Abstract
The availability of reliable biomarkers of aging is important not only to monitor the effect of interventions and predict the timing of pathologies associated with aging but also to understand the mechanisms and devise appropriate countermeasures. Blood cells provide an easily available tissue and gene expression profiles from whole blood samples appear to mirror disease states and some aspects of the aging process itself. We report here a microarray analysis of whole blood samples from two cohorts of healthy adult and elderly subjects, aged 43±3 and 68±4 years, respectively, to monitor gene expression changes in the initial phase of the senescence process. A number of significant changes were found in the elderly compared to the adult group, including decreased levels of transcripts coding for components of the mitochondrial respiratory chain, which correlate with a parallel decline in the maximum rate of oxygen consumption (VO2max), as monitored in the same subjects. In addition, blood cells show age-related changes in the expression of several markers of immunosenescence, inflammation and oxidative stress. These findings support the notion that the immune system has a major role in tissue homeostasis and repair, which appears to be impaired since early stages of the aging process.
Keywords: aging, immunosenescence, microarray, blood cells, exercise
INTRODUCTION
The demonstration that some disease states are mirrored by gene expression profiles in blood cells has stimulated the analyses of gene expression changes in blood cells in different physiological and pathological conditions, including aging [1, 2]. A number of studies focused on candidate genes, while others used a genome-wide approach, such as microarray analyses, that provide an unbiased way to investigate the expression of the whole transcriptome. Age-related changes have been the object of a limited number of microarray analyses, however the different designs of available studies does not allow one to draw definitive conclusions about the existence of a specific “aging-signature” in blood cells. We report here a study on the effect of aging in whole blood cells in a selected cohort of healthy subjects from two specific age groups, taking advantage of different bioinformatics approaches for data analysis.
The scope and objectives of this work differ from those of previous studies. For example, one study involved two heterogeneous cohorts, including both males and females and spanning a wide age range (23 to 77), and identified only 16 genes showing a significant positive or negative correlation with age [3]. Another study was specifically focused on the identification of genes related to increased longevity and thus compared gene expression profiles in blood cells from nonagenarians with their middle-age offspring using the partners of the offspring as population controls [4].
In contrast, we focused on a restricted age segment, by comparing subjects in their mid 40s with those in their mid 60s, in order to identify gene expression changes in this initial period of the senescence process. Our preliminary functional studies revealed that the maximum oxygen uptake (VO2max), which reflects aerobic fitness, is significantly decreased in this specific phase of the life cycle, in agreement with previous studies [5, 6]. While microarray analysis provides an unbiased view of gene expression profiles reflecting the whole genome, we were especially interested in two specific types of changes. The first concerns genes coding for mitochondrial energy metabolism, to see whether changes in blood cells mirror those found in other tissues and may thus correlate with the global decrease in maximum oxygen uptake. The second concerns the effect of aging on immune cells and immune function, in relation with the notion of immunosenescence, i.e. the functional deterioration and remodeling of the immune system, which negatively impacts both innate and adaptive immunity [7]. Indeed, aging affects various aspects of innate immunity, with a decline in the function of NK cells [8], neutrophils and monocytes [9, 10], leading to impaired phagocytosis and chemotaxis [3]. Adaptive immunity is also affected, with decreased numbers and proportions of CD4+ and CD8+ naïve T cells [11, 12] and accumulation of late-stage differentiated effector memory CD4+ and CD8+ T cells [13] and of Th17 cells [14]. Aging is also associated with a decline in naïve B cell production, leading to an accumulation of memory B cells and a less robust response to antigen [5].
The interest in immune cell changes in gene expression during aging is further stimulated by new studies supporting the notion that immunological mechanisms are not only essential in the response to pathogenic microbes and tumor cells, but have a wider homeostatic role in tissue repair by affecting stem cell function in different tissues. For example, it has been shown that reduced macrophage amount and changes in monocyte/macrophage polarization play a pivotal role in skeletal muscle regeneration [15]. Aging results in a decline in the number of macrophages present in the skeletal muscle and in a defective regulation of their function [16]. Furthermore, recent studies revealed that regulatory T cells (Tregs) stimulate the regeneration of mouse skeletal muscle by promoting the activation of the muscle stem cells, the so called satellite cells [17], and that the impaired regeneration of mouse skeletal muscle during aging is in part due to the reduced recruitment, proliferation, and retention of Tregs in injured muscle [18]. This finding presumably reflects changes in gene expression between Tregs from young and old mice, with particular reference to genes coding for chemokine receptors [19]. Interestingly, these changes are reversible, as the supplementation with specific interleukins, such as IL-33, induces an increased Treg population and enhances muscle regeneration in injured muscles of old mice [18].
The demonstration that specific immune cell populations have a major role in tissue homeostasis supports the possibility that age-dependent changes in immune function may not only mirror but in fact contribute to the aging process of the whole body, thus providing a further stimulus for in depth study of the immune function during aging. The detailed analysis of age-related changes in blood cell gene expression is a step in this direction.
In order to characterize the genes and pathways specifically involved in the process of immuno-senescence, and to further validate the use of whole blood derived RNA as a kaleidoscope through which one can observe the direct effect of aging on blood cells we recruited two groups of healthy men in the local area of Verona. The first group consisted of adult/middle-aged males around 46 years old and the second group was comprised of elderly males around 68 years old. We chose these two age windows because several aspects related to the decline of physiological functions and metabolic responses are preserved in adults/middle-aged, yet decline in the elderly [11]. Our results show that several of the effects of aging are detectable even from samples obtained from within this narrow and relatively close age window (~46 vs ~68). We observed differentially expressed genes and pathways related to immunosenescence, inflammation and systemic aging. Furthermore, we report that transcriptional signatures of blood cells related to mitochondrial function are positively associated with the aerobic capacity of the subjects.
RESULTS
Clinical and anthropometric characteristics of the two groups
Characteristics of the participants in the two groups of healthy individuals are reported in Table 1. The adult and elderly groups consisted of 11 and 9 subjects respectively, with average age of 46 ±3 and 68±4 years. All volunteers were physically active, although not performing specific training programs. In agreement with previous studies the two groups had significantly different values of glucose and C reactive protein (CRP) [20, 21], which were significantly higher in the elderly (Table1). The blood cell profile was also different with an age-related expansion of the neutrophils, eosinophils granulocyte compartment, including both neutrophils, eosinophils and basophils. However, all parameters were still within the normal physiological range.
Table 1. Summary of the characteristics of the subjects enrolled in the study.
| Adults | Eldelry | p. value | |
|---|---|---|---|
| Subjects number | 11 | 9 | |
| Gender | male | male | |
| Age | 46 ± 3 | 68 ± 4 | * |
| BMI (kg/m2) | 25.3 ± 1.9 | 27 ± 2.6 | |
| sBP (mmHg) | 121 ± 11 | 127 ± 16 | |
| dBP (mmHg) | 84 ± 9 | 81 ± 7 | |
| AST (IU/L) | 30 ± 6 | 28 ± 6 | |
| ALT (IU/L) | 29 ± 9.4 | 23 ± 9 | |
| Glucose (mg/dL) | 86 ± 9 | 102 ± 11 | *** |
| Cholesterol (mg/dL) | 217 ±36 | 202 ± 39 | |
| HDL (mg/dL) | 56 ± 16 | 52 ± 8 | |
| Triglycerides (mg/dL) | 99 ± 30 | 93 ± 27 | |
| C reactive protein (CRP)(mg/L) | 0.8 ± 0,7 | 2.5 ± 2.3 | ** |
| RBC (104/μL) | 506 ± 58 | 494 ± 44 | |
| Platelet (104/μL) | 24.8 ± 4.8 | 26.7 ± 7.0 | |
| WBC (/μL) | 6290 ± 1245 | 6440 ± 1060 | |
| Neutrophil (/μL) | 3296 ± 955 | 3906 ± 698 | * |
| Eosinophil (/μL) | 342 ± 189 | 214 ± 156 | * |
| Basophil (/μL) | 46 ± 16 | 26 ± 15 | * |
| Monocyte (/μL) | 338 ± 86 | 323 ± 56 | |
| Lymphocyte (/μL) | 2141 ± 709 | 1920 ± 720 | |
| Monocyte (/μL) | 338 ± 86 | 323 ± 56 |
Data are expressed as mean ± SD
Student's T test p. value < 0.05
p. value < 0.01
p. value < 0.001.
Identification of genes differentially expressed in blood samples from adults and elderly subjects
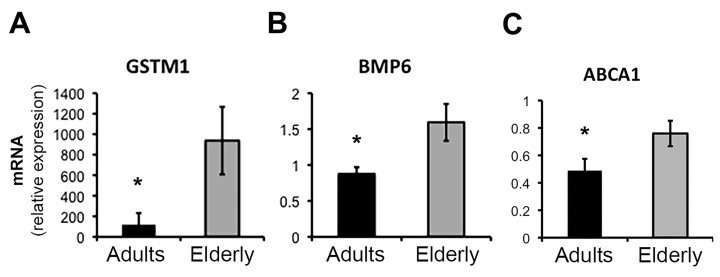
Total RNA was isolated from whole blood samples obtained in the morning, after overnight fasting. Gene expression was determined using whole-transcript arrays (Affymetrix HuGene 1.0 ST) and expression data were analyzed both at gene and pathways level. The elderly group showed increased the expression of 492 genes and decreased the expression of 418 genes (ANOVA p <0.05) (Table S1), by using a more stringent criteria (p < 0.01) the total number of differentially expressed genes was reduced to a more discrete 180 genes (112 upregulated e 68 down-regulated) (Table S1 and Figure 1). Only 4 genes were dysregulated more than 2-fold (GSTM1, CCDC23, TREML3P up-regulated; IGHV1-18 down-regulated) with aging. Changes of the expression levels of a group of representative genes were validated by qPCR (Figure 2A-C). Analysis of the gene list using DAVID [12] showed that the genes up-regulated in the elderly group shows that they are associated with categories such as Immunity and defense, Lipid, Fatty acid and steroid metabolism, Detoxification, Intracellular signaling cascade; whereas the down-regulated genes were associated with Protein biosynthesis and Protein folding (Table S2).
Figure 1. Heatmap of differen-tially expressed genes between blood samples of Adult and Elderly groups.
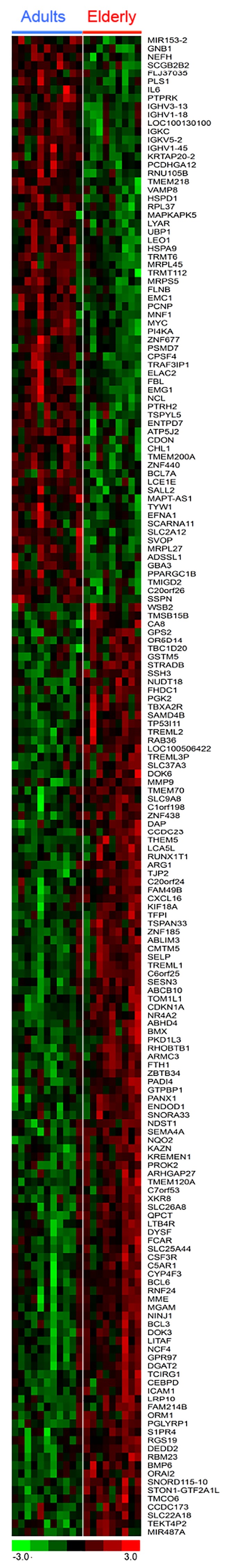
Expression profile of 180 genes that differed between Adults and Elderly (p < 0.01). Each gene is represented by a row; each subject by a column. The largest cluster of probes show enhanced expression (transition from green to red), and another cluster show reduced expression (transition from red to green) in the Elderly as compared to Adult. The full set of genes is reported in Table S1.
Figure 2. Real-time PCR (qPCR) validation of microarray results for selected genes.
Quantitative analysis by qPCR of the expression levels of GSTM1 (A), BMP6 (B), and ABCA1 (C) genes in samples from the Adult (n=11) and Elderly (n=9) groups. Data were normalized against GAPDH housekeeping gene. Error bars, SEM. * p < 0.05.
Age-related alteration of pathways related to immune function
We noted age-related dysregulation of several factors involved in differentiation pathways of immune cells, such as the nuclear receptor NR4A2, the BMX kinase and the two antagonists BMP6 and NOG, which were up- and down-regulated respectively in the elderly group (Figure 1 and 2B).
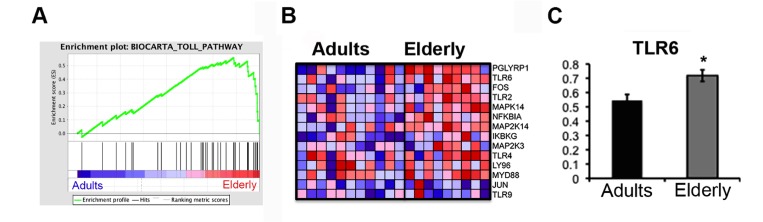
To gain insight into the biological mechanisms involved in immunosenescence we performed gene set enrichment analysis (GSEA), a computational method that determines whether an apriori defined set of genes shows statistically significant, concordant differences between biological states [13]. This method revealed a significant age-related enrichment (FDR<0.05) of categories related to biological pathways involved in signaling, immunity and cellular metabolism, such as the MET pathway, that has been associated with activated monocytes [14], with the Rho pathway and Toll like receptors (TLRs) signaling among the most over-represented pathways in the elderly group (Table 2). In this gene set we detected three different pattern recognition receptors (TLR4, TLR6, TLR9), the adaptor protein Myd88, the regulatory factors NEMO (IKBKG) and NF-kBIa. The higher expression level of TLR6 was confirmed by quantitative PCR (Figure 3). Using gene sets from the Immunological Signatures database, we noted the related enrichment in the elderly group for genes up-regulated in monocytes following LPS treatment (NES=2.47, FDR<0.0001) (Table 2).
Table 2. GSEA analysis of pathways positively and negatively associated with ageing.
| GROUP | Data Base | Pathway | NES | FDR (<0.05) |
|---|---|---|---|---|
| ELDERLY | Biocarta | INTEGRIN PATHWAY | 2.17 | 0.010 |
| RHO PATHWAY | 2.10 | 0.014 | ||
| MET PATHWAY | 2.04 | 0.017 | ||
| TOLL PATHWAY | 2.03 | 0.014 | ||
| VDR PATHWAY | 2.04 | 0.021 | ||
| GRANULOCYTES PATHWAY | 1.96 | 0.028 | ||
| PTEN PATHWAY | 1.93 | 0.033 | ||
| Kegg | ABC TRANSPORTERS | 2.11 | 0.009 | |
| Immunological Signatures | LOW LPS VS CTRL TREATED MONOCYTES UP | 2.47 | <0.0001 | |
| LOW LPS VS VEHICLE TREATED MONOCYTES UP | 2.30 | <0.0001 | ||
| ADULTS | Reactome | TRANSLATION | −2.27 | <0.0001 |
| mRNA SPLICING | −1.84 | 0.030 | ||
| RESPIRATORY ELECTRON TRANSPORT | −1.85 | 0.026 | ||
| Kegg | RIBOSOME | −2.18 | 0.001 | |
| AMINOACYL TRNA BIOSYNTHESIS | −1.77 | 0.040 | ||
| SPLICEOSOME | −1.80 | 0.058 |
Gene sets significantly enriched in the Elderly (positive NES) or in the Adult groups (negative NES) are reported. NES, normalized enrichment score; FDR q-val, probability for false discovery rate.
Figure 3. The Toll Pathway gene set is enriched in the Elderly group.
Gene set enrichment analysis (GSEA) was performed with microarray data. (A) Enrichment plots for the Biocarta Toll Pathway gene set are shown. Genes related to the Toll Pathway most strongly associated with the Elderly phenotype are represented on the far right. (B) The heatmap shows the relative gene expression (red = high, blue = low) for each gene of the core enrichment for the samples of the two groups (Adults, Elderly). (C) qPCR analysis of the expression levels of the TLR6 gene in samples from the Adult (n=11) and Elderly (n=9) groups. Data were normalized against GAPDH housekeeping gene. Error bars, SEM. * p < 0.05.
Finally, using as reference the KEGG database we found that the class of ABC transporters, a family of proteins that utilize ATP hydrolysis to transport a wide variety of substrates across various cellular membranes, was enriched in the elderly group. In this gene set ABCC4, ABCA1 and ABCG1 showed the highest level of up-regulation in the elderly, with ABCA1 changes confirmed by qPCR (Figure 2C).
Aging is accompanied by reduced expression of mitochondrial genes
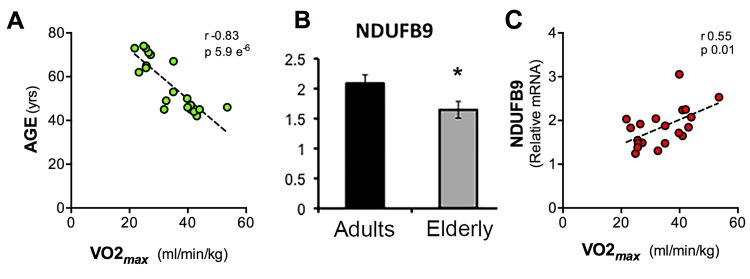
KEGG and Reactome pathways revealed that many genes coding for subunits of the electron transport chain (ETC) complexes are significantly down-regulated in the elderly group (FDR= 0,026; NES= −1,85) (Table 2). In particular the transcripts of 33 mitochondrial genes of nuclear origin were under-represented in the elderly (Supplementary Figure 1), with reduced levels of the complex I component NDUFB9 also confirmed by qPCR (Figure 4B).
Figure 4. Scatter charts of values of Age, VO2max and NDUFB9 expression levels.
Graphical representation of the correlation between VO2max and Age (A), expression levels of NDUFB9 assessed by qPCR and normalized against GAPDH (B), correlation between or VO2max and NDUFB9 gene expression levels detected by qPCR (C). (Pearson correlation, p = 5.9 e−6; r = 0.83 (A), p = 0.01; r = 0.55 (B), error bars, SEM * p < 0.05).
We asked whether these changes observed at the transcriptional levels in blood cells are associated with a global functional parameter of aerobic fitness, such as the maximum rate of oxygen consumption (VO2max), which declines in the elderly group (Figure 4A). We ran the GSEA using VO2max values as a reference parameter and were able to identify a number of pathways positively and negatively associated with VO2max (Table S3). Interestingly, a group of gene sets coding for mitochondrial respiratory chain components was positively and significantly associated with VO2max. For example, NDUFB9 expression levels, as determined by qPCR, were decreased in the elderly group (Figure 4B) and were significantly correlated with VO2max (r 0.55, p 0.01) (Figure 4C).
Additional age-dependent changes in gene expression
According to gene sets from the KEGG and Reactome database, elderly subjects showed reduced content of transcripts associated with protein synthesis (Table 2). In particular, one of the most under-represented gene sets in the elderly group was “Translation” (NES −2.27, FDR <0.0001). In this category we found several transcripts coding for numerous ribosomal proteins and the transcripts of 6 different subunits of the eukaryotic initiation factor 3 (eIF3A, eIF3B, eIF3D, eIF3G, eIF3I and eIF3K).
Transcripts of genes in the management of cellular oxidative stress and detoxification were consistently increased in blood cells of elderly subjects, including GSTM1, GSTT1, GSTM5, NQO2, PRDX5, GPX3 (Figure 2A and Table S1).
Finally in agreement with a recent study investigating age-related changes in gene expression from whole peripheral blood [3], CD248 and SLC4A10, coding respectively for the transmembrane glycoprotein endosialin and for a sodium bicarbonate transporter, were significantly down-regulated in the elderly group (ANOVA p < 0.05; Figure 1 and Table S1).
DISCUSSION
Haematological markers of inflammation, immuno-senescence and frailty
Comparative haematological analysis of blood samples from the adult/middle-aged and elderly groups highlight various elements suggestive of systemic inflammation, such as the increased number of neutrophils and CRP levels in the elderly group. These elements have also been proposed as markers for immunosenescence and frailty in comparisons between young and old persons, and in a cohort of 85 year old individuals [22]. Although there is still a debate about whether the number of neutrophils changes with age, recent studies associated an age-related increase in the abundance of neutrophils to low grade inflammation and frailty [23, 24]. Interestingly, when elevated beyond the physiological range, increased neutrophil number is correlated with increased mortality during in the following 2 years, a phenomenon linked to other markers of inflammation such as increased serum levels of IL-6 or CRP [25].
Age-related changes of factors involved in differentiation and cell stress responses
Our microarray-based comparison of blood transcripts from middle-aged and elderly groups also highlighted various biomarkers implicated in senescence of the immune system, and in the expansion of the granulocyte compartment.
An important finding of this study is the up-regulation of a group of transcripts encoding for proteins with protective roles against oxidative stress, in support of the free-radical theory of aging [26] and the notion that successfully managing oxidative stress can help extend lifespan [27]. In particular, the expression level of GSTM1 has a protective effect, and was associated with reduced mortality in participants of ilSIRENTE study [28], whereas GSTT1 was associated with lower cancer risk, longevity and aging in human granulosa cells [29]. Conversely reduced expression of GSTM1 is associated with prostate cancer and leukemia [30] and its copy number profile is correlated with prognosis [31].
The elderly group also showed dysregulation of factors involved in the differentiation of lymphocytes. In particular, transcripts for nuclear receptor NR4A2 are up-regulated in the elderly group. NR4A2 has been implicated both in the control of differentiation of T helper 17 cells [32], but it is also involved in lipid loading and inflammatory responses in macrophages at atherosclerotic lesions [33].
Our data also suggest a progressive imbalance of the Bmp6/Noggin ratio that may negatively affect proliferation, differentiation and activity of many cell types, and may be related to the pathophysiology of neurodegenerative diseases. Interestingly, there was an inverse relationship between BMP6, which was increased in the elderly group, and Noggin, which was expressed at higher levels in the adult/middle-aged group. BMP6 codes for Bone morphogenetic protein 6, a member of the TGFbeta family that has recently been indicated as an inhibitor of both Ig production and proliferation in memory and naive B cells [34]. BMP6-mediated inhibiton of proliferation can be reversed by treatment with its extracellular natural antagonist Noggin, coded by NOG. Interestingly, BMP6 has been recently associated with Alzheimer disease and reduced neurogenesis both in human patients and in transgenic mice [35].
We also noted an age-related increase of transcripts encoding for the Bone marrow kinase on chromosome X (BMX), a cytosolic kinase expressed in different tissues and in several types of cancer [36]. During the inflammatory response BMX modulates the secretion of pro-inflammatory cytokines induced by TNF, IL-1β, and TLR4. BMX genetic ablation in mutant mice resulted in protection from arthritis [36], suggesting that its up-regulation in the elderly blood cells contribute to the onset of chronic inflammation and may represent a novel therapeutic target in contexts outside of cancer.
Age-related signaling pathways in blood cells
GSEA analysis highlighted a number of pathways associated with aging. The finding that genes of the Rho signaling pathway are up-regulated in relation with human aging opens the scenario to a wide range of hypotheses and speculations. Rho is a small GTPase that plays a pivotal role in the transduction of mechanical stimuli generated by specific transmembrane proteins (integrins and stretch-activated cell-surface receptors) [37], however in endothelial cells also oxidative stress can also activate the Rho signaling pathway, inducing an increase of arginase I activity [38]. Thus, since oxidative stress and arginase levels are increased during aging, it is possible to conceive that Rho signaling is more active in aged cells and play a relevant role in vascular function/dysfunction [38]. Furthermore changes in extracellular matrix (ECM) stiffness can modulate Rho signaling, and aging is associated with increased endothelial permeability as a function of stiffness of the ECM, and increased Rho activity [39]. However, there is not much data about Rho signaling in hematopoietic cells, although several studies point to RhoA as an important player in the regulation of cell adhesion and differentiation. In particular, RhoA has been implicated in the proliferation and differentiation of pre-T cells, as well as in the migration and activation of T-lymphocytes [40]. It is attractive to speculate that the Rho pathway could also become more active with aging as a response to changes in the microenvironment.
Toll-like receptors (TLRs) are pattern recognition receptors that play a key role in alerting the immune system to the presence of microbial infections. Once activated, the TLRs trigger an inflammatory cascade through the activation of NF-kB-mediated transcription and the consequent production of cytokines and type I interferon, shaping both the innate and adaptive immune responses [41]. TLRs are expressed by monocytes, NK cells, DCs, and B and T lymphocytes [42]. Recent studies indicate that aging influences the function of pattern recognition receptors (PRRs), although results are sometimes controversial, possibly due to different experimental protocols and recruitment criteria [43]. Our data show increased expression levels of TLR6, TLR4 and TLR9. TLR6 and TLR4 recognize lipopeptides and LPS, respectively, although FFAs and elevated glucose levels can also activate TLRs [44]. Changes in serum composition during aging resulting from altered metabolism, glycemia and lipidemia may thus tune systemic inflammation, and eventually contribute to the development of chronic diseases (insulin resistance, atherosclerosis, Alzheimer's disease). TLR4-Myd88 signaling is involved in the development of insulin resistance in high fat diet fed mice [45], reinforcing the notion that lipid-receptors enable innate immunity to sense the lipid and glucose environment and to modulate the inflammatory responses [46]. This is consistent with the enrichment in the elderly group of two immunological signatures for genes up-regulated in monocytes following LPS treatment (Table 2) and with the up-regulation of the BMX kinase, suggesting that monocytes in the elderly group may be in a pro-inflammatory state.
The Met-pathway, also up-regulated in the elderly, is triggered by the pleiotropic cytokine HGF (Hepatocyte Growth Factor) and its tyrosine-kinase receptor c-Met [47]. The HGF receptor is expressed in endothelial cells, erythroid precursors and has also recently been characterized in macrophages [14]. In human monocytes the endotoxin LPS and the pro-inflammatory cytokine IL-1β can induce the expression of both HGF and c-Met. In a sort of autoregulatory loop HGF induces the up-regulation of HGF, its receptor (c-Met) and other pro-inflammatory cytokines [14]. In experimental autoimmune encephalomyelitis (EAE) this pathway promotes proliferation of classically activated macrophages, suggesting a pro-inflammatory role for HGF [48]. These results further suggest that aging favors a pro-inflammatory profile of monocytes and macrophages also through autocrine/paracrine loops mediated also by HGF. It has been suggested that HGF may have a negative effect on muscle regenerative capacity, since high levels of HGF induce satellite cell quiescence by stimulating myostatin expression [49]. Thus sustained systemic levels of HGF may constitute a link between immunosenescence and sarcopenia, although further studies are needed to investigate this relationship.
Finally, the age-dependent increase in the ABC transporters, ABCA1 and ABCG1, is of interest because they mediate active cholesterol efflux toward a variety of exogenous acceptors, including HDL, LDL, liposomes, and cyclodextrin. ABC transporters are particularly relevant for macrophages, since combined deficiency of ABCA1 and ABCG1 promotes foam cells accumulation and accelerates atherosclerosis [50], and also impairs macrophage migration [51]. ABCA1 and ABCG1 transporters thus play a synergistic role in preventing atherosclerosis and cardiovascular disease, making them a useful marker for healthy aging [50].
Indicators of reduced protein synthesis in aged blood cells
We also report several biological pathways that were down-regulated in the elderly group consisting of sets of genes related to the biosynthetic capacity and metabolism. From the GSEA analysis one of the most depleted gene sets in the elderly is “Translation” (Reactome), including a large number of genes coding for ribosomal proteins and factors involved in the phases of initiation and elongation of translation. Six genes coding for components of the highly conserved large scaffold complex eIF3 were down-regulated, suggesting that binding of the mRNA and the interactions with the 40S ribosomal subunit could become progressively impaired or less efficient with aging. In mammalians eIF3 plays a crucial role in several steps of the initiation of mRNA translation affecting cell growth as well as cancer [52]. The balance between muscle hypertrophy and atrophy is also controlled by eIF3 [53] and one wonders whether the consequent impaired protein synthesis, contributing to the onset and progression of sarcopenia in skeletal muscle, may reflect a more general age-related impairment of the translation process affecting also the blood cells.
Mitochondrial gene expression
An additional gene set was found down-regulated by aging: the “Electron Transport Chain” (ETC). The idea that mitochondria play a causative role in the progression of aging was first proposed in 1956 with the “free radical theory” by Harman [26], and altered mitochondrial function is a well characterized consequence of aging. The ETC, that drives the transfer of electrons from NADH and FADH2 to molecular oxygen to produce ATP, is composed of 5 complexes organized in supercomplexes. Alterations of these supramolecular engines, by mutations or alteration of proper import and/or folding of the component proteins, or imbalance of stoichiometry, can impair mitochondrial function and contribute to age-related degeneration of several tissues, such as brain, lymphocytes and heart [54]. In this context, our data extend the control phases for the correct assembly of the ETC also to the transcriptional level, showing that a group of nuclear genes encoding for elements of the ETC are generally down-regulated by aging. These observations are in agreement with the demonstration of decreased ETC enzyme activities in aging rat brain and lymphocytes [54], and with age-dependent transcriptome and proteome mitochondrial changes in heart [55] and skeletal muscles [56]. In our samples the complex most severely affected at the transcriptional level is complex I. In rats it has been shown that the activities of the various ETC complexes (I-V) undergo a decline with increasing age, and that this decay follows a parallel pattern in brain, lymphocytes and skeletal muscle [54, 57]. Using a multi-disciplinary approach it has been shown that senescence implies the down-regulation of the expression levels of transcripts correlated with reduced activity of ETC complexes in myocardium mitochondria [55]. Aging causes a decay of whole body aerobic function, as determined by maximal oxygen consumption and in humans this decline is accelerated after 60 years of age. The age-related decline in VO2max, at whole body level, is the results of adaptations occurring both at central and peripheral level, including the reduction in maximal heart rate and lean body mass [6]. Although we cannot take in the relative contribution of each system, mitochondrial dysfunction in different tissues contributes to the age-associated reduction of VO2max and it is of interest that this dysfunction is reflected in the decreased levels of transcripts of respiratory complexes in blood cells.
Comparison with previous studies
In this study we chose to perform microarray analysis on whole blood samples to avoid alteration in gene expression during the separation of the various cell types [58]. Two previous studies have reported gene expression profiling of whole blood cells in relation with age. Nakamura and colleagues analyzed whole blood RNA [3] in a large cohort of subjects, both male and female, of age between 23 and 77. They found 16 genes strongly correlated with aging (11 up-regulated and 5 downregulated). Of these SLC4A10 and CD248 showed the strongest correlation with age. Our dataset confirms this result, since CD248 and SLC4A10 were both significantly down-regulated in the elderly group. CD248 encodes for the transmembrane glycoprotein endosialin, which has been associated to stromal cells in multiple types of cancer but its expression in human blood cells is restricted to naïve CD8+ T-cells, in which it acts as an inhibitor of cellular proliferation maintaining naive T-cells in a quiescent state [59]. Its lower expression in the elderly would indicate a lower abundance of naive T-cells in this population, a well-characterized feature of immunosenescence.
Another study had a more specific objective, namely to identify genes associated with familial longevity [4]. In this study blood samples were obtained from participants of the Leiden Longevity Study, comprising nonagerian sibling pairs, their middle-aged offspring and the partners of the offspring as population controls. A number of genes that represent a potential “longevity-signature” were thus identified [4]. In that report the pathway most closely associated with longevity was the Rho signaling pathway, associated to cellular growth and cytoskeletal organization, and by its connection with the mTOR pathway has been linked to lifespan and health. One of the major limitations of this study is the limited size of the subjects involved, however other studies in which highthroughput technologies combined to physical exercise/inactivity interventions were applied used more limited samples [60-63].
In our study we focused on a more restricted age difference (46 vs 68 years) and used exclusively male subjects. Our results support the notion that crucial changes in gene expression take place in blood cells in this relatively limited time period. Interestingly, some of these changes correlate with global functional changes, such as VO2max.
CONCLUSIONS
This study shows that many changes in different pathways and genes occur in a relatively restricted age window, corresponding to the early phase of senescence. Interestingly, similar age-dependent changes in gene expression were also found in many other tissues. As such, blood can serve as a relatively non-invasive surrogate for biomarker discovery. While further studies with larger sample size are required to support this conclusion, our data are consistent with the notion that blood cells can provide a key to understanding systemic events.
METHODS
Protection of human subjects
Human subjects research was conducted accordingly to the principles of the Declaration of Helsinki. The study described in this work were in compliance with protocols approved by Institutional Review Board of the University of Verona. All subjects gave their informed consent, and biological specimens and all data collected were anonymized.
Subjects enrollment, health data collection
For this study twenty healthy men between the ages of 45-55 (n=11) and 65-75 (n=9) years old were recruited in the local area of Verona. They completed a medical-history questionnaire. Exclusion criteria considered are: cardiovascular, respiratory, neurological, endocrine and inflammatory diseases, diabetes or other metabolic disorders, medications affecting the cardiovascular function. Participant characteristics are presented in Table 1.
Blood sampling
The subjects were asked to avoid vigorous physical activity in the 24 h before blood sampling and to fasten from the evening meal until the morning, when samples were obtained.
Blood was sampled from antecubital vein of each subject while seated in two different occasions, which were separated by at least 7 days. To preserve RNA quality and integrity 3 ml of blood have been collected intoTEMPUS Blood RNA tubes (ABI, Foster City, CA, USA) containing 6 ml Applied BioSystems RNA stabilization reagent.
Haematological testing
All the samples were processed for routine hematological testing immediately after collection (<15min) on the same Advia 2120i hematology system (Siemens Healthcare Diagnostics, Deerfield, IL, USA) using standard local procedures at GB Rossi Hospital, Verona, Italy.
The parameters tested included red blood cells count (RBC), haematocrit (HCT), haemoglobin (HGB), mean red cell volume (MCV), mean red cell haemoglobin content (MCHC), red blood cell distribution width (RDW), white blood cells (WBC) count, and WBC differential, including lymphocytes, monocytes, neutrophils, eosinophils, basophils and large unstained cells, platelet count, mean platelet volume. The instrument was calibrated against appropriate proprietary reference standard material and verified with the use of proprietary controls.
Clinical chemistry and immunochemistry test
The clinical chemistry and immunochemistry tests were performed on serum aliquots on the same instrument Cobas® 6000 < c501 > and < e601 > module (Roche Diagnostics GmbH, Penzberg, Germany), according to the manufacturer's specifications and using proprietary reagents. The panel of tests included the following: glucose, total cholesterol, HDL cholesterol, triglycerides, total protein, albumin, urea, creatinine, uric acid, alkaline phosphatase, amylase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), C-reactive protein (CRP). The instrument was calibrated against appropriate proprietary reference standard materials and verified with the use of proprietary quality controls.
Blood pressure
Blood pressure at rest in sitting position was measured before the evaluation of VO2max in the brachial artery by means of a blood pressure monitor (Tango+, SunTech Medical, USA).
Total RNA preparation and RNA pooling
Total RNA was isolated from each of the two blood samples using Tempus Spin RNA Isolation Kit (Applied Biosystems) as specified in the manufacturer's guidelines. Quality of the purified RNA from was verified on an Agilent® 2100 Bioanalyzer (Agilent Technologies, CA); RNA concentrations were determined using a NanoDrop® ND-1000 spectrophotometer (NanoDrop Technologies, DE). To improve the detection sensitivity of transcripts on microarrays globin mRNA was depleted from a portion of each total RNA sample isolated from TEMPUS Blood RNA tubes using the GLOBINclear™-Human kit (Ambion, TX). To reduce variations due to confounding events, such as spontaneous up- and down-regulation of genes, the two samples of clean RNA from each subject were pooled into one single sample (1ug+1ug RNA).
cRNA preparation, hybridization, staining, and scanning
The cRNA labeling and hybridizations were performed according to protocols from Affymetrix Inc. (Santa Clara, CA). Briefly, 100 ng of total RNA from whole blood and Globin depleted samples was converted to cRNA and then to sense-strand biotin-dUTP-labeled cDNA using Ambion WT Expression Kit according to the manufacturer's recommended protocols. The single stranded cDNA was then fragmented to ~40-70 nt size by incubating in fragmentation buffer. Fragmented cDNA was assessed for relative length on Agilent 2100 Bioanalyzer and hybridized to Affymetrix Human Gene ST 1.0 chips for 16 hours, washed, stained on an Affymetrix fluidics station and scanned using Affymetrix GeneChip scanner 3000.
Mircoarray data analysis
Microarray probe fluorescence signals were converted to expression values using robust multiarray average procedure RMA [64] of Bioconductor affy package. Specifically, fluorescence intensities have been background adjusted, normalized using quantile normalization, and expression values for a total of 19 684 custom probe sets calculated using median polish summarization and custom chip definition files for Human Gene 1.0 ST arrays based on Entrez genes (hugene10st_Hs_ENTREZG version 15.1.0) [65]. Quality control was performed using ArrayQualityMetrics [66], and based on the quality assessment all 20 samples were deemed suitable for further analysis.
All data analyses were performed in R version 3.1.2 using Bioconductor libraries of BioC 2.3 and R statistical packages. Raw data are available at Gene Expression Omnibus (GEO) GSE67220.
Genes with statistically significant differential expression between adults and elderly subjects were identified using two-way analysis of variance (ANOVA, p<0.05).
Quantitative real time PCR
For quantitative Real Time-PCR assays, total RNA was characterized by electrophoresis (Agilent 2100 Bioanalyzer, CA). 400 ng of RNA was converted to cDNA using random primers and Superscript III (Invitrogen, CA). Amplification was carried out in triplicates with an 7900 HT Fast Real Time PCR System (Applied Biosystems) using SYBR green chemistry (Fast SYBR green master mix Applied Biosystems) and a standard 2-step protocol. The primers specific for each gene were designed and analyzed with Primer3 (freeware) and Vector NTI (Invitrogen, freeware). Identity of the amplicons was confirmed by their dissociation profiles and gel analysis.
Quantitative PCR standard curves were constructed by using serial dilutions of muscle cDNAs, using at least 4 dilution points and the efficiency of all primer sets was between 1,8 and 2,2. The data were normalized against Gapdh housekeeping gene. Primers are listed in Table S3.
Evaluation of VO2max
All the tests were performed at approximately the same time of the day (22–25°C, 55–65% relative humidity). The subjects were asked to avoid vigorous physical activity in the 24 h before the test and to avoid food intake in the 8 hours before reaching the laboratory. Here they were given a standardized light meal 30 min before starting the test. VO2max and ventilatory thresholds were evaluated during a ramp test performed on an electronically braked cycle ergometer (Excalibur Sport, Lode, Groningen, The Netherlands). The subjects were familiarised with the tasks and asked to perform a maximal incremental ramp test to determine the VO2max [67]. The cycle ergometer seat and handlebar positions were customised for each subject. The ergometer was operated by a metabolic cart (Quark b2, Cosmed, Rome, Italy) that also allowed continuous, breath-by-breath measures of gas exchange and ventilation (at the mouth) and HR. The ramp test consisted of 3 min at rest, 5 min of warm-up exercise at 50 W, followed by a continuous increase in the workload by 15-20 W per minute until voluntary exhaustion. The accepted criteria for maximal effort were: respiratory exchange ratio >1.1 and heart rate (HR) >90% of the predicted maximum based on age.
Over-representation analysis
Over-representation analysis was performed using the Gene Set Enrichment Analysis (GSEA) software (http://www.broadinstitute.org/gsea/msigdg/gsea/msigd). GSEA was applied on linear expression data of the entire dataset. To determine which set of genes shows statistically significant, concordant differences between elderly and adult subjects, we performed GSEA using Signal2Noise as metric and 1,000 permutations of gene set. Gene sets were defined as significantly enriched if the False Discovery Rate (FDR) was < 5%.
To determine VO2max –max ermine VOs < 5%. 5 VO2max value of each subject was used as continuous phenotype labels, and the Pearson's correlation as the metric to select gene sets with expression patterns resembling those encoded in the phenotype labels. Finally, in this case, gene sets were defined as significantly enriched if the False Discovery Rate (FDR) was <5.
Statistical analysis
All data are reported as the mean ± SEM or SD as indicated in the legend. Statistical analyses between the two groups were performed with unpaired Student's t-test. A P value of < 0.05 was considered significant.
SUPPLEMENTARY DATA TABLES AND FIGURE
Acknowledgments
We thank N. Tamassia for suggestions and comments.
Footnotes
FUNDING
This work was supported by grants from the Italian Space Agency contract DCMC GO-1B1127-003 to CC, the European Space Agency (ESA) MAP Project # 4000102580 to CC, European Commission Integrated Project MYOAGE no. 223576 to SS, and FSE.
CONFLICTS OF INTEREST
The authors declare no competing financial interests.
REFERENCES
- 1.Sullivan PF, Fan C, Perou CM. Evaluating the comparability of gene expression in blood and brain. American journal of medical genetics Part B, Neuropsychiatric genetics. 2006;141B:261–68. doi: 10.1002/ajmg.b.30272. [DOI] [PubMed] [Google Scholar]
- 2.Passtoors WM, Beekman M, Gunn D, Boer JM, Heijmans BT, Westendorp RG, Zwaan BJ, Slagboom PE. Genomic studies in ageing research: the need to integrate genetic and gene expression approaches. J Intern Med. 2008;263:153–66. doi: 10.1111/j.1365-2796.2007.01904.x. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura S, Kawai K, Takeshita Y, Honda M, Takamura T, Kaneko S, Matoba R, Matsubara K. Identification of blood biomarkers of aging by transcript profiling of whole blood. Biochem Biophys Res Commun. 2012;418:313–18. doi: 10.1016/j.bbrc.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Passtoors WM, Boer JM, Goeman JJ, Akker EB, Deelen J, Zwaan BJ, Scarborough A, Breggen R, Vossen RH, Houwing-Duistermaat JJ, Ommen GJ, Westendorp RG, van Heemst D, et al. Transcriptional profiling of human familial longevity indicates a role for ASF1A and IL7R. PLoS One. 2012;7:e27759. doi: 10.1371/journal.pone.0027759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babcock MA, Paterson DH, Cunningham DA. Influence of ageing on aerobic parameters determined from a ramp test. Eur J Appl Physiol Occup Physiol. 1992;65:138–43. doi: 10.1007/BF00705071. [DOI] [PubMed] [Google Scholar]
- 6.Hawkins S, Wiswell R. Rate and mechanism of maximal oxygen consumption decline with aging: implications for exercise training. Sports Med. 2003;33:877–88. doi: 10.2165/00007256-200333120-00002. [DOI] [PubMed] [Google Scholar]
- 7.Pawelec G, Solana R, Remarque E, Mariani E. Impact of aging on innate immunity. J Leukoc Biol. 1998;64:703–12. doi: 10.1002/jlb.64.6.703. [DOI] [PubMed] [Google Scholar]
- 8.Abbatecola AM, Ferrucci L, Grella R, Bandinelli S, Bonafè M, Barbieri M, Corsi AM, Lauretani F, Franceschi C, Paolisso G. Diverse effect of inflammatory markers on insulin resistance and insulin-resistance syndrome in the elderly. J Am Geriatr Soc. 2004;52:399–404. doi: 10.1111/j.1532-5415.2004.52112.x. [DOI] [PubMed] [Google Scholar]
- 9.Joehanes R, Johnson AD, Barb JJ, Raghavachari N, Liu P, Woodhouse KA, O'Donnell CJ, Munson PJ, Levy D. Gene expression analysis of whole blood, peripheral blood mononuclear cells, and lymphoblastoid cell lines from the Framingham Heart Study. Physiol Genomics. 2012;44:59–75. doi: 10.1152/physiol-genomics.00130.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005;579:2035–39. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 11.Wilson MM, Morley JE. Invited review: Aging and energy balance. J Appl Physiol (1985) 2003;95:1728–36. doi: 10.1152/japplphysiol.00313.2003. [DOI] [PubMed] [Google Scholar]
- 12.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 13.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galimi F, Cottone E, Vigna E, Arena N, Boccaccio C, Giordano S, Naldini L, Comoglio PM. Hepatocyte growth factor is a regulator of monocyte-macrophage function. J Immunol. 2001;166:1241–47. doi: 10.4049/jimmunol.166.2.124. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Melton DW, Porter L, Sarwar ZU, McManus LM, Shireman PK. Altered macrophage phenotype transition impairs skeletal muscle regeneration. Am J Pathol. 2014;184:1167–84. doi: 10.1016/j.ajpath.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Przybyla B, Gurley C, Harvey JF, Bearden E, Kortebein P, Evans WJ, Sullivan DH, Peterson CA, Dennis RA. Aging alters macrophage properties in human skeletal muscle both at rest and in response to acute resistance exercise. Exp Gerontol. 2006;41:320–27. doi: 10.1016/j.exger.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, Mathis D. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–95. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, Benoist C, Mathis D. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity. 2016;44:355–67. doi: 10.1016/j.immuni.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mo R, Chen J, Han Y, Bueno-Cannizares C, Misek DE, Lescure PA, Hanash S, Yung RL. T cell chemokine receptor expression in aging. J Immunol. 2003;170:895–904. doi: 10.4049/jimmunol.170.2.895. [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal M, Doberne L, Greenfield M, Widstrom A, Reaven GM. Effect of age on glucose tolerance, insulin secretion, and in vivo insulin action. J Am Geriatr Soc. 1982;30:562–67. doi: 10.1111/j.1532-5415.1982.tb05662.x. [DOI] [PubMed] [Google Scholar]
- 21.Ballou SP, Lozanski FB, Hodder S, Rzewnicki DL, Mion LC, Sipe JD, Ford AB, Kushner I. Quantitative and qualitative alterations of acute-phase proteins in healthy elderly persons. Age Ageing. 1996;25:224–30. doi: 10.1093/ageing/25.3.224. [DOI] [PubMed] [Google Scholar]
- 22.Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol. 2010;22:507–13. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Garrido J, Navarro-Martínez R, Buigues-González C, Martínez-Martínez M, Ruiz-Ros V, Cauli O. The value of neutrophil and lymphocyte count in frail older women. Exp Gerontol. 2014;54:35–41. doi: 10.1016/j.exger.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Compté N, Bailly B, De Breucker S, Goriely S, Pepersack T. Study of the association of total and differential white blood cell counts with geriatric conditions, cardio-vascular diseases, seric IL-6 levels and telomere length. Exp Gerontol. 2015;61:105–12. doi: 10.1016/j.exger.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Ferrando-Martínez S, Ruiz-Mateos E, Hernández A, Gutiérrez E, Rodríguez-Méndez MM, Ordoñez A, Leal M. Age-related deregulation of naive T cell homeostasis in elderly humans. Age (Dordr) 2011;33:197–207. doi: 10.1007/s11357-010-9170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 27.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–26. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onder G, Capoluongo E, Giovannini S, Concolino P, Russo A, Liperoti R, Bernabei R, Landi F. Interaction between GSTM1 genotype and IL-6 on mortality in older adults: results from the ilSIRENTE study. Cytokine. 2011;53:301–05. doi: 10.1016/j.cyto.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz JR, Fiuza-Luces C, Buxens A, Cano-Nieto A, Gomez-Gallego F, Santiago C, Rodri-guez-Romo G, Garatachea N, Lao JI, Moran M, Lucia A. Are centenarians genetically predis-posed to lower disease risk? Age (Dordr) 2012;34:1269–83. doi: 10.1007/s11357-011-9296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lodygin D, Epanchintsev A, Menssen A, Diebold J, Hermeking H. Functional epigenomics identifies genes frequently silenced in prostate cancer. Cancer Res. 2005;65:4218–27. doi: 10.1158/0008-5472.CAN-04-4407. [DOI] [PubMed] [Google Scholar]
- 31.Usvasalo A, Ninomiya S, Räty R, Hollmén J, Saarinen-Pihkala UM, Elonen E, Knuutila S. Focal 9p instability in hematologic neoplasias revealed by comparative genomic hybridization and single-nucleotide poly-morphism microarray analyses. Genes Chromosomes Cancer. 2010;49:309–18. doi: 10.1002/gcc.20741. [DOI] [PubMed] [Google Scholar]
- 32.Raveney BJ, Oki S, Yamamura T. Nuclear receptor NR4A2 orchestrates Th17 cell-mediated autoimmune inflammation via IL-21 signalling. PLoS One. 2013;8:e56595. doi: 10.1371/journal.pone.0056595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonta PI, van Tiel CM, Vos M, Pols TW, van Thienen JV, Ferreira V, Arkenbout EK, Seppen J, Spek CA, van der Poll T, Pannekoek H, de Vries CJ. Nuclear receptors Nur77, Nurr1, and NOR-1 expressed in atherosclerotic lesion macrophages reduce lipid loading and inflammatory responses. Arterioscler Thromb Vasc Biol. 2006;26:2288–94. doi: 10.1161/01.ATV.0000238346.84458.5d. [DOI] [PubMed] [Google Scholar]
- 34.Kersten C, Sivertsen EA, Hystad ME, Forfang L, Smeland EB, Myklebust JH. BMP-6 inhibits growth of mature human B cells; induction of Smad phosphorylation and upregulation of Id1. BMC Immunol. 2005;6:9. doi: 10.1186/1471-2172-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crews L, Adame A, Patrick C, Delaney A, Pham E, Rockenstein E, Hansen L, Masliah E. Increased BMP6 levels in the brains of Alzheimer's disease patients and APP transgenic mice are accompanied by impaired neurogenesis. J Neurosci. 2010;30:12252–62. doi: 10.1523/JNEUROSCI.1305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cenni B, Gutmann S, Gottar-Guillier M. BMX and its role in inflammation, cardiovascular disease, and cancer. Int Rev Immunol. 2012;31:166–73. doi: 10.3109/08830185.2012.663838. [DOI] [PubMed] [Google Scholar]
- 37.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11:633–43. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chandra S, Romero MJ, Shatanawi A, Alkilany AM, Caldwell RB, Caldwell RW. Oxidative species increase arginase activity in endothelial cells through the RhoA/Rho kinase pathway. Br J Pharmacol. 2012;165:506–19. doi: 10.1111/j.1476-5381.2011.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer CB, Reinhart-King CA. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med. 2011;3:112ra122. doi: 10.1126/scitranslmed.3002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rougerie P, Delon J. Rho GTPases: masters of T lymphocyte migration and activation. Immunol Lett. 2012;142:1–13. doi: 10.1016/j.imlet.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 41.van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends Immunol. 2006;27:49–55. doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 43.Shaw AC, Panda A, Joshi SR, Qian F, Allore HG, Montgomery RR. Dysregulation of human Toll-like receptor function in aging. Ageing Res Rev. 2011;10:346–53. doi: 10.1016/j.arr.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masters SL, Latz E, O'Neill LA. The inflammasome in atherosclerosis and type 2 diabetes. Sci Transl Med. 2011;3:81ps17. doi: 10.1126/scitranslmed.3001902. [DOI] [PubMed] [Google Scholar]
- 45.Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, Verma IM, Olefsky JM. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–29. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagy L, Szanto A, Szatmari I, Széles L. Nuclear hormone receptors enable macrophages and dendritic cells to sense their lipid environment and shape their immune response. Physiol Rev. 2012;92:739–89. doi: 10.1152/physrev.00004.2011. [DOI] [PubMed] [Google Scholar]
- 47.Benvenuti S, Comoglio PM. The MET receptor tyrosine kinase in invasion and metastasis. J Cell Physiol. 2007;213:316–25. doi: 10.1002/jcp.21183. [DOI] [PubMed] [Google Scholar]
- 48.Moransard M, Sawitzky M, Fontana A, Suter T. Expression of the HGF receptor c-met by macrophages in experimental autoimmune encephalomyelitis. Glia. 2010;58:559–71. doi: 10.1002/glia.20945. [DOI] [PubMed] [Google Scholar]
- 49.Chazaud B. Dual effect of HGF on satellite/myogenic cell quiescence. Focus on “High concentrations of HGF inhibit skeletal muscle satellite cell proliferation in vitro by inducing expression of myostatin: a possible mechanism for reestablishing satellite cell quiescence in vivo”. Am J Physiol Cell Physiol. 2010;298:C448–49. doi: 10.1152/ajpcell.00561.2009. [DOI] [PubMed] [Google Scholar]
- 50.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–08. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pagler TA, Wang M, Mondal M, Murphy AJ, Westerterp M, Moore KJ, Maxfield FR, Tall AR. Deletion of ABCA1 and ABCG1 impairs macrophage migration because of increased Rac1 signaling. Circ Res. 2011;108:194–200. doi: 10.1161/CIRCRESANA.110.228619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hinnebusch AG. eIF3: a versatile scaffold for translation initiation complexes. Trends Biochem Sci. 2006;31:553–62. doi: 10.1016/j.tibs.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Sanchez AM, Csibi A, Raibon A, Docquier A, Lagirand-Cantaloube J, Leibovitch MP, Leibovitch SA, Bernardi H. eIF3f: a central regulator of the antagonism atrophy/hypertrophy in skeletal muscle. Int J Biochem Cell Biol. 2013;45:2158–62. doi: 10.1016/j.biocel.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandhu SK, Kaur G. Mitochondrial electron transport chain complexes in aging rat brain and lymphocytes. Biogerontology. 2003;4:19–29. doi: 10.1023/A:1022473219044. [DOI] [PubMed] [Google Scholar]
- 55.Preston CC, Oberlin AS, Holmuhamedov EL, Gupta A, Sagar S, Syed RH, Siddiqui SA, Raghavakaimal S, Terzic A, Jahangir A. Aging-induced alterations in gene transcripts and functional activity of mitochondrial oxidative phosphorylation complexes in the heart. Mech Ageing Dev. 2008;129:304–12. doi: 10.1016/j.mad.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ibebunjo C, Chick JM, Kendall T, Eash JK, Li C, Zhang Y, Vickers C, Wu Z, Clarke BA, Shi J, Cruz J, Fournier B, Brachat S, et al. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol Cell Biol. 2013;33:194–212. doi: 10.1128/MCB.01036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 1999;372:399–407. doi: 10.1006/abbi.1999.1508. [DOI] [PubMed] [Google Scholar]
- 58.Lundahl J, Halldén G, Hallgren M, Sköld CM, Hed J. Altered expression of CD11b/CD18 and CD62L on human monocytes after cell preparation procedures. J Immunol Methods. 1995;180:93–100. doi: 10.1016/0022-1759(94)00303-E. [DOI] [PubMed] [Google Scholar]
- 59.Hardie DL, Baldwin MJ, Naylor A, Haworth OJ, Hou TZ, Lax S, Curnow SJ, Willcox N, MacFadyen J, Isacke CM, Buckley CD. The stromal cell antigen CD248 (endosialin) is expressed on naive CD8+ human T cells and regulates proliferation. Immunology. 2011;133:288–95. doi: 10.1111/j.1365-2567.2011.03437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Connolly PH, Caiozzo VJ, Zaldivar F, Nemet D, Larson J, Hung SP, Heck JD, Hatfield GW, Cooper DM. Effects of exercise on gene expression in human peripheral blood mononuclear cells. J Appl Physiol (1985) 2004;97:1461–69. doi: 10.1152/japplphysiol.00316.2004. [DOI] [PubMed] [Google Scholar]
- 61.Zieker D, Zieker J, Dietzsch J, Burnet M, Northoff H, Fehrenbach E. CDNA-microarray analysis as a research tool for expression profiling in human peripheral blood following exercise. Exerc Immunol Rev. 2005;11:86–96. [PubMed] [Google Scholar]
- 62.Chopard A, Lecunff M, Danger R, Lamirault G, Bihouee A, Teusan R, Jasmin BJ, Marini JF, Leger JJ. Large-scale mRNA analysis of female skeletal muscles during 60 days of bed rest with and without exercise or dietary protein supplementation as countermeasures. Physiol Genomics. 2009;38:291–302. doi: 10.1152/physiolgenomics.00036.2009. [DOI] [PubMed] [Google Scholar]
- 63.Radom-Aizik S, Zaldivar F, Jr, Leu SY, Cooper DM. Brief bout of exercise alters gene expression in peripheral blood mononuclear cells of early- and late-pubertal males. Pediatr Res. 2009;65:447–52. doi: 10.1203/PDR.0b013e3181993473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 65.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci USA. 2001;98:31–36. doi: 10.1073/pnas.98.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kauffmann A, Gentleman R, Huber W. arrayQualityMetrics--a bioconductor package for quality assessment of microarray data. Bioinformatics. 2009;25:415–16. doi: 10.1093/bioinformatics/btn647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wasserman K. Critical capillary PO2 and the role of lactate production in oxyhemoglobin dissociation during exercise. Adv Exp Med Biol. 1999;471:321–33. doi: 10.1007/978-1-4615-4717-4_39. [DOI] [PubMed] [Google Scholar]
- 68.Appay V, Sauce D. Naive T cells: the crux of cellular immune aging? Exp Gerontol. 2014;54:90–93. doi: 10.1016/j.exger.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 69.Fülöp T, Larbi A, Pawelec G. Human T cell aging and the impact of persistent viral infections. Front Immunol. 2013;4:271. doi: 10.3389/fimmu.2013.2013.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gayoso I, Sanchez-Correa B, Campos C, Alonso C, Pera A, Casado JG, Morgado S, Tarazona R, Solana R. Immunosenescence of human natural killer cells. J Innate Immun. 2011;3:337–43. doi: 10.1159/00328005. [DOI] [PubMed] [Google Scholar]
- 71.Panda A, Arjona A, Sapey E, Bai F, Fikrig E, Montgomery RR, Lord JM, Shaw AC. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol. 2009;30:325–33. doi: 10.1016/j.it.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmitt V, Rink L, Uciechowski P. The Th17/Treg balance is disturbed during aging. Exp Gerontol. 2013;48:1379–86. doi: 10.1016/j.exger.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 73.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–94. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 74.van Lier RA, ten Berge IJ, Gamadia LE. Human CD8(+) T-cell differentiation in response to viruses. Nat Rev Immunol. 2003;3:931–39. doi: 10.1038/nri1254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.