Abstract
Posttraumatic stress disorder (PTSD) and mild traumatic brain injury (mTBI) are highly prevalent among Veterans of the conflicts in Iraq and Afghanistan. These conditions are associated with common and unique neuropsychological and neuroanatomical changes. This review synthesizes neuropsychological and neuroimaging studies for both of these disorders and studies examining their co-occurrence. Recommendations for future research, including utilizing combined neuropsychological and advanced neuroimaging techniques to study these disorders alone and in concert, are presented. It is clear from the dearth of literature that more attention in the literature should be given to examining temporal relationships between PTSD and mTBI, risk and resilience factors associated with both disorders and their co-occurrence, and mTBI-specific factors such as time since injury and severity of injury, utilizing comprehensive, yet targeted cognitive tasks.
Since the start of the global war on terrorism, American military service members have deployed 3.3 million times as part of Operations Enduring and Iraqi Freedom (OEF/OIF; Sheppard, Malatras & Israel, 2010). Post-traumatic stress disorder (PTSD) and mild traumatic brain injury (mTBI) are two of the most common consequences of warzone deployment. Although resilience is the most common outcome, as many as 20% of returning military personnel are affected with PTSD (Hoge et al., 2004). Further, approximately 15-20% returning service members reported a probable TBI during their deployment ((e.g., Belanger, Uomoto, & Vanderploeg, 2009; Tanielian & Jaycox, 2009; Terrio et al., 2009; Hoge et al., 2008; Schneiderman, Braver, & Kang, 2008; Vasterling et al., 2006). Thus, the recovery and reintegration of a significant minority of returning military personnel will be affected by these conditions. These conditions also affect families, and the personal, public, and societal costs of these disorders make them a major public health concern. As a result, management of these conditions is a high priority for the Department of Defense (DoD) and the Department of Veterans Affairs (VA).The aim of the current review is to summarize the literature on neurocognitive effects of PTSD and TBI following warzone deployments. The main focus is on OEF/OIF Veterans; however, we draw upon the literature from other war theatres as well as civilian samples in instances where the literature on OEF/OIF Veterans is limited. This review begins with a conceptualization of PTSD from a neurocognitive perspective. The relation between PTSD and specific areas of neuropsychological functioning are reviewed, including intellectual functioning/IQ, memory, attention and executive functioning. The review then explores the neuropsychological effects of traumatic brain injury (TBI), with a specific focus on mTBI. The effect of the co-occurrence of mTBI and PTSD on neurocognition is also reviewed. Finally, the literature on structural and functional neuroimaging of PTSD, TBI, and their co-occurrence is examined, including studies utilizing both neuroimaging and neuropsychological assessment. What will become apparent in the course of this review is the dearth of literature in this important area.
Neuropsychology of PTSD
Although PTSD is typically viewed as a disorder of dysregulated fear conditioning, neuroscognitive conceptualizations are plausible and well-documented in the literature (Rubin, Bernsten, & Johansen, 2008; Vasterling, Verfaellie, 2009). Abnormalities in memory (e.g., intrusive memories, avoidance of trauma-related memories) and attention (impaired concentration, hypervigilance) are central to the clinical presentation of PTSD and are included among the diagnostic criteria (APA, 2000). People with PTSD appear to process threat-related stimuli differently than neutral stimuli (for reviews, see Bar-Haim, Lamy, Pergamin, Bakermans-Kraneburg, & van IJzendoom, 2007; Buckley, Blanchard, & Neill, 2000; Constans, 2005). In addition, studies utilizing neuropsychological tests involving emotionally neutral information (e.g., words or photographs that do not evoke any particular emotional content) have shown that PTSD is most robustly associated with impairments on tasks assessing overall intellectual ability, attention, memory, and executive functioning (Vasterling, Verfaellie, & Sullivan, 2009; Vasterling & Brailey, 2005; Johnson & Asbjørnsen, 2008; Brewin, Kleiner, Vasterling, & Field, 2007; Brewin, Andrews, & Valentine, 2000). Although neuropsychological impairment associated with PTSD is typically mild to moderate in magnitude, often falling within the normal range, these impairments may be of high clinical relevance (Vasterling & Verfaellie, 2009). Intellectual Functioning/IQ.). Lower intellectual functioning was associated with risk for PTSD in a meta-analysis of 6 studies with a total of 1,149, and this relationship represented a small mean effect size (r = 18; Brewin et al., 2000). Two studies found that the relationship between lower IQ and PTSD remained even after controlling for level of combat exposure (Macklin et al., 1998; McNally & Shin, 1995). This relationship between lower IQ and PTSD was supported by subsequent studies with Veterans (Gilbertson, Gurvits, Lasko, Orr, & Pitman, 2001; Vasterling et al., 2002; Gale, Deary, Boyle, Barefoot, Mortensen, & Batty, 2008; Kremen, et al., 2007). Verbal IQ appears particularly sensitive to risk for PTSD (Vasterling, Brailey, Constans, Borges, & Sutker, 1997;; Gil et al., 1990).
Memory
Two meta-analyses found a robust relationship between concurrent PTSD symptoms and memory impairment (Brewin et al., 2007; Johnsen & Asbjørnsen, 2008). Brewin et al. (2007) found that the overall magnitude of the association was small to moderate, and was stronger for verbal than for visual memory, affecting immediate and delayed recall equally, even after controlling for the presence of concurrent head injuries. Johnsen and Asbjørnsen (2008) examined verbal memory only and found a medium effect comparing people with PTSD to normal controls. Both meta-analyses found that effect sizes were smaller when trauma-exposed, rather than non-traumatized control groups were used. Both meta-analyses concluded that the association between PTSD and memory impairment was present in both military and civilian samples, whereas Johnsen and Asbjørnsen (2008) concluded that the strongest effects were observed among war Veterans.
Attention and Executive Functioning
These constructs are reviewed together because of significant conceptual overlap (Lezak, 1995). In two studies that tested a four-domain model of attention (Mirsky, Anthony, Duncan, Ahearn, & Kellam, 1991), Gulf War and Vietnam Veterans with PTSD performed worse than warzone-exposed Veterans without PTSD on sustained attention and encoding tasks, but not on focus-execute or a shifting task (Vasterling et al., 1998; 2002). These findings are representative of other studies with war Veterans in which PTSD was associated with deficits on encoding (e.g., Gurvits, Lasko, Schacter, Kuhne, Orr, & Pitman, 1993; Uddo, Vasterling, Brailey, & Sutker, 1993; Barrett, Green, Morris, Giles, & Croft, 1996; Vasterling, Brailey, Constans, Borges, & Sutker, 1997; Beckham, Crawford, & Feldman, 1998; Gilbertson et al., 2001), but not set-shifting (Gurvits et al., 1993; Sullivan, Krengel, Proctor, Devine, Heeren, & White, 2003) or focus-execute tasks (Litz et al., 1996). Findings regarding sustained attention have been mixed, with studies finding PTSD-related deficits in Veterans (Semple et al., 1996) and civilians (e.g., McFarlane, Weber, & Clark, 1993) while other studies of Veterans found no relation (Sullivan et al., 2003; Vasterling et al., 2000). These mixed findings may be related to differences across sustained attention tasks. PTSD symptom severity was associated with a pattern of cognitive intrusion (i.e., commission errors, failure to inhibit inappropriate responses) across a range of tasks (Vasterling et al., 1998). Similarly, an association was found between PTSD and perseverative responses suggestive of ventromedial prefrontal dysfunction (Koenen et al., 2001), suggesting that PTSD may be associated with executive dysfunction.
Owing to increased awareness of the prevalence of TBI and high rates of co-occurrence between PTSD and TBI among OEF/OIF Veterans (Hoge et al., 2008; Carlson et al., 2011), few studies have examined the effect of PTSD on neuropsychological test performance independent of the effect of TBI. The Neurocognition Deployment Health Study (NDHS) examined cognitive functioning among soldiers before and after deployment to Iraq (OIF) and found an interaction between PTSD symptom severity and time since deployment on sustained attention (Marx et al., 2009a). Among soldiers who returned from Iraq more than a year prior to re-assessment (n = 164), greater PTSD severity was associated with worse attention after controlling for pre-deployment performance, whereas no effect of PTSD was found for Soldiers who returned more recently (n = 104). There was no effect of PTSD on verbal memory, visual memory, or response time, and no effect of level of combat exposure, depression, head injury, or recent alcohol consumption on any of these tasks. These findings suggest that more chronic PTSD symptoms exert a larger and potentially an increasing influence on attentional impairment a year after deployment to Iraq compared to other factors that may influence neuropsychological functioning.
Based on prior findings suggesting that pre-trauma neurocognitive tasks tapping hippocampal and prefrontal functions may moderate the development of PTSD symptoms among war Veterans (Gilbertson et al., 2006) and civilians (Parslow & Jorm, 2007), a subsequent NDHS report (Marx, Doron-Lamarca, Proctor, & Vasterling, 2009b) examined whether pre-deployment neurocognitive functioning predicted post-deployment PTSD symptoms. Marx et al. (2009b) examined 668 soldiers and found that worse performance on immediate visual memory was the only pre-deployment neurocognitive variable that predicted higher levels of residualized post-deployment PTSD symptoms after covarying level of combat exposure. This suggests that better ability to form visual images, which may facilitate rehearsal and habituation in response to a traumatic event, may protect against the development of PTSD. Pre-deployment delayed visual memory, verbal memory, working memory, sustained attention, and inhibition did not predict residualized post-deployment PTSD symptoms in this study. In summary, PTSD adversely affects neurocognition and vice versa. These effects are evident even in the absence of traumatic brain injury (TBI). Below, the effects of TBI, and in particular mild TBI (mTBI), are reviewed.
Neuropsychology of mTBI
Mild TBI is the diagnosis used to describe the experience of a brief alteration of mental status (e.g., confusion, disorientation), loss of consciousness for less than 30 minutes, and/or post-traumatic amnesia (loss of memory for events immediately before, during, or after an injury) for less than 24 hours following an impact to or forceful motion of the head [National Center for Injury Prevention and Control (NCIPC), 2003]. This type of injury is typically a closed-head injury, sustained as a result of events such as a motor vehicle accident, direct or indirect exposure to an explosion, or a fall, as opposed to a penetrating injury. mTBI, also referred to as concussion, is common in the general population (1-2%, Remedy Health Media, 2010), and as mentioned above, is particularly prevalent in Veterans returning from military operations in Iraq and Afghanistan, with estimates from 15-20%).
Studying the neuropsychological effect of mTBI is complicated by several issues including: (a) a wide range in severity of mTBIs; (b) the variability in remission of cognitive symptoms (discussed below); and (c) evidence suggesting that few individuals with mTBI are formally assessed with neuropsychological measures to quantify deficits because most mTBIs are treated in non-hospital medical settings (e.g., outpatient clinics) or not at all (e.g., Cassidy et al., 2004; NCIPC, 2003). Studies of OEF/OIF military Veterans are complicated by the variable and often lengthy duration between the time of injury(ies) and formal neuropsychological assessment, high rates of co-occurrence between mTBI and PTSD in this population (e.g., Carlson et al., 2011; Hoge et al., 2008), symptom overlap between mTBI and PTSD, as well as the potential influence of post-secondary gain (e.g., Nelson, Hoelzle, McGuire, Gerrier-Auerbach, Charlesworth, & Sponheim, 2010).
Prognosis and Recovery
Whereas most individuals experience at least some cognitive difficulties following mTBI (Belanger, Curtiss, Demery, Lebowitz, & Vanderploeg, 2005), there is significant variability in the duration and course of post-concussion recovery. Evidence clearly suggests that, for most people, the cognitive effects of mTBI resolve within days to three months post-injury (for meta-analyses see: Belanger et al., 2005; Frenchman et al., 2005; Iverson, 2005; Schretlen & Shapiro, 2003). Yet, despite generally good long-term prognosis for individuals who experience a mTBI, a subset report the subjective experience of chronic cognitive deficits, especially in attention and memory (e.g., Dikmen, Machamer, Fann, & Temkin, 2010; Vanderploeg, et al., 2009; Iverson, 2005; Hartlage, Durant-Wilson, & Patch, 2001; Binder, Rohling, & Larrabee, 1997; Rimel, Giordani, Barth, Boll, & Jane, 1981).
Domains of cognitive functioning affected by mTBI
Post-injury reductions in information processing speed have been identified as the single greatest predictor of neuropsychological functioning during the acute stages of recovery following mTBI (Frenchman, Fox, & Mayberry, 2005). In addition, attention, specifically concentration and divided attention, as well as learning/memory processes, are the primary cognitive symptoms following a concussion/mTBI (APA, 2000). Executive functioning (EF) is another key area to assess in an objective neuropsychological examination of mTBI (e.g., Demery, Larson, Dixit, Bauer, & Pearlsteim, 2010), as EF deficits often result in cognitive, behavioral, and/or emotional difficulties following mTBI. In terms of behavioral and emotional symptoms, headaches, vertigo/dizziness, becoming easily fatigued, disruptions in sleep, irritability/aggression, anxiety, depression, affective lability, apathy, and changes in personality also have been identified as characteristic post-concussion difficulties (APA, 2000). Notably, many of these symptoms overlap with the diagnostic criteria for PTSD, complicating assessment and diagnosis.
The Effect of Effort
As with civilian samples (e.g., Heilbronner et al., 2009; Belanger et al., 2005), evidence suggests the importance of examining the effects of evaluation context and effort during testing on neuropsychological performance in OEF/OIF Veterans with deployment-related mTBI (Nelson et al., 2010; Armistead-Jehle, 2010; Whitney, Shepard, Williams, Davis, & Adams, 2009). A survey of studies reveals that some Veterans demonstrate insufficient effort during neuropsychological testing, with estimates ranging from 17% of a sample with a history of mTBI performing poorly on at least two effort tests (Whitney et al., 2009) to 50% performing poorly on at least one effort test (Armistead-Jehle, 2010). Nelson and colleagues (2010) compared testing effort (measured by the Rey Fifteen Item Test, the California Verbal Learning Test-II, the Victoria Symptom Validity Test, and the Overall Test Battery Measure) and neuropsychological performance among four groups of Veterans: OEF/OIF Veterans with and without a history of mTBI tested in a research setting, OEF/OIF Veterans with mTBI tested or in a forensic setting, and non-OEF/OIF Veterans with mTBI tested in a forensic setting. The vast majority of all Veterans tested within a forensic setting (84.1%) demonstrated insufficient effort on at least one of three indicators (rates did not differ by OEF/OIF status), far exceeding the 10.7% insufficient effort rate observed in OEF/OIF Veterans tested for research purposes (Nelson et al., 2010). In total, effort accounted for 20-33% of the variance in cognitive performance for Veterans tested in a forensic setting, compared to just 1-8% of the variance for research participants (Nelson et al., 2010). These findings underscore the importance of incorporating tests of effort in assessments of neuropsychological functioning in Veterans being evaluated for cognitive deficits following mTBI, particularly within clinical and forensic settings.
Interestingly, self-reported cognitive symptoms following mTBI are not strongly associated with neuropsychological performance in OEF/OIF Veterans (Spencer, Drag, Walker, & Bieliauskas, 2010), and in this study, only a small proportion of Veterans failed effort testing measured by the Rey Fifteen Item Test. Specifically, subjective ratings of attention/concentration and thinking/organization were unrelated to objective tests of these domains. Self-reported ratings of memory impairment were only modestly correlated (r = −.20) with verbal delayed recall and were unrelated to verbal immediate recall and delayed recall of non-verbal information (Spencer et al., 2010). Moreover, self-reported cognitive problems were positively correlated with the number of symptoms of depression, PTSD, and anxiety these Veterans endorsed (Spencer et al., 2010). These findings highlight the influence of psychological distress on subjective perceptions of cognitive difficulties following mTBI. These findings also suggest that standardized clinical neuropsychological tests may not be sufficiently sensitive to detect subtle reductions in cognitive abilities following mTBI that may, nonetheless, contribute to distress.
Neuropsychology of co-occurring PTSD/mTBI
Although there are many overlapping deficits seen when the two are assessed individually, some studies have found deficits that are thought to be unique to their co-occurrence. These include differing levels of severity of deficits associated with either PTSD or mTBI, as well as additional impairments not typically associated with either.
Prevalence
There are a number of estimates regarding the co-occurrence of PTSD and mTBI. Hoge, et al. (2008) indicated that, out of their sample, 43.9% of Army infantry Soldiers who lost consciousness and 27.3% with an altered mental state reported symptoms congruent with PTSD. This equated to approximately 5% of their sample meeting criteria for both PTSD and TBI. Similarly, in another study, results indicated that approximately one-third of Veterans who experienced a TBI also reported symptoms of PTSD (Tanielian & Jaycox, 2008).
Methodological issues
Studying the influence of co-occurring PTSD and mTBI on neuropsychological functioning has proven challenging. Findings are conflicting, especially as to whether or not the co-occurrence of PTSD and mTBI leads to deficits over and above their individual effects (Gordon, et al., 2011). Additionally, not all studies have used a no-diagnosis control group for comparison. For example, Brenner et al. (2010) found no differences between a group with PTSD only and a group with co-occurring PTSD and mTBI on measures of processing speed, inhibition, abstract concept formation, set shifting and maintenance, immediate memory, delayed recall, visual search, tracking, sustained attention, and working memory.
Attention/Memory/Executive Functioning
Although few data are available, there has been some interesting neuropsychological evidence indicating neuropsychological deficits unique to patients with co-occurring PTSD/mTBI. One study indicated lower Stroop Word Reading scores in Veterans with co-occurring PTSD/TBI compared to Veterans diagnosed with PTSD only (Brenner, at al., 2010), and a co-occurring group scored lower on set-shifting, an executive function (Barrett et al., 1996). Another study demonstrated verbal processing speed deficits in both PTSD and comorbid diagnosis groups as compared to control and TBI only groups (Campbell, et al., 2009). This study (Campbell et al., 2009) also found executive functioning deficits in PTSD alone compared to a group with co-occurring PTSD and mTBI.
Effort
One study compared the relative influence of self-reported TBI and PTSD on processing speed and executive functioning in OEF/OIF combat Veterans with TBI only (average 9.7 months post-head injury), co-occurring TBI and PTSD (average 28.4 months post-head injury), or PTSD only (Campbell et al., 2009). Of note, after excluding 19% of the sample for insufficient effort, Veterans with mild to moderate TBI alone did not perform more poorly on these measures than Veterans with PTSD alone (Campbell et al., 2009).
Despite methodological differences, these studies suggest that, in the long-term, the cognitive effects of exposure to mTBI are comparable to that observed in Veterans who endorse significant symptoms of PTSD, and the co-occurrence, where studied, is associated with greater cognitive difficulties.
Structural and Functional Neuroimaging of PTSD and TBI in OEF/OIF Veterans
Structural Neuroimaging Studies
Structural Neuroimaging of PTSD
Many structural neuroimaging studies have examined PTSD since Bremner and colleagues’ (1995) seminal magnetic resonance imaging (MRI) study, although few have examined neuroanatomical correlates of combat-related PTSD among OEF/OIF Veterans. In civilians samples, there is substantial evidence that adults with PTSD have smaller hippocampal volumes versus those without PTSD (Hedges & Woon, 2010; Karl et al., 2006; Kitayama et al., 2005; Smith, 2005), though these differences may be present premorbidly (Kimble, 2008; Gilbertson et al. 2002; Woodward, et al., 2006, 2009). PTSD vs. no-PTSD volumetric differences in other brain structures have not been reliably demonstrated. Karl and colleagues’ (2006) meta-analysis reported lower amygdala volume, whereas Woon and Hedges’ (2010) larger meta-analysis failed to replicate this finding. Lower anterior cingulate cortex (ACC; Karl et al., 2006; Woodward et al., 2005, 2006) and medial prefrontal cortex (mPFC) volumes of patients with PTSD have been reported (Shin, Rauch, & Pitman, 2005; Milad & Rauch, 2007; Rauch et al., 2003; Yamasue et al., 2003), though differences in these regions are not as well-substantiated as the hippocampus differences.
Two recent studies in Veterans are noteworthy. Wang and colleagues (2010) used high resolution MRI at 4T to examine possible differences in hippocampal subfields between Veterans with and without PTSD (23% Vietnam, 16% Gulf War, 61% OEF/OIF). This study was the first to demonstrate an association between PTSD and selective volume loss in the cornu ammonis 3 (CA3) and dentate gyrus subfields of the hippocampus in humans, which replicates prior work with animals (e.g., McKittrick et al., 2000; Pham, Nacher, Hof, & McEwen, 2003). In a second study using a subsample of the same participants, Schuff and colleagues (2011) used diffusion tensor imaging (DTI) to further assess possible microstructural differences between Veterans with and without PTSD. They found that Veterans with PTSD had reduced fractional anisotropy (FA) in areas near the ACC, prefrontal cortex (PFC), precentral gyrus, and posterior angular gyrus, demonstrating less integrity of white matter near these areas. This pattern of deficits is particularly intriguing given corroborating evidence from functional neuroimaging studies that suggest an affective-cognitive network imbalance in PTSD (see below).
Taken together, these findings suggest that volumetric differences within the limbic system may underlie some of the affective and cognitive processing symptomatolgy of PTSD as well as the autonomic effects observed. In addition to being anatomically linked, the neurofunctional roles of these brain structures contribute to affective processing (Gordon et al., 2011; Williams et al., 2006), memory consolidation (Squire, 1992; Roozendaal et al., 2009; Segev, Ramot, & Akirav, 2012), memory formation (Buzsáki , Chen, & Gage, 1990; Squire, 1992), and stimulus monitoring (Mayer et al., 2011), in addition to their contributions to interoception (for review, please see Cameron, 2001). Thus it is likely that these neuroanatomical findings affect the neurofunctional properties and capacity of these structures to perform their operations effectively, particularly with regard to cognitive and affective processing (Figure 1).
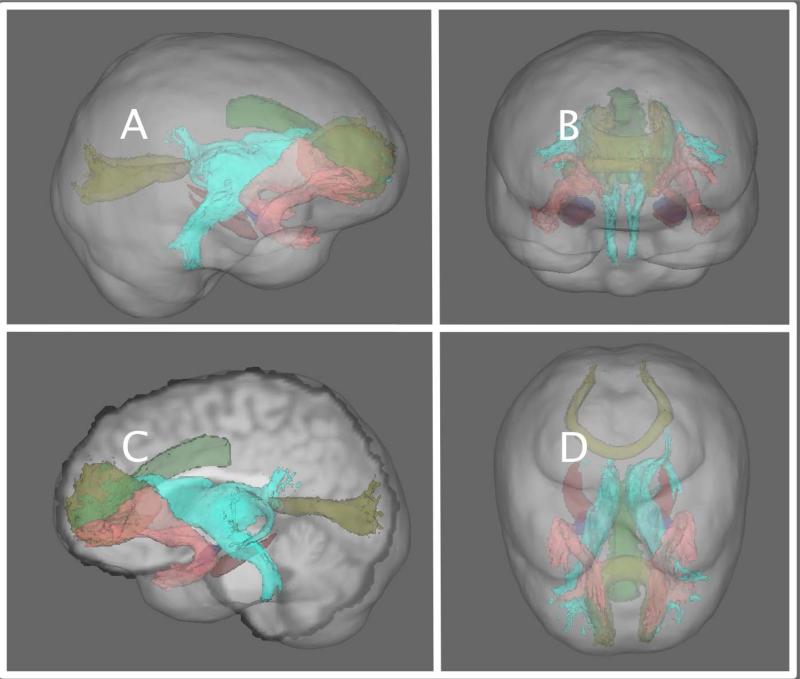
Figure 1.
Depiction of neural structures thought to be involved in combat-related PTSD. Here, the amygdala is depicted in red, the hippocampus in blue, and the anterior cingulate in green. The white matter tracts associated with PTSD are the uncinate fasciculus (shown as pink), the forceps minor and major (in yellow), and the anterior thalamic radiation (shown in cyan). Panels A and C are sagittal depictions. Panel B is an anterior view, and Panel D is a superior view of the cognitive-affective network implicated in PTSD.
Structural Neuroimaging of TBI
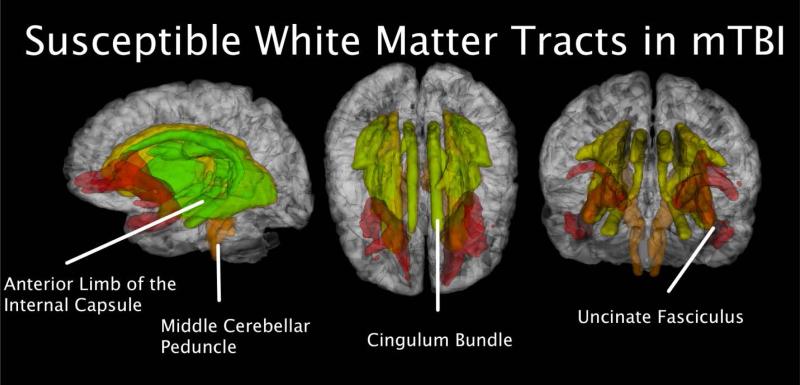
It is well established that conventional structural imaging techniques, such as computed tomography (CT) and MRI, are unlikely to advance our understanding of combat-related mTBI, as these methods are generally considered to lack sufficient sensitivity to detect the subtle abnormalities that characterize mTBI (Mac Donald, et al., 2011). However, studies of civilians have demonstrated the utility of DTI for examining white matter abnormalities in TBI (e.g., Kraus, Susmaras, Caughlin, Walker, Sweeney, & Little, 2007). In that vein, the limited research on TBI in OEF/OIF Veterans that has been conducted to date has primarily relied upon DTI and high resolution MRI. In one of the only large scale DTI studies of TBI conducted among OEF/OIF Veterans to date, Mac Donald and colleagues (2011) reported that DTI was able to detect a significantly greater number of abnormalities for 18 of 63 OEF/OIF Veterans with TBI than would be expected by chance (p < .001). Specifically, relative to control participants, OEF/OIF Veterans with TBI showed abnormalities in the middle cerebellar peduncles, cingulum bundles, and right orbitofrontal white matter, regions that have been implicated in the TBI based on intracranial wave physics (Taylor & Ford, 2009), despite normal MRI findings (Figure 2). They also noted abnormalities in the uncinate fasciculus and the anterior limb of the internal capsule. The former has shown aberrant properties in PTSD, suggesting a potential underlying culprit in the high comorbidity rates seen in OEF/OIF Veterans. These results were deemed to be consistent and reliable based on 6- and 12-month follow-up scans of 47 TBI participants which showed persistent abnormalities in these same regions. These data highlight the need for additional TBI research to be conducted with OEF/OIF Veterans using more advanced structural neuroimaging techniques, such as DTI and high field, high resolution MRI. This is particularly important research among Veterans from the current war theatres, as the nature and frequency of TBIs (e.g., recurrent blast exposure) differ substantially from typical head injury exposures in the general population.
Figure 2.
White matters tracts susceptible to mTBI. These include the anterior limb of the internal capsule, the cingulum bundle, the middle cerebellar peduncle, and the uncincate fasciculus.
Structural Neuroimaging of Co-occurring PTSD & TBI
Structural studies have recently begun to examine the co-occurrence of PTSD and TBI. In one of the few studies to date examine structural differences among Veterans with PTSD, TBI, or both (war theater was not specified), Brenner and colleagues (2009) found that participants diagnosed with TBI only were more likely to have MRI head- trauma-related findings [i.e., encephalamalacia or gradiant echo (GE) identified hemosiderin deposits1] when compared to patients that had co-occurring comorbid PTSD and TBI. Sponheim and colleagues (2011) used DTI to identify neuroanatomical correlates of neurofunctional abnormalities [assessed using electroencephalogram (EEG)] in nine blast-injured OEF/OIF Veterans compared with matched healthy civilians. They found that decreased neuronal synchronicity was correlated with measures of decreased white matter integrity (increased FA) in the frontal lobe above and beyond the variance explained by PTSD symptoms or medications. The consistency of frontal lobe dysfunction coupled with aberrant neuroanatomical findings across modalities (i.e., EEG, fMRI, DTI) suggests that the frontal lobe is a key component in the underlying neuropathophysiology of both combat-related TBI and PTSD.
The study conducted by Sponheim and colleagues (2011) is one of the only structural imaging studies of TBI that used PTSD as a covariate. Thus, additional research aimed at using
DTI and other advanced structural imaging techniques to disentangle the complex relationship between PTSD and TBI among OEF/OIF Veterans is needed.
Functional Neuroimaging Studies
Functional neuroimaging—namely functional MRI (fMRI) and positron emission tomography (PET)—are flexible imaging techniques that capture changes in neural activity within the brain. Functional neuroimaging techniques can be used to assess population differences in the way that diseased brains process and evaluate stimuli relative to those who are unaffected. They also enable researchers to examine differences in connectivity and the neural processes that underlie complex cognitive functions. Thus, functional neuroimaging techniques hold great promise for understanding the changes that occur within the brains of OEF/OIF military personnel who sustain TBIs and/or develop PTSD as part of their deployments. Furthermore, functional neuroimaging techniques may eventually improve clinical care by identifying regional brain abnormalities that could serve as targets for therapeutic interventions.
Functional Neuroimaging of PTSD
Neuroanatomical and neurobiological models of PTSD suggest that alterations within the limbic system and memory networks may underlie the disorder. However, few studies to date have examined the neurofunctional correlates of combat-related PTSD among OEF/OIF Veterans. In one of the few large studies of post-9/11 Veterans, Hayes and colleagues (2011) found behavioral and neurofunctional differences between Veterans with PTSD and trauma-exposed controls. Specifically, they observed lower amygdala and hippocampal activity during encoding of trauma-related stimuli, supporting the role of the medial temporal lobes in PTSD. Morey and colleagues (2008) had participants perform an fMRI task during which they alternated between viewing neutral or combat-related images and a cognitive task to identify network changes in regions that subserve cognitive and emotional processes. Compared to the neutral pictures, activation for the emotionally laden pictures in the ventromedial emotional processing stream comprised of the ventromedial prefrontal cortex, inferior frontal gyrus, and ventral anterior cingulate was associated with PTSD symptom severity. Interestingly, activations during the cognitive task in the middle frontal gyrus, dorsal anterior cingulate, and inferior parietal lobule were negatively correlated with PTSD symptoms. Additionally, Schuff and colleagues (2011) demonstrated disrupted functional connectivity in the frontal lobes using EEG. Together, these findings corroborate with neuroanatomical results suggesting aberrant frontolimbic circuitry as a signature for the disorder.A different approach to understanding combat-related PTSD would be to characterize neurofunctional markers of resilience. Vythilingham and colleagues (2009) compared civilians to special forces Soldiers deemed resilient to the development of PTSD. During a monetary incentive delay task, known to activate the reward-senstive ventral striatum (Knutson et al., 2000), they found differences in neural activity within the subgenual prefrontal cortex and the nucleus accumbens area unrelated to performance measures. Specifically, they found that civilians had increased activation of these regions during anticipation of high reward conditions compared to low reward conditions, while the soldiers had no differences in brain activation between conditions. Thus, one can conclude that resilience may be marked by a neuronal ‘numbing’ of the reward system, an invariable level of neural activity unresponsive to reward valence. Alternatively, resilience may be marked by extreme high or low baseline activity, creating ceiling or floor effects. Although the authors readily admitted the shortcomings of the study (i.e., using non-trauma exposed civilians as a control group), using a novel cohort of this type warrants additional attention, as studies designed to identify factors that contribute to resiliency may also help to identify soldiers at greatest risk for post-deployment difficulties. Further, fMRI has been used to document treatment response. Preliminary findings from Roy and colleagues (2010) showed that successful PTSD treatment (e.g., through the use of exposure therapy delivered via virtual reality or prolonged imaginal exposure) was associated with decreased activation in the amygdala, subcallosal gyrus, and lateral prefrontal cortex. Importantly, these regions are similar to those identified as hyperactive in previous studies of OEF/OIF Veterans (e.g., Morey, et al., 2008), suggesting that successful treatment for PTSD might be associated with a dampening of hyper-sensitivity of these regions. Combined with studies examining the correlation between PTSD symptoms and neurofunctional responses, a distributed network emerges in which structures subserving emotion appear to be negatively correlated to symptoms of combat-related PTSD, while those subserving both cognition and emotion seem to be positively correlated (Figure 3). Given these findings, it is important that we begin to understand the relationships within the fronto-limbic circuit to better understand the mechanisms underlying this complex disorder.
Figure 3.
Neural structures that have been positively (red) or negatively (blue) associated with PTSD symptomatology. Regions in black have shown mixed results in the literature.
Functional Neuroimaging of mTBI
TBI—particularly combat-related TBI—has been relatively understudied within the functional neuroimaging community to date. A recent meta-analysis of studies examining civilians who have sustained mTBI found that, relative to healthy controls without TBI, patients exhibit differential activation in several prefrontal, temporal, and parietal regions, including the superior and middle frontal gyri, the superior temporal gyrus, and the superior and inferior parietal lobules (Simmons & Matthews, in press). In one of the few studies of its kind, Peskind and colleagues (2011) used PET to compare twelve Iraq war Veterans with mTBI and persistent PCS (ten of whom also met DSM-IV criteria for PTSD) with twelve cognitively normal community volunteers. The Veterans with PCS exhibited a decreased cerebral metabolic rate of glucose in the cerebellum, portions of the brainstem (e.g., pons), and the medial temporal lobe, suggesting that hypometabolism in these areas might underlie the persistent PCS often observed among OEF/OIF Veterans who sustain mTBIs. However, the differences in medication status and age between the Veteran and control groups, as well as the potential confound of PTSD on the Veteran TBI group causes some hesitation in interpreting these results. Despite these caveats, this study represents an important advancement in the way that research on returning war Veterans is approached. Furthermore, these results are consistent with white matter tractography differences observed in mTBI, and in line with current theories on the physical properties of TBI injuries.
Functional Neuroimaging of Comorbid PTSD & TBI
There are few functional neuroimaging studies of the co-occurrence of TBI and PTSD. Using information from the BrainMap database (Fox & Lancaster, 2002; Laird, et al., 2009; Laird, Lancaster, & Fox, 2005), Simmons and Matthews (2011) used meta-analytic techniques to identify regions of overlap between PTSD and mTBI in the published literature. Separate meta-analyses were performed on PTSD and mTBI. Only a few regions were identified in both analyses, with the greatest overlap occurring in the middle frontal gyrus. Importantly, differences in middle frontal gyrus activation were in opposite directions. Specifically, participants with PTSD demonstrated more activation in the middle frontal gyrus relative to controls, whereas participants with mTBI demonstrated compromised activation, although the authors suggest that this difference may be due to differences in study design and tasks. Overlap was also observed to a lesser extent in the caudate and ACC. However, as with the overlap found in the middle frontal gyrus, these results should be interpreted with caution in relation to combat-related PTSD given that the findings are based on a diverse set of non-Veteran participants.
Combined Neuroimaging and Neuropsychological Assessment Studies
Multimodal research is a necessary precursor to advancing our understanding of complex neurological and psychiatric disorders like TBI and PTSD. One promising approach is the combination of neuroimaging and neuropsychological assessment. Use of these complementary approaches in tandem has potential to more efficiently elucidate the pathophysiological mechanisms underlying PTSD and TBI. As noted above, one of the most consistent findings among Veterans with PTSD has been reduced hippocampal volume. Given the hippocampus’ well-established role in long-term memory consolidation (Kandel, Schwartz, & Jessell, 1991), it has been suggested that these deficits underlie the memory impairments sometimes observed among patients with PTSD (Horner & Hamner, 2002; Shin et al., 2005). Support for this position comes from early MRI studies demonstrating correlations between hippocampal volume and verbal memory scores. For example, Bremner et al. (1995) reported that decreased right hippocampal volume was associated with deficits in short term verbal memory among Vietnam combat Veterans. Results in this area have not been entirely consistent (e.g., Stein et al., 1997), however, and many of these studies were not conducted with combat-exposed Veterans. Thus, the degree to which these findings can be extrapolated to OEF/OIF Veterans is unclear, and the likelihood that the complex, diverse set of deficits observed are a result of a single neural component are unlikely. Rather, it is more probable that the neuroanatomical deficiencies result in disrupted functional capacity both locally (i.e., within the structure itself), as well as globally (i.e., between the structure and its neuroanatomical connections).
The study conducted by Peskind and colleagues (2011) described above also included a neuropsychological assessment in addition to the PET scan. This study found that Veterans with mTBI showed subtle impairments in verbal fluency, cognitive processing speed, attention, and working memory (as measured by the Sentence Repetition, Ruff 2 & 7 speed, and Categories) as well as decreased cerebral metabolic rate of glucose in the cerebellum, portions of the brainstem (e.g., pons), and the medial temporal lobe. The authors noted that this pattern of results was quite consistent with a “cerebellar cognitive affective syndrome” that has been found among patients presenting with cerebellar lesions (Schmahmann, 2004). It is important to recall that 10 of 12 participants in this study had co-occurring PTSD. This further highlights the need to understand the neural relationships between structures of interest in PTSD and TBI to better delineate the neural hallmarks of the disorder.
More recently, Brenner and colleagues (2009) found that participants who sustained a TBI were more likely to have MRI head trauma-related findings when compared to patients with co-occurring PTSD and TBI. This study also included a neuropsychological assessment battery, although only minimal differences on the neuropsychological measures were noted between Veterans with TBI, PTSD, or both. For example, no significant group differences were found on the Halstead Impairment Index (HII), Paced Auditory Serial Addition Task (PASAT), Rey Auditory Verbal Learning Test (RAVLT), or Continuous Performance Test-II (CPT-II) scores, tests of attention (PASAT and CPT-II) and verbal learning and memory (RAVLT). There were, however, significant differences on the Booklet Category Test (BCT), a test of executive functioning. Specifically, the results indicated that patients with TBI only performed better than patients with PTSD or a co-occurring diagnosis on this assessment. While the comprehensive neuropsychological inventory in the Brenner and colleagues (2009) study is a particular strength, the inclusion of only conventional MRI measurements hindered its effect on our understanding of TBI and PTSD. Utilizing more sensitive and robust measurements, such as DTI and functional connectivity (rather than just activation studies) will likely lead to advancements in our understanding of the neurophysiological basis of both disorders. Thus, while the inclusion of neuropsychological tests is important, it is equally important to take advantage of the technological and statistical advances within the neuroimaging community. For example, Levin and colleagues (2010) used DTI along with assessments of verbal memory, PTSD, and PCS to compare OEF/OIF Veterans with TBI to a control group of Veterans without TBI. They reported that Veterans with TBI demonstrated less efficient verbal memory, and that the verbal memory deficits were unrelated to PTSD severity. Interestingly, DTI revealed that total words recalled consistently was positively correlated with fractional anisotropy (FA; the amount of diffusion of water in white matter, in which higher FA means lower white matter integrity) in the left and right posterior internal capsule and left corticospinal tract. No other group differences were found for nonverbal memory or decision making. In addition, DTI measures were uncorrelated with symptom measures. Thus, utilizing advanced imaging techniques and neuropsychological batteries will increase our sensitivity to detect subtle differences in neural networks, propelling our understanding of these disorders forward.
CONCLUSIONS
Despite many neuropsychological deficits falling in the normal to mild range in PTSD and mTBI, the subjective presence of differences in cognitive and emotional functioning can be quite distressing to Veterans upon their return from deployment. Although extensive research has documented associations between PTSD and mTBI and neurocognitive impairment, there is currently no established neuropsychological profile for either disorder, or their comorbidity, that aids in differential diagnosis (see Table 1 for a summary of neuropsychological findings in these disorders). The relative paucity of research exploring the specific co-morbidity leaves us with little to confidently state regarding the mechanisms of action for the pattern of mild to moderate deficits. It is of note that, currently, not enough information is available to make conclusions about severity of deficits seen in the comorbid diagnosis of PTSD and mTBI across all cognitive domains. Our review of the few studies that examine neuropsychological function in the co-occurrence of these disorders have produced mixed findings, but the balance of the studies indicate that participants with PTSD and mTBI together do worse on neuropsychological measures than those with either alone.
Table 1.
Neuropsychological Deficits in PTSD, mTBI, and PTSD/mTBI
| Cognitive Domain | PTSD | mTBI | PTSD/mTBI |
|---|---|---|---|
| Attention | Moderate - Severe | Mild | Mild - Moderate |
| Inhibition | Moderate | Mild - Moderate | Moderate |
| Processing Speed | Mild | Mild | Mild |
| Set-Shiiting | Mild | Mild | Moderate |
| Verbal Fluency | Mild-Moderate | Mild | --- |
| Verbal Memory | Mild-Moderate | Mild | --- |
| Visual Memory | Mild | Mild - Moderate | --- |
| Working Memory | Mild-Moderate | Mild | --- |
Of particular import are the conceptual and methodological issues regarding the temporal, sequential and synergistic effects of having a neuropathologic event (mTBI) and traumatic exposure that results in at least one Axis I diagnosis (PTSD). We cannot yet say whether cognitive impairments represent risk/resilience factors; perhaps cognitive impairments are most frequently consequences of traumatic exposure. Alternatively, the smattering of mild to moderate impairments may be clinical correlates of these disorders. Regarding PTSD, the preponderance of the preliminary data suggests that the relation is bi-directional: some cognitive variables moderate the impact of subsequent trauma exposure on PTSD, whereas others may develop concurrently with PTSD or change over time as a result of PTSD (Marx et al., 2009b; Vasterling & Verfaellie, 2009). The degree of specificity of the relation between PTSD and cognitive functioning relative to the effects of trauma exposure and commonly co-occurring conditions (e.g., head injury, depression, substance use) is a complex issue. Some have questioned the integrity of an association between PTSD and neuropsychological impairment once key methodological factors have been carefully considered (Danckwerts & Leathem, 2003; Crowell, Kieffer, Siders, & Vanderploeg, 2002). However, at least one study of Soldiers deployed to Iraq identified relatively specific effects of PTSD on attention (e.g., Marx et al., 2009a).
Overall, the current state of the literature suggests that the neuropsychology of mTBI in Veterans is similar to what is known about the effects of mTBI in the general population. Following mTBI, many Veterans will experience acute cognitive (e.g., attention, learning/memory, processing speed) and/or emotional/behavioral (e.g., irritability, disruptions in sleep) difficulties that will typically resolve in the first three months following injury. In addition to the challenges associated with obtaining a clear understanding of the acute cognitive effects of mTBI within the general population, studies of Veterans are also made difficult by the often significant time lapse between the injury and opportunity for formal assessment, symptom overlap between mTBI and PTSD, as well as other factors influencing testing effort and symptom reporting. The clinical utility of formal neuropsychological assessment to aid in diagnosis and treatment planning for Veterans with PTSD and/or TBI has yet to be firmly established. It may also be the case that formal neuropsychological tests alone may be insufficient to detect subtle cognitive changes that could lead to subjective distress. Assessment of psychological symptoms and effort testing are crucial components of comprehensive evaluation of these disorders.
There is a pressing need for additional prospective, longitudinal studies, as the majority of data to date have been cross-sectional. We must follow Veterans with mono- and dual-consequences of combat exposure in order to study the directionality of the influences of PTSD and mTBI on cognitive symptoms, especially as aging and additional injuries may change the course of symptoms. Careful attention to the assessment of other meaningful co-occurring diagnoses that further cloud the picture is imperative in future research efforts. Addressing the knowledge gap will ultimately facilitate efficient matching of interventions to individuals.
Future Directions
As we approach the second decade of Iraq/Afghanistan deployments, it is vital that imaging studies specific to the OEF/OIF war theater personnel emerge, particularly those identifying the abberant neural patterns, whether structural or functional, associated with two of the most common combat morbidities. These studies should also include evaluation of our current war, Operation New Dawn, which may have parallels and differences with the two prior wars. Given the unique length and number of deployments, combined with the multiple exposures to blast and trauma incidents, such studies are necessary to advance our understanding of the neuropsychological processes, courses of disease, and therapeutic remedies that may ultimately impact the quality of care we can deliver to Veterans. Though recommendations for improved research methodologies have existed in the literature for a couple of years (Van Boven, et al., 2009), few studies adopting these regimens have been identified, despite the mounting need. Objective and specific neuroimaging based biomarkers for both disorders would be invaluable for improving diagnosis, subsequent symptom monitoring, and treatment. Additional studies utilizing newer and more advanced imaging technologies will be critical for making such biomarkers a reality (Mac Donald, et al., 2011). Moreover, there is a critical need for studies that use advanced imaging techniques to compare Veterans with PTSD only, TBI only, and co-occurring PTSD-TBI, as such an approach appears essential for disentangling this complex relationship. A second area of high priority research concerns the use of fMRI and other functional imaging techniques to document treatment response, as an improved understanding of the neural mechanisms that underlie successful treatment is essential for the development of novel therapeutic targets.
Another priority area concerns the use of meta-analytic tools that enable researchers to begin piecing together consistent patterns of differential activity between diseased and healthy populations (e.g., Simmons & Matthews, 2011). To date, these studies have consistently combined combat-related PTSD with all other types of PTSD, as well as mTBI resulting from blast injuries with mTBI associated with other types of head injuries, despite the accumulation of data suggesting that the different mechanisms of injuries may have different consequences (French, 2010). The lack of studies that more precisely identify and control severity of injury in TBI, time since injury, and whether participants are reporting subjective cognitive symptoms, other psychiatric conditions such as depression, and post-concussive symptoms also significantly limit the conclusions that can be drawn. Until a sufficient number of studies are conducted with combat Veterans to power separate analyses in well-controlled samples, we will be forced to continue to rely upon existing models developed within the civilian arena.
Finally, due to the increased availability and use of advanced neuroimaging techniques, the clinical focus of neuropsychological assessment has increasingly turned to understanding the functional implications of injuries and the manner in which treatment influences neurological systems (Abutalebi, Doering, Della Rosa, & Mariën, 2008; Noggle, Davis, & Barisa, 2008; Ricker & Arenth, 2008). However, we believe that there is currently a pressing need for multimodal research studies that combine advanced neuroimaging techniques with sturdy neuropsychological assessment batteries. Such approaches are likely to yield far more useful information than designs that use single assessment methodologies. Ultimately, it is hoped that these and other novel neuroimaging designs will lead to pivotal advances in the diagnosis and treatment of PTSD and TBI among returning Veterans.
Acknowledgements
This research was supported by the Department of Veterans Affairs (VA) VISN 17 Center of Excellence for Research on Returning War Veterans, a Merit Award (I01RX000304) to Sandra B. Morissette, Ph.D. from the Rehabilitation Research and Development Service of the VA Office of Research and Development entitled, “Functional Outcomes in OEF/OIF Veterans with PTSD and Alcohol Misuse,” a DVA VISN 17 New Investigator Award to Nathan A. Kimbrel, Ph.D. entitled, “Genetic and Environmental Effects on PTSD, Depression, and Alcohol Misuse,” and a DVA VISN 17 New Investigator Award to Eric C. Meyer, Ph.D. entitled, “Experiential Avoidance, Neurocognition, and Functional Outcomes in PTSD.”
Footnotes
Hemosiderin deposits are iron deposits thought to be left behind after a bleed in the brain. Because this iron is magnetic, it can be detected via MRI. Suceptibility Weighted Imaging (SWI) or other field-dependent relaxivity imaging also potentially could be used to detect microbleeds (TBIlaw.com).
References
- Abutalebi J, Doering LB, Della Rosa PA, Mariën P. Structural and functional neuroimaging in neuropsychology: A concise overview. In: Mariën P, Abutalebi J, editors. Neuropsychological research: A review. Psychology Press; New York, NY US: 2008. pp. 51–72. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 4th Edition Text Revision (DSM-IV-TR) American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Armistead-Jehle P. Symptom validity test performance in U.S. veterans referred for evaluation of mild TBI. Applied Neurpsychology. 2010;17:52–59. doi: 10.1080/09084280903526182. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg, van IJzendoom MH. Thereat related attention bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–12. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Barrett DH, Green ML, Morris R, Giles WH, Croft JB. Cognitive functioning and posttraumatic stress disorder. American Journal of Psychiatry. 1996;259:2701–2707. doi: 10.1176/ajp.153.11.1492. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Crawford AL, Feldman ME. Trail Making Test performance in Vietnam combat veterans with and without posttraumatic stress disorder. Journal of Traumatic stress. 1998;11:811–819. doi: 10.1023/A:1024409903617. [DOI] [PubMed] [Google Scholar]
- Belanger HG, Curtiss G, Demery JA, Lebowitz BK, Vanderploeg RD. Factors moderating neuropsychological outcomes following mild traumatic brain injury: A meta-analysis. Journal of the International Neuropsychological Society. 2005;11:215–227. doi: 10.1017/S1355617705050277. [DOI] [PubMed] [Google Scholar]
- Belanger HG, Uomoto JM, Vanderploeg RD. The Veterans Health Administration's (VHA's) Polytrauma System of Care for mild traumatic brain injury: Costs, benefits, and controversies. Journal of Head Trauma Rehabilitation. 2009;24(1):4–13. doi: 10.1097/HTR.0b013e3181957032. [DOI] [PubMed] [Google Scholar]
- Bendlin BB, Ries ML, Lazar M, Alexander AL, Dempsey RJ, Rowley HA, Johnson SC. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. NeuroImage. 2008;42(2):503–514. doi: 10.1016/j.neuroimage.2008.04.254. doi: 10.1016/j.neuroimage.2008.04.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED. Anterior and middle cranial fossa in traumatic brain injury: Relevant neuroanatomy and neuropathology in the study of neuropsychological outcome. Neuropsychology. 2007;21:515–531. doi: 10.1037/0894-4105.21.5.515. [DOI] [PubMed] [Google Scholar]
- Binder LM, Rohling ML, Larrabee GJ. A review of mild head trauma, Part I: Meta-analytic review of neuropsychological studies. Journal of Clinical & Experimental Neuropsychology. 1997;19:421–431. doi: 10.1080/01688639708403870. [DOI] [PubMed] [Google Scholar]
- Brenner LA, Ladley-O'Brian SE, Harwood JEF, Filley CM, Kelly JP, Homaifar BY, et al. An Exploratory Study of Neuroimaging, Neurologic, and Neuropsychological Findings in Veterans With Traumatic Brain Injury and/or Posttraumatic Stress Disorder. Military Medicine. 2009;174(4):347–352. doi: 10.7205/milmed-d-01-5808. [DOI] [PubMed] [Google Scholar]
- Brenner LA, Terrio H, Homaifar BY, Gutierrez PM, Staves PJ, Harwood JEF, Warden D. Neuropsychological test performance in soldiers with blast-related mild TBI. Neuropsychology. 2010;24(2):160–167. doi: 10.1037/a0017966. doi: 10.1037/a0017966. [DOI] [PubMed] [Google Scholar]
- Brewin C, Andrews B, Valentine J. Meta-analysis of risk factors for PTSD in trauma-exposed adults. Journal of Consulting and Clinical Psychology. 2000;68:748–766. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Kleiner JS, Vasterling JJ, Field AP. Memory for Emotionally Neutral Information in Posttraumatic Stress Disorder: A Meta-Analytic Investigation. Journal of Abnormal Psychology. 2007;116(3):448–463. doi: 10.1037/0021-843X.116.3.448. [DOI] [PubMed] [Google Scholar]
- Buckley TC, Blanchard EB, Neill WT. Information processing and PTSD: A review of the empirical literature. Clinical Psychology Review. 2000;28:1041–1065. doi: 10.1016/s0272-7358(99)00030-6. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Chen LS, Gage FH. Spatial organization of physiological activity in the hippocampal region: relevance to memory formation. Prog Brain Res. 1990;83:257–268. doi: 10.1016/s0079-6123(08)61255-8. [DOI] [PubMed] [Google Scholar]
- Cameron OG. Interoception: The inside story - A model for psychosomatic processes. Psychosomatic Medicine. 2001;63:697–710. doi: 10.1097/00006842-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Campbell TA, Nelson LA, Lumpkin R, Yoash-Gantz RE, Pickett TC, McCornick CL. Neuropsychological measures of processing speed and executive functioning in combat Veterans with PTSD, TBI, and comorbid TBI/PTSD. Psychiatric Annals. 2009;39(8):796–803. [Google Scholar]
- Carlson KF, Kehle SM, Meis L, Greer N, MacDonald R, Rutks I, Sayer NA, Dobscha SK, Wilt TJ. Prevalence, assessment, and treatment of mild traumatic brain injury and posttraumatic stress disorder: A systematic review of the evidence. Journal of Head Trauma and Rehabilitation. 2011;26:103–115. doi: 10.1097/HTR.0b013e3181e50ef1. [DOI] [PubMed] [Google Scholar]
- Cassidy JD, Carroll LJ, Peloso PM, Borg J, von Holst H, Holm L, et al. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Journal of Rehabilitative Medicine. 2004;36(Supplement 43):28–60. doi: 10.1080/16501960410023732. [DOI] [PubMed] [Google Scholar]
- Constans JI. Information-processing biases in PTSD. In: Vasterling JJ, Brewin CR, editors. Neuropsychology of PTSD: Biological, cognitive, and clinical perspectives. Guilford Press; New York: 2005. pp. 105–130. [Google Scholar]
- Danckwerts A, Leathem J. Questioning the link between PTSD and cognitive dysfunction. Neuropsychology Review. 2003;13:221–235. doi: 10.1023/b:nerv.0000009485.76839.b7. [DOI] [PubMed] [Google Scholar]
- Demery JA, Larson MJ, Dixit NK, Bauer RM, Perlstein WM. Operational characteristics of executive functioning following traumatic brain injury. The Clinical Neuropsychologist. 2010;24:1292–1308. doi: 10.1080/13854046.2010.528452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikmen S, Machamer J, Fann JR, Temkin NR. Rates of symptom reporting following traumatic brain injury. Journal of the International Neuropsychological Society. 2010;16:401–411. doi: 10.1017/S1355617710000196. [DOI] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL. Opinion: Mapping context and content: the BrainMap model. Nature Reviews Neuroscience. 2002;3(4):319–321. doi: 10.1038/nrn789. [DOI] [PubMed] [Google Scholar]
- French LM. Military traumatic brain injury: An examination of important differences. Annals of the New York Academy of Sciences. 2010;1208:38–45. doi: 10.1111/j.1749-6632.2010.05696.x. [DOI] [PubMed] [Google Scholar]
- Frenchman KA, Fox AM, Mayberry MT. Neuropsychological studies of mild traumatic brain injury: A meta-analytic review of research since 1995. Journal of Clinical and Experimental Neuropsychology. 2005;27:334–351. doi: 10.1080/13803390490520328. [DOI] [PubMed] [Google Scholar]
- Gale CR, Deary IJ, Boyle SH, Barefott J, Mortensen LH, Batty GD. Cognitive ability in early adulthood and risk of 5 specific disorders in middle age: The Vietnam experience study. Archives of General Psychiatry. 2008;65:1410–1418. doi: 10.1001/archpsyc.65.12.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarelli TA, Graham DI. Neuropathology. In: Silver JM, McAllister TW, Yudofsky SC, editors. Textbook of traumatic brain injury. American Psychiatric Publishing, Inc.; Washington, DC: 2005. pp. 27–50. [Google Scholar]
- Gil T, Calev A, Greenberg D, Kugelmass S, Lerer B. Cognitive functioning in post-traumatic stress disorder. Journal of Traumatic Stress. 1990;3:29–45. [Google Scholar]
- Gilbertson MW, Gurvits TV, Lasko NB, Orr SP, Pitman RK. Multivariate assessment of explicit memory functioning in combat veterans with posttraumatic stress disorder. Journal of Traumatic stress. 2001;14:413–432. doi: 10.1023/A:1011181305501. [DOI] [PubMed] [Google Scholar]
- Gilbertson M, Paulus L, Williston S, Gurvits T, Lasko N, Pitman R, et al. Neurocognitive function in monozygotic twins discordant for combat exposure: Relationship to posttraumatic stress disorder. Journal of Abnormal Psychology. 2006;115:484–495. doi: 10.1037/0021-843X.115.3.484. [DOI] [PubMed] [Google Scholar]
- Gordon SN, Fitzpatrick PJ, Hilsabeck RC. No effect of PTSD and other psychiatric disorders on cognitive functioning in veterans with mild TBI. The Clinical Neuropsychologist. 2011;25(3):337–347. doi: 10.1080/13854046.2010.550634. doi: 10.1080/13854046.2010.550634. [DOI] [PubMed] [Google Scholar]
- Gordon I, Voos AC, Bennett RH, Bolling DZ, Pelphrey KA, Kaiser MD. Brain mechanisms for processing affective touch. Human Brain Mapping. doi: 10.1002/hbm.21480. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvits TV, Lasko NB, Schacter SC, Kuhne AA, Orr SP, Pitman RK. Neurological status of Vietnam veterans with chronic posttraumatic stress disorder. Journal of Neuropsychiatry and Clinical Neurosciences. 1993;5:183–188. doi: 10.1176/jnp.5.2.183. [DOI] [PubMed] [Google Scholar]
- Hartlage LC, Durant-Wilson D, Patch PC. Persistent neurobehavioral problems following mild traumatic brain injury. Archives of Clinical Neuropsychology. 2001;16:561–570. [PubMed] [Google Scholar]
- Heilbronner RL, Sweet JJ, Morgan JE, Larrabee GJ, Millis SR, Conference Participants American Academy of Clinical Neuropsychology consensus conference statement on the neuropsychological assessment of effort, response bias, and malingering. The Clinical Neuropsychologist. 2009;23:1093–1129. doi: 10.1080/13854040903155063. [DOI] [PubMed] [Google Scholar]
- Hofman PA, Stapert SZ, van Kroonenburgh MJ. MR imaging, single-photon emission CT, and neurocognitive performance after mild traumatic brain injury. American Journal of Neuroradiology. 2001;22:441–449. [PMC free article] [PubMed] [Google Scholar]
- Hoge C, Castro C, Messer S, McGurk D, Cotting D, Koffman R. Combat duty in Iraq and Afghanistan, mental health care problems, and barriers to care. New England J of Medicine. 2004;351:13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas J, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. The New England Journal of Medicine. 2008;358(5):453–463. doi: 10.1056/NEJMoa072972. doi:10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Horner MD, Hamner MB. Neurocognitive Functioning in Posttraumatic Stress Disorder. [Article]. Neuropsychology Review. 2002;12(1):15–30. doi: 10.1023/a:1015439106231. [DOI] [PubMed] [Google Scholar]
- Iverson GL. Outcome from mild traumatic brain injury. Current Opinion in Psychiatry. 2005;18:301–317. doi: 10.1097/01.yco.0000165601.29047.ae. [DOI] [PubMed] [Google Scholar]
- Iverson GL. Complicated vs uncomplicated mild traumatic brain injury: Acute neuropsychological outcome. [Article]. Brain Injury. 2006;20(13/14):1335–1344. doi: 10.1080/02699050601082156. [DOI] [PubMed] [Google Scholar]
- Iverson GL. Outcome from mild traumatic brain injury. Current Opinion in Psychiatry. 2005;18:301–317. doi: 10.1097/01.yco.0000165601.29047.ae. [DOI] [PubMed] [Google Scholar]
- Johnsen GE, Asbjornsen AE. Consistent impaired verbal memory in PTSD: A meta-analysis. Journal of Affective Disorders. 2008;111:74–82. doi: 10.1016/j.jad.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. McGraw Hill; 1991. [Google Scholar]
- Koenen KC, Driver KL, Oscar-Berman M, Wolfe J, Folsm S, Schlesinger L. Measures of prefrontal system dysfunction in post-traumatic stress disorder. Brain and Cognition. 2001;45:64–78. doi: 10.1006/brcg.2000.1256. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130(Pt 10):2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Kremen W, Koenen KC, Boake C, Purcell S, Eisen SA, Franz CE, Lyons MJ. Pretrauma cognitive ability and risk for posttraumatic stress disorder. Archives of General Psychiatry. 2007;64:361–368. doi: 10.1001/archpsyc.64.3.361. [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, et al. ALE meta-analysis workflows via the BrainMap database: Progress towards a probabilistic functional brain atlas. Frontiers in Neuroinformatics. 2009;3:1–11. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Lancaster JL, Fox PT. BrainMap: the social evolution of a human brain mapping database. Neuroinformatics. 2005;3(1):65–78. doi: 10.1385/ni:3:1:065. [DOI] [PubMed] [Google Scholar]
- Levin H, Kraus MF. The frontal lobes and traumatic brain injury. Journal of Neuropsychiatry and Clinical Neuroscience. 1994;6(4):443–454. doi: 10.1176/jnp.6.4.443. [DOI] [PubMed] [Google Scholar]
- Levin HS, Wilde E, Troyanskaya M, Petersen NJ, Scheibel R, Newsome M, et al. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. Journal of Neurotrauma. 2010;27:683–694. doi: 10.1089/neu.2009.1073. [DOI] [PubMed] [Google Scholar]
- Lezak M. Neuropsychological assessment. 3rd ed. Oxford University Press; New York: 1995. [Google Scholar]
- Litz BT, Weathers FW, Monaco V, Herman DS, Wulfsohn M, Keane TM. Attention, arousal, and memory in posttraumatic stress disorder. Journal of Traumatic Stress. 1996;9:497–520. doi: 10.1007/BF02103661. [DOI] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, et al. Detection of blast-related traumatic brain injury in U. S. military personnel. New England Journal of Medicine. 2011;364(22):2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklin ML, Metzger LP, Litz BT, McNally RJ, Lasko NB, Orr SP, Pitman RK. Lower Precombat Intelligence Is a Risk Factor for Posttraumatic Stress Disorder. Journal of Consulting and Clinical Psychology. 1998;66:323–326. doi: 10.1037//0022-006x.66.2.323. [DOI] [PubMed] [Google Scholar]
- Marx B, Brailey K, Proctor S, MacDonald H, Graefe A, Amorso P, Vasterling J. Association of time since deployment, combat intensity, and posttraumatic stress symptoms with neuropsychological outcomes following Iraq war deployment. Archives of General Psychiatry. 2009a;66:996–1004. doi: 10.1001/archgenpsychiatry.2009.109. [DOI] [PubMed] [Google Scholar]
- Marx BP, Doron-Lamarca S, Proctor SP, Vasterling PP. The influence of pre-deployment neurocgnitive functioning on post-deployment PTSD symptom outcomes among Iraq-deployed Army Soldiers. Journal of the international Neuropsychological Society. 2009b;15:840–852. doi: 10.1017/S1355617709990488. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Teshiba TM, Franco AR, Ling J, Shane MS, Stephen JM, Jung RE. Modeling conflict and error in the medial frontal cortex. Human Brain Mapping. doi: 10.1002/hbm.21405. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane AC, Weber DL, Clark CR. Abnormal stimulus processing in post-traumatic stress disorder. Biological Psychiatry. 1993;5:817–826. doi: 10.1016/0006-3223(93)90088-u. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Shin LM. Association of intelligence with severity of posttraumatic stress disorder symptoms in Vietnam combat veterans. American Journal of Psychiatry. 1995;152:936–938. doi: 10.1176/ajp.152.6.936. [DOI] [PubMed] [Google Scholar]
- Miotto EC, Cinalli FZ, Serrao VT, Benute GG, Lucia MCS, Scaff M. Cognitive deficits in patients with mild to moderate traumatic brain injury. Arquivos de Neuro-Psiquiatria. 2010;68(6):862–868. doi: 10.1590/s0004-282x2010000600006. doi: 10.1590/s0004-282x2010000600006. [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Anthony BJ, Duncan CC, Ahearn MB, Kellam SG. Analysis of the elements of attention: A neuropsychological approach. Neuropsychology Review. 1991;2:109–145. doi: 10.1007/BF01109051. [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Cooper DA, LaBar KS, McCarthy G. Neural systems for executive and emotional processing are modulated by symptoms of posttraumatic stress disorder in Iraq War Veterans. Psychiatry Research: Neuroimaging. 2008;162(1):59–72. doi: 10.1016/j.pscychresns.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Injury Prevention and Control . Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Centers for Disease Control and Prevention; Atlanta, GA: 2003. [Google Scholar]
- Nelson NW, Hoelzle JB, McGuire KA, Gerrier-Auerbach AG, Charlesworth MJ, Sponheim SR. Evaluation context impacts neuropsychological performance in OEF/OIF Veterans with reported combat-related concussion. Archives of Clinical Neuropsychology. 2010;25:713–723. doi: 10.1093/arclin/acq075. [DOI] [PubMed] [Google Scholar]
- Noggle CA, Davis AS, Barisa M. Neuropsychology and neuroimaging: Integrating and understanding structure and function in clinical practice. In: D'Amato RC, Hartlage LC, editors. Essentials of neuropsychological assessment: Treatment planning for rehabilitation. 2nd ed. Springer Publishing Co.; New York, NY US: 2008. pp. 79–102. [Google Scholar]
- Parslow RA, Jorm AF. Pretrauma and posttrauma neurocognitive functioning and PTSD symptoms in a community sample of young adults. The American Journal of Psychiatry. 2007;164:509–515. doi: 10.1176/ajp.2007.164.3.509. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Petrie EC, Cross DJ, Pagulayan K, McCraw K, Hoff D, et al. Cerebrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq war Veterans with persistent post-concussive symptoms. NeuroImage. 54(Supplement 1):S76–S82. doi: 10.1016/j.neuroimage.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remedy Health Media Healthcommunities.com [April 2011];“Traumatic Brain Injury (TBI) Overview, Incidence and Prevalence”. 2010 on http://www.neurologychannel.com/tbi/index.shtml.
- Ricker JH, Arenth PM. Functional neuroimaging in clinical neuropsychology. In: Morgan JE, Ricker JH, editors. Textbook of clinical neuropsychology. Psychology Press; New York, NY US: 2008. pp. 840–847. [Google Scholar]
- Rimel RW, Giordani B, Barth JT, Boll TJ, Jane JA. Disability caused by minor head injury. Neurosurgery. 1981;9:221–228. [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, Van der Zee EA, Lee S, McGaugh JL, McIntyre CK. Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdale. Journal of Neuroscience. 2009;29(45):14299–14308. doi: 10.1523/JNEUROSCI.3626-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld JV, Ford NL. Bomb blast, mild traumatic brain injury and psychiatric morbidity: A review. Injury. 2010;41(5):437–443. doi: 10.1016/j.injury.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Roy MJ, Francis J, Friedlander J, Banks-Williams L, Lande RG, Taylor P, et al. Improvement in cerebral function with treatment of posttraumatic stress disorder. Annals of New York Academy of Sciences. 2010;1208:142–149. doi: 10.1111/j.1749-6632.2010.05689.x. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Bernsten D, Johansen MK. A memory based model of posttraumatic stress disorder: Evaluating basic assumptions underlying the PTSD diagnosis. Psychological Review. 2008;115:985–1011. doi: 10.1037/a0013397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J. Neuropsychiatr. Clin. Neurosci. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schneiderman AI, Braver ER, Kang HK. Understanding Sequelae of Injury Mechanisms and Mild Traumatic Brain Injury Incurred during the Conflicts in Iraq and Afghanistan: Persistent Postconcussive Symptoms and Posttraumatic Stress Disorder. 2008 doi: 10.1093/aje/kwn068. [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, Shapiro AM. A quantitative review of the effects of traumatic brain injury on cognitive functioning. International Review of Psychiatry. 2003;15:545–561. doi: 10.1080/09540260310001606728. [DOI] [PubMed] [Google Scholar]
- Schuff N, Zhang Y, Zhan W, Lenoci M, Ching C, Boreta L, et al. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: An MRI study. NeuroImage. 2011;54(Supplement 1):S62–S68. doi: 10.1016/j.neuroimage.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev A, Ramot A, Akirav I. Stress hormones receptors in the amygdale mediate the effects of stress on the consolidation, but not the retrieval, of a non aversive spatial task. PLoS One. 2012;7(1):e29988. doi: 10.1371/journal.pone.0029988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple WE, Goyer PF, McCormick R, Compton-Toth B, Morris E, Donovan B, Schulz SC. Attention and regional cerebral blood flow in posttraumatic stress disorder patients with substance abuse histories. Psychiatry Research: Neuroimaging. 1996;67:17–28. doi: 10.1016/0925-4927(96)02735-7. [DOI] [PubMed] [Google Scholar]
- Sheppard SC, Malatras JW, Israel AC. The impact of deployment on U.S. military families. American Psychologist. 2010;65:599–609. doi: 10.1037/a0020332. [DOI] [PubMed] [Google Scholar]
- Simmons AN, Matthews SC. Neural circuitry of PTSD with or without mild traumatic brain injury: A meta-analysis. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.03.016. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- Spencer RJ, Drag LL, Walker SJ, Beiliauskas LA. Self-reported cognitive symptoms following mild traumatic brain injury are poorly associated with neuropsychological performance in OEF/OIF veterans. Journal of Rehabilitation Research & Development. 2010;47(6):521–530. doi: 10.1682/jrrd.2009.11.0181. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, McGuire KA, Kang SS, Davenport ND, Aviyente S, Bernat EM, et al. Evidence of disrupted functional connectivity in the brain after combat-related blast injury. NeuroImage. 2011;54(Supplement 1):S21–S29. doi: 10.1016/j.neuroimage.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psych. Rev. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stroop Campbell, T. A., Nelson LA, Lumpkin R, Yoash-Gantz RE, Pickett TC, McCormick CL. Neuropsychological measures of processing speed and executive functioning in combat veterans with PTSD, TBI, and comorbid TBI/PTSD. Psychiatric Annals. 2009;39(8):796–803. doi: 10.3928/00485713-20090728-01. [Google Scholar]
- Sullivan K, Krengel M, Proctor SP, Devine S, Heeren T, White RF. Cognitive functioning in treatment-seeking Gulf War veterans: Pyridostigmine bromide use and PTSD. Journal of Psychopathology and Behavioral Assessment. 2003;25:95–103. [Google Scholar]
- TBIlaw.com. [January 29, 2012];Congressional NFL hearings: Dr. Randall Benson testifies about neuroimaging advances-Susceptibility weighted imaging. In www.tbilaw.com/blog/2010/01/congressional-nfl-hearings-dr-ronald-benson-testifies-about-neuroimaging-advances-susceptibility-weighted-imaging.html.
- Tanielian T, Jay LH. Invisible wounds of war: Psychological and cognitive injuries, their consequences, and services to assist recovery. RAND Corporation; CA: 2008. [Google Scholar]
- Taylor PA, Ford CC. Simulation of blast-induced early time intracranial wave physics leading to traumatic brain injury. J Biomech Eng. 2009;131:061007. doi: 10.1115/1.3118765. [DOI] [PubMed] [Google Scholar]
- Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. Journal of Head Trauma Rehabilitation. 2009;24(1):14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- Uddo M, Vasterling JJ, Brailey K, Sutker PB. Memory and attention in combat-related post-traumatic stress disorder (PTSD). Journal of Psychopathology and Behavioral Assessment. 1993;15:43–52. [Google Scholar]
- Van Boven RW, Harrington GS, Hackney DB, Ebel A, Gauger G, Bremner JD, et al. Advances in neuroimaging of traumatic brain injury and posttraumatic stress disorder. Journal of Rehabilitation Research & Development. 2009;46:717–756. doi: 10.1682/jrrd.2008.12.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderploeg RD, Belanger HG, Curtiss G. Mild Traumatic Brain Injury and Posttraumatic Stress Disorder and Their Associations With Health Symptoms. Archives of Physical Medicine & Rehabilitation. 2009;90(7):1084–1093. doi: 10.1016/j.apmr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Brailey K, Constans JI, Borges A, Sutker PB. Assessment of intellectual resources in Gulf War veterans: Relationship to PTSD. Assessment. 1997;4:51–59. [Google Scholar]
- Vasterling JJ, Brailey K, Constans JI, Sutker PB. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- Vasterling Brailey, K., Sutker PB. Olfactory identification in combat-related posttraumatic stress disorder. Jounral of Traumatic Stress. 2000;13:241–253. doi: 10.1023/A:1007754611030. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN, Jr., Sutker PB. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Brailey K. Neuropsychological findings in adults with PTSD. In: Vasterling JJ, Brewin CR, editors. Neuropsychology of PTSD: Biological, cognitive, and clinical perspectives. Guilford Press; New York: 2005. pp. 178–207. [Google Scholar]
- Vasterling JJ, Proctor SP, Amoroso P, Kane R, Heeren T, White RF. Neuropsychological Outcomes of Army Personnel Following Deployment to the Iraq War. JAMA: Journal of the American Medical Association. 2006;296(5):519–529. doi: 10.1001/jama.296.5.519. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Verfaellie M. Posttraumatic stress disorder: A neurocognitive perspective. Journal of the International Neuropsychological Society. 2009;15:826–829. doi: 10.1017/S1355617709990683. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Verfaellie M, Sullivan KD. Mild traumatic brain injury and posttraumatic stress disorder in returning veterans: Perspectives from cognitive neuroscience. Clinical Psychology Review. 2009;29:674–684. doi: 10.1016/j.cpr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Nelson EE, Scaramozza M, Waldeck T, Hazlett G, Southwick SM, et al. Reward circuitry in resilience to severe trauma: An fMRI investigation of resilient special forces soldiers. Psychiatry Research: Neuroimaging. 2009;172(1):75–77. doi: 10.1016/j.pscychresns.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden DL, French LM, Shupenko L, Fargus J, Riedy G, Erickson ME, et al. Case report of a soldier with primary blast brain injury. NeuroImage. 2009;47(Supplement 2):T152–T153. doi: 10.1016/j.neuroimage.2009.01.060. [DOI] [PubMed] [Google Scholar]
- Whitney KA, Shepard PH, Williams AL, Davis JJ, Adams KM. The Medical Symptom Validity Test in the evaluation of Operation Iraqi Freedom/Enduring Freedom soldiers: A preliminary study. Archives of Clinical Neuropsychology. 2009;24:145–152. doi: 10.1093/arclin/acp020. [DOI] [PubMed] [Google Scholar]
- Williams LM, Liddell BJ, Kemp AH, Bryant RA, Meares RA, Peduto AS, Gordon E. Amygdala-prefrontal dissociation of subliminal and supraliminal fear. Human Brain Mapping. 27(8):652–661. doi: 10.1002/hbm.20208. [DOI] [PMC free article] [PubMed] [Google Scholar]