Abstract
Objective
Short-term weight loss is accompanied by bone loss in postmenopausal women. The longer-term impact on bone in the reduced overweight/obese woman compared to those who regain their weight was examined in this study using a case-control design.
Methods
Postmenopausal women (n = 42, body mass index of 28.3 ± 2.8 kg/m2; 60.7 ± 5.5 y) were recruited 2 years after the start of a 6 month weight loss trial and those who maintained their weight (WL-M) were matched to a cohort who regained weight (WL-R). Serum hormones and bone markers were measured in a subset. Bone mineral density (BMD) at the femoral neck (FN), trochanter, spine, radius, and total body and soft tissue composition were taken at baseline, 0.5 and 2 years.
Results
During WL, both groups lost 9.3 ± 3.4% body weight with no significant difference between groups. After weight loss, weight change was −0.1 ± 2.7 % and 6.0 ± 3.3% in the WL-M (n=22) and WL-R (n=20) groups, respectively. After 2 years, both groups lost BMD at the FN and trochanter (p ≤ 0.01), whereas only the WL-M group reduced BMD at the 1/3 radius (p < 0.001). There was a greater BMD loss at the trochanter (−6.8 ± 5.7%) and the 1/3 radius (−4.5 ± 3.3%) in the WL-M compared to the WL-R group after 2 years. Multiple linear regression showed that change in leg fat mass (but not trunk fat) contributed to trochanter BMD loss (p <0.05).
Conclusions
After 2 years, there is no BMD recovery of weight reduction-induced bone loss, irrespective of weight-regain. These data suggest that the period after weight loss may be an important point in time to prevent bone loss for both those who maintain or regain weight.
Keywords: Bone, body composition, caloric restriction, postmenopausal, weight loss, weight cycle
INTRODUCTION
Obese and overweight people are strongly encouraged to lose 5–15% weight to reduce the risk of co-morbid conditions. During the menopausal transition, women tend to gain weight and visceral adiposity, and therefore many of these women who were never overweight previously, are now encouraged to reduce weight to improve health. However, studies show that weight loss in even obese and overweight women results in loss of bone mineral density (BMD) of approximately 1–2% with 10% weight reduction, and can partially be attenuated with certain interventions (1–4). Loss of bone due to weight reduction is more consistently shown in older compared to younger individuals (4–7). In large retrospective studies, weight reduction or variability has been associated with higher hip bone loss and fracture, whether it was voluntary or involuntary weight loss (8–10).
Overweight and obese individuals who lose weight either maintain a reduced-obese state or regain the lost weight primarily as fat mass (11). Women who are restrained eaters who chronically diet, or who “weight-cycle” (weight loss, followed by regain) may be at greater risk of low bone mass or osteoporosis (10;12;13). A limited number of previous trials have examined the response to weight regain after weight reduction in a single group of women to show that it leads to partial recovery of bone at some anatomical sites (14–17). In this trial, we use a case-control design to examine whether the rate of bone loss differs in reduced-obese postmenopausal women who maintain their lost weight for two years compared to those who regain weight.
METHODS
Participants
Postmenopausal women who successfully completed a 6-month weight loss protocol in our laboratories were eligible for recruitment for this study. Participants were contacted approximately two years after initial inclusion in a 6-month weight reduction program that was either reported previously (1;2;18) or who were part of small unpublished pilot studies from 2002–2008. In order to be eligible, postmenopausal women had to be healthy without evidence of osteoporosis, metabolic bone disease, thyroid disorders, immune disease, heart attack or stroke in the past 6 months, kidney stones, diabetes, active cancers, or cancer therapy within the past 12 months. Participants were excluded if they changed their usual daily intake of supplemental calcium or multi-vitamin/mineral, started a new exercise program, or were on medications known to influence bone metabolism including hormone replacement therapy. These studies were approved by the Rutgers University Institutional Review Board and all participants signed an informed consent.
Protocol
Participants were measured at three time points; baseline (time 0), six months of weight reduction (0.5 y), and final (2 y). During the weight loss (0–0.5 y) period, participants underwent 6 months of weight loss interventions in our laboratories. In this protocol, participants were counseled once weekly for the first 2 months and then twice monthly thereafter, by a registered dietitian to reduce usual intake by 500–600 kcal/d, while maintaining usual physical activity levels, described previously (1;2;18). During the 6 month intervention, volunteers were given a multivitamin containing 400 IU Vitamin D and total calcium intake was at least 1000 mg/d in all women. Upon completion of the intervention, all participants were counseled to consume approximately 1.2 g Ca and 400 IU Vitamin D daily, through diet and supplementation. Following weight loss, there was a no intervention period (0.5–2.0 y) and participants were categorized according to weight change for this final measurement. Women were recruited for those who maintained their weight and then were age matched to a cohort who did not meet these criteria and regained their body weight (WL-R) in a case control design. To be eligible for the weight-loss maintainer (WL-M) group, weight regain needed to be less than 25%, whereas the regainers (WL-R) were defined as those who regained more than this amount.
Bone and body composition measurements and serum markers
Weight and height was measured to the nearest 0.25 kg and 0.25 cm, respectively at baseline, after weight loss (0.5 year), and at the two year final measurement with a balance beam scale and stadiometer, respectively; (Detecto, Webb City, MO). Bone mineral density was measured at the femoral neck, trochanter, spine, total body, and 1/3 and ultradistal (UD) radius by dual energy x-ray absorptiometry (DXA, GE Lunar, Madison, WI, USA; coefficient of variation <1% for all sites). Scans were performed by using enCORE 2004 software (version 8.10.027; GE Lunar). Bone mineral content at each site was also measured. Fat free soft tissue (FFST), total fat mass, trunk fat and leg fat were measured by DXA using total body scans and the manufacturer’s standard cut lines for leg and trunk regions. Calcium intake was estimated using 3-day food records and analyzed using the USDA data base (Food Works Software 10.1, Long Valley, NJ).
Fasting morning blood samples were collected in the entire population at baseline and in a subset (n=22) after 2 years. Bone formation markers, osteocalcin (OC) (BTI; Stoughton, MA, CV < 9%) was measured by RIA. Serum N-telopeptide of type I collagen (NTx) was measured by ELISA (Osteomark; Princeton, NJ, CV <4.6%). Intact PTH, 25OHD and estradiol were analyzed by radioimmunoassay (RIA). The CV was <6.8% for PTH (DSL, TX; Scantibodies, CA), <12.5% for 25OHD (DiaSorin, MI), < 12.2 % for estradiol (DSL, Webster, TX). Our laboratory participates in Vitamin D external quality assessment scheme (DEQAS) to monitor the performance of the RIA used for assessment of 25OHD.
Statistical Analysis
Changes in body composition, hormones and bone markers at the three time points (baseline, 0.5 and 2 years) between the groups (WL-R and WL-M) were analyzed by a two-factor repeated measures ANOVA, and test if the F test was significant post-hoc analysis was performed using Tukey’s pairwise multiple comparison. Changes in bone and markers between groups from baseline to final measurement were analyzed using one-way ANOVA. Annual BMD loss was determined at each site by dividing the percent change in BMD by the number of months during the entire study period for each individual. Multiple regression analysis was used to assess how the change in independent variables (age, leg fat, trunk fat, FFST) over time influenced change in BMD at each site. To determine whether weight loss and weight regain will have a similar effect on BMD, a power analysis was performed with α set at 0.05, with the value of β set at 0.90 using trochanter BMD change during weight loss or maintenance (5), This analysis indicated that 15 participants per group would be necessary to avoid a type II error, and we included at least 5 additional participants per group to account for two possible baseline covariates. Values are expressed as mean ± SD except in figures that include standard error of the mean to improve visual clarity. Analysis was performed with SAS statistical software (SAS Institute Inc. 9.2, Cary, NC).
RESULTS
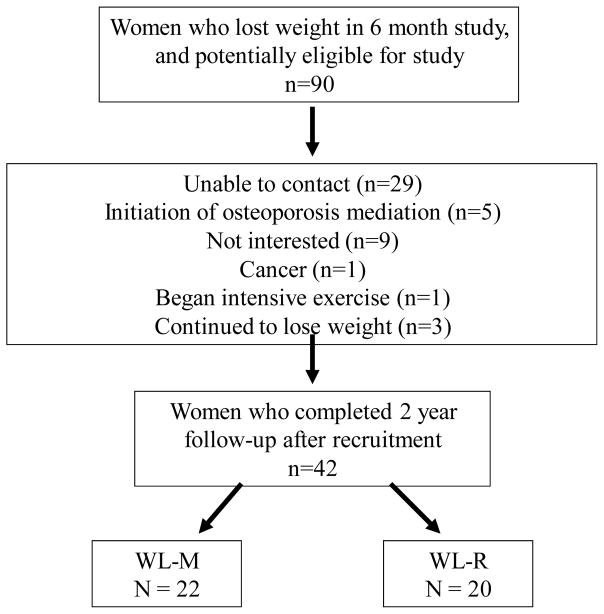
Ninety women who previously completed weight loss interventions in our laboratory were contacted for this study. Sixty-one responded to these inquiries and nineteen were excluded (due to initiation of osteoporosis medications, initiation of a rigorous exercise program, diagnosis with cancer, initiation of medication for diabetes or declined to participate) (Figure 1). Forty-two postmenopausal women agreed and met criteria to be included in this follow-up study. Participants were Whites (n=40) and African Americans (n=2). Measurements were taken at an average of 22 ± 6 months after initiation of weight loss. At baseline, there were no significant differences in age, weight, BMI, years since menopause, bone, or soft tissue data between groups (Table 1). In both groups of women, 12–15% had surgical or drug-induced menopause. For the women who either refused to participate or could not be contacted, we analyzed a subset (n=31), and their age, BMI and weight loss did not differ from those included in this study.
Figure 1.
Recruitment Flow Chart
Table 1.
Baseline and percent change women who lost weight and either maintained (WL-M) or regained the weight (WL-R)
| WL-M (n=22) | WL-R (n=20) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Time period | Baseline1 | 6-mo | 2 years | Baseline | 6-mo | 2 years | |
|
| |||||||
|
% Change
|
% Change
|
||||||
| Age (years) | 61.3 ± 5.6 | 60.1 ± 5.2 | |||||
| YSM (years) | 10.5 ± 7.7 | 11.4 ± 7.3 | |||||
| BMI ( kg/m2) | 27.7 ± 2.6 | 28.9 ± 3.0 | |||||
| Body Weight (kg) | 73.1 ± 7.1 | −10.2 ± 3.2 † | −10.3 ± 4.2 † | 75.3 ± 7.0 | −8.2 ± 3.3 † | −2.8 ± 3.3 † ** | |
| FFST (kg) | 36.0 ± 3.3 | −3.3 ± 4.3 † | −3.8 ± 5.5 † | 36.4 ± 3.9 | −3.4 ± 5.5 † | −1.9 ± 6.4 | |
| Fat Mass (kg) | 30.7 ± 4.8 | −18.2 ± 8.7 † | −16.6 ± 10.5 † | 33.0 ± 5.1 | −13.6 ± 6.7 † | −5.4 ± 6.6 † ** | |
| Trunk Fat (kg) | 13.1 ± 2.4 | −18.2 ± 14.0 † | −19.1 ± 17.4 † | 14.5 ± 2.5 | −14.1 ± 6.7 † | −4.9 ± 11.5** | |
| Leg Fat (kg) | 10.8 ± 1.9 | −13.9 ± 11.1 † | −16.9 ± 13.0 † | 11.2 ± 2.4 | −12.0 ± 7.6 † | −2.5 ± 9.9** | |
|
| |||||||
| BMD (g/cm2) | |||||||
| Femoral Neck | 0.86 ± 0.11 | −0.5 ± 3.1 | −2.9 ± 4.3† | 0.87 ± 0.09 | −0.1 ± 3.2 | −2.2 ± 3.3† | |
| Trochanter | 0.73 ± 0.11 | −4.1 ± 4.4 † | −6.8 ± 5.7 † | 0.75 ± 0.11 | −2.6 ± 4.7 † | −2.5 ± 6.1 †** | |
| UD-Radius | 0.31 ± 0.04 | −1.5 ± 3.9 † | −1.0 ± 4.6 | 0.32 ± 0.05 | 0.7 ± 3.4 * | −2.0 ± 3.9 | |
| 1/3 Radius | 0.63 ± 0.09 | −1.8 ± 2.3 † | −4.5 ± 3.3 † | 0.64 ± 0.07 | −0.9 ± 2.9 | −1.6 ± 3.8 †** | |
| Spine | 0.99 ± 0.09 | −3.3 ± 5.3 † | −1.3 ± 6.4 | 0.99 ± 0.13 | −2.9 ± 4.7 † | −2.5 ± 6.3 † | |
| Total Body | 1.11 ± 0.08 | −1.1 ± 1.8 † | −1.5 ± 1.7 † | 1.12 ± 0.08 | −0.4 ± 1.7 | −0.5 ± 1.9* | |
|
| |||||||
| BMC (g) | |||||||
| Femoral Neck | 4.40 ± 0.83 | −1.3 ± 6.2 | −1.4 ± 5.2 | 4.34 ± 0.43 | 1.4 ± 5.4 | −1.5 ± 6.2 | |
| Trochanter | 8.48 ± 1.82 | −2.3 ± 8.8 | −6.0 ± 9.0 † | 8.97 ± 2.24 | −3.5 ± 8.8 | −1.3 ± 10.7** | |
| UD-Radius | 1.11 ± 0.16 | −1.3 ± 9.1 | −4.7 ± 12.4 | 1.13 ± 0.17 | 1.7 ± 12.1 | −4.1 ± 15.7 | |
| 1/3 Radius | 1.57 ± 0.21 | −2.8 ± 2.6 † | −4.1 ± 3.2 † | 1.53 ± 0.19 | −1.8 ± 4.3 † | −1.3 ± 5.6** | |
| Total Body | 2276 ± 271 | −1.6 ± 5.3 | −2.7 ± 5.1 † | 2309 | ± 257 | −0.6 ± 3.9 | −1.7 ± 4.3 |
Data are mean ± S.D
No significant differences among groups were observed for any variable at baseline.
Differs from WL-M group, p < 0.05;
p≤0.09 (same time period);
Differs from baseline, p < 0.05;
p ≤ 0.09
Abbreviations: WL-M (weight loss maintained); WL-R (weight loss and then regained); 0 - 0.5 y, weight loss; 0 – 2 y, baseline to final; BMD , bone mineral density; FFST, fat free soft tissue; UD, ultradistal; YSM, years since menopause
Dietary intake
Baseline total calcium intake (diet and supplement) was 948 ± 352 mg/d and did not differ significantly between the WL-M and WL-R groups. In the parent study, (0–6 mo) there were equal number (n=13) of women assigned to normal Ca intake in each weight loss group, and the remaining were assigned to the higher Ca intake in the WL-M (n=9) and WL-R (n=7) groups. Total calcium intake during the weight loss intervention was 1275 ± 406 mg/d in the WL-M group (n=22) and 1194 ± 389 mg/d in the WL-R group (n=20) and did not differ significantly between the two groups. In addition, total calcium intake after 2 years (1211 ± 512 mg/d) also did not differ between groups. Participants consumed 400 IU/d of vitamin D in their multi-vitamin throughout the 2 year period, and a small amount in their diet during active weight loss (64 ± 43 IU/d) and during the no intervention period (88 ± 84 IU/d) that did not vary significantly between groups.
Body Weight and soft tissue
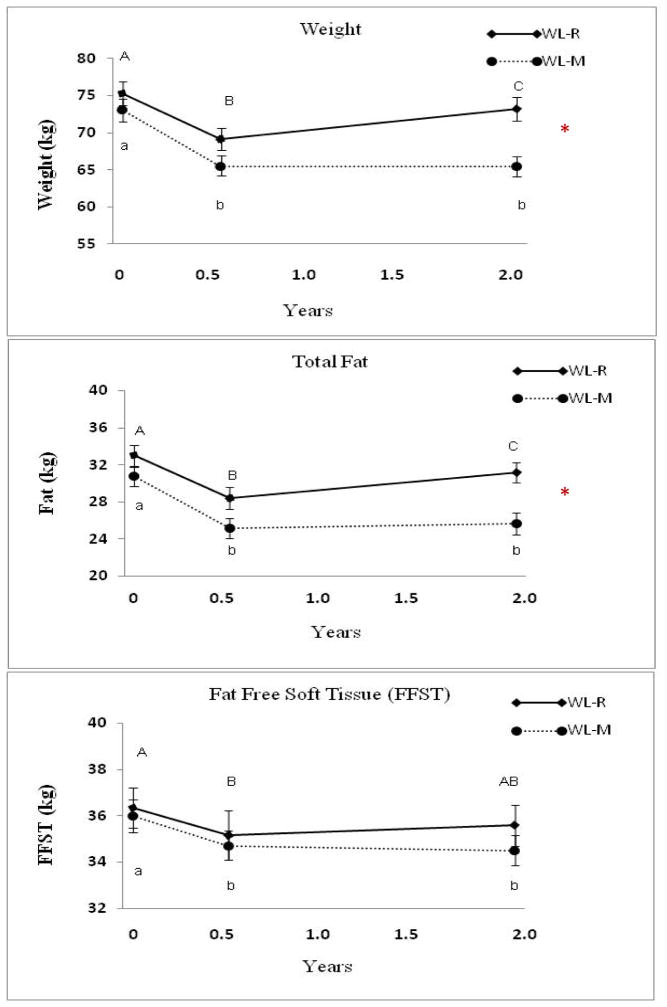
After the six month weight loss protocol, there were no significant differences in weight loss (9.3 ± 3.5%) in the WL-R and WL-M groups. However, weight loss differed significantly between groups over two years (Table 1). During the post-weight loss period (6 months to 2 years), the WL-M group lost an average of 0.1 ± 2.0 kg while the WL-R group gained 4.1 ± 2.3 kg (p ≤ 0.0001; Figure 2). There were also significant differences between groups for total, trunk and leg fat, with greater gain in the WL-R compared to WL-M group (p < 0.01) (Table 1). Fat-free soft tissue decreased after 2 years in all women (−1.2 ± 2.2 kg or −2.9 ± 5.6%), and did not differ significantly between groups. Weight and fat mass (total, trunk, leg) were significantly different between the groups at 2 years (p<0.001) with higher values in the WL-R group compared to WL-M group.
Figure 2.
Weight (kg), fat and fat-free soft tissue at baseline, the end of the weight loss intervention (0.5 y) and after 2 years in the Weight Loss Maintenance (WL-M, n= 22) and Weight Loss Gain (WL-R, n=20) groups. Data (Mean ± SEM). * Repeated Measures ANOVA differs between groups, p < 0.05. The only time point that differed between groups was at 2 years for weight and fat (p < 0.001).
Values with different letters are significantly different, p < 0.05. Upper and lower case letters are used to denote WL-R and WL-M, respectively.
Bone mineral density and content and serum markers
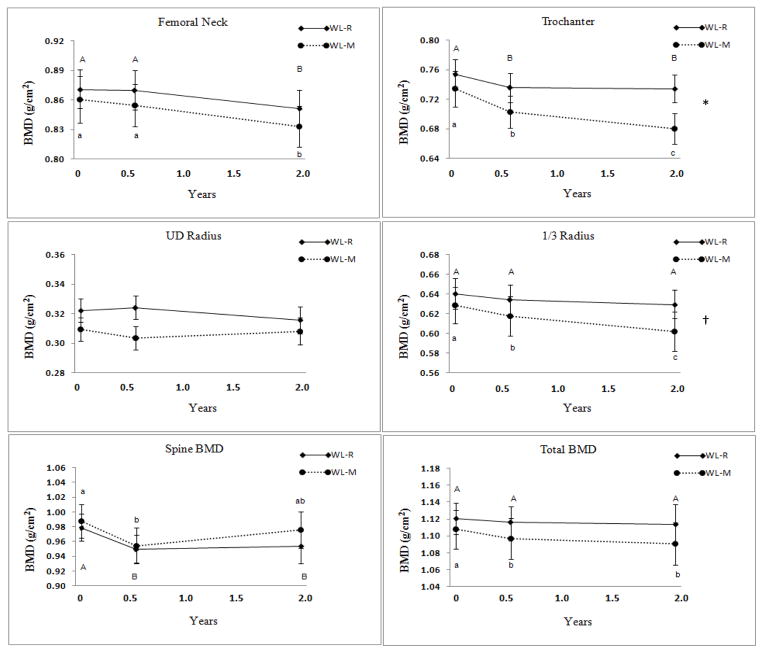
After 6 months of weight loss, BMD decreased in both groups (p ≤ 0.01) at the trochanter (−3.3 ± 4.6 %) and spine (−3.1± 5.8%). In the WL-M group, BMD also decreased at the 1/3 radius and the total body (Table 1). Bone mineral content decreased only at the 1/3 radius for both groups during the 6 months of weight loss. While there was a trend for greater BMD loss at the UD radius during the weight loss period in the WL-M compared to the WL-R group, no other sites showed differences between groups (Table 1).
During the post-intervention period (6 months to 2 years), there was a further decrease in BMD at the femoral neck in both groups (Figure 3). The trochanter and 1/3 radius BMD continued to decrease during the post-intervention period in the WL-M group. Repeated measures ANOVA showed significantly greater BMD loss over time (2 years) between groups at the trochanter (P < 0.02) and a trend at the 1/3 radius (Table 1, Figure 3). Over 2 years, BMD decreased significantly at most sites and in both groups (Figure 3). Bone mineral content decreased more at the 1/3 radius and trochanter in the WL-M than WL-R group (p < 0.05) after 2 years (Table 1).
Figure 3.
Bone mineral density (BMD) at baseline, the end of the weight loss intervention (0.5 y) and the follow-up (2 y) in theWeight Loss Maintenance (WL-M, n= 22) and Weight Loss Gain (WL-R, n=20) groups. (Mean ± SEM); * Repeated Measures ANOVA differs between groups, (P < 0.02); † p < 0.08. There are no significant between group differences at any time point.
Values with different letters are significantly different. Upper and lower case letters are used to denote WL-R and WL-M, respectively.
Bone turnover markers and hormone values did not differ between the groups at baseline in the entire population or subset (Supplemental Table 1). Over the 2 year period, the bone resorption marker, serum NTX, increased more in the WL-M than WL-R group (p < 0.05). In addition, serum PTH tended to increase more in the WL-M than WL-R group (p < 0.09). Changes in serum 25OHD, estradiol and osteocalcin did not differ between the WL-M and WL-R groups.
Predictors of the change in BMD over 2 years
In order to identify how age and soft tissue influence BMD, we examined whether leg and trunk fat or fat-free soft tissue was important in predicting the relationship using multiple regression analyses (Table 2). Age and body composition (leg or trunk fat or fat-free soft tissue) served as explanatory variables for each of the dependent variables. As expected, age did not have a significant independent association with bone loss since there was a relatively narrow age range (53–72 y) (Table 2). The changes in trochanter BMD were largely explained by changes in leg fat (p <0.05) and also tended to be explained by fat-free soft tissue (p < 0.07). Similarly leg fat tended to explain changes in total BMD (p <0.08).
Table 2.
Multiple Regression Model of relative contribution of age and change in body composition on change in BMD for all women over 2 years (n=42)1
| Variable (g/cm2)1 | Explanatory Variable2 | β coefficient | P value | Model R2 (%) |
|---|---|---|---|---|
| Trochanter BMD | Fat-free soft tissue | 0.3250 | 0.066 | 19.5 |
| Leg Fat | 0.3370 | 0.043 | ||
| Age | −0.0511 | 0.749 | ||
| Trunk fat | 0.0072 | 0.998 | ||
| Total BMD | Fat-free soft tissue | −0.206 | 0.558 | 14.9 |
| Leg Fat | −0.302 | 0.073 | ||
| Age | 0.063 | 0.808 | ||
| Trunk fat | 0.058 | 0.836 |
Variables without significant findings or trend are not shown (femoral neck, total spine, UD radius, 1/3 radius).
DISCUSSION
It is well established that weight reduction leads to the loss of bone (19), and this study was designed to determine whether it continues differently in women who maintain or regain the lost weight. We show that overweight and obese postmenopausal women who undergo ~10% weight reduction and regain ~70% of the weight over 18 months, have attenuated trochanter and 1/3 radius BMD loss compared to those who maintain a reduced body weight. In women who regain weight, the bone lost during weight reduction is permanent, but at 2 years, it does not exceed the 0.5–1% rate of annual BMD loss expected depending on the site due to normal aging (20–25). These findings suggest that only those women who undergo short-term weight reduction and maintain the lost weight for two years, continue to lose more BMD at several sites than those who regain the weight.
A few previous studies have addressed the influence of weight loss and weight regain on site-specific BMD (14–17). The results of these studies have been contradictory, possibly due to mixed genders, younger ages of the participants or shorter study designs (14–16) . In one study, pre-menopausal women were examined during a 3-month very low energy diet (13.2 kg loss), followed by 9 months of randomized controlled walking, and then followed for 2 years after 62% weight regain (15). The authors report reduced lumbar spine and femoral neck BMD over 2 years for the entire group, but neither site correlated with weight change (15). Avenell et al (14) studied BMD in 16 postmenopausal women for 6 months of weight loss followed by 6 months of complete weight regain and found greater loss of BMD at the lumbar spine, but not femoral neck, than weight-stable women (14). In another study, 16 frail obese older adults were followed up 30 months after a one year weight loss trial, and it was found that weight remained below baseline and that hip BMD decreased, but there no change in lumbar spine and whole body BMD (17). In this same study, physical performance and metabolic profile remained improved after 30 months (17). In one other study, 23 postmenopausal women were examined one year after 6 months of 5% weight loss (16). The decrease in lumbar spine and hip BMD due to weight loss showed no further decrease during 1 year of weight regain (93% fat mass gain). It was concluded that bone did not recover with weight regain. However, the absence of “bone recovery” is less surprising since it would not be expected with fat mass gain (16) and in fact BMD loss would be expected due to aging. Our findings show that not only is there no bone recovery with weight regain, but that BMD loss continues or begins at some bone sites. For example, there was no femoral neck BMD loss after 6 months, but there was a significant 2% loss after 2 years. This delayed response to weight loss may be due to the bone remodeling transient that may take up to 2 years to complete (26). The annual bone loss in weight-stable postmenopausal women is up to 1%/year (20–25), which is similar to those who regain their weight in the current trial showing loss at the femoral neck (−1.1%/y), trochanter (−1.3%/y) and UD radius (−1.0%/y). In addition, women who successfully maintained a lower body weight for 2 years (WL-M group) showed an even higher annual BMD loss at the trochanter (~3.3%/y) and 1/3 radius (−2.5%/y). There is also elevated bone resorption at 2 years for women who maintained weight loss compared to those who regained body weight and this is consistent with findings of higher bone resorption shown 9 months after a short-term (3 mo) weight loss (27). These findings clarify that bone loss is permanent and continues at a faster rate for at least 2 years for those who do not regain weight. In addition, while the current study observed only one weight loss cycle, bone loss would be expected to be greater with subsequent weight cycling, as shown in rodents (12) . Furthermore, retrospective studies show that multiple episodes of weight loss and regain (weight cycle) increase fracture risk at some sites (10;15;28;29) which would suggest that even with a normal annual rate of bone loss observed in the “regain” group over 2 years, bone quality may be compromised. It is possible that the bone loss during the post-weight loss period is delayed due to the bone remodeling transient or systemic factors altered by lowering body weight (19;26), yet it should be noted that some anatomical sites showed faster, rather than slower bone loss, during this post-weight loss period.
The current study demonstrates that both a loss of fat-free soft tissue and leg fat is a predictor of bone loss, whereas there was no relationship with trunk fat, representing a site with greater visceral fat. One recent three month weight loss study in young men and women (30) examined how soft tissue compartments explain BMD loss with and without six months of weight regain. The authors conclude that changes in soft tissue composition had a little contribution to changes in BMD with weight loss (30). However, there was no bone loss with weight loss in these participants, possibly due to the short-term intervention and/or the young age of the participants (19), so understanding how loss of bone is regulated by soft tissue changes could not be addressed. Our results are encouraging because they suggest that fat loss in a predominantly visceral region is not associated with bone loss. Nevertheless, this hypothesis using DXA technology should be confirmed using magnetic resonance imaging and/or quantitative computed tomography to distinguish between different fat depots. Also, it is possible that loss of fat-free soft tissue is exacerbated with each weight loss period (31), especially in older individuals (32), and this at least partially explains the lower BMD found in weight-cyclers.
This study is limited in its interpretation since it is a case-control design rather than randomized controlled trial such that women in the two groups were self-selected. In addition, BMD measurement errors are a concern in the obese population due to excess and homogeneity of fat tissue surrounding the bone (33–36) and/or due to changes in soft tissue with weight reduction. However, there are a few reasons that we are less concerned about this potential error. First, the results show bone changes at both central and peripheral bone sites that have more and less adiposity, respectively. In addition, adipose tissue change in this moderate weight loss/regain study is less than in studies showing bone measurement errors (33;34). For example, it has been found that 6 kg or more of fat layering on the region being measured (spine or hip) will artificially increase BMD, but the error was not found using smaller amounts of fat (37). In the current study, total body fat gain in the wt regain group was only 2.8 kg and therefore was well below the threshold where error would be expected, and the BMD loss during weight stability (0.5–2 years) in the WL-M group cannot be attributed to changes in fat tissue. In addition, the absence of a control weight maintenance group is also a limitation, however age related bone loss has been extensively studied in the literature (20–25) and thus this is a smaller concern.
One strength of this study is that participants were measured on identical instrumentation with the same certified radiology technician over 2 years for all the three measurements. Also, although participants had different calcium supplementation assignments during the six month weight loss, intake was at or above the recommended level in all individuals, there was a relatively even distribution of both levels of calcium between groups, and both had similar calcium intake during months 6–24. It is also possible since this dataset is biased since it excludes certain women who were not eligible for follow-up (i.e., 5 women were excluded due to initiation of osteoporosis medication and 9 who were not interested). However, it was encouraging that nearly all who were contacted agreed to participate if they met the inclusion criteria, and those who we were unable to contact or did not participate had a similar age, BMI and weight loss to those included in the study. One further limitation is that we did not monitor physical activity during months 6–24 and depended on participants to remember whether there were any extreme changes in physical activity (the reason for exclusion of one woman during screening). It would be expected that those who maintained their body weight (vs. regained) would have been more physically active to maintain their lower weight and this would have a beneficial effect on bone, yet BMD loss was greater or similar in the group who successfully maintained weight loss.
CONCLUSIONS
This study shows that weight reduction-induced bone loss that is apparent immediately after weight loss either continues or first begins at the trochanter, femoral neck and radius in postmenopausal women. Weight regain does not result in recovery of bone, but it does prevent greater loss at the trochanter and at 1/3 radius compared to reduced-obese women. It should be noted that even after weight loss, about 50% women in the WL-M group still met the overweight criteria. Thus, weight loss would be recommended for these individuals who typically would not be considered at risk of osteoporosis based on their overweight status. There is concern that repeated dieting would further bone loss and osteoporosis risk in this population compared to obese women who have been weight-stable, possibly due to poor bone quality (38)(39) but specific studies have not been conducted. Hence, questions about weight history should be considered when evaluating risk of osteoporosis. Current recommendations appropriately encourage weight loss in overweight individuals to reduce the risk of co-morbidities. Until future prospective studies address how to prevent bone loss after successful weight reduction, therapies that have been shown to ameliorate bone loss during weight reduction such as adequate calcium and vitamin D, a higher protein intake and increased bone-loading exercise should also be encouraged after weight stabilizes. Future studies determining whether the BMD changes are in cortical or trabecular bone or affect geometry after weight loss remains an important question in the prevention of fracture risk.
Supplementary Material
Acknowledgments
Funding support: NIH AG12161
We wish to thank Drs. Riedt and Cifuentes who helped to recruit and conduct the weight loss protocol in these volunteers, and the volunteers for their commitment to these studies.
Footnotes
Conflicts of interest: None
Reference List
- 1.Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res. 2005;20:455–63. doi: 10.1359/JBMR.041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sukumar D, Ambia-Sobhan H, Zurfluh R, Schlussel Y, Stahl TJ, Gordon CL, et al. Areal and volumetric bone mineral density and geometry at two levels of protein intake during caloric restriction: A randomized, controlled trial. J Bone Miner Res. 2011;26:1339–1348. doi: 10.1002/jbmr.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villareal DT, Shah K, Banks MR, Sinacore DR, Klein S. Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: a one-year randomized controlled trial. J Clin Endocrinol Metab. 2008;93:2181–2187. doi: 10.1210/jc.2007-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gozansky WS, Van Pelt RE, Jankowski CM, Schwartz RS, Kohrt WM. Protection of bone mass by estrogens and raloxifene during exercise-induced weight Loss. J Clin Endocrinol Metab. 2005;90:52–59. doi: 10.1210/jc.2004-0275. [DOI] [PubMed] [Google Scholar]
- 5.Riedt CS, Schlussel Y, von Thun N, Ambia-Sobhan H, Stahl T, Field MP, et al. Premenopausal overweight women do not lose bone during moderate weight loss with adequate or higher calcium intake. Am J Clin Nutr. 2007;85:972–980. doi: 10.1093/ajcn/85.4.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shapses SA, Von Thun NL, Heymsfield SB, Ricci TA, Ospina M, Pierson RN, Jr, et al. Bone turnover and density in obese premenopausal women during moderate weight loss and calcium supplementation. J Bone Miner Res. 2001;16:1329–1336. doi: 10.1359/jbmr.2001.16.7.1329. [DOI] [PubMed] [Google Scholar]
- 7.Redman LM, Rood J, Anton SD, Champagne C, Smith SR, Ravussin E. Calorie restriction and bone health in young, overweight individuals. Arch Intern Med. 2008;168:1859–1866. doi: 10.1001/archinte.168.17.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ensrud KE, Fullman RL, Barrett-Connor E, Cauley JA, Stefanick ML, Fink HA, et al. Voluntary weight reduction in older men increases hip bone loss: the osteoporotic fractures in men study. J Clin Endocrinol Metab. 2005;90:1998–2004. doi: 10.1210/jc.2004-1805. [DOI] [PubMed] [Google Scholar]
- 9.Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc. 2003;51:1740–1747. doi: 10.1046/j.1532-5415.2003.51558.x. [DOI] [PubMed] [Google Scholar]
- 10.Meyer HE, Tverdal A, Selmer R. Weight variability, weight change and the incidence of hip fracture: a prospective study of 39,000 middle-aged Norwegians. Osteoporos Int. 1998;8:373–378. doi: 10.1007/s001980050077. [DOI] [PubMed] [Google Scholar]
- 11.Beavers KM, Lyles MF, Davis CC, Wang X, Beavers DP, Nicklas BJ. Is lost lean mass from intentional weight loss recovered during weight regain in postmenopausal women? Am J Clin Nutr. 2011;94:767–774. doi: 10.3945/ajcn.110.004895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogden JD, Kemp FW, Huang AE, Shapses SA, Ambia-Sobhan H, Jagpal S, et al. Bone mineral density and content during weight cycling in female rats: effects of dietary amylase-resistant starch. Nutr Metab (Lond) 2008;5:34. doi: 10.1186/1743-7075-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacon L, Stern JS, Keim NL, Van Loan MD. Low bone mass in premenopausal chronic dieting obese women. Eur J Clin Nutr. 2004;58:966–971. doi: 10.1038/sj.ejcn.1601922. [DOI] [PubMed] [Google Scholar]
- 14.Avenell A, Richmond PR, Lean ME, Reid DM. Bone loss associated with a high fibre weight reduction diet in postmenopausal women. Eur J Clin Nutr. 1994;48:561–566. [PubMed] [Google Scholar]
- 15.Fogelholm GM, Sievanen HT, Kukkonen-Harjula TK, Pasanen ME. Bone mineral density during reduction, maintenance and regain of body weight in premenopausal, obese women. Osteoporos Int. 2001;12:199–206. doi: 10.1007/s001980170130. [DOI] [PubMed] [Google Scholar]
- 16.Villalon KL, Gozansky WS, Van Pelt RE, Wolfe P, Jankowski CM, Schwartz RS, et al. A Losing Battle: Weight Regain Does Not Restore Weight Loss-Induced Bone Loss in Postmenopausal Women. Obesity (Silver Spring) 2011;19:2345–50. doi: 10.1038/oby.2011.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waters DL, Vawter R, Qualls C, Chode S, Armamento-Villareal R, Villareal DT. Long-term maintenance of weight loss after lifestyle intervention in frail, obese older adults. J Nutr Health Aging. 2013;17:3–7. doi: 10.1007/s12603-012-0421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricci TA, Heymsfield SB, Pierson RN, Jr, Stahl T, Chowdhury HA, Shapses SA. Moderate energy restriction increases bone resorption in obese postmenopausal women. Am J Clin Nutr. 2001;73:347–352. doi: 10.1093/ajcn/73.2.347. [DOI] [PubMed] [Google Scholar]
- 19.Shapses SA, Sukumar D. Bone metabolism in obesity and weight loss. Annu Rev Nutr. 2012;32:287–309. doi: 10.1146/annurev.nutr.012809.104655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makovey J, Naganathan V, Seibel M, Sambrook P. Gender differences in plasma ghrelin and its relations to body composition and bone - an opposite-sex twin study. Clin Endocrinol (Oxf) 2007;66:530–537. doi: 10.1111/j.1365-2265.2007.02768.x. [DOI] [PubMed] [Google Scholar]
- 21.Sirola J, Kroger H, Honkanen R, Jurvelin JS, Sandini L, Tuppurainen MT, et al. Factors affecting bone loss around menopause in women without HRT: a prospective study. Maturitas. 2003;45:159–167. doi: 10.1016/s0378-5122(03)00150-6. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen TV, Sambrook PN, Eisman JA. Bone loss, physical activity, and weight change in elderly women: the Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res. 1998;13:1458–1467. doi: 10.1359/jbmr.1998.13.9.1458. [DOI] [PubMed] [Google Scholar]
- 23.Young R, May H, Murphy S, Grey C, Compston JE. Rates of bone loss in peri- and postmenopausal women: a 4 year, prospective, population-based study. Clin Sci (Lond) 1996;91:307–312. doi: 10.1042/cs0910307. [DOI] [PubMed] [Google Scholar]
- 24.Sornay-Rendu E, Munoz F, Duboeuf F, Delmas PD. Rate of forearm bone loss is associated with an increased risk of fracture independently of bone mass in postmenopausal women: the OFELY study. J Bone Miner Res. 2005;20:1929–1935. doi: 10.1359/JBMR.050704. [DOI] [PubMed] [Google Scholar]
- 25.Daly RM, Ahlborg HG, Ringsberg K, Gardsell P, Sernbo I, Karlsson MK. Association between changes in habitual physical activity and changes in bone density, muscle strength, and functional performance in elderly men and women. J Am Geriatr Soc. 2008;56:2252–2260. doi: 10.1111/j.1532-5415.2008.02039.x. [DOI] [PubMed] [Google Scholar]
- 26.Heaney RP. The bone-remodeling transient: implications for the interpretation of clinical studies of bone mass change. J Bone Miner Res. 1994;9:1515–1523. doi: 10.1002/jbmr.5650091003. [DOI] [PubMed] [Google Scholar]
- 27.Hinton PS, LeCheminant JD, Smith BK, Rector RS, Donnelly JE. Weight loss-induced alterations in serum markers of bone turnover persist during weight maintenance in obese men and women. J Am Coll Nutr. 2009;28:565–573. doi: 10.1080/07315724.2009.10719788. [DOI] [PubMed] [Google Scholar]
- 28.Sogaard AJ, Meyer HE, Tonstad S, Haheim LL, Holme I. Weight cycling and risk of forearm fractures: a 28-year follow-up of men in the Oslo Study. Am J Epidemiol. 2008;167:1005–1013. doi: 10.1093/aje/kwm384. [DOI] [PubMed] [Google Scholar]
- 29.Gallagher KI, Jakicic JM, Kiel DP, Page ML, Ferguson ES, Marcus BH. Impact of weight-cycling history on bone density in obese women. Obes Res. 2002;10:896–902. doi: 10.1038/oby.2002.123. [DOI] [PubMed] [Google Scholar]
- 30.Bosy-Westphal A, Later W, Schautz B, Lagerpusch M, Goele K, Heller M, et al. Impact of intra- and extra-osseous soft tissue composition on changes in bone mineral density with weight loss and regain. Obesity (Silver Spring) 2011;19:1503–1510. doi: 10.1038/oby.2011.40. [DOI] [PubMed] [Google Scholar]
- 31.Lee JS, Visser M, Tylavsky FA, Kritchevsky SB, Schwartz AV, Sahyoun N, et al. Weight loss and regain and effects on body composition: the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2010;65:78–83. doi: 10.1093/gerona/glp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taaffe DR, Cauley JA, Danielson M, Nevitt MC, Lang TF, Bauer DC, et al. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2001;16:1343–1352. doi: 10.1359/jbmr.2001.16.7.1343. [DOI] [PubMed] [Google Scholar]
- 33.Tothill P. Dual-energy x-ray absorptiometry measurements of total-body bone mineral during weight change. J Clin Densitom. 2005;8:31–38. doi: 10.1385/jcd:8:1:031. [DOI] [PubMed] [Google Scholar]
- 34.Bolotin HH. Analytic and quantitative exposition of patient-specific systematic inaccuracies inherent in planar DXA-derived in vivo BMD measurements. Med Phys. 1998;25:139–151. doi: 10.1118/1.598175. [DOI] [PubMed] [Google Scholar]
- 35.Blake GM, Fogelman I. How important are BMD accuracy errors for the clinical interpretation of DXA scans? J Bone Miner Res. 2008;23:457–462. doi: 10.1359/jbmr.071119. [DOI] [PubMed] [Google Scholar]
- 36.Svendsen OL, Hassager C, Skodt V, Christiansen C. Impact of soft tissue on in vivo accuracy of bone mineral measurements in the spine, hip, and forearm: a human cadaver study. J Bone Miner Res. 1995;10:868–873. doi: 10.1002/jbmr.5650100607. [DOI] [PubMed] [Google Scholar]
- 37.Yu EW, Thomas BJ, Brown JK, Finkelstein JS. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res. 2012;27:119–124. doi: 10.1002/jbmr.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Premaor MO, Pilbrow L, Tonkin C, Parker RA, Compston J. Obesity and fractures in postmenopausal women. J Bone Miner Res. 2010;25:292–297. doi: 10.1359/jbmr.091004. [DOI] [PubMed] [Google Scholar]
- 39.Sukumar D, Schlussel Y, Riedt CS, Gordon C, Stahl T, Shapses SA. Obesity alters cortical and trabecular bone density and geometry in women. Osteoporos Int. 2011;22:635–645. doi: 10.1007/s00198-010-1305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.