Abstract
Because cells growing in a three-dimensional (3-D) environment have the potential to bridge many gaps of cell cultivation in 2-D environments (e.g., flasks or dishes). In fact, it is widely recognized that cells grown in flasks or dishes tend to de-differentiate and lose specialized features of the tissues from which they were derived. Currently, there are mainly two types of 3-D culture systems where the cells are seeded into scaffolds mimicking the native extracellular matrix (ECM): (a) static models and (b) models using bioreactors. The first breakthrough was the static 3-D models. 3-D models using bioreactors such as the rotating-wall-vessel (RWV) bioreactors are a more recent development. The original concept of the RWV bioreactors was developed at NASA's Johnson Space Center in the early 1990s and is believed to overcome the limitations of static models such as the development of hypoxic, necrotic cores. The RWV bioreactors might circumvent this problem by providing fluid dynamics that allow the efficient diffusion of nutrients and oxygen. These bioreactors consist of a rotator base that serves to support and rotate two different formats of culture vessels that differ by their aeration source type: (1) Slow Turning Lateral Vessels (STLVs) with a co-axial oxygenator in the center, or (2) High Aspect Ratio Vessels (HARVs) with oxygenation via a flat, silicone rubber gas transfer membrane. These vessels allow efficient gas transfer while avoiding bubble formation and consequent turbulence. These conditions result in laminar flow and minimal shear force that models reduced gravity (microgravity) inside the culture vessel. Here we describe the development of a multicellular 3-D organotypic model of the human intestinal mucosa composed of an intestinal epithelial cell line and primary human lymphocytes, endothelial cells and fibroblasts cultured under microgravity provided by the RWV bioreactor.
Keywords: Bioengineering, Issue 113, Human, three-dimensional (3-D), microgravity, rotating wall vessel (RWV), intestine, epithelial cells
Introduction
The first breakthrough in building a 3-D model was reported in the early of 1980s when scientists started to investigate different types of the scaffold (e.g., laminin, collagen type I, collagen IV, and fibronectin) and cocktails of growth factors to improve cell-to-cell and ECM interactions of "static" 3-D models1-7. Since then, the main problem with these models has been limitations in the transfer of nutrients and oxygen within the medium and tissue constructs8. In contrast to cells in the in vivo environment that receives a steady flow of nutrients and oxygen from surrounding networks of blood vessels, the static nature of these models hinders the effective distribution of them to the cells. For example, cell aggregates generated in in vitro static models that exceed a few millimeters in size will invariably develop hypoxic, necrotic cores9. The RWV bioreactors might circumvent this problem by providing fluid dynamics that allow the efficient diffusion of nutrients and oxygen 10-12. However, to date, work using RWV bioreactors have been limited to the inclusion of one or two cell types 13-17. Moreover, instead of a spatial orientation similar to native tissues, those cells formed cell aggregates. The main reason for these limitations has been the lack of a scaffold able to incorporate cells in an integrated fashion. The scaffolds used in the RWV bioreactors to date consist, with few exceptions 16-18, mainly of synthetic microbeads, tubular cylinders or small sheets 13-15,19-23. These are stiff materials whose composition and flexibility cannot be manipulated, and to which cells are attached to their surface. Thus, it is unlikely that these models will provide a system in which to evaluate, in an integrated fashion, the various cell components such as stromal cells (e.g., fibroblasts, immune and endothelial cells) that should be dispersed within the scaffold to closely mimic human tissue.
Here we describe the development of a multicellular 3-D organotypic model of the human intestinal mucosa composed of an intestinal epithelial cell line and primary human lymphocytes, endothelial cells, and fibroblasts24. These cells were cultured under microgravity provide by the RWV bioreactor 13,25-30. In our 3-D model, the ECM possesses many distinct properties, such as an osmolality similar to the culture medium (e.g., negligible diffusional restraints during culture) and the capability to incorporate cells and other relevant extracellular matrix proteins, as well as the appropriate stiffness to be used in bioreactors24. Biological systems are very complex, and over the past few years, there has been a shift in the focus of mucosal research toward the examination of cell interactions with their surroundings rather than studying them in isolation. In particular, the importance of cell-cell interactions in influencing intestinal cell survival and differentiation is well documented 31-34. Specifically, the communication between epithelial cells and their niche has a profound influence on the epithelial cell expansion and differentiation 35. Indeed, it is widely accepted that not only cell-to-cell but also cell-to-ECM interactions are critical to the maintenance and differentiation of epithelial cells in 3-D culture models. Previous studies have demonstrated that gut ECM proteins such as collagen I 24,36,37, laminin 38 and fibronectin 39 are instrumental in influencing intestinal epithelial cells to acquire spatial orientation similar to the native mucosa. Thus, the development of new technologies, like our 3-D model24, that can mimic the phenotypic diversity of the gut is required if researchers intend to recreate the complex cellular and structural architecture and function of the gut microenvironment. These models represent an important tool in the development and evaluation of new oral drugs and vaccine candidates.
Protocol
Ethics statement: All blood specimens were collected from volunteers that participated in protocol number HP-00040025-1. The University of Maryland Institutional Review Board approved this protocol and authorized the collection of blood specimens from healthy volunteers for the studies included in this manuscript. The purpose of this study was explained to volunteers, and all volunteers gave informed, signed consent before the blood draw.
Note: See Table 1 for medium supplement preparation. See Table 2 for the preparation of the 3-D culture media.
1. Preparation of Culture Vessels
Unscrew the cap of the fill port of the 50 ml-vessel and fill with 50 ml of sterile culture medium (e.g., RPMI). Perform all procedures under sterile conditions in a laminar hood.
Replace the cap and wipe any spilled medium on any surface with 70% ethanol.
Allow the vessel to incubate for at least 15 min to O/N at RT.
Use the fill port to discard the culture medium and add 30 ml of 3-D culture medium.
Wipe any spilled medium on any surface with 70% ethanol.
2. Preparation of the Cells
- Human epithelial cell line (HCT-8)24,40.
- Culture cells in RPMI supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin, 50 µg/ml gentamicin, 2 mM L-glutamine, 1 mM sodium pyruvate, 10 mM HEPES buffer and 10% heat-inactivated fetal bovine serum (FBS) (supplement 1). At 70% confluency, split cells at a ratio of 1:5.
- Human colonic fibroblasts (CCD-18Co cells)24,41.
- Culture cells in Basal Eagle's medium enriched with supplement 1. At confluency, split cells at a ratio of 1:3.
- Human Umbilical Vein Endothelial (HUVEC) cells24,42.
- Culture cells in Endothelial Basal Medium (EBM) medium enriched with 100 µg/ml heparin, 3 μg/ml Endothelial Cell Growth Supplement (ECGS), and supplement 1. At 70% confluency, split cells at a ratio of 1:2.
- Lymphocytes/Monocytes
- Isolate Peripheral blood mononuclear cells (PBMC) from blood by density gradient centrifugation using standard techniques43.
- Briefly, using a 10 ml-pipette, carefully add 30 ml of diluted blood (1:1 blood: Phosphate Buffered Saline (PBS)) into a 50 ml-conical tube containing 10 ml of density centrifugation media. After the centrifugation step, lymphocytes and monocytes will reside in the "white" layer between the density centrifugation media and plasma layers.
- Collect the white layer using a transfer pipette. Avoid granulocyte contamination by limiting the collection of density centrifugation media. After washing43, use PBMC as is or cryopreserved in liquid N2. Note: It is important to highlight that PBMC largely consists of lymphocytes and monocytes, with a small proportion of dendritic cells and other cell types.
Grow all cells under standard culture conditions at 37 °C in an atmosphere of 5% CO2.
3. Preparation of Collagen-embedded Cells
Determine the number of inserts needed based on the numbers of experimental conditions to be set-up. Place the appropriate number of inserts into the wells of a 6-well plate.
- Prepare a suspension of HUVEC and fibroblasts:
- Wash the flasks twice in PBS and detach the cells using trypsin, 0.25% (1x) with 0.05% trypsin-tetrasodium ethylenediaminetetraacetate (Trypsin-EDTA). Add enough trypsin solution to cover the entirety of the cell culture surface area. Detachment usually occurs within 5 to 15 min
- Collect detached cells in a 50 ml-tube containing 30 ml of Dulbecco's Modified Eagle's medium (DMEM) -30% FBS medium.
- Centrifuge the tube at 500 x g for 10 min.
- Resuspend the cells in 30 ml of DMEM-30% FBS medium.
- Count the total number of viable cells by diluting 20 μl of the resuspended cells with 20 μl of Trypan blue dye. Load the hemocytometer with the cell mixture. Viable PBMCs will be white clear; nonviable PBMC will acquire the dye and become blue.
- Resuspend each cell type in DMEM-30% FBS medium at a concentration of 5 - 8 x 107 cells ml- 1.
- Prepare cell-containing collagen gels Note: Each vessel will require the preparation of ~5 ml of cell-containing collagen gel.
- In a 50 ml-conical tube prepare the collagen mixture by adding 1 x DMEM media, supplemented with 50 µg/ml gentamicin, 2 mM L-glutamine and 10% heat-inactivated FBS plus 3 mg/ml bovine collagen-I, 10 μg/ml laminin, 40 μg/ml collagen IV, 10 μg/ml fibronectin, 2 μg/ml heparin sulfate proteoglycan and 15 mM NaOH (to reach the physiological pH).
- Close the tube and mix by inverting several times until the mixture is fully homogenized. Avoid bubble formation. IMPORTANT: Keep the mixture on ice to prevent gelification.
- Critical step: If the mixture develops an acidic appearance (yellowish color), add a few drops of sterile 1 N NaOH to neutralize the mixture.
- Add concentrated HUVEC and fibroblasts at a density of 1.0 - 1.2 and 1.5 - 2.0 x 106 cells/ml, respectively to the enriched-collagen mixture (described above). Close the tubes and mix by inverting several times until the mixture is fully homogenized. Avoid bubble formation. IMPORTANT: Keep the mixture on ice to prevent gelification.
- Add 4 - 5 ml of the cell-containing collagen gel to each insert, previously loaded into a 6 well-plate, and allowed to gelify in the hood with little or no movement for 1 hr.
- After 1 hr, transfer the 6-well plate to a 37 °C, 5% CO2 incubator for 1 - 2 additional hours.
- Return the 6-well plate to the hood, and with the help of a sterile forceps and scalpel aseptically cut-off the membrane from the insert detaching the cell-containing gel from the membrane. Cut the detached gel into small squares (~5 x 5 mm).
- Add the small cell-containing squares into a 50-ml HARV using the fill port.
- Prepare a suspension of HCT-8 epithelial cells:
- Wash the flasks twice in PBS and detach the cells using trypsin, 0.25% (1x) with Trypsin-EDTA treatment. Add enough trypsin solution to cover the entirety of the cell culture surface area. Detachment usually occurs within 10 to 15 min.
- Collect detached cells in 30 ml of DMEM-30% FBS medium.
- Centrifuge DMEM-30% FBS medium containing tube at 500 x g for 10 min.
- Resuspend the cells in 30 ml of DMEM-30% FBS medium.
- Count the total number of viable cells as described in 3.2.5.
- Resuspend the HCT-8 epithelial cells in DMEM-30% FBS medium at a concentration of 107 cells ml- 1.
- Add 1 ml of the concentrated HCT-8 epithelial cells into the 50-ml HARV using the fill port.
By using the fill port, add additional 3-D culture medium until the vessel is nearly full (~20 ml).
Replace the cap and wipe the lip of the fill port with 70% ethanol.
Place a syringe in each syringe port of the RWV: place a 5 ml-syringe containing 3 - 4 ml of 3-D culture medium in one port and a 5 ml-empty syringe in the other port.
Remove any visible bubbles by pulling into the empty syringe while approximately the same volume of medium is injected through the other syringe port.
Place the vessels in the bioreactor, turn on the power and set the speed to about 13 to 14 rotations per minute. Note: Critical step: Rotation rate may need to be adjusted a few times during an experiment to maintain the cells orbiting within the vessel, thereby avoiding contact with the vessel walls.
Culture cells at 37 °C, 5% CO2 under microgravity, provided by the RWV bioreactor.
Change culture medium every 3 - 4 days by replacing approximately 30 ml with fresh 3-D culture medium.
After 4 days (±1 day), remove the vessels from the bioreactor and add lymphocytes/monocytes to the vessels (2 x 107/vessel).
Place the vessels back in the bioreactor at 37 °C, 5% CO2 for an additional 5 days (±1 day).
After the additional 5 days (day 9 of the culture), add lymphocytes/monocytes to the vessels (2 x 107/vessel) and continue the cultures for up to 12 additional days.
Change culture medium every 3 - 4 days by replacing approximately 30 ml with fresh 3-D culture medium.
4. Harvesting 3-D Cultures for Histology
With the aid of a transfer pipette harvest each small gel fragment, now called "construct," into a six-well plate containing 10 ml of 10% formalin buffer. Leave for 3 hr at RT or for 12 - 16 hr at 4 °C.
Prepare the tissue processing and embedding cassettes by labeling and adding two pieces of biopsy foam pads into each cassette.
Immerse the cassettes in 10% formalin buffer.
With the aid of forceps place the fixed-constructs between the two foam pads.
Immerse the cassettes in 10% formalin buffer.
Proceed with standard procedures for embedding, sectioning, and staining44.
Representative Results
Previously we have engineered a multicellular 3-D organotypic model of the human intestinal mucosa comprised of an intestinal epithelial cell line and primary human lymphocytes, endothelial cells and fibroblasts cultured under microgravity conditions24 (Figure 1). Fibroblasts and endothelial cells were embedded in a collagen I matrix enriched with additional gut basement membrane proteins45 (i.e., laminin, collagen IV, fibronectin and heparin sulfate proteoglycan) and added to RWV bioreactors. After 10 - 15 days, histological staining analysis demonstrated the presence of villus-like structures in the constructs. Approximately 60 - 80% of these epithelial cells were organized as a monolayer of polarized cells with their nuclei located in a basal position near the ECM, which is a major feature of well-differentiated cells (Figure 2).
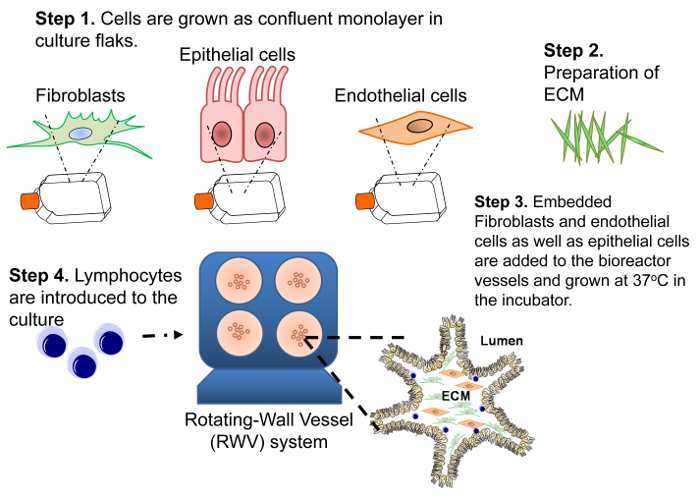 Figure 1: Diagram of the Construction of the 3-D Model. Four steps are necessary to build the 3-D system. First, to obtain the required number of cells to generate the 3-D model, a human intestinal enterocyte epithelial cell line (HCT-8) and primary human endothelial cells and fibroblasts are grown as 2-D confluent monolayers. Second, the ECM composed of collagen-I matrix enriched with additional gut basement membrane proteins (i.e., laminin, collagen IV, fibronectin and heparin sulfate proteoglycan) is prepared. Third, fibroblasts and endothelial cells are embedded in a collagen-I mixture and added to RWV bioreactors followed by the addition HCT-8 epithelial cells. Fourth, primary lymphocytes are added to the culture at days 4 and 9 (±1 day). Please click here to view a larger version of this figure.
Figure 1: Diagram of the Construction of the 3-D Model. Four steps are necessary to build the 3-D system. First, to obtain the required number of cells to generate the 3-D model, a human intestinal enterocyte epithelial cell line (HCT-8) and primary human endothelial cells and fibroblasts are grown as 2-D confluent monolayers. Second, the ECM composed of collagen-I matrix enriched with additional gut basement membrane proteins (i.e., laminin, collagen IV, fibronectin and heparin sulfate proteoglycan) is prepared. Third, fibroblasts and endothelial cells are embedded in a collagen-I mixture and added to RWV bioreactors followed by the addition HCT-8 epithelial cells. Fourth, primary lymphocytes are added to the culture at days 4 and 9 (±1 day). Please click here to view a larger version of this figure.
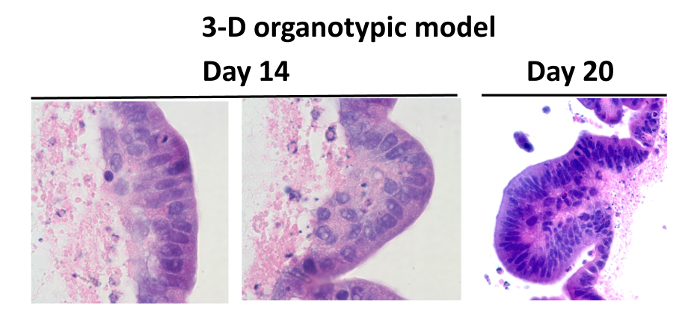 Figure 2: Comparison Between the Normal Human Intestine and the Organotypic Models. Hematoxylin and eosin staining of cells cultured in the 3-D microgravity model: tissues were stained purple and scaffold stained pink. Cells from the 3-D model were cultured for 14 ("a" and "b") and 20 days (c). Twenty days after seeding, total cell numbers are maintained, and cells were well differentiated showing villus-like features. Images are displayed at 100X ("a" and "b") and 40X ("c") magnification. Please click here to view a larger version of this figure.
Figure 2: Comparison Between the Normal Human Intestine and the Organotypic Models. Hematoxylin and eosin staining of cells cultured in the 3-D microgravity model: tissues were stained purple and scaffold stained pink. Cells from the 3-D model were cultured for 14 ("a" and "b") and 20 days (c). Twenty days after seeding, total cell numbers are maintained, and cells were well differentiated showing villus-like features. Images are displayed at 100X ("a" and "b") and 40X ("c") magnification. Please click here to view a larger version of this figure.
| Product Name | Pkg. Size | Reconstitution | Working Concentration | Storage |
| Fibroblast Growth Factor-Basic (bFGF) | 25 mg | To prepare 25 μg/ml stock solution; add 25 μg of FGF into 1 ml of sterile medium (RPMI plus 1% FCS), swirl to dissolve, add 100 μl/aliquot | 5 ng/ml | powder -20 oC, working aliquots -20 oC, avoid repeat freeze/thaw |
| Stem Cell Factor (SCF) | 10 mg | To prepare 10 μg/ml stock solution; add 10 μg of SCF into 1 ml of sterile medium (RPMI plus 1% FCS), swirl to dissolve, add 250 μl/aliquot | 5 ng/ml | powder -20 oC, working aliquots -20 oC, avoid repeat freeze/thaw |
| Hepatocyte Growth Factor (HGF) | 5 mg | To prepare 5 μg/ml stock solution; add 5 μg of HGF into 1 ml of sterile medium (RPMI plus 1% FCS), swirl to dissolve, add 200 μl/ aliquot | 2 ng/ml | powder -20 oC, working aliquots -20 oC, avoid repeat freeze/thaw |
| Endothelin 3 | 50 mg | To prepare 50 μg/ml stock solution; add 50 μg of Endotelin into 1 ml of sterile medium (RPMI plus 1% FCS), swirl to dissolve, add 100 μl/aliquot | 10 ng/ml | powder -20 oC, working aliquots -20 oC, avoid repeat freeze/thaw |
| Laminin | 1 mg | Thaw slowly at 2 - 8 oC, swirl and add 100 μl/aliquot | 10 mg/ml | liquid -20 oC, working aliquots -20 oC, avoid repeat freeze/thaw |
| Vascular Endothelial Growth Factor (VEGF) | 10 mg | To prepare 5 μg/ml stock solution; add 10 μg of VEGF into 2 ml of sterile water, swirl to dissolve, add 100 μl / aliquot | 1 ng/ml | powder -20 oC, working aliquots -20 oC, avoid repeat freeze/thaw |
| Leukemia Inhibitory Factor (LIF) | 50 mg | To prepare 20 μg/ml stock solution; add 50 μg of LIF into 2.5 ml of sterile water, swirl to dissolve, add 100 μl/aliquot | 4 ng/ml | powder -20 oC, working aliquots -20 oC, avoid repeat freeze/thaw |
| Adenine | 25 g | To prepare 0.18 M stock solution; add 121.5 mg og adedine into 50 ml of 0.05 M HCl (250 μl of 37.1% HCl into 50 ml of water)), swirl to dissolve, filter sterilize and add 1 ml/aliquot | 1.8 x 10-3 M | powder 4 oC, working aliquots -20 oC, avoid repeat freeze/thaw |
| Insulin | 50 mg | To prepare 5 mg/ml stock solution; add 50 mg of insulin into 10 ml of 0.005 M HCl (5 μl of 37.1% HCl into 10 ml of water)), swirl to dissolve, filter sterilize and add 0.5 ml/aliquot | 5 mg/ml | powder -20 oC, working aliquots -20 oC, avoid repeat freeze/thaw |
| T3 | 100 mg | To prepare 2 x 10-8 M stock solution; add 3.4 mg of T3 into 25 ml of 1 M NaOH, dilute 0.1 ml of this solution into 9.9 ml of PBS, dilute again 0.1 ml of this solution into 9.9 ml of PBS, swirl to dissolve, filter sterilize and add 0.5 ml/aliquot | 2 x 10-11 M | powder -20 oC, working aliquots -20 oC, avoid repeat freeze/thaw |
| Cholera Toxin | 5 mg | To prepare 10-7 M stock solution; add 5 mg of Cholera toxin into 5 ml sterile ddH2O (store at -20 oC); dilute 50 μl of this solution into 5 ml ddH2O, swirl to homogenise, filter sterilize and add 0.5 ml/aliquot | 10-10 M | powder 4 - 8 oC, working aliquots -20 oC, avoid repeat freeze/thaw |
| Fibronectin | 1 mg | To prepare 1 mg/ml stock solution; add 1 mg of fibronectin into 1 ml of sterile distiled water. Allow 30 min. for material to go into solution. DO NOT AGITATE OR SWIRL. Add 100 μl/aliquot | 10 mg/ml | powder 4 - 8 oC, working aliquots -20 oC, avoid repeat freeze/thaw |
| apo-Transferrin | 100 mg | To prepare 5 mg/ml stock solution (1,000x); add 100 mg of transferrin into 20 ml of PBS swirl to dissolve, filter sterilize and add 0.5 ml/aliquot | 5 mg/ml | powder -20 oC, working aliquots -20 oC, avoid repeat freeze/thaw |
| Heparin | 50 KU | To prepare 50 mg/ml stock solution (1,000x); add 50 mg of heparin into 1 ml of water swirl to dissolve, filter sterilize and add 1 ml/aliquot | 0.1 mg/ml | powder -20 oC, working aliquots -20 oC, avoid repeat freeze/thaw |
| Heparan sulfate proteoglycan | 1 mg | To prepare 0.1 mg/ml stock solution; add 1 mg of heparan sulfate into 10 ml of sterile water swirl to dissolve, and add 0.2 ml/aliquot | 2 mg/ml | powder -20 oC, working aliquots -20 oC, avoid repeat freeze/thaw |
| Collagen IV | 5 mg | To prepare 2 mg/ml stock solutin: add 2 mg of collagen V into 2.5 ml of sterile 0.25% acetic acid. Allow 1 - 2 hr for material to go into solution. Swirl for better dillution. Add 400 μl/aliquot | 80 mg/ml | powder -20 oC, working aliquots -20 oC, avoid repeat freeze/thaw |
Table 1: Defined Medium Supplement Preparation.
| F-12 Medium Supplemented with | |||
| Reagent | Stock Solution | Final Solution | Amount |
| Fetal Calf serum | 10% | 50 ml | |
| Sodium pyruvate | 100 mM | 1 mM | 5 ml |
| L-Glutamine | 200 mM | 2 mM | 5 ml |
| Hepes | 1 M | 10 mM | 5 ml |
| Gentamicin | 50 mg/ml | 50 μg/ml | 500 μl |
| Penicillin/streptomycin | 10,000 U/ml/10 mg/ml | 100 U/ml/100 μg/ml | 5 ml |
| Insulin | 5 mg/ml | 5 μg/ml | 500 μl |
| T3 | 2 x 10- 8 M | 2 x 10-11 M | 500 μl |
| Adenine | 1.8 x 10- 1 M | 1.8 x 10- 3 M | 5 ml |
| Transferrin | 5 mg/ml | 5 μg/ml | 500 μl |
| heparin | 50 mg/ml | 0.1 mg/ml | 1 ml |
| ECGS | 3 mg/ml | 3 μg/ml | 5 ml |
| bFGF | 25 μg/ml | 5 ng/ml | 100 μl |
| SCF | 10 μg/ml | 5 ng/ml | 250 μl |
| HGF | 5 μg/ml | 2 ng/ml | 200 μl |
| Endothelin 3 | 50 μg/ml | 10 ng/ml | 200 μl |
| LIF | 20 μg/ml | 4 ng/ml | 100 μl |
| VEGF | 5 μg/ml | 1 ng/ml | 100 μl |
| Choleran toxin | 10-7 M | 10-10 M | 500 μl |
| Note: The amount cited above is for preparation of 500 ml of 3-D culture media. Media must be stored at 4 °C for no more than 2 weeks |
Table 2: Preparation of 3-D Culture Media.
Discussion
In this manuscript, we describe the development of a bioengineered model of the human intestinal mucosa comprised of multiples cell types including primary human lymphocytes, fibroblasts, and endothelial cells, as well as intestinal epithelial cell lines24. In this 3-D model, cells are cultured within a collagen-rich extracellular matrix under microgravity conditions24.
As described previously, the major features of this model are: (i) the ability to mimic the epithelial tissue monolayer organization, (ii) the induction of appropriate polarity of epithelial cells, tight junctions, desmosomes and microvilli, (iii) a long-term culture (up to 20 days) with high viability of the primary cells (i.e., fibroblasts and endothelial cells), (iv) the expression the tissue-like differentiation markers including villin, cytokeratin, E-cadherin and mucin, (v) the capability to produce considerable amounts of cytokines (e.g., IL-8) and alkaline phosphatase upon antigenic stimulation, (vi) the transport of nutrients such as glucose (i.e., expression of disaccharidases, and presence of sugar transporters), and (vii) the multi-lineage differentiation of intestinal epithelial cells (i.e., absorptive enterocyte, globet and M cells)24.
It is important to highlight that to achieve reproducible results using our 3-D model the investigator must adhere to good cell culture guidelines46-48. It is crucial to systematically control cells for viability, mycoplasma contamination and changes in cell growth behavior. If a problem is identified, first ensure that no unauthorized changes have been introduced to the protocol. If the problem persists, switch to a new batch of the various medium components (including serum) and/or cells. A limitation of our 3-D model is the use of tumorigenic HCT-8 epithelial cell line. However, it is important to consider that the presence of HCT-8 line offers the advantage of being available commercially. Moreover, these epithelial cells do not express either classical or non-classical human leukocyte antigen (HLA)-class I molecules49,50. Thus, they allow the culture of epithelial cells with PBMC of different HLA-class I haplotypes in the absence of epithelial cell-PBMC alloreactivity. Furthermore, when comparing this system with systems such as the gut stem cell organoids51-53, this model offers many benefits. Although gut stem cell organoids provide invaluable information about cell biology and intestinal differentiation 51-53, this model permits direct apical exposure to nutrients, drugs and pathogens. This model also provides easy access to luminal contents such anti-microbial peptides and cytokines. In contrast, organoids are compact units with a luminal surface facing inwards. Consequently, there is a restricted amount of product that can be introduced into the organoid lumen 54.
We believe that our multicellular 3-D organotypic model of the human intestinal mucosa has wide-ranging potential as a tool for discovery in both health and disease, including interaction with pathogens, antigen trafficking, and inflammatory and metabolic processes24. Finally, due to the multicellular nature of our 3-D model, our model could enable gain- and loss-studies using immune cell types that are likely to influence epithelial cell behavior in vivo.
Disclosures
The authors declare that a US Non-Provisional Patent Application has been filed in the U.S.Patent and Trademark Office (Number: 13/360,539).
Acknowledgments
This work was supported, in part, by NIAID, NIH, DHHS federal research grants R01 AI036525 and U19 AI082655 (CCHI) to MBS and by NIH grant DK048373 to AF. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy And Infectious Diseases or the National Institutes of Health.
References
- Aumailley M, Timpl R. Attachment of cells to basement membrane collagen type IV. J Cell Biol. 1986;103:1569–1575. doi: 10.1083/jcb.103.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buset M, Winawer S, Friedman E. Defining conditions to promote the attachment of adult human colonic epithelial cells. In Vitro Cell Dev Biol. 1987;23:403–412. doi: 10.1007/BF02623855. [DOI] [PubMed] [Google Scholar]
- Fitzgerald TJ, Repesh LA, Blanco DR, Miller JN. Attachment of Treponema pallidum to fibronectin, laminin, collagen IV, and collagen I, and blockage of attachment by immune rabbit IgG. Br J Vener Dis. 1984;60:357–363. doi: 10.1136/sti.60.6.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetschy JF, Ulrich G, Aunis D, Ciesielski-Treska J. Fibronectin and collagens modulate the proliferation and morphology of astroglial cells in culture. Int J Dev Neurosci. 1987;5:63–70. doi: 10.1016/0736-5748(87)90049-9. [DOI] [PubMed] [Google Scholar]
- Paye M, Lapiere CM. The lack of attachment of transformed embryonic lung epithelial cells to collagen I is corrected by fibronectin and FXIII. J Cell Sci. 1986;86:95–107. doi: 10.1242/jcs.86.1.95. [DOI] [PubMed] [Google Scholar]
- Pourreau-Schneider N, et al. Estrogen response of MCF-7 cells grown on diverse substrates and in suspension culture: promotion of morphological heterogeneity, modulation of progestin receptor induction; cell-substrate interactions on collagen gels. J Steroid Biochem. 1984;21:763–771. doi: 10.1016/0022-4731(84)90042-6. [DOI] [PubMed] [Google Scholar]
- Pratt BM, Harris AS, Morrow JS, Madri JA. Mechanisms of cytoskeletal regulation. Modulation of aortic endothelial cell spectrin by the extracellular matrix. Am J Pathol. 1984;117:349–354. [PMC free article] [PubMed] [Google Scholar]
- Jessup JM, et al. Microgravity culture reduces apoptosis and increases the differentiation of a human colorectal carcinoma cell line. In vitro cellular & developmental biology - Animal. 2000;36:367–373. doi: 10.1290/1071-2690(2000)036<0367:mcraai>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sutherland RM, et al. Oxygenation and differentiation in multicellular spheroids of human colon carcinoma. Cancer Res. 1986;46:5320–5329. [PubMed] [Google Scholar]
- Unsworth BR, Lelkes PI. Growing tissues in microgravity. Nat Med. 1998;4:901–907. doi: 10.1038/nm0898-901. [DOI] [PubMed] [Google Scholar]
- Zwezdaryk KJ, Warner JA, Machado HL, Morris CA, Honerzu Bentrup K. Rotating cell culture systems for human cell culture: human trophoblast cells as a model. Journal of visualized experiments : JoVE. 2012. [DOI] [PMC free article] [PubMed]
- Radtke AL, Herbst-Kralovetz MM. Culturing and Applications of Rotating Wall Vessel Bioreactor Derived 3D Epithelial Cell Models. Journal of visualized experiments : JoVE. 2012. [DOI] [PMC free article] [PubMed]
- Barrila J, et al. Organotypic 3D cell culture models: using the rotating wall vessel to study host-pathogen interactions. Nat Rev Microbiol. 2010;8:791–801. doi: 10.1038/nrmicro2423. [DOI] [PubMed] [Google Scholar]
- Honer zu Bentrup K, et al. Three-dimensional organotypic models of human colonic epithelium to study the early stages of enteric salmonellosis. Microbes and infection / Institut Pasteur. 2006;8:1813–1825. doi: 10.1016/j.micinf.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Nickerson CA, et al. Three-dimensional tissue assemblies: novel models for the study of Salmonella enterica serovar Typhimurium pathogenesis. Infect Immun. 2001;69:7106–7120. doi: 10.1128/IAI.69.11.7106-7120.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara Warren C, et al. Detection of epithelial-cell injury, and quantification of infection, in the HCT-8 organoid model of cryptosporidiosis. J Infect Dis. 2008;198:143–149. doi: 10.1086/588819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho HM, Teel LD, Goping G, O'Brien AD. A three-dimensional tissue culture model for the study of attach and efface lesion formation by enteropathogenic and enterohaemorrhagic Escherichia coli. Cell Microbiol. 2005;7:1771–1781. doi: 10.1111/j.1462-5822.2004.00594.x. [DOI] [PubMed] [Google Scholar]
- Lin HJ, O'Shaughnessy TJ, Kelly J, Ma W. Neural stem cell differentiation in a cell-collagen-bioreactor culture system. Brain Res. Dev Brain Res. 2004;153:163–173. doi: 10.1016/j.devbrainres.2004.08.010. [DOI] [PubMed] [Google Scholar]
- He L, et al. Increased proliferation and adhesion properties of human dental pulp stem cells in PLGA scaffolds via simulated microgravity. Intl Endo J. 2015. [DOI] [PubMed]
- Straub TM, et al. In vitro cell culture infectivity assay for human noroviruses. Emerg Infect Dis. 2007;13:396–403. doi: 10.3201/eid1303.060549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst-Kralovetz MM, et al. Lack of norovirus replication and histo-blood group antigen expression in 3-dimensional intestinal epithelial cells. Emerg Infect Dis. 2013;19:431–438. doi: 10.3201/eid1903.121029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin TJ, Schroeder WF, Wolf DA, Moyer MP. Rotating-wall vessel coculture of small intestine as a prelude to tissue modeling: aspects of simulated microgravity. Proc Soc Exp Biol Med. 1993;202:181–192. doi: 10.3181/00379727-202-43525. [DOI] [PubMed] [Google Scholar]
- Goodwin TJ, Jessup JM, Wolf DA. Morphologic differentiation of colon carcinoma cell lines HT-29 and HT-29KM in rotating-wall vessels. In Vitro Cell Dev Biol. 1992;28:47–60. doi: 10.1007/BF02631079. [DOI] [PubMed] [Google Scholar]
- Salerno-Goncalves R, Fasano A, Sztein MB. Engineering of a multicellular organotypic model of the human intestinal mucosa. Gastroenterology. 2011;141:18–20. doi: 10.1053/j.gastro.2011.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond TG, Hammond JM. Optimized suspension culture: the rotating-wall vessel. Am J Physiol Renal Physiol. 2001;281:12–25. doi: 10.1152/ajprenal.2001.281.1.F12. [DOI] [PubMed] [Google Scholar]
- Cherry RS, Papoutsakis ET. Physical mechanisms of cell damage in microcarrier cell culture bioreactors. Biotech and Bioeng. 1988;32:1001–1014. doi: 10.1002/bit.260320808. [DOI] [PubMed] [Google Scholar]
- Bergmann S, Steinert M. From Single Cells to Engineered and Explanted Tissues: New Perspectives in Bacterial Infection Biology. Int Rev Cell Mol Biol. 2015;319:1–44. doi: 10.1016/bs.ircmb.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Xu B, et al. Simulated microgravity affects ciprofloxacin susceptibility and expression of acrAB-tolC genes in E. coli ATCC25922. Int J Clin Exp Pathol. 2015;8:7945–7952. [PMC free article] [PubMed] [Google Scholar]
- Han C, Jiang C, Yu C, Shen H. Differentiation of transforming growth factor beta1-induced mesenchymal stem cells into nucleus pulposus-like cells under simulated microgravity conditions. Cell Mol Biol (Noisy-le-Grand, France) 2015;61:50–55. [PubMed] [Google Scholar]
- Wang C, et al. Microgravity activates p38 MAPK-C/EBPbeta pathway to regulate the expression of arginase and inflammatory cytokines in macrophages. Inflamm Res. 2015;64:303–311. doi: 10.1007/s00011-015-0811-3. [DOI] [PubMed] [Google Scholar]
- Iliev ID, et al. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481–1489. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- Kerneis S, Bogdanova A, Kraehenbuhl JP, Pringault E. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- Sato T, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2010. [DOI] [PMC free article] [PubMed]
- Lei NY, et al. Intestinal subepithelial myofibroblasts support the growth of intestinal epithelial stem cells. PLoS One. 2014;9:e84651. doi: 10.1371/journal.pone.0084651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voog J, Jones DL. Stem cells and the niche: a dynamic duo. Cell Stem Cell. 2010;6:103–115. doi: 10.1016/j.stem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabaji Z, et al. Use of collagen gel as an alternative extracellular matrix for the in vitro and in vivo growth of murine small intestinal epithelium. Tissue Eng Part C Methods. 2013;19:961–969. doi: 10.1089/ten.tec.2012.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabaji Z, et al. Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium. PLoS One. 2014;9:e107814. doi: 10.1371/journal.pone.0107814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodin S, Antonsson L, Hovatta O, Tryggvason K. Monolayer culturing and cloning of human pluripotent stem cells on laminin-521-based matrices under xeno-free and chemically defined conditions. Nat Protoc. 2014;9:2354–2368. doi: 10.1038/nprot.2014.159. [DOI] [PubMed] [Google Scholar]
- Foulke-Abel J, et al. Exp Biol Med. Vol. 239. Maywood, N.J: 2014. Human enteroids as an ex-vivo model of host-pathogen interactions in the gastrointestinal tract; pp. 1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins WA, Watrach AM, Schmale JD, Schultz RM, Harris JA. Cultural and antigenic properties of newly established cell strains derived from adenocarcinomas of the human colon and rectum. J Natl Cancer Inst. 1974;52:1101–1110. doi: 10.1093/jnci/52.4.1101. [DOI] [PubMed] [Google Scholar]
- Hinterleitner TA, Saada JI, Berschneider HM, Powell DW, Valentich JD. IL-1 stimulates intestinal myofibroblast COX gene expression and augments activation of Cl- secretion in T84 cells. Am J Physiol. 1996;271:1262–1268. doi: 10.1152/ajpcell.1996.271.4.C1262. [DOI] [PubMed] [Google Scholar]
- Hoshi H, McKeehan WL. Brain- and liver cell-derived factors are required for growth of human endothelial cells in serum-free culture. Proc Natl Acad Sci U S A. 1984;81:6413–6417. doi: 10.1073/pnas.81.20.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno-Goncalves R, Pasetti MF, Sztein MB. Characterization of CD8(+) Effector T Cell Responses in Volunteers Immunized with Salmonella enterica. Serovar Typhi Strain Ty21a Typhoid Vaccine. J Immunol. 2002;169:2196–2203. doi: 10.4049/jimmunol.169.4.2196. [DOI] [PubMed] [Google Scholar]
- Poggioli T, Sarathchandra P, Rosenthal N, Santini MP. Intramyocardial cell delivery: observations in murine hearts. J Vis Exp. 2014. p. e51064. [DOI] [PMC free article] [PubMed]
- Eastburn DJ, Mostov KE. Laying the foundation for epithelia: insights into polarized basement membrane deposition. EMBO Rep. 2010;11:329–330. doi: 10.1038/embor.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coecke S, et al. Guidance on good cell culture practice. a report of the second ECVAM task force on good cell culture practice. Altern Lab Anim. 2005;33:261–287. doi: 10.1177/026119290503300313. [DOI] [PubMed] [Google Scholar]
- Geraghty RJ, et al. Guidelines for the use of cell lines in biomedical research. Br J Cancer. 2014;111:1021–1046. doi: 10.1038/bjc.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung T, et al. Good Cell Culture Practice. ECVAM Good Cell Culture Practice Task Force Report 1. Altern Lab Anim. 2002;30:407–414. doi: 10.1177/026119290203000404. [DOI] [PubMed] [Google Scholar]
- Blanchet O, et al. Altered binding of regulatory factors to HLA class I enhancer sequence in human tumor cell lines lacking class I antigen expression. Proc Natl Acad Sci U S A. 1992;89:3488–3492. doi: 10.1073/pnas.89.8.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmisano GL, et al. HLA-E surface expression is independent of the availability of HLA class I signal sequence-derived peptides in human tumor cell lines. Hum Immunol. 2005;66:1–12. doi: 10.1016/j.humimm.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Ootani A, et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15:701–706. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Wilson SS, Tocchi A, Holly MK, Parks WC, Smith JG. A small intestinal organoid model of non-invasive enteric pathogen-epithelial cell interactions. Mucosal Immunol. 2014. [DOI] [PMC free article] [PubMed]


