03).
Participants with reduced growth and an early decline, as compared with those who had normal growth, had lower FEV1 lung function at enrollment (odds ratio, 0.85 per 1% change in the predicted value; P<0.001), a lower bronchodilator response (odds ratio, 0.91 per 1% change; P<0.001), and increased airway hyperresponsiveness (odds ratio, 0.66 per unit change in log-transformed milligrams per milliliter; P = 0.008); were more likely to be male (odds ratio, 3.07; P<0.001); were younger at enrollment (odds ratio, 0.62 per year of age; P<0.001); and had a lower level of parental education (odds ratio, 0.43 for at least a college degree vs. a lower level; P = 0.01), a greater number of positive skin tests at enrollment (odds ratio for ≥3 positive tests vs. <3, 2.42; P = 0.03), and a lower incidence of hospitalization during the trial (odds ratio, 0.26 per any hospitalization; P = 0.02). There were no significant differences between any of the three abnormal-pattern groups and the normal-pattern group with respect to the assigned treatment in the CAMP trial or the proportion of participants who had smoked 100 or more cigarettes by the age of 18 years.
At their last spirometric session (mean age, 26.0±1.8 years), participants with reduced lung-growth patterns were more likely to meet GOLD spirometric staging criteria for COPD than were participants with normal lung-growth patterns (16% of the reduced-growth group and 21% of the group with reduced-growth and an early decline vs. 1% of the normal-growth group and 5% of the group with normal growth and an early decline, P<0.001) (Table 2). A total of 36% of the combined reduced-growth groups and 8% of the combined normal-growth groups were classified as having COPD according to the modified GOLD spirometric criterion of a post-bronchodilator FEV1:FVC ratio that was below the lower limit of the normal range (P<0.001).25,26
Table 2.
Global Initiative for Chronic Obstructive Lung Disease (GOLD) Classification According to the Pattern of Lung-Function Growth and Decline.*
| GOLD Classification | Normal Growth (N = 170) |
Normal Growth and Early Decline (N = 178) |
Reduced Growth (N = 160) |
Reduced Growth and Early Decline (N = 176) |
Total (N = 684) |
|---|---|---|---|---|---|
| Met GOLD criterion: postbronchodilator FEV1:FVC <0.70 — no. (%) | |||||
| No | 168 (99) | 169 (95) | 135 (84) | 139 (79) | 611 (89) |
| Yes | |||||
| Stage 1, FEV1 ≥80% of predicted value | 2 (1) | 8 (4) | 18 (11) | 16 (9) | 44 (6) |
| Stage 2, FEV1 ≥50% and <80% of predicted value | 0 | 1 (1) | 7 (4) | 20 (11) | 28 (4) |
| Stage 3, FEV1 ≥30% and <50% of predicted value | 0 | 0 | 0 | 1 (1) | 1 (<1) |
| P value† | — | 0.05 | <0.001 | <0.001 | <0.001 |
| Met modified GOLD criterion: postbronchodilator FEV1:FVC <LLN — no. (%)‡ | |||||
| No | 160 (94) | 159 (89) | 103 (64) | 113 (64) | 535 (78) |
| Yes | |||||
| Stage 1, FEV1 ≥80% of predicted value | 10 (6) | 18 (10) | 47 (29) | 38 (22) | 113 (17) |
| Stage 2, FEV1 ≥50% and <80% of predicted value | 0 | 1 (1) | 10 (6) | 24 (14) | 35 (5) |
| Stage 3, FEV1 ≥30% and <50% of predicted value | 0 | 0 | 0 | 1 (1) | 1 (<1) |
| P value† | — | 0.10 | <0.001 | <0.001 | <0.001 |
Each participant’s spirometric measurement from his or her last visit in the follow-up period was classified according to the lung-function criteria for COPD staging in the GOLD classification. The mean age at the last visit was 26.0±1.8 years (range, 23 to 30). GOLD staging is based on postbronchodilator FEV1 criteria. GOLD stage 1 indicates mild COPD, stage 2 moderate COPD, and stage 3 severe COPD.
P values (two-sided) are for the comparison with normal growth and were determined with the use of the exact Mantel–Haenszel chi-square test for trend.
LLN denotes lower limit of the normal range, defined as the 5th percentile value in the third National Health and Nutrition Examination Survey.
DISCUSSION
We classified study participants with persistent childhood asthma according to trajectories in the growth and decline of FEV1 lung function. The identification of these trajectories was performed to match the long-standing model of lung-function growth and decline (Fig. 1)1 and was informed by previous work identifying a plateau phase starting by the age of 20 years, followed by a decline starting by the age of 25 years in outbred populations.27 In the two groups with an early decline in lung function, the mean age when lung function began to decline was 21.1 years for participants with normal growth and 21.3 years for those with reduced growth, indicating that early decline is an important contribution to the long-term deficit in lung function that is frequently observed in patients with asthma.28 The average participant was followed until a mean age of 26.0 years, making the determination of reduced growth versus normal growth more robust than the determination of early decline versus no decline; the latter assessment would be aided by a longer follow-up period.
Among the children with mild-to-moderate, persistent asthma in our study, 75% had abnormal patterns of lung growth and decline in early adulthood. Many of the risk factors we identified for reduced growth of lung function have been reported previously, including maternal smoking during gestation,29 reduced lung function at enrollment,30 and increased airway hyperresponsiveness.31 Among these factors, reduced lung function at baseline was the strongest predictor of longitudinal lung-function impairment. Younger age at enrollment was a strong independent predictor of reduced growth (Table S7 in Supplementary Appendix 1), but this is probably because children with more severe asthma have more severe symptoms earlier, and such children may have been enrolled in CAMP at an earlier age than those with less severe asthma.
Many studies suggest that asthma may lead to COPD or persistent airflow obstruction32 or is a risk factor for COPD.14,33 That 11% of the CAMP cohort and 18% of the participants who had reduced lung-function growth with or without an early decline met the case definition for COPD, on the basis of postbronchodilator spirometric criteria at an age of less than 30 years, is indicative of this connection. We have previously reported that a majority of the CAMP cohort had airflow obstruction.34 Our data support the hypothesis that both reduced growth and an early decline are trajectories leading to an asthma–COPD overlap syndrome35 and complement the recent observation that in older patients, a rapid decline in lung function can lead to COPD.36 Disease progression from asthma to COPD can be due to smoking,37 but in our cohort, smoking exposure was too low to evaluate this relationship conclusively (Table 1).
Our study has several limitations. First, our results do not establish asthma as either the cause or the effect of each pattern of lung-function growth and decline; they merely establish its co-occurrence. Second, a longer follow-up period would provide a more accurate characterization of the lung-function plateau period and subsequent decline, allowing for a characterization of the timing and precursors of decline in all the study participants, not only in those with an early decline. Although we focus on participants with spirometric measurements at a minimum age of 23 years, this represents a tradeoff between including more participants in our analysis and making potentially more accurate determinations of the extent of the lung-function plateau phase and subsequent decline. Third, additional risk factors, including genetic factors, prematurity, childhood respiratory infections, and environmental exposures, were not available for analysis in the present study but would be interesting to assess in future studies. Finally, our assignments of longitudinal lung-function patterns in this cohort may not be applicable to patients with mild, intermittent asthma.
Impaired lung function at enrollment and male sex were the most significant predictors of abnormal longitudinal patterns of lung-function growth and decline. A pattern of reduced growth is evident early in childhood and can be expected to persist into adulthood. A total of 52% of patients with mild-to-moderate, persistent asthma have early lung-function decline, and 51% of those have no plateau phase. Identification of an abnormal trajectory by means of early and ongoing serial FEV1 monitoring may help identify children and young adults who are at risk for abnormal lung-function growth that might lead to chronic airflow obstruction in adulthood. Whether there are interventions that can modify the outcome will require further research.
Supplementary Material
Acknowledgments
Supported by grants from the Parker B. Francis Foundation (to Dr. McGeachie), the National Institutes of Health (U01HL075408, to Ms. Yates, Ms. Sternberg, Mr. Van Natta, and Drs. Wise and Tonascia; R01HL127200 and R21HL120794, to Dr. Zhou; P01-HL083069 and U01-HL065899, to Dr. Weiss; U01-HL105569, to Dr. Wise; K08-HL097029 and R01-HL113264, to Dr. Cho; T32-HL007427, to Dr. Croteau-Chonka; K08-HL102265 and R01-HL124233, to Dr. Castaldi; R01-HL075478, R01-HL089856, and P01-HL105339, to Dr. Silverman; U01-HL091075, to Dr. Strunk; K25 HL091124, to Dr. Kho; and U01-HL065899 and R01-NR013391, to Dr. Tantisira); the National Human Genome Research Institute (HG003143 and HG007010); and the Human Frontier Science Program Organization.
APPENDIX
The authors’ full names and academic degrees are as follows: Michael J. McGeachie, Ph.D., Katherine P. Yates, Sc.M., Xiaobo Zhou, Ph.D., Feng Guo, Ph.D., Alice L. Sternberg, Sc.M., Mark L. Van Natta, M.H.S., Robert A. Wise, M.D., Stanley J. Szefler, M.D., Sunita Sharma, M.D., Alvin T. Kho, Ph.D., Michael H. Cho, M.D., Damien C. Croteau-Chonka, Ph.D., Peter J. Castaldi, M.D., Gaurav Jain, M.S., Amartya Sanyal, Ph.D., Ye Zhan, Bryan R. Lajoie, Ph.D., Job Dekker, Ph.D., John Stamatoyannopoulos, M.D., Ronina A. Covar, M.D., Robert S. Zeiger, M.D., Ph.D., N. Franklin Adkinson, M.D., Paul V. Williams, M.D., H. William Kelly, Pharm.D., Hartmut Grasemann, M.D., Judith M. Vonk, Ph.D., Gerard H. Koppelman, M.D., Dirkje S. Postma, M.D., Benjamin A. Raby, M.D., Isaac Houston, Ph.D., Quan Lu, Ph.D., Anne L. Fuhlbrigge, M.D., Kelan G. Tantisira, M.D., Edwin K. Silverman, M.D., Ph.D., James Tonascia, Ph.D., Scott T. Weiss, M.D., and Robert C. Strunk, M.D.
The authors’ affiliations are as follows: the Channing Division of Network Medicine and the Department of Medicine (M.J.M., X.Z., F.G., A.T.K., M.H.C., D.C.C.-C., P.J.C., B.A.R., I.H., A.L.F., K.G.T., E.K.S., S.T.W.) and the Division of Pulmonary and Critical Care Medicine (A.L.F.), Brigham and Women’s Hospital, Boston Children’s Hospital (A.T.K.), and the Program in Molecular and Integrative Physiological Sciences, Departments of Environmental Health and Genetics and Complex Diseases, Harvard T.H. Chan School of Public Health (Q.L.), Boston; Johns Hopkins Bloomberg School of Public Health (K.P.Y., A.L.S., M.L.V.N., J.T.) and Johns Hopkins University School of Medicine (R.A.W., N.F.A.), Baltimore; National Jewish Health, Children’s Hospital Colorado, and University of Colorado Denver School of Medicine (S.J.S., R.A.C.), Division of Pulmonary Sciences and Critical Care Medicine, Department of Medicine, University of Colorado (S.S.), and University of Colorado (R.A.C.), Denver; Program in Systems Biology, Department of Biochemistry and Molecular Pharmacology (G.J., A.S., Y.Z., B.R.L., J.D.), and Howard Hughes Medical Institute (J.D.), University of Massachusetts Medical School, Worcester; School of Biological Sciences, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore (A.S.); Genome Sciences, School of Medicine, University of Washington (J.S.), and ASTHMA Inc. Clinical Research Center and Northwest Asthma and Allergy Center (P.V.W.), Seattle; University of California at San Diego, Pediatrics, La Jolla, and Kaiser Permanente Southern California Region, San Diego (R.S.Z.); University of New Mexico Health Sciences Center, Albuquerque (H.W.K.); the Division of Respiratory Medicine, Department of Pediatrics, the Hospital for Sick Children and University of Toronto, Toronto (H.G.); Groningen Research Institute for Asthma and COPD (J.M.V., G.H.K., D.S.P.), the Departments of Epidemiology (J.M.V.) and Pulmonology (D.S.P.), University of Groningen, University Medical Center Groningen, and the Department of Pediatric Pulmonology and Pediatric Allergology, Beatrix Children’s Hospital (G.H.K.), Groningen, the Netherlands; and the Division of Allergy, Immunology, and Pulmonary Medicine, Washington University School of Medicine, St. Louis (R.C.S.).
Footnotes
REFERENCES
- 1.Speizer FE, Tager IB. Epidemiology of chronic mucus hypersecretion and obstructive airways disease. Epidemiol Rev. 1979;1:124–42. doi: 10.1093/oxfordjournals.epirev.a036206. [DOI] [PubMed] [Google Scholar]
- 2.Weiss ST, Speizer FE. Epidemiology and natural history. In: Weiss EB, Stein M, editors. Bronchial asthma: mechanisms and therapeutics. 3rd ed Little, Brown; Boston: 1993. p. 15. [Google Scholar]
- 3.Fletcher C. The natural history of chronic bronchitis and emphysema: an eight-year study of early chronic obstructive lung disease in working men in London. Oxford University Press; New York: 1976. [Google Scholar]
- 4.Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–22. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 5.Hospers JJ, Postma DS, Rijcken B, Weiss ST, Schouten JP. Histamine airway hyper-responsiveness and mortality from chronic obstructive pulmonary disease: a cohort study. Lancet. 2000;356:1313–7. doi: 10.1016/S0140-6736(00)02815-4. [DOI] [PubMed] [Google Scholar]
- 6.Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med. 2013;1:728–42. doi: 10.1016/S2213-2600(13)70118-8. [DOI] [PubMed] [Google Scholar]
- 7.Grol MH, Gerritsen J, Vonk JM, et al. Risk factors for growth and decline of lung function in asthmatic individuals up to age 42 years: a 30-year follow-up study. Am J Respir Crit Care Med. 1999;160:1830–7. doi: 10.1164/ajrccm.160.6.9812100. [DOI] [PubMed] [Google Scholar]
- 8.Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339:1194–200. doi: 10.1056/NEJM199810223391703. [DOI] [PubMed] [Google Scholar]
- 9.James AL, Palmer LJ, Kicic E, et al. Decline in lung function in the Busselton Health Study: the effects of asthma and cigarette smoking. Am J Respir Crit Care Med. 2005;171:109–14. doi: 10.1164/rccm.200402-230OC. [DOI] [PubMed] [Google Scholar]
- 10.Tantisira KG, Fuhlbrigge AL, Tonascia J, et al. Bronchodilation and bronchoconstriction: predictors of future lung function in childhood asthma. J Allergy Clin Immunol. 2006;117:1264–71. doi: 10.1016/j.jaci.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 11.Hallberg J, Anderson M, Wickman M, Svartengren M. Factors in infancy and childhood related to reduced lung function in asthmatic children: a birth cohort study (BAMSE) Pediatr Pulmonol. 2010;45:341–8. doi: 10.1002/ppul.21190. [DOI] [PubMed] [Google Scholar]
- 12.Ulrik CS. Outcome of asthma: longitudinal changes in lung function. Eur Respir J. 1999;13:904–18. doi: 10.1034/j.1399-3003.1999.13d35.x. [DOI] [PubMed] [Google Scholar]
- 13.Jamrozik E, Knuiman MW, James A, Divitini M, Musk AW. Risk factors for adult-onset asthma: a 14-year longitudinal study. Respirology. 2009;14:814–21. doi: 10.1111/j.1440-1843.2009.01562.x. [DOI] [PubMed] [Google Scholar]
- 14.Svanes C, Sunyer J, Plana E, et al. Early life origins of chronic obstructive pulmonary disease. Thorax. 2010;65:14–20. doi: 10.1136/thx.2008.112136. [DOI] [PubMed] [Google Scholar]
- 15.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–64. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Childhood Asthma Management Program Research Group The Childhood Asthma Management Program (CAMP): design, rationale, and methods. Control Clin Trials. 1999;20:91–120. [PubMed] [Google Scholar]
- 17.The Childhood Asthma Management Program Research Group Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054–63. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 18.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 19.Cleveland WS. LOWESS: a program for smoothing scatterplots by robust locally weighted regression. Am Stat. 1981;35:54. [Google Scholar]
- 20.Hosmer DW, Lemeshow S, May S. Applied survival analysis: regression modeling of time-to-event data. 2nd ed Wiley-Interscience; Hoboken, NJ: 2008. [Google Scholar]
- 21.Hosmer DW, Lemeshow S, Sturdivant RX. Applied logistic regression. 3rd ed Wiley; Hoboken, NJ: 2013. [Google Scholar]
- 22.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 23.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 24.Fleiss JL, Levin BA, Paik MC. Statistical methods for rates and proportions. 3rd ed Wiley-Interscience; Hoboken, NJ: 2003. [Google Scholar]
- 25.Swanney MP, Ruppel G, Enright PL, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63:1046–51. doi: 10.1136/thx.2008.098483. [DOI] [PubMed] [Google Scholar]
- 26.Mohamed Hoesein FA, Zanen P, Lammers JW. Lower limit of normal or FEV1/FVC < 0.70 in diagnosing COPD: an evidence-based review. Respir Med. 2011;105:907–15. doi: 10.1016/j.rmed.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Mensinga TT, Schouten JP, Rijcken B, Weiss ST. Determinants of maximally attained level of pulmonary function. Am J Respir Crit Care Med. 2004;169:941–9. doi: 10.1164/rccm.2201011. [DOI] [PubMed] [Google Scholar]
- 28.Guerra S, Sherrill DL, Kurzius-Spencer M, et al. The course of persistent airflow limitation in subjects with and without asthma. Respir Med. 2008;102:1473–82. doi: 10.1016/j.rmed.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollams EM, de Klerk NH, Holt PG, Sly PD. Persistent effects of maternal smoking during pregnancy on lung function and asthma in adolescents. Am J Respir Crit Care Med. 2014;189:401–7. doi: 10.1164/rccm.201302-0323OC. [DOI] [PubMed] [Google Scholar]
- 30.Huang S, Vasquez MM, Halonen M, Martinez FD, Guerra S. Asthma, airflow limitation and mortality risk in the general population. Eur Respir J. 2015;45:338–46. doi: 10.1183/09031936.00108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harmsen L, Ulrik CS, Porsbjerg C, Thomsen SF, Holst C, Backer V. Airway hyperresponsiveness and development of lung function in adolescence and adulthood. Respir Med. 2014;108:752–7. doi: 10.1016/j.rmed.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Vonk JM, Jongepier H, Panhuysen CI, Schouten JP, Bleecker ER, Postma DS. Risk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow up. Thorax. 2003;58:322–7. doi: 10.1136/thorax.58.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tai A, Tran H, Roberts M, Clarke N, Wilson J, Robertson CF. The association between childhood asthma and adult chronic obstructive pulmonary disease. Thorax. 2014;69:805–10. doi: 10.1136/thoraxjnl-2013-204815. [DOI] [PubMed] [Google Scholar]
- 34.Strunk RC, Weiss ST, Yates KP, Tonascia J, Zeiger RS, Szefler SJ. Mild to moderate asthma affects lung growth in children and adolescents. J Allergy Clin Immunol. 2006;118:1040–7. doi: 10.1016/j.jaci.2006.07.053. [DOI] [PubMed] [Google Scholar]
- 35.Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64:728–35. doi: 10.1136/thx.2008.108027. [DOI] [PubMed] [Google Scholar]
- 36.Lange P, Celli B, Agustí A, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–22. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 37.Tamimi A, Serdarevic D, Hanania NA. The effects of cigarette smoke on airway inflammation in asthma and COPD: therapeutic implications. Respir Med. 2012;106:319–28. doi: 10.1016/j.rmed.2011.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.