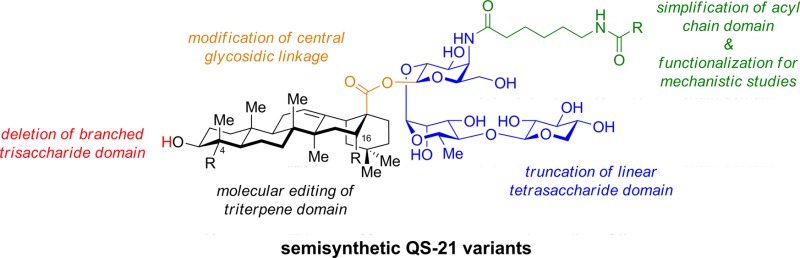
Conspectus
Vaccines based on molecular subunit antigens are increasingly being investigated due to their improved safety and more precise targeting compared to classical whole-pathogen vaccines. However, subunit vaccines are inherently less immunogenic; thus, coadministration of an adjuvant to increase the immunogenicity of the antigen is often necessary to elicit a potent immune response. QS-21, an immunostimulatory saponin natural product, has been used as an adjuvant in conjunction with various vaccines in numerous clinical trials, but suffers from several inherent liabilities, including scarcity, chemical instability, and dose-limiting toxicity. Moreover, little is known about its mechanism of action. Over a decade-long effort, beginning at the University of Illinois at Urbana-Champaign and continuing at the Memorial Sloan Kettering Cancer Center (MSKCC), the group of Prof. David Y. Gin accomplished the total synthesis of QS-21 and developed a practical semisynthetic approach to novel variants that overcome the liabilities of the natural product. First, semisynthetic QS-21 variants were designed with stable amide linkages in the acyl chain domain that exhibited comparable in vivo adjuvant activity and lower toxicity than the natural product. Further modifications in the acyl chain domain and truncation of the linear tetrasaccharide domain led to identification of a trisaccharide variant with a simple carboxylic acid side chain that retained potent adjuvant activity, albeit with reemergence of toxicity. Conversely, an acyl chain analogue terminating in a free amine was inactive but enabled chemoselective functionalization with radiolabeled and fluorescent tags, yielding adjuvant-active saponin probes that, unlike inactive congeners, accumulated in the lymph nodes in vaccinated mice and internalized into dendritic cells. Subtle variations in length, stereochemistry, and conformational flexibility around the central glycosidic linkage provided QS-21 variants with adjuvant activities that correlated with specific conformations found in molecular dynamics simulations. Notably, deletion of the entire branched trisaccharide domain afforded potent, truncated saponin variants with negligible toxicity and improved synthetic access, facilitating subsequent investigation of the triterpene core. The triterpene C4-aldehyde substituent, previously proposed to be important for QS-21 adjuvant activity, proved to be dispensable in these truncated saponin variants, while the presence of the C16 hydroxyl group enhanced activity. Novel adjuvant conjugates incorporating the small-molecule immunopotentiator tucaresol at the acyl chain terminus afforded adjuvant-active variants but without significant synergistic enhancement of activity. Finally, a new divergent synthetic approach was developed to provide versatile and streamlined access to additional linear oligosaccharide domain variants with modified sugars and regiochemistries, opening the door to the rapid generation of diverse, synthetically accessible analogues. In this Account, we summarize these multidisciplinary studies at the interface of chemistry, immunology, and medicine, which have provided critical information on the structure–activity relationships (SAR) of this Quillaja saponin class; access to novel, potent, nontoxic adjuvants for use in subunit vaccines; and a powerful platform for investigations into the mechanisms of saponin immunopotentiation.
1. Introduction
Modern subunit vaccines comprising homogeneous molecular antigens are being developed to prevent and treat a variety of human diseases.1 While these subunit vaccines allow more precise targeting and improved safety compared to classical whole-pathogen vaccines, they are poorly immunogenic and must be coadministered with an adjuvant to elicit a potent immune response. However, few adjuvants are of sufficient potency and acceptable toxicity for clinical use.2 Aluminum salts, either alone (alum) or in proprietary mixtures (AS04),3 and oil-in-water emulsions containing squalene (MF59, AS03)4 have been used as adjuvants in a number of vaccines, but have relatively low potency and significant side effects, respectively. Therefore, there remains a great need for novel adjuvants to enable implementation of subunit vaccines.5
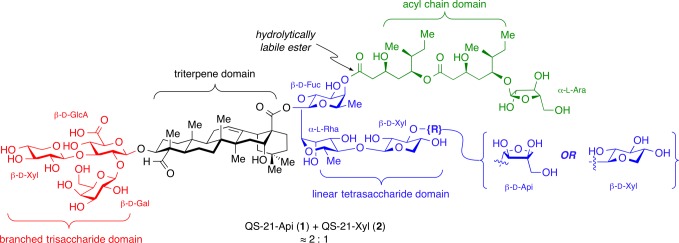
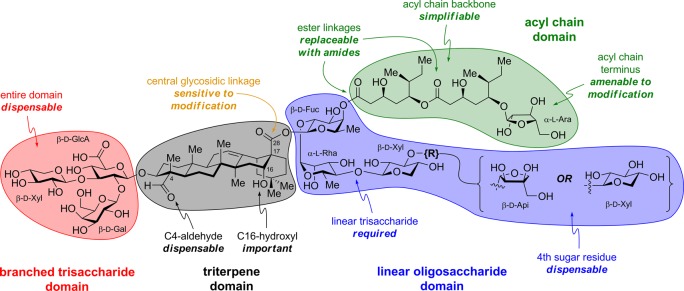
The saponin natural product QS-21 is one of the most potent adjuvants known. Isolated from Quillaja saponaria tree bark, it is composed of four structural domains: a branched trisaccharide, a quillaic acid triterpene, a bridging linear tetrasaccharide, and a pseudodimeric acyl chain (Figure 1). QS-21 stimulates both antibody-based humoral immune responses (Th2) and cellular immunity (Th1), including production of antigen-specific cytotoxic T-lymphocytes.6 Vaccines containing QS-21, either alone in purified form or as a major component of adjuvant mixtures (e.g., Quil A, ISCOMs, ISCOMATRIX, AS01, AS02),7 have been investigated in clinical trials for cancers (melanoma, sarcoma, breast, prostate, ovarian, lung),6 infectious diseases (hepatitis, HIV, malaria, tuberculosis)8 and Alzheimer’s disease.9
Figure 1.
Structure of QS-21 and four structural domains.
Despite its remarkable potency and extensive clinical investigation, QS-21 suffers from several limitations. First, access to homogeneous QS-21 is limited due to an exceedingly low-yielding isolation and heterogeneity of crude extracts from Quillaja saponaria.10 Indeed, QS-21 is not a single molecule but an ≈2:1 mixture of two isomeric constituents, QS-21-Api (1) and QS-21-Xyl (2),11 that differ at the terminal sugar of the linear tetrasaccharide domain. Second, QS-21 is associated with clinical toxicity including swelling and erythema at the injection site, and systemic flu-like symptoms.6 Third, QS-21 undergoes spontaneous hydrolysis of the acyl chain domain ester linkages,12 producing adjuvant-inactive and hemolytic byproducts, complicating formulation and storage. Finally, the mechanisms of action of QS-21 are poorly understood,13 hindering rational design of improved variants and optimal matching of adjuvants with vaccine antigens based on desired immunological end points. Along these lines, it is generally agreed that QS-21 is not a ligand for Toll-like receptors that stimulate innate immunity,14 and does not operate by a depot effect, whereby the adjuvant increases the lifetime of the antigen and its presentation to the immune system.15 It has been hypothesized that QS-21 may facilitate antigen uptake by antigen-presenting cells by binding to cell-surface lectins through its carbohydrate domains, leading to production of specific cytokines that activate cellular and/or humoral responses.16 The triterpene domain C4-aldehyde substituent has been proposed to form a Schiff base with amino groups on T-cell surface receptors, providing costimulation for T-cell activation.16,17 QS-21 has been shown to activate the NLRP3 inflammasome in vitro, but this activation decreased the effects of a HIV gp120/QS-21 vaccine in vivo.18
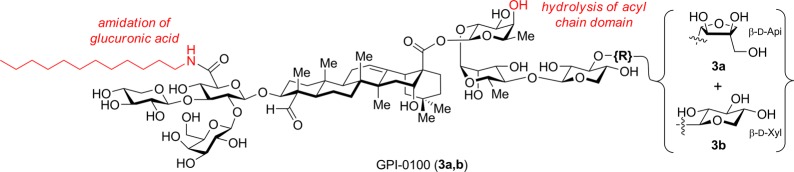
The inherent liabilities of QS-21 highlight the need for improved analogues. Due to the structural complexity of and difficulty in derivatizing the natural product, only a few QS-21 analogues have been reported previously.17,19 Of note, GPI-0100, prepared from QS-21-containing bark extracts by saponification of the acyl chain domain and installation of a dodecylamide in the branched trisaccharide domain (Figure 2),19 retained potent adjuvant activity, but required 20-fold higher doses than QS-21 for optimal efficacy in a breast cancer clinical trial, resulting in significant hepatotoxicity.6 Together with its heterogeneity, this precluded further clinical use of GPI-0100. Recently, this heterogeneity was addressed by Wang and co-workers, who synthesized the two proposed immunoactive components of GPI-0100 and confirmed the preclinical adjuvant activity of xylose isomer 3b.20
Figure 2.
Structures and semisynthetic modifications of main active components of GPI-0100.
Herein, we describe efforts in the laboratory of Prof. David Y. Gin to develop improved variants of QS-21 that overcome the inherent limitations of the natural product. Through extensive structure–activity relationship (SAR) studies spanning all four domains of QS-21, novel semisynthetic variants with potent adjuvant activity and low toxicity in vivo were identified. Moreover, development of structurally related pairs of adjuvant-active and -attenuated saponins enabled biodistribution and fluorescence imaging studies that provided insights into the mechanisms of saponin immunopotentiation.
2. Development of Improved Synthetic Saponin Adjuvants
2.1. Total Synthesis of QS-21 and Semisynthetic Approach to QS-21 Variants
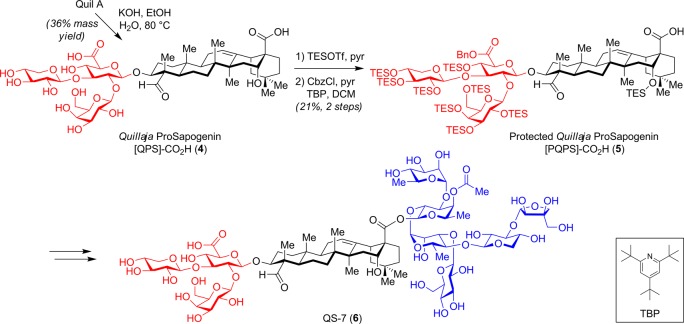
Only a few efforts toward the synthesis of Quillaja saponins have been reported.20−23 Gin and co-workers accomplished the only total syntheses of QS-21-Api (1)24,25 and QS-21-Xyl26 (2) (Figure 1), in 76 steps, providing access to homogeneous material. In a mouse vaccination model using the weakly immunogenic glycolipid GD3 (melanoma, sarcoma, neuroblastoma antigen) conjugated to the highly immunogenic KLH carrier protein (GD3-KLH), the synthetic compounds and natural QS-21 exhibited similar adjuvant activities.27 In studies toward the related saponin QS-7 (6),28 Deng et al. developed a semisynthetic approach involving isolation of the branched trisaccharide–triterpene portion (prosapogenin, [QPS]-CO2H, 4) from commercial Quil A extracts followed by selective protection and functionalization (Scheme 1). This methodology was then applied to a 100 mg scale synthesis of QS-21 (56 steps) for a melanoma vaccine clinical trial.6 This semisynthetic technology also opened the door to the synthesis of diverse QS-21 variants to investigate SAR and identify improved analogues.
Scheme 1. Semisynthesis of QS-7 from Partially Purified Quillaja saponaria Extract Quil A.
2.2. Development of Stable, Simplified Amide-Based Acyl Chain Variants
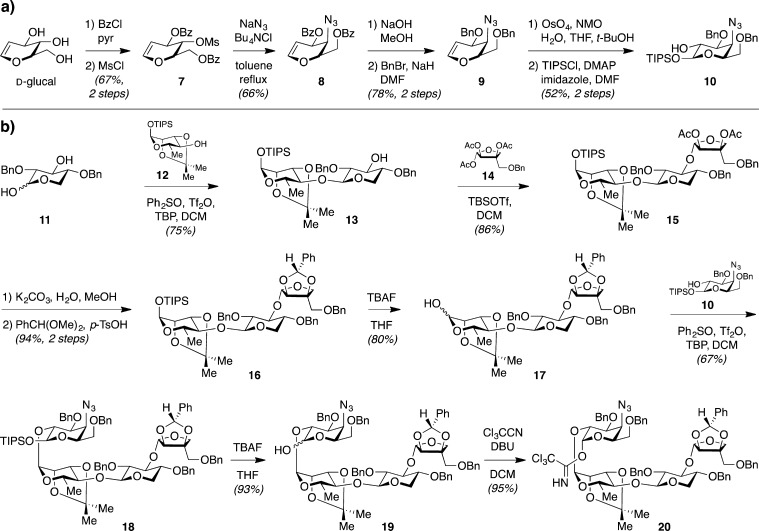
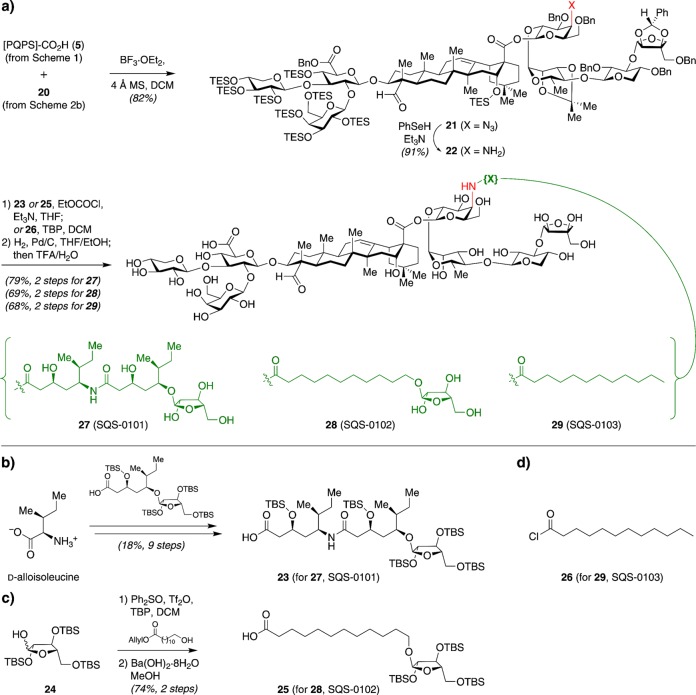
Adams et al. first addressed the chemical instability of QS-21 by replacing the hydrolytically labile esters in the acyl chain domain with amide linkages.29 This required replacement of the bridging fucose residue in the linear tetrasaccharide domain with 4-amino-4-deoxygalactose. Thus, protected 4-azido-4-deoxygalactose 10 was synthesized from d-glucal (Scheme 2a), then coupled with trisaccharide hemiacetal 17 by dehydrative glycosylation30 (Scheme 2b). The modified tetrasaccharide 20 was coupled to the protected Quillaja prosapogenin ([PQPS]-CO2H, 5)28 via Schmidt glycosylation to provide trisaccharide–triterpene–tetrasaccharide construct 21 (Scheme 3a). The azide was reduced to the requisite amine 22 under mild phenylselenol conditions providing an advanced intermediate on which to append diverse acyl chains. The first and most conservative structural variant, 27 (SQS-0101), incorporated amide linkages within the elaborate QS-21 side chain, whereas the other two variants, 28 (SQS-0102) and 29 (SQS-0103), incorporated simplified acyl chains (Scheme 3).
Scheme 2. Synthesis of Modified Linear Tetrasaccharide Domain via (a) Preparation of Protected 4-Azido-4-deoxygalactose 10 and (b) Carbohydrate Assembly.
Scheme 3. Synthesis of Acyl Chain Domain Variants with Amide Linkages by (a) Acylation of Amine 22 with (b–d) the Corresponding Acyl Chain Analogues.
The immunopotentiating activities of these saponin variants were evaluated in collaboration with Philip Livingston and Govind Ragupathi at MSKCC in a preclinical mouse vaccination model. Groups of five female C57BL/6J mice were injected subcutaneously with the GD3-KLH conjugate vaccine and the saponin of interest on days 0, 7, and 14, followed by a booster on day 65. Antibody responses to both GD3 and KLH, including both IgM (low-affinity antibodies initially produced by B cells in early, short-term, T-cell independent response) and IgG (high-affinity antibodies produced after T-helper cell-activated affinity maturation and class switching in persistent, long-term response) for GD3, were assessed by ELISA on day 72. Bisamide 27 (SQS-0101) elicited responses comparable to or higher than natural QS-21, with considerably less toxicity as assessed by median weight loss at days 1, 2, 3, and 7 after the first vaccination (Figure 3a–c). Arabinosyl aliphatic amide 28 (SQS-0102) and dodecanoic amide 29 (SQS-0103) also elicited similar antibody responses but the former was associated with marked weight loss while the latter was nontoxic (Figure 3d).29 Subtyping of anti-GD3 IgG antibodies for the IgG1 and IgG2 subtypes, indicative of T-helper type 2 (Th2) humoral and T-helper type 1 (Th1) cell-mediated immune responses, respectively, revealed predominance of IgG2b for all three saponin variants, similar to natural QS-21. These antibodies were also able to effect binding and lysis of a GD3-positive melanoma cell line, again consistent with the activity of natural QS-21.
Figure 3.
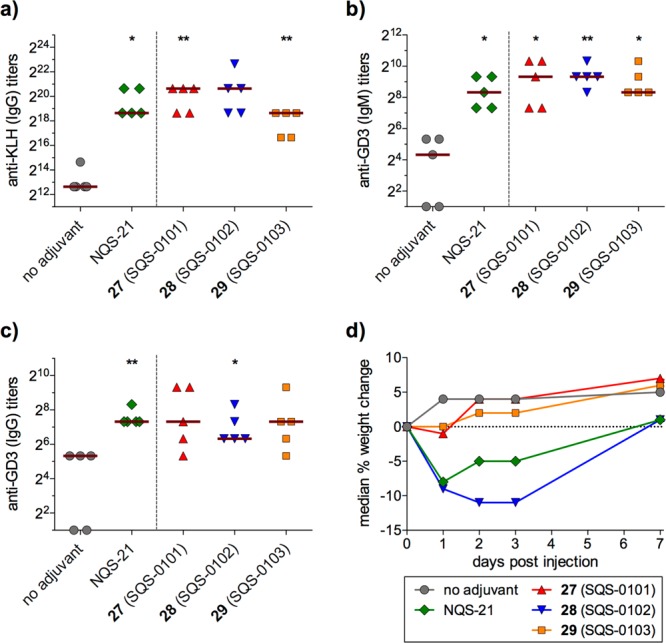
Immunological evaluation of acyl chain domain variants with amide linkages. (a–c) Antibody titers after three vaccinations and booster and (d) median weight loss after first vaccination. Mice vaccinated with GD3-KLH (10 μg) and saponin (10 μg); horizontal bars indicate median titers; statistical significance compared to no-adjuvant control assessed using two-tailed unpaired Student’s t test with 95% confidence interval, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. NQS-21 = natural QS-21.
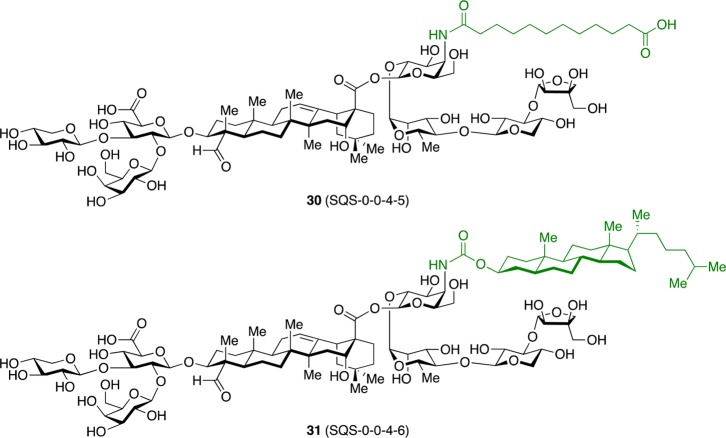
These results demonstrated the feasibility of developing simplified saponin adjuvants with retained or enhanced activity and attenuated toxicity. However, these three variants were still suboptimal, with 27 (SQS-0101) requiring a 54-step synthesis, 28 (SQS-0102) showing increased toxicity, and 29 (SQS-0103) exhibiting poor aqueous solubility. Thus, Chea et al. investigated additional acyl chain domain variants (Figure 4).31 Dodecanedioic acyl variant 30 (SQS-0-0-4-5) was designed to improve water solubility while cholestanyl variant 31 (SQS-0-0-4-6) was devised to probe a hypothesis that interaction of QS-21 with cell membrane-bound cholesterol may be important in initiating adjuvant activity. Mouse vaccinations with GD3-KLH and the immunogenic peptide MUC1 (prostate and breast cancer antigen, nonglycosylated tandem repeat) conjugated to KLH (MUC1-KLH) revealed that 30 elicited antibody responses comparable to natural and synthetic QS-21 across all antigens, while 31 was less active (Figure 5a–c). Notably, no weight loss was observed with either saponin (Figure 5d). Thus, dodecanedioic acyl variant 30 was selected as a lead structure for additional SAR studies.
Figure 4.
Structures of additional acyl chain domain variants. Four-number SQS (synthetic Quillaja saponin) codes designate structural variants in each of the four corresponding structural domains of QS-21, left to right, with 0 assigned to the natural product structure.
Figure 5.
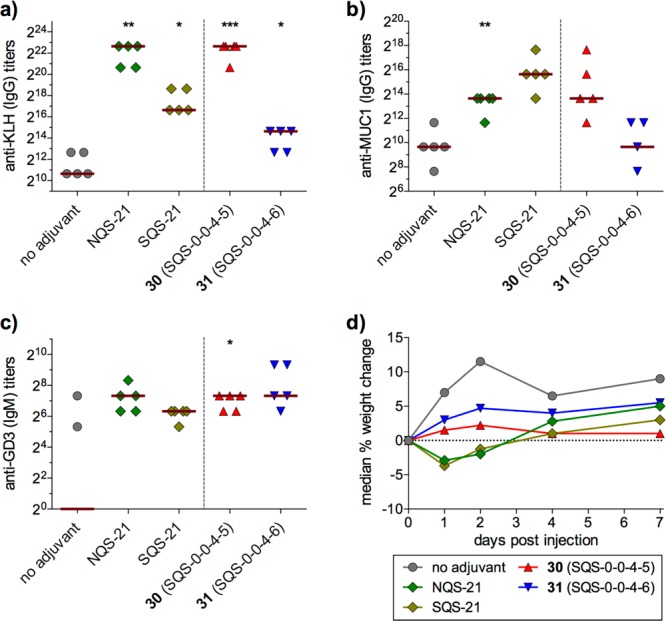
Immunological evaluation of additional acyl chain domain variants (5 μg GD3-KLH, 5 μg MUC1-KLH, 10 μg saponin). SQS-21 = synthetic QS-21 (2:1 mixture of 1 and 2).
2.3. Stepwise Truncation of Linear Tetrasaccharide Domain
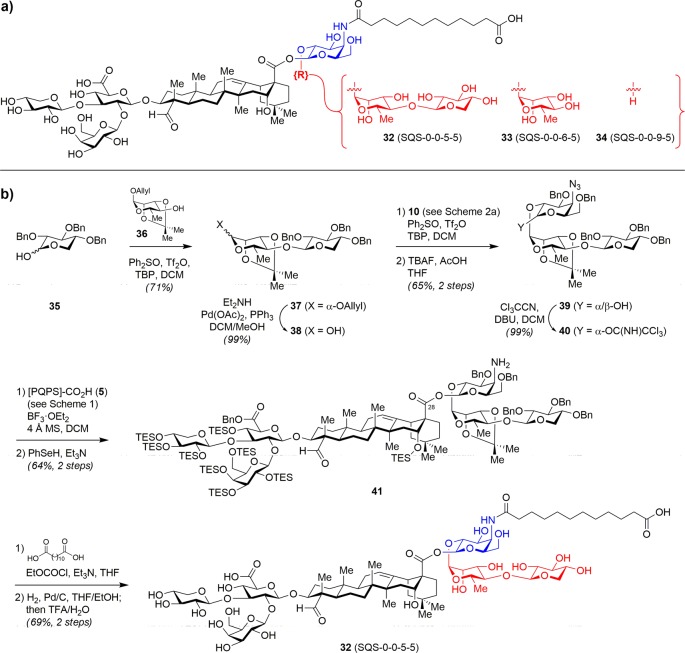
Chea et al. next explored stepwise truncation of the linear tetrasaccharide domain using trisaccharide variant 32 (SQS-0-0-5-5), disaccharide variant 33 (SQS-0-0-6-5), and monosaccharide variant 34 (SQS-0-0-9-5) (Figure 6a).31 The trisaccharide imidate 40 lacking the fourth (apiose/xylose) residue was synthesized in 16 steps, coupled to 5 ([PQPS]-CO2H), and the azide was reduced to afford amine 41 (Figure 6b). Acylation with a dodecanedioic acyl chain followed by global deprotection provided trisaccharide variant 32. The further-truncated variants 33 and 34 were prepared analogously (not shown).
Figure 6.
(a) Structures of progressively truncated linear tetrasaccharide domain variants. (b) Representative synthesis of linear trisaccharide variant 32.
Immunological evaluation of these saponins was carried out with a four-component vaccine with GD3-KLH, MUC1-KLH, and ovalbumin (OVA), a reliable immunogen that induces antibody and T-cell responses. Trisaccharide variant 32 (SQS-0-0-5-5) generated antibody titers comparable to QS-21, while disaccharide 33 (SQS-0-0-6-5) and monosaccharide 34 (SQS-0-0-9-5) elicited progressively attenuated responses overall (Figure 7a–d). All three analogues exhibited acute toxicity, with one to two mice dying in each cohort, although weight loss decreased with truncation (Figure 7e). Despite this reemergence of toxicity, trisaccharide variant 32 could be synthesized more efficiently (24 steps) than the parent tetrasaccharide variant 30 (SQS-0-0-4-5) (36 steps).
Figure 7.
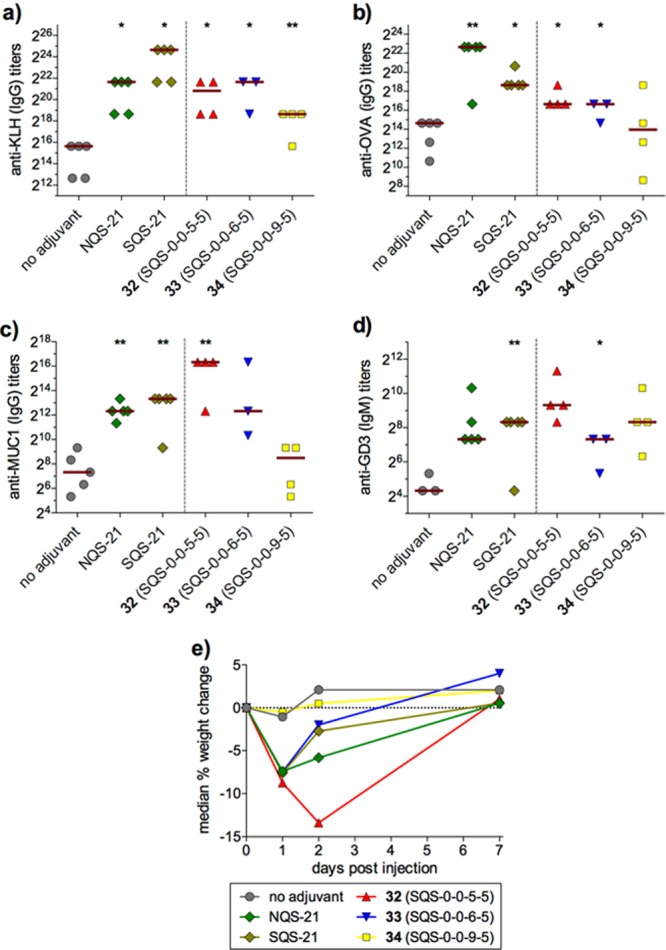
Immunological evaluation of truncated linear tetrasaccharide domain variants (5 μg GD3-KLH, 2.5 μg MUC1-KLH, 20 μg OVA, 20 μg saponin).
2.4. Development of Functionalized Acyl Chain Domain Variants
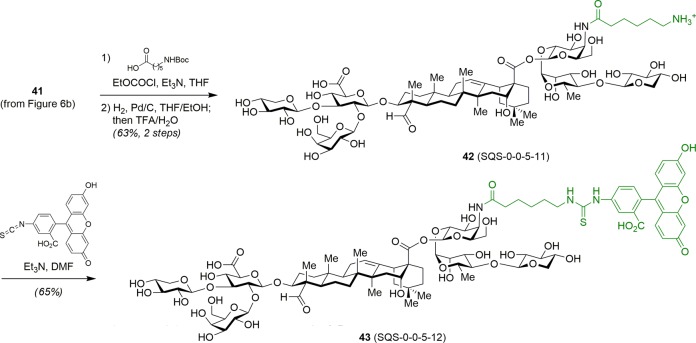
Chea et al. next investigated the effects of ionic charge in the acyl chain domain upon adjuvant activity with cationic 6-aminohexanoyl variant 42 (SQS-0-0-5-11) (Scheme 4).31 This amine-containing saponin also enabled late-stage, chemoselective functionalization with reporter groups, as in fluorescent saponin 43 (SQS-0-0-5-12). Immunological evaluation with MUC1-KLH revealed that, while the negative charge of the dodecanedioic acyl variant 32 (SQS-0-0-5-5) was accommodated, the positive charge in the 6-aminohexanoyl variant 42 resulted in decreased activity (Figure 8).31 Strikingly, acylation of the acyl chain domain amine with fluorescein isothiocyanate restored activity in 43. In contrast, installation of other fluorophores, such as BODIPY (SQS-0-0-5-17) and Cascade Blue (SQS-0-0-5-15), resulted in attenuated adjuvant activity (not shown).
Scheme 4. Synthesis of Functionalized Acyl Chain Domain Variants.
Figure 8.
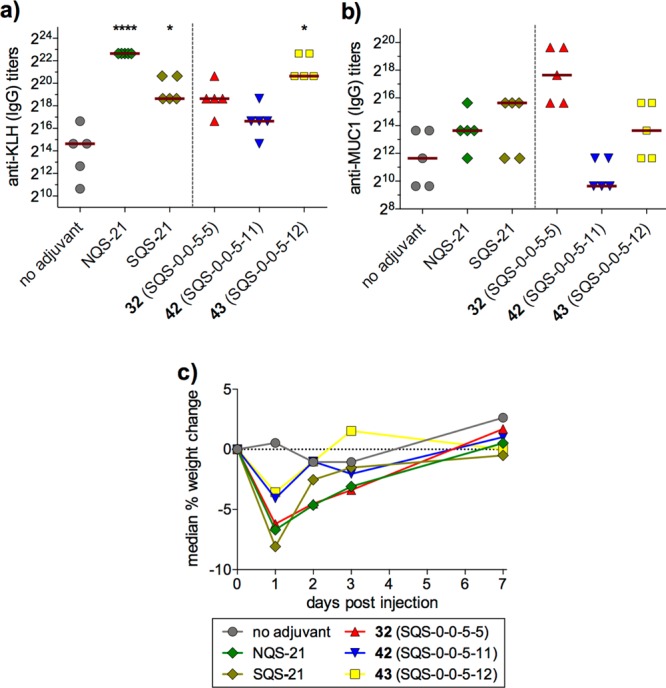
Immunological evaluation of functionalized acyl chain domain variants (2.5 μg MUC1-KLH, 10 μg saponin).
2.5. Modification of Length, Stereochemistry, and Flexibility in Central Glycosidic Linkage
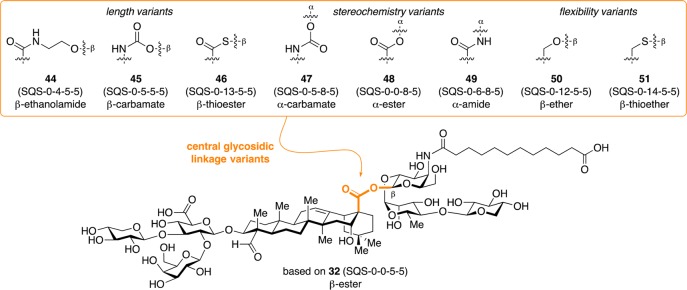
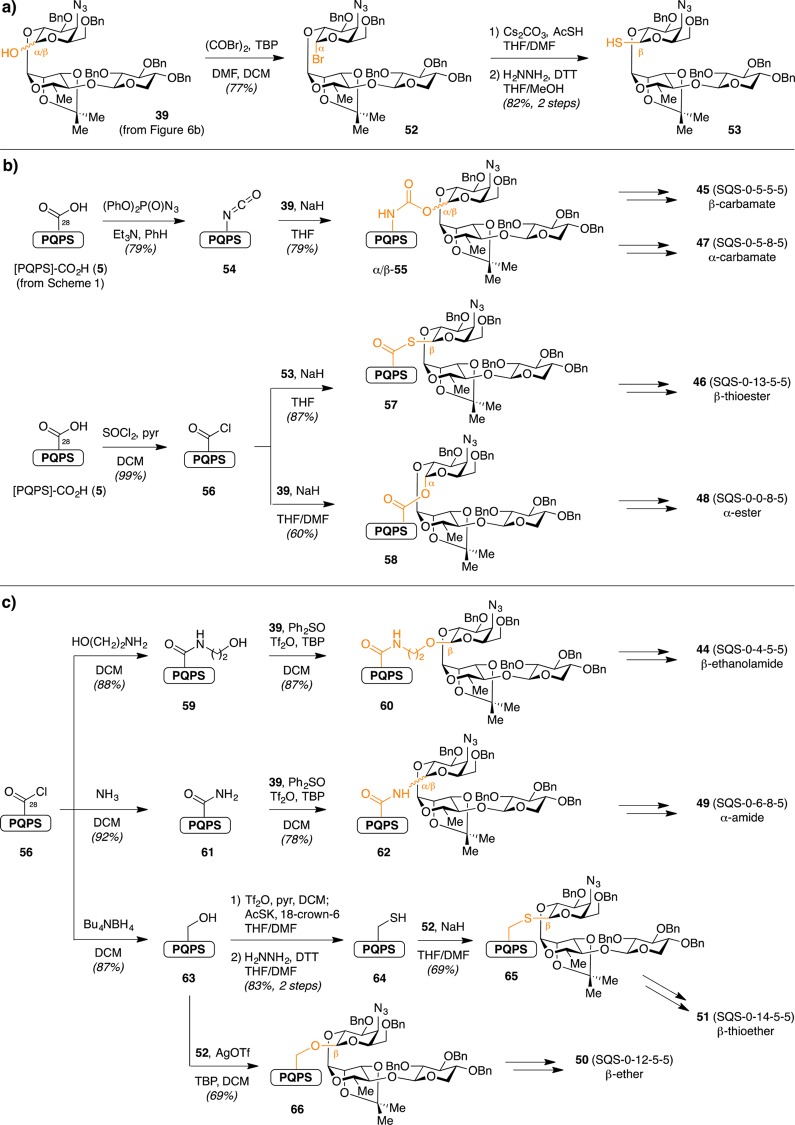
Building upon lead compound 32 (SQS-0-0-5-5), Walkowicz et al. investigated the role of the central glycosidic linkage.32 These analogues probed the effects of linker length (β-ethanolamide 44 [SQS-0-4-5-5], β-carbamate 45 [SQS-0-5-5-5], β-thioester 46 [SQS-0-13-5-5]), stereochemistry (α-carbamate 47 [SQS-0-5-8-5], α-ester 48 [SQS-0-0-8-5], α-amide 49 [SQS-0-6-8-5]), and flexibility (β-ether 50 [SQS-0-12-5-5], β-thioether 51 [SQS-0-14-5-5]) (Figure 9) on adjuvant activity and toxicity. The syntheses were accomplished using two complementary strategies in which the glycosyl donor (Scheme 5a) was used either as a nucleophile in a polarity-reversed coupling (Scheme 5b) or as an electrophile in a traditional glycosylation (Scheme 5c). Reduction of the resulting azides to the corresponding amines, acylation with dodecanedioic acid monobenzyl ester, and global deprotection provided the saponin variants (not shown).
Figure 9.
Structures of central glycosidic linkage variants.
Scheme 5. Synthesis of Central Glycosidic Linkage Variants via (a) Preparation of Linear Trisaccharide Glycosyl Donors and (b) Installation on [PQPS]-CO2H Core (5) Using Glycosyl Acceptor as an Electrophile or (c) Nucleophile.
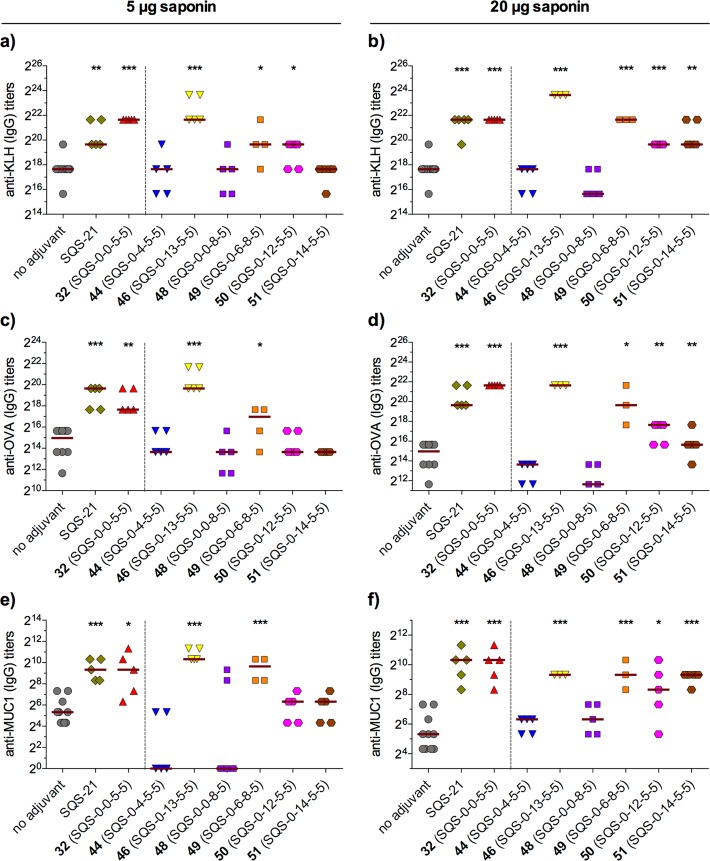
In mice vaccinated with GD3-KLH, MUC1-KLH, and OVA, these central linker variants exhibited distinct adjuvant activities, despite their relatively conservative modifications (Figure 10). Increased linker distance in β-ethanolamide 44 (SQS-0-4-5-5) and β-carbamate 45 (SQS-0-5-5-5) led to loss of activity, while a smaller increase in β-thioester 46 (SQS-0-13-5-5) was accommodated. Mice treated with thioester 46 exhibited no weight loss at a 5 μg dose, but dose-limiting toxicity at a 20 μg dose (not shown). Stereochemical inversion at the anomeric position within the glycosidic linkage abrogated adjuvant activity in α-carbamate 47 (SQS-0-5-8-5) and α-ester 48 (SQS-0-0-8-5), while α-amide 49 (SQS-0-6-8-5) retained activity but was toxic at both 5 and 20 μg doses (not shown). Increasing linker flexibility in β-ether 50 (SQS-0-12-5-5) and β-thioether 51 (SQS-0-14-5-5) resulted in attenuated activity.
Figure 10.
Immunological evaluation of central linkage variants (5 μg GD3-KLH, 2.5 μg MUC1-KLH, 20 μg OVA, 5 or 20 μg saponin). Anti-GD3 responses and data for inactive α/β-carbamates (45, 47) not shown.
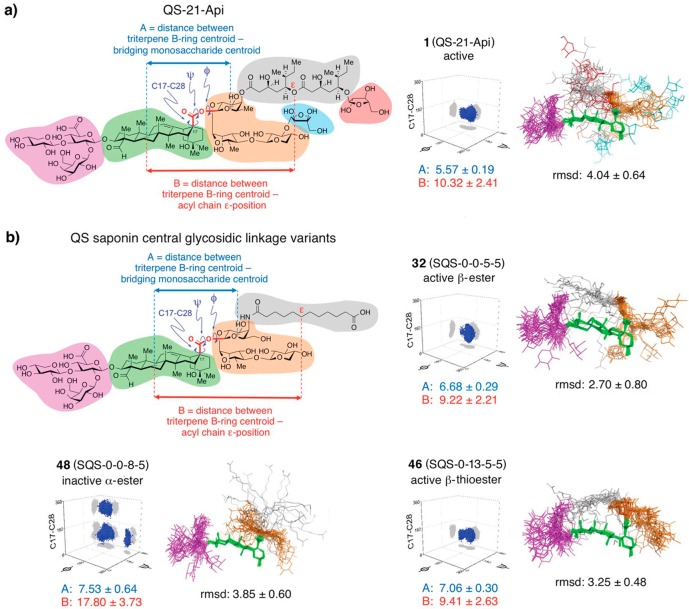
Strikingly, these differences in adjuvant activity correlated with specific conformational preferences identified in molecular dynamics simulations of the saponin variants.32 The most potent analogues, β-ester 32 (SQS-0-0-5-5) and β-thioester 46 (SQS-0-13-5-5), exhibited relatively rigid conformations, similar to QS-21-Api (1), with the acyl chain folded back over the triterpene, suggesting a preferred, active conformation (Figure 11). In contrast, inactive variants such as α-ester 48 (SQS-0-0-8-5) adopted distinct and less-ordered conformations. These conformational preferences were quantitatively characterized by measuring dihedral angles within the central linkage, which were similar and tightly clustered in the active saponins, in contrast to the inactive variants. This provided a molecular rationale for why subtle linker modifications lead to major changes in overall saponin conformation. Collectively, these results suggest an important role for saponin conformation in adjuvant activity, perhaps contributing to proper distribution, subcellular localization, and/or molecular recognition by a putative cellular target.
Figure 11.
Conformational ensembles and central glycosidic linkage dihedral angle distributions (arbitrary axis zero-points) from unrestrained molecular dynamics simulations of (a) QS-21-Api (1) and (b) representative saponin variants, distinguishing active and inactive saponins.
2.6. Development of Aryl Iodide Acyl Chain Variants and Deletion of Branched Trisaccharide Domain
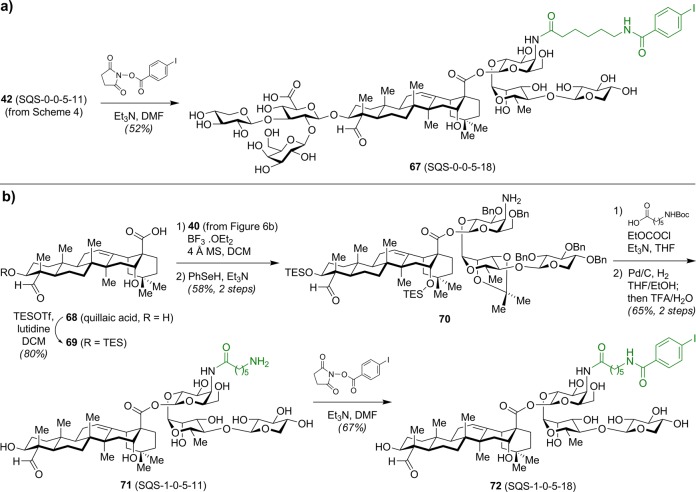
With a view toward developing radiolabeled probes (subsection 2.8), Fernández-Tejada et al. synthesized aryl iodide variant 67 (SQS-0-0-5-18) by 4-iodobenzoylation of the acyl chain domain amine in 42 (Scheme 6a).33 A truncated congener, 72 (SQS-1-0-5-18), lacking the entire left-hand branched trisaccharide domain, was also prepared from quillaic acid, which was readily isolated and purified in gram scale from commercial Quil A (Scheme 6b).25 Selective silylation followed by glycosylation with trisaccharide imidate 40 and reduction of the azide provided the triterpene-linear trisaccharide amine scaffold 70. Acylation with the 6-aminohexanoic acyl chain, global deprotection, and chemoselective 4-iodobenzoylation of the acyl chain domain amine in 71 then afforded truncated variant 72.
Scheme 6. Synthesis of Aryl Iodide Acyl Chain Domain Variants.
Immunological evaluation with MUC1-KLH and OVA revealed that aryl iodide 67 (SQS-0-0-5-18) elicited antibody responses comparable to QS-21, with considerably lower weight loss (Figure 12). Strikingly, the truncated variant 72 (SQS-1-0-5-18) lacking the branched trisaccharide domain elicited antibody responses comparable to QS-21 and 67, albeit at a higher dose. Moreover, mice treated with truncated variant 72 exhibited no weight loss at a 20 μg dose and only 1% weight loss (on day 1) at 50 μg.33 Discovery that the branched trisaccharide domain is not required for adjuvant activity, and that its deletion reduces toxicity, provided a major structural simplification and an improved activity/toxicity profile compared to QS-21.
Figure 12.
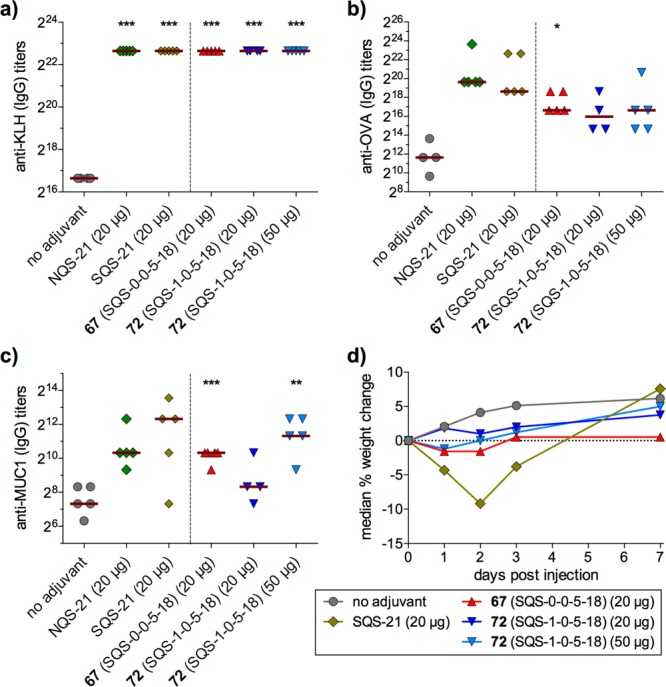
Immunological evaluation of aryl iodide acyl chain domain variants (2.5 μg MUC1-KLH, 20 μg OVA, 20 or 50 μg saponin).
2.7. Molecular Editing of Triterpene Domain
The dispensability of the branched trisaccharide domain for adjuvant activity facilitated access to additional saponin variants with specific modifications in the triterpene domain.33 The triterpene C4-aldehyde substituent has been proposed to be important for activity based on the reduced adjuvant activity of QS-21 derivatives in which this aldehyde is replaced with an amine.17 However, in addition to removing the aldehyde, this modification introduces a positive charge that could interfere with saponin biodistribution, subcellular localization, or noncovalent target binding (cf. inactive acyl chain domain amine variant 42 [SQS-0-0-5-11]).31 Thus, Fernández-Tejada et al. pursued more conservative structural modifications, replacing the C4-aldehyde substituent in quillaic acid variant 72 (SQS-1-0-5-18) with a hydroxymethyl group in caullophylogenin variant 73 (SQS-1-11-5-18) or a methyl group in echinocystic acid variant 74 (SQS-1-8-5-18). The triterpene C16-hydroxyl group was also independently deleted in the corresponding gypsogenin 75 (SQS-1-9-5-18), hederagenin 76 (SQS-1-10-5-18), and oleanolic acid 77 (SQS-1-7-5-18) variants (Figure 13).
Figure 13.
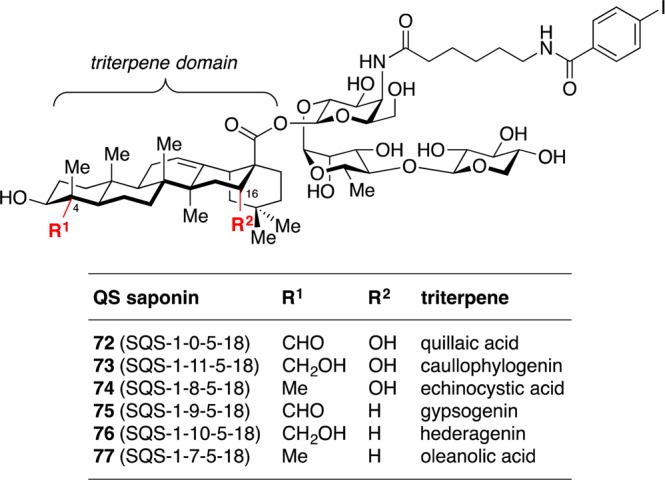
Structures of triterpene domain variants with independent modifications of C4-aldehyde and C16-hydroxyl group.
Synthesis of the echinocystic acid (74, SQS-1-8-5-18), hederagenin (76, SQS-1-10-5-18), and oleanolic acid (77, SQS-1-7-5-18) variants started from the corresponding commercially available triterpenes, and followed a similar sequence to that described above for quillaic acid variant 72 (SQS-1-0-5-18). Gypsogenin variant 75 (SQS-1-9-5-18) was also synthesized from hederagenin while caullophylogenin variant 73 (SQS-1-11-5-18) was accessed from an advanced intermediate en route to 72 (not shown). In mouse vaccinations with GD3-KLH, MUC1-KLH, and OVA, echinocystic acid variant 74 elicited antibody titers similar to or higher than QS-21, closely followed by caullophylogenin variant 73 (Figure 14). Notably, both of these variants lack the C4-aldehyde substituent, bringing the Schiff base mechanistic hypothesis into question, at least in the context of these saponin variants. Meanwhile, gypsogenin (75), hederagenin (76), and oleanolic acid (77, not shown) variants, which lack the C16-hydroxyl, induced attenuated antibody responses. This suggests an important role for this functionality in adjuvant activity, perhaps affecting saponin conformation and/or target binding. In all cases, no weight loss was observed (not shown).33 These results reveal the dispensability of the C4-aldehyde group for potent immunostimulatory effects and point to a previously unappreciated role for the C16-hydroxyl group in enhancing activity. Indeed, other saponin adjuvants that lack a C4-aldehyde substituent but possess a C16-hydroxyl group have been reported.34,35 Moreover, the potent adjuvant activity, non-toxicity, and improved synthetic accessibility (23 total steps) of echinocystic acid variant 74 compared to QS-21 makes it a promising candidate for further development. Despite the important structural differences between this echinocystic acid variant and QS-21, it elicited both IgG1 and IgG2b antibody subclasses, indicative of the ability to induce both Th2 and Th1 responses, respectively, a hallmark of the natural product.
Figure 14.
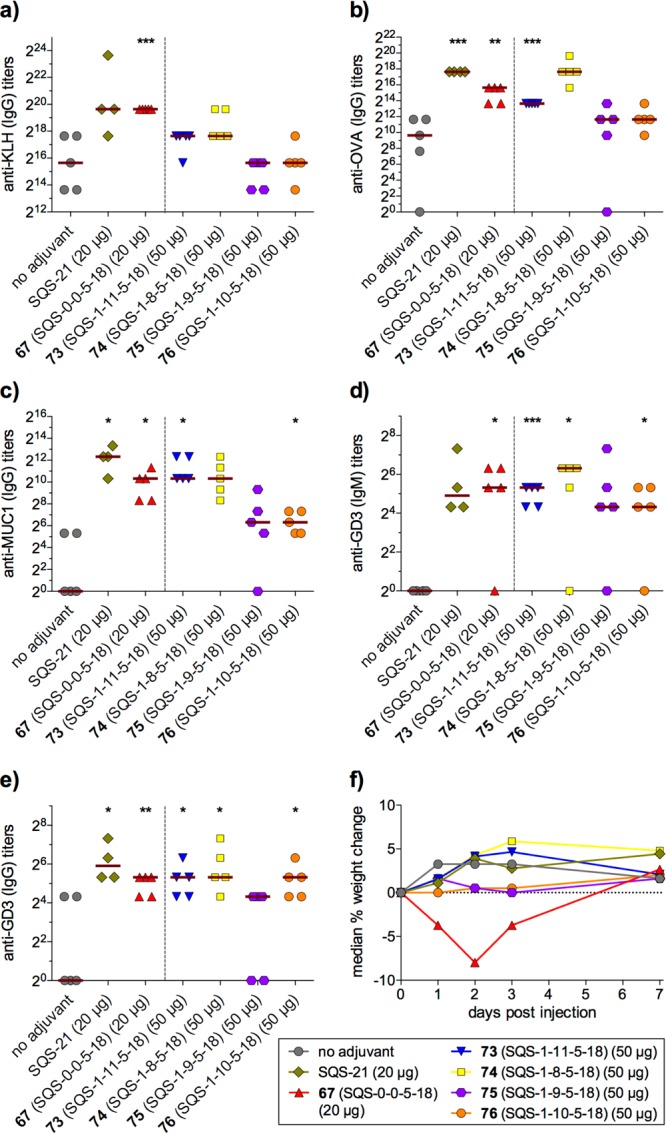
Immunological evaluation of triterpene domain variants (5 μg GD3-KLH, 2.5 μg MUC1-KLH, 20 μg OVA, 20 or 50 μg saponin). Data for attenuated oleanolic acid variant 77 (SQS-1-7-5-18) not shown.
2.8. Mechanistic Studies with Radiolabeled and Fluorescent Saponin Probes
Having established the potent adjuvant activity and low toxicity of aryl iodide variants 67 (SQS-0-0-5-18) and 72 (SQS-1-0-5-18), [131I]-radiolabeled congeners were generated (not shown)33 in collaboration with Jason Lewis and Nagavarakishore Pillarsetty at MSKCC. Biodistribution studies were carried out in mice coadministered with OVA. The active variant [131I]-67 ([131I]-SQS-0-0-5-18) was recovered at significantly higher levels at the injection site and nearest draining lymph nodes at 24 h post injection compared to a structurally similar but inactive variant, [131I]-SQS-0-3-7-18 (not shown), which lacks the linear tetrasaccharide domain (Figure 15a). Radioactivity was also retained at these two sites at 72 and 96 h for [131I]-67, but not in other tissues and organs (where large differences were also initially observed at 24 h). In contrast, for the inactive saponin, [131I]-SQS-0-3-7-18, radioactivity cleared from all tissues by 72 and 96 h post injection. Similar biodistribution results were obtained using two truncated variants that lack the branched trisaccharide domain, active quillaic acid variant [131I]-72 ([131I]-SQS-1-0-5-18) and attenuated oleanolic acid variant [131I]-77 ([131I]-SQS-1-7-5-18) (not shown). This provided a positive correlation between adjuvant activity and this specific biodistribution profile.
Figure 15.
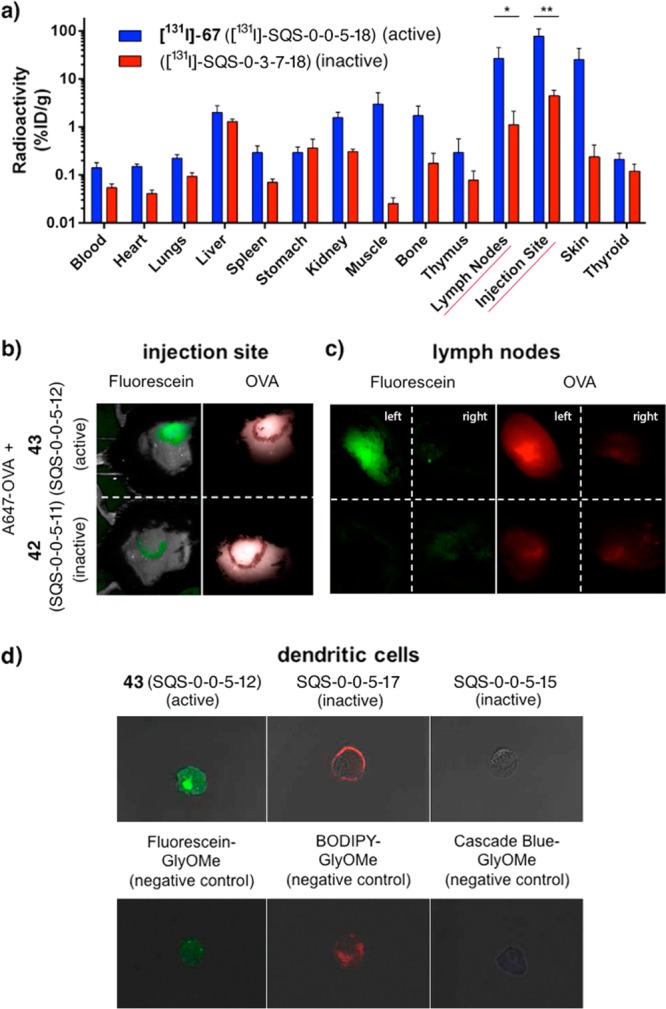
Mechanistic studies of saponin variants. (a) Biodistribution of active and inactive radioiodinated saponins in mice (24 h post injection; 20 μg OVA, 20 μg unlabeled saponin, ≈25 μCi radiolabeled saponin); statistical significance shown only for injection site and lymph nodes. (b) In vivo fluorescence imaging of active fluorescein-labeled saponin 43 and inactive nonfluorescent precursor 42, each coadministered with Alexa-467-OVA (A647-OVA), in mice (24 h post injection; 20 μg A647-OVA, 10 μg saponin) and (c) fluorescence imaging of dissected lymph nodes (mice injected in left flank; right lymph nodes are negative controls). (d) Confocal microscopy imaging of subcellular localization of active and inactive fluorescent saponins and fluorescent glycine methyl ester (GlyOMe) negative controls in immature dendritic cells.
Other Quillaja saponin-containing adjuvant mixtures have been reported to impact the biodistribution of coadministered antigens.36,37 To probe this possibility, biodistribution of [131I]-OVA in the presence or absence of active aryl iodide variant 67 (SQS-0-0-5-18) was also investigated. However, high thyroid uptake of radioactivity was observed, indicative of rapid deiodination of OVA. Thus, in a complementary approach, in vivo fluorescence imaging studies were performed with fluorescein-labeled active adjuvant 43 (SQS-0-0-5-12), in collaboration with Jeffrey Gardner at MSKCC. In mice injected with 43 and Alexa-647-labeled OVA (A647-OVA), both the adjuvant and antigen localized at the injection site and nearest draining lymph node at 24 h post injection (Figure 15b,c). In contrast, when coadministered with the inactive, nonfluorescent precursor 42 (SQS-0-0-5-11), A647-OVA was retained only at the injection site with no accumulation in the lymph nodes. Immunohistochemical analysis of dissected lymph nodes indicated subnodal localization of the active adjuvant 43 (SQS-0-0-5-12) to the cortex of the draining lymph nodes, and flow cytometric analysis revealed that internalization of 43 (SQS-0-0-5-12) was specific to dendritic cells. In an earlier confocal microscopy study, Chea et al. showed internalization of 43 to a distinct cellular compartment of immature dendritic cells, in contrast to related attenuated variants bearing BODIPY (SQS-0-0-5-17) and Cascade Blue (SQS-0-0-5-15) fluorophores, and other fluorescent negative controls (Figure 15d).31 Taken together, these results suggest a role for active adjuvants in the trafficking of OVA by antigen-presenting cells to the draining lymph nodes, a known site of immune cell maturation, and provide early insights into the mechanisms of saponin immunopotentation.
2.9. Investigation of Saponin–Tucaresol Conjugates
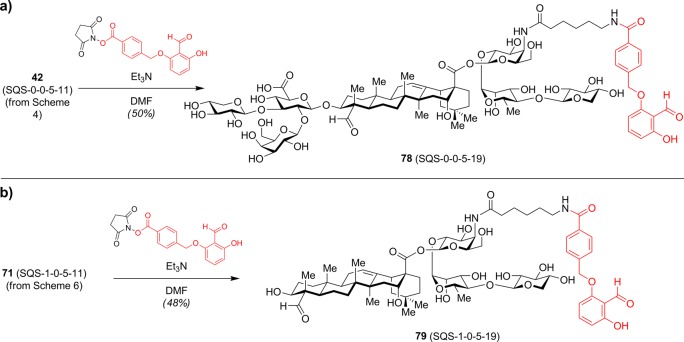
By analogy to aldehyde-containing adjuvants such as tucaresol,38 QS-21 has been suggested to interact with putative T-cell surface receptors through its C4-aldehyde substituent, providing a costimulatory signal leading to T-cell activation.16,17 To investigate potential synergies between QS-21 and tucaresol, Fernández-Tejada et al. developed saponin–tucaresol conjugate 78 (SQS-0-0-5-19) and its truncated congener 79 (SQS-1-0-5-19) (Scheme 7).39 However, in mouse vaccinations with MUC1-KLH (2.5 μg) and OVA (20 μg), incorporation of tucaresol did not significantly enhance the adjuvant activity of these saponins (20 and 50 μg) compared to aryl iodide variants 67 (SQS-0-0-5-18) and 72 (SQS-1-0-5-18) (not shown). Aryl iodide variant 67 also exhibited similar adjuvant activity with or without equimolar tucaresol.
Scheme 7. Synthesis of Saponin–Tucaresol Conjugates.
2.10. Streamlined, Divergent Synthesis of Linear Oligosaccharide Domain Variants
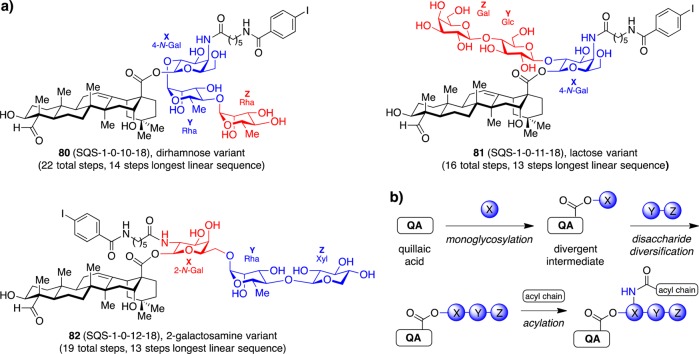
To develop more streamlined synthetic access to saponin variants, Fernández-Tejada et al. designed additional linear oligosaccharide domain variants, based on aryl iodide variant 72 (SQS-1-0-5-18), using readily available carbohydrate precursors. Dirhamnose variant 80 (SQS-1-0-10-18) and lactose variant 81 (SQS-1-0-11-18) incorporated modifications of individual sugars and linkages while 2-galactosamine variant 82 (SQS-1-0-12-18) altered the regiochemistry at the bridging monosaccharide (Figure 16).40 While the trisaccharide was assembled then coupled en bloc to the triterpene domain in the parent saponin 72 (SQS-1-0-5-18; 23 total steps, 16 for the linear trisaccharide) and dirhamnose variant 80 (22 total steps), a divergent strategy was developed for lactose variant 81 (16 total steps) and 2-galactosamine variant 82 (19 total steps), involving stepwise monoglycosylation of the triterpene domain with a single bridging sugar followed by elongation with the desired terminal disaccharide. This late-stage diversification should facilitate rapid preparation of diverse linear oligosaccharide variants to identify synthetically streamlined saponin adjuvants in the future.
Figure 16.
Streamlined synthetic access using (a) linear oligosaccharide domain variants derived from readily available carbohydrate precursors and (b) a divergent synthetic strategy.
3. Conclusions
Despite being one of the most promising investigational immunoadjuvants, clinical advancement of QS-21 has been constrained due to its inherent liabilities, including scarcity, chemical instability, and toxicity. To realize the full potential of subunit vaccines, improved adjuvants that overcome these liabilities are required. Toward this end, the Gin lab first developed synthetic technologies to access QS-21, then leveraged them to prepare nearly 50 saponin analogues, providing detailed SAR (Figure 17) and identifying variants with potent adjuvant activity, increased stability, and decreased toxicity. Several are promising candidates for further preclinical development, such as echinocystic acid variant 74 (SQS-1-8-5-18). Development of radiolabeled and fluorescent probes also enabled investigations into the enigmatic mechanisms of action of these saponin adjuvants. Correlation of three-dimensional structure with adjuvant activity and the pronounced SAR within this family suggest that these saponins may act by interacting with discrete molecular targets, in contrast to nonspecific inflammatory mechanisms attributed to other adjuvants.3 Although the Gin lab research program has now concluded, it has provided efficient synthetic approaches and valuable mechanistic insights that should facilitate future development of improved adjuvants for use in subunit vaccines to address a variety of human diseases.
Figure 17.
Summary of saponin structure–adjuvant activity relationships.
Acknowledgments
We thank all past members of the Gin laboratory, especially Michelle M. Adams, Eric K. Chea, and William E. Walkowicz, and our collaborators, Philip O. Livingston, Govind Ragupathi, Payal Damani, Constantine George, Jason S. Lewis, Nagavarakishore Pillarsetty, and Jeffrey R. Gardner, for their contributions to the work reviewed here. We also thank Samuel J. Danishefsky for scientific advice and helpful discussions.
Biographies
Alberto Fernández-Tejada obtained his PhD in Chemistry from the University of La Rioja in 2009 with Prof. Jesús M. Peregrina and Dr. Francisco Corzana. In 2010, he began postdoctoral studies with Prof. David Y. Gin at MSKCC, and moved to the lab of Prof. Samuel J. Danishefsky after Prof. Gin’s death in 2011. In 2014, he joined the group of Prof. Jesús Jiménez-Barbero at CIB-CSIC (Madrid), then CIC bioGUNE (Bilbao). Since 2015, he has worked with Prof. Ben G. Davis at the University of Oxford. In 2017, he will begin his independent career as a Group Leader at CIC bioGUNE. His research interests lie in chemical immunology and glycobiology, including the synthesis, biological evaluation, and conformational analysis of carbohydrates and glycoconjugates.
Derek S. Tan received his BS in Chemistry from Stanford University in 1995, working with Prof. Dale G. Drueckhammer. He went on to graduate studies with Prof. Stuart L. Schreiber at Harvard University and received his PhD in Chemistry in 2000. He then joined the lab of Prof. Samuel J. Danishefsky at MSKCC for his postdoctoral studies. He began his independent career at MSKCC in 2002, where he is now a Member and Chairman of the Chemical Biology Program and a Tri-Institutional Professor. He is also Director of the Tri-Institutional PhD Program in Chemical Biology. After the untimely death of his close colleague, Prof. David Y. Gin, in 2011, he had the privilege of working with the Gin lab through the completion of their studies.
David Y. Gin (1967–2011) received his BSc in Chemistry from the University of British Columbia in 1989, where he studied terpene synthesis with Prof. Thomas Money. He obtained his PhD in Chemistry at Caltech in 1994 with Prof. Andrew G. Myers, where he completed the total synthesis of the tunicamycin antibiotics. He then held a two-year NSERC postdoctoral appointment at Harvard University with Prof. E. J. Corey, where he completed the total synthesis of ecteinascidin 743. He began his independent career in 1996 at the University of Illinois at Urbana–Champaign, where he remained for 10 years. In 2006, he moved to MSKCC, where he was a Member and Tri-Institutional Professor in the Molecular Pharmacology & Chemistry Program. He was known for his methodologies for carbohydrate assembly, heterocycloaddition approaches to polycyclic alkaloids, and synthetic and biological studies of the vaccine adjuvant QS-21. Prof. Gin passed away unexpectedly on March 22, 2011 at the age of 43. He was an exceptional scientist, colleague, mentor, and friend who is greatly missed by the entire community.
Author Present Address
§ A.F.-T.: Department of Chemistry, University of Oxford, Mansfield Road, OX1 3TA, Oxford, United Kingdom.
Author Contributions
A.F.-T. and D.S.T. wrote the manuscript.
A.F.-T. acknowledges the Spanish Ministry of Education/Fulbright Commission (ME–Fulbright fellowship) and European Commission (Marie Curie Individual Fellowship) for postdoctoral funding. Financial support for the Gin lab was provided by the National Institutes of Health (R01 AI085622, R01 GM058833, P30 CA008748–Thompson), William and Alice Goodwin and the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center of MSKCC.
The authors declare the following competing financial interests: A.F.-T., D.S.T., and D.Y.G. are coinventors on patents and patent applications based on this work. D.Y.G. was a cofounder of, and his estate holds financial interests in, Adjuvance Technologies, Inc., which has licensed certain technologies described herein.
Author Status
∥ D.Y.G.: Deceased March 22, 2011.
Dedication
Dedicated to the memory of our mentor and colleague, David Y. Gin (1967–2011) on the 5-year memorial of his passing.
References
- Jones L. H. Recent Advances in the Molecular Design of Synthetic Vaccines. Nat. Chem. 2015, 7, 952–960. 10.1038/nchem.2396. [DOI] [PubMed] [Google Scholar]
- Reed S. G.; Orr M. T.; Fox C. B. Key Roles of Adjuvants in Modern Vaccines. Nat. Med. 2013, 19, 1597–1608. 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- HogenEsch H. Mechanism of Immunopotentiation and Safety of Aluminum Adjuvants. Front. Immunol. 2013, 3, 406. 10.3389/fimmu.2012.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. B.; Haensler J. An Update on Safety and Immunogenicity of Vaccines Containing Emulsion-based Adjuvants. Expert Rev. Vaccines 2013, 12, 747–758. 10.1586/14760584.2013.811188. [DOI] [PubMed] [Google Scholar]
- O’Hagan D. T.; Fox C. B. New Generation Adjuvants – from Empiricism to Rational Design. Vaccine 2015, 33 (Suppl 2), B14–20. 10.1016/j.vaccine.2015.01.088. [DOI] [PubMed] [Google Scholar]
- Ragupathi G.; Gardner J. R.; Livingston P. O.; Gin D. Y. Natural and Synthetic Saponin Adjuvant QS-21 for Vaccines against Cancer. Expert Rev. Vaccines 2011, 10, 463–470. 10.1586/erv.11.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alving C. R.; Peachman K. K.; Rao M.; Reed S. G. Adjuvants for Human Vaccines. Curr. Opin. Immunol. 2012, 24, 310–315. 10.1016/j.coi.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.; Qiu L.; Wang X.; Zou X.; Lu M.; Yin J. Carbohydrate-based Vaccine Adjuvants – Discovery and Development. Expert Opin. Drug Discovery 2015, 10, 1133–1144. 10.1517/17460441.2015.1067198. [DOI] [PubMed] [Google Scholar]
- Marciani D. J. A Retrospective Analysis of the Alzheimer’s Disease – The Critical Need for New Development Strategies. J. Neurochem. 2016, 137, 687–700. 10.1111/jnc.13608. [DOI] [PubMed] [Google Scholar]
- Kensil C. R.; Patel U.; Lennick M.; Marciani D. Separation and Characterization of Saponins with Adjuvant Activity from Quillaja Saponaria Molina Cortex. J. Immunol. 1991, 146, 431–437. [PubMed] [Google Scholar]
- Soltysik S.; Bedore D. A.; Kensil C. R. Adjuvant Activity of QS-21 Isomers. Ann. N. Y. Acad. Sci. 1993, 690, 392–395. 10.1111/j.1749-6632.1993.tb44041.x. [DOI] [PubMed] [Google Scholar]
- Cleland J. L.; Kensil C. R.; Lim A.; Jacobsen N. E.; Basa L.; Spellman M.; Wheeler D. A.; Wu J.-Y.; Powell M. F. The Isomerization and Formulation Stability of the Vaccine Adjuvant QS-21. J. Pharm. Sci. 1996, 85, 22–28. 10.1021/js9503136. [DOI] [PubMed] [Google Scholar]
- Petrovsky N.; Cooper P. D. Carbohydrate-based Immune Adjuvants. Expert Rev. Vaccines 2011, 10, 523–537. 10.1586/erv.11.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pink J. R.; Kieny M.-P. 4th Meeting on Novel Adjuvants Currently in/close to Human Clinical Testing, World Health Organization. Vaccine 2004, 22, 2097–2102. 10.1016/j.vaccine.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Kensil C. R. Saponins as Vaccine Adjuvants. Crit. Rev. Ther. Drug Carrier Syst. 1996, 13, 1–55. [PubMed] [Google Scholar]
- Marciani D. J. Vaccine Adjuvants: Role and Mechanisms of Action in Vaccine Immunogenicity. Drug Discovery Today 2003, 8, 934–943. 10.1016/S1359-6446(03)02864-2. [DOI] [PubMed] [Google Scholar]
- Soltysik S.; Wu J.-Y.; Recchia J.; Wheeler D. A.; Newman M. J.; Coughlin R. T.; Kensil C. R. Structure/function Studies of QS-21 Adjuvant: Assessment of Triterpene Aldehyde and Glucuronic Acid Roles in Adjuvant Function. Vaccine 1995, 13, 1403–1410. 10.1016/0264-410X(95)00077-E. [DOI] [PubMed] [Google Scholar]
- Marty-Roix R.; Vladimer G. I.; Pouliot K.; Weng D.; Buglione-Corbett R.; West K.; MacMicking J. D.; Chee J. D.; Wang S.; Lu S.; Lien E. Identification of QS-21 as an Inflammasome-activating Molecular Component of Saponin Adjuvants. J. Biol. Chem. 2016, 291, 1123–1136. 10.1074/jbc.M115.683011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciani D. J.; Press J. B.; Reynolds R. C.; Pathak A. K.; Pathak V.; Gundy L. E.; Farmer J. T.; Koratich M. S.; May R. D. Development of Semisynthetic Triterpenoid Saponin Derivatives with Immune Stimulating Activity. Vaccine 2000, 18, 3141–3151. 10.1016/S0264-410X(00)00118-3. [DOI] [PubMed] [Google Scholar]
- Wang P.; Dai Q.; Thogaripally P.; Zhang P.; Michalek S. M. Synthesis of QS-21-based Immunoadjuvants. J. Org. Chem. 2013, 78, 11525–11534. 10.1021/jo402118j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. J.; Gin D. Y. Synthesis of the Trisaccharide Portion of the Immunological Adjuvant QS-21A via Sulfonium-mediated Oxidative and Dehydrative Glycosylation. Org. Lett. 2001, 3, 1801–1804. 10.1021/ol015651u. [DOI] [PubMed] [Google Scholar]
- Zhu X.; Yu B.; Hui Y.; Schmidt R. R. Synthesis of the Trisaccharide and Tetrasaccharide Moieties of the Potent Immunoadjuvant QS-21. Eur. J. Org. Chem. 2004, 2004, 965–973. 10.1002/ejoc.200300580. [DOI] [Google Scholar]
- Zhu X.; Yu B.; Hui Y.; Higuchi R.; Kusano T.; Miyamoto T. Synthesis and Absolute Stereochemistry of the Acyl Moiety of Quillaja Saponins. Tetrahedron Lett. 2000, 41, 717–719. 10.1016/S0040-4039(99)02124-3. [DOI] [Google Scholar]
- Wang P.; Kim Y. J.; Navarro-Villalobos M.; Rohde B. D.; Gin D. Y. Synthesis of the Potent Immunostimulatory Adjuvant QS-21A. J. Am. Chem. Soc. 2005, 127, 3256–3257. 10.1021/ja0422007. [DOI] [PubMed] [Google Scholar]
- Kim Y. J.; Wang P.; Navarro-Villalobos M.; Rohde B. D.; Derryberry J.; Gin D. Y. Synthetic Studies of Complex Immunostimulants from Quillaja Saponaria: Synthesis of the Potent Clinical Immunoadjuvant QS-21Aapi. J. Am. Chem. Soc. 2006, 128, 11906–11915. 10.1021/ja062364i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng K.; Adams M. M.; Damani P.; Livingston P. O.; Ragupathi G.; Gin D. Y. Synthesis of QS-21-Xylose: Establishment of the Immunopotentiating Activity of Synthetic QS-21 Adjuvant with a Melanoma Vaccine. Angew. Chem., Int. Ed. 2008, 47, 6395–6398. 10.1002/anie.200801885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragupathi G.; Damani P.; Deng K.; Adams M. M.; Hang J.; George C.; Livingston P. O.; Gin D. Y. Preclinical Evaluation of the Synthetic Adjuvant SQS-21 and its Constituent Isomeric Saponins. Vaccine 2010, 28, 4260–4267. 10.1016/j.vaccine.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng K.; Adams M. M.; Gin D. Y. Synthesis and Structure Verification of the Vaccine Adjuvant QS-7-Api. Synthetic Access to Homogeneous Quillaja Saponaria Immunostimulants. J. Am. Chem. Soc. 2008, 130, 5860–5861. 10.1021/ja801008m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams M. M.; Damani P.; Perl N. R.; Won A.; Hong F.; Livingston P. O.; Ragupathi G.; Gin D. Y. Design and Synthesis of Potent Quillaja Saponin Vaccine Adjuvants. J. Am. Chem. Soc. 2010, 132, 1939–1945. 10.1021/ja9082842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia B. A.; Poole J. L.; Gin D. Y. Direct Glycosylations with 1-Hydroxy Glycosyl Donors Using Trifluoromethanesulfonic Anhydride and Diphenyl Sulfoxide. J. Am. Chem. Soc. 1997, 119, 7597–7598. 10.1021/ja971067y. [DOI] [Google Scholar]
- Chea E. K.; Fernández-Tejada A.; Damani P.; Adams M. M.; Gardner J. R.; Livingston P. O.; Ragupathi G.; Gin D. Y. Synthesis and Preclinical Evaluation of QS-21 Variants Leading to Simplified Vaccine Adjuvants and Mechanistic Probes. J. Am. Chem. Soc. 2012, 134, 13448–13457. 10.1021/ja305121q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkowicz W. E.; Fernández-Tejada A.; George C.; Corzana F.; Jiménez-Barbero J.; Ragupathi G.; Tan D. S.; Gin D. Y. Quillaja Saponin Variants with Central Glycosidic Linkage Modifications Exhibit Distinct Conformations and Adjuvant Activities. Chem. Sci. 2016, 7, 2371–2380. 10.1039/C5SC02978C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Tejada A.; Chea E. K.; George C.; Pillarsetty N.; Gardner J. R.; Livingston P. O.; Ragupathi G.; Lewis J. S.; Tan D. S.; Gin D. Y. Development of a Minimal Saponin Vaccine Adjuvant Based on QS-21. Nat. Chem. 2014, 6, 635–643. 10.1038/nchem.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nico D.; Santos F. N.; Borja-Cabrera G. P.; Palatnik M.; Palatnik de Sousa C. B. Assessment of the Monoterpene, Glycidic and Triterpene-moieties’ Contributions to the Adjuvant Function of the CP05 Saponin of Calliandra Pulcherrima Benth During Vaccination Against Experimental Visceral Leishmaniasis. Vaccine 2007, 25, 649–658. 10.1016/j.vaccine.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Oda K.; Matsuda H.; Murakami T.; Katayama S.; Ohgitani T.; Yoshikawa M. Adjuvant and Haemolytic Activities of 47 Saponins Derived from Medicinal and Food Plants. Biol. Chem. 2000, 381, 67–74. 10.1515/BC.2000.009. [DOI] [PubMed] [Google Scholar]
- Duewell P.; Kisser U.; Heckelsmiller K.; Hoves S.; Stoitzner P.; Koernig S.; Morelli A. B.; Clausen B. E.; Dauer M.; Eigler A.; Anz D.; Bourquin C.; Maraskovsky E.; Endres S.; Schnurr M. ISCOMATRIX Adjuvant Combines Immune Activation with Antigen Delivery to Dendritic Cells in Vivo Leading to Effective Cross-priming of CD8+ T Cells. J. Immunol. 2011, 187, 55–63. 10.4049/jimmunol.1004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. T.; Goss-Sampson M.; Bomford R. Adjuvant Activity of Saponin: Antigen Localization Studies. Int. Arch. Allergy Immunol. 2004, 77, 409–412. 10.1159/000233817. [DOI] [PubMed] [Google Scholar]
- Rhodes J. Discovery of Immunopotentiatory Drugs: Current and Future Strategies. Clin. Exp. Immunol. 2002, 130, 363–369. 10.1046/j.1365-2249.2002.02016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Tejada A.; Chea E. K.; George C.; Gardner J. R.; Livingston P. O.; Ragupathi G.; Tan D. S.; Gin D. Y. Design, Synthesis, and Immunologic Evaluation of Vaccine Adjuvant Conjugates Based on QS-21 and Tucaresol. Bioorg. Med. Chem. 2014, 22, 5917–5923. 10.1016/j.bmc.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Tejada A.; Tan D. S.; Gin D. Y. Versatile Strategy for the Divergent Synthesis of Linear Oligosaccharide Domain Variants of Quillaja Saponin Vaccine Adjuvants. Chem. Commun. 2015, 51, 13949–13952. 10.1039/C5CC05244K. [DOI] [PMC free article] [PubMed] [Google Scholar]