Abstract
The lysosome is the main catabolic hub of the cell. Owing to its role in fundamental processes such as autophagy, plasma membrane repair, mTOR signaling, and maintenance of cellular homeostasis, the lysosome has a profound influence on cellular metabolism and human health. Indeed, inefficient or impaired lysosomal function has been implicated in the pathogenesis of a number of degenerative diseases affecting various organs and tissues, most notably the brain, liver, and muscle. The discovery of the coordinated lysosomal expression and regulation (CLEAR) genetic program and its master controller, transcription factor EB (TFEB), has provided an unprecedented tool to study and manipulate lysosomal function. Most lysosome-based processes—including macromolecule degradation, autophagy, lysosomal exocytosis, and proteostasis—are under the transcriptional control of TFEB. Interestingly, impaired TFEB signaling has been suggested to be a contributing factor in the pathogenesis of several degenerative storage diseases. Preclinical studies based on TFEB exogenous expression to reinstate TFEB activity or promote CLEAR network–based lysosomal enhancement have highlighted TFEB as a candidate therapeutic target for the treatment of various degenerative storage diseases.
Keywords: transcription factor EB, TFEB, lysosome enhancement, degenerative storage diseases, degradative pathways, autophagy, proteinopathies
Introduction
The first evidence of a genetic program that oversees cellular catabolic needs by coordinating the biogenesis of lysosomes was provided in 2009 with the characterization of the basic helix-loop-helix leucine zipper transcription factor EB (TFEB) as a master regulator of lysosomal function.1 Owing to progress in understanding the involvement of lysosomal catabolic pathways in the regulation of cell metabolism in physiological and pathological conditions, the identification of TFEB has triggered intense research efforts in the investigation of lysosomal regulation and TFEB involvement in disease pathogenesis in conditions as diverse as neurodegenerative disease and cancer. Additional studies are exploring the range of cellular and organismal processes in which TFEB plays essential roles. Finally, TFEB is being investigated as a practical and convenient tool to modulate lysosome-dependent degradative processes in cell biology studies and, interestingly, as an instrument to test the potential of lysosomal enhancement as a therapeutic strategy for an increasing number of degenerative diseases. This review focuses on the progresses made thus far in understanding lysosomal regulation by TFEB, the processes it governs, and its possible contribution to the pathogenesis of various degenerative storage diseases. Also discussed are recent results from preclinical studies based on TFEB exogenous expression in cellular and animal models of proteinopathies and storage disorders.
TFEB promotes lysosomal biogenesis and function
Following the hypothesis that lysosomal pathways may be coordinated at the level of gene expression, sequence analysis of the promoters of lysosomal genes indicated the enrichment of a palindromic motif (TCACGTGA) in the proximity of their transcription start sites. A genome-wide search established that this motif was generally associated with genes with lysosomal function, and a meta-analysis of a vast set of expression datasets showed that these genes have indeed coordinated expression. The enriched motif was therefore named coordinated lysosomal expression and regulation (CLEAR) element.1 A similar approach was previously used to discover a regulatory element associated with the expression of nuclear genes encoding mitochondrial proteins in Drosophila.2, 3
Out of four candidate transcription factors tested, TFEB was able to induce the expression of lysosomal genes and increase lysosomal proliferation in cultured cells.1 TFEB was identified as a target of microRNA-128 (miR-128), and TFEB silencing obtained using a miR-128 mimic showed downregulation of CLEAR lysosomal genes, thus demonstrating that active TFEB is required for the expression of CLEAR genes.1 Furthermore, chromatin immunoprecipitation (ChIP) analysis confirmed direct binding of TFEB to lysosomal promoters.1 A genome-wide expression analysis of genes induced by TFEB revealed that various classes of genes serving the lysosomal system—including those encoding autophagy proteins, transporters of lysosomal enzymes, and proteins protecting the cell from the leakage of such enzymes—were also CLEAR genes under the transcriptional control of TFEB.1 Proof of principle that hitherto unrecognized lysosomal genes could be identified by testing uncharacterized TFEB targets was provided with the identification of C1orf85/GLMP as a novel lysosomal membrane protein.1 A subsequent analysis that integrated TFEB ChIP-seq analysis with genome-wide mapping of CLEAR sites, co-expression meta-analysis of lysosomal genes, and transcriptional induction by TFEB determined that the CLEAR network comprises approximately 400 putative direct TFEB targets and also led to the identification of nine novel lysosomal proteins.4 Lysosomal genes that are under the control of TFEB tend to have clusters (either tandems or tetramers) of CLEAR motifs at their promoters. CLEAR genes control expression, import, and activity of lysosomal enzymes involved in the degradation of proteins, glycosaminoglycans, complex lipids, and glycogen.1, 4 Interestingly, non-lysosomal enzymes involved in the degradation of hemoglobin and chitin were also found to be under the control of TFEB.4 TFEB was also found to modulate the expression of genes involved in processes such as lysosomal acidification, exocytosis, and endocytosis.1, 4 Additional genes and pathways that are part of the CLEAR network include components of the innate immune system, the transcriptional coactivator PGC1α—which is involved in mitochondrial biogenesis and the insulin signaling pathway—and components of AMP-activated protein kinase (AMPK) and mechanistic target of rapamycin (mTOR) signaling.4
Subsequent work showed that TFEB promotes the formation of autophagosomes and their fusion with lysosomes through the upregulation of several key autophagy and lysosomal genes, a process that is initiated by nutrient starvation and executed by inhibition of extracellular signal-regulated kinase 2 (ERK2)-mediated phosphorylation of TFEB at Ser142.5 TFEB and its paralogs, TFE3 and microphthalmia-associated transcription factor (MITF), are also required for the execution of mitophagy—a type of autophagy that selectively eliminates damaged mitochondria; these TFs act downstream of the mitophagy regulators, PINK1 and Parkin, and mitophagy execution requires core components of autophagosome formations, such as Atg9A and Atg5.6 TFEB-mediated autophagy induction was found to be antagonized by ZKSCAN3, a master transcriptional repressor of autophagy.7
Exogenous expression of human TFEB in the mouse liver revealed activation of lipid catabolism and oxidation pathways and repression of lipid biosynthetic processes.8 One of the most upregulated genes was found to be PGC1α, which was confirmed to be a direct TFEB transcriptional target under starvation conditions. Indeed, TFEB overexpression in livers lacking either PGC1α or its nuclear receptor, peroxisome proliferator-activated receptor α (PPARα), resulted in diminished activation of lipid metabolism genes, indicating that PGC1α–PPARα signaling mediates the effects of TFEB on lipid metabolism.8 TFEB availability in the liver of mice challenged with a high-fat diet correlated with the capacity to activate lipid degradation, which depended on a functional autophagic pathway. Significantly, TFEB liver overexpression in a genetic model of obesity improved metabolic syndrome phenotypes.8
Trafficking of lysosomal enzymes from the endoplasmic reticulum (ER)—the site of their synthesis—to the endolysosomal system through the Golgi complex is an essential step of lysosomal biogenesis. Missense mutations in lysosomal enzymes often disrupt protein folding, subsequently interfering with their proper transport to the lysosome and leading to their degradation. TFEB overexpression could rescue protein trafficking and maturation of the common L444P mutant of glucosylceramidase, the protein mutated in Gaucher’s disease, by enhancing the cell’s folding and trafficking networks, highlighting the role played by TFEB in the control of lysosomal proteostasis.9
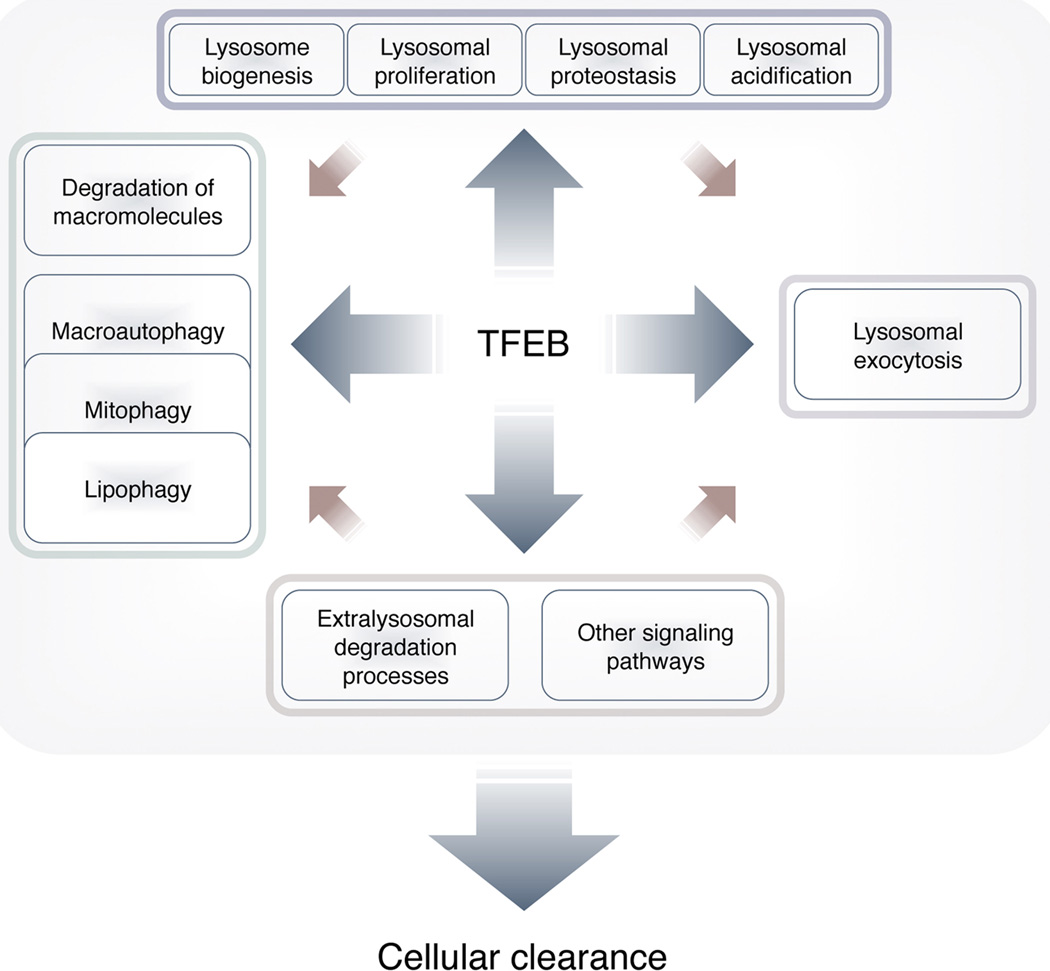
Collectively, these findings have provided a coherent picture of the TFEB/CLEAR network as a regulatory hub that controls cellular clearance through modulation of lysosomal biogenesis and proliferation and relevant lysosomal degradative functions, including degradation of macromolecules, autophagy, mitophagy, and lipophagy (Fig. 1).
Figure 1.
TFEB-regulated processes. TFEB positively regulates the transcription of genes involved in all steps of lysosome biogenesis. TFEB promotes lysosomal proliferation, acidification, and exocytosis, and induces genes involved in autophagic pathways. Together, TFEB-modulated processes promote clearance of lysosomal and autophagic substrates.
Transcriptional and posttranscriptional regulation of TFEB
TFEB mRNA levels are either up- or downregulated by various endogenous mechanisms. For example, miR-128 has a target sequence in TFEB 3’UTR, and miR-128–mediated downregulation of TFEB levels has been shown in human cells1 and in the rat brain.10 Conversely, lysosomal stress induced by the addition of sucrose to the media of cultured cells was shown to upregulate TFEB expression and promote TFEB translocation from a cytosolic location to the nucleus.1 This observation provided the first evidence for endogenous activation of TFEB as an adaptive response to lysosomal stress, which was subsequently confirmed by the analysis of mouse embryonic fibroblasts from various models of lysosomal storage disorders showing increased TFEB nuclear accumulation.1 Increased TFEB expression and transcriptional activation of the CLEAR network have also been detected in an induced pluripotent stem (iPS) cell model of Huntington disease,11 a proteinopathy characterized by accumulation of cellular inclusions of polyQ-expanded huntingtin, and in the brain of mice lacking presenilin expression (a protein involved in lysosomal homeostasis) in excitatory neurons.12 These findings underline the sensitivity of the lysosomal-autophagy system to stress signals due to accumulated or undegraded material. TFEB nuclear translocation has also been observed as an adaptive response to a variety of stress conditions, including exposure to arsenic,13 lysosomal stressors,13–17 oxidative stress,18, 19 transition metals,20 ethanol,21 and the senescence inducer doxorubicin.22
Starvation studies in cells and mouse tissues have demonstrated that TFEB regulates its own transcription by binding to CLEAR sites located in its first intron.8 TFEB expression levels are also modulated by PGC1α,23 a master activator of mitochondrial proliferation, which is, in turn, a TFEB direct target.8 PGC1α binds to one of the promoters of TFEB,23 and its regulation of TFEB is essential to TFEB activation for mitophagy.24 PGC1α regulation of TFEB transcription is achieved via PPARα/retinoid X receptor α (RXRα) heterodimer co-binding of TFEB promoter and can be mimicked by the PPARα agonist, gemfibrozil.25 Additional identified transcriptional regulators of TFEB are the fed-state sensing nuclear receptor farnesoid X receptor (FXR) and the fasting transcriptional activator cAMP response element–binding protein (CREB), which inhibits and induces TFEB expression in the liver in a fed or fasting state, respectively.26
TFEB nuclear translocation is modulated by posttranslational modifications, such as phosphorylation at specific serine residues. ERK2 was first identified as a controller of TFEB subcellular localization by phosphorylation at Ser142, resulting in TFEB cytosolic retention, a process abolished by nutrient starvation.5 Subsequently, mTOR complex 1 (mTORC1), a kinase complex that functions as a switch between cellular anabolic and catabolic pathways, was also identified as a regulator of TFEB subcellular location via direct phosphorylation of specific TFEB residues. In cells cultured in nutrient-poor conditions, knockout/down of tuberous sclerosis 2 (Tsc2), an upstream regulator of mTORC1, resulted in partial dephosphorylation and nuclear relocation of endogenous TFEB, which was reverted by rapamycin administration and required a serine-rich region at the TFEB C-terminus.27 In nutrient-replete cells, inhibition of mTORC1 promoted nuclear accumulation of TFEB by abrogation of mTORC1 phosphorylation of TFEB at Ser14228 and Ser211.29, 30 Phosphorylation of TFEB at Ser211 promotes TFEB interaction with the YWHA/14-3-3 proteins,29, 30 which determine TFEB cytosolic retention by masking a nuclear localization signal located between amino acids 241 and 252.30 The interaction between TFEB and mTORC1 occurs at the lysosomal surface and is indeed abolished by dominant-negative mutations in Rag GTPases (Rags),28 proteins that mediate the recruitment of mTORC1 to the lysosomal membrane. Conversely, constitutively active versions of Rags lead to TFEB retention in the cytoplasm, irrespective of the nutrient status of the cell.28 TFEB localization to lysosomes requires the first 30 TFEB amino acids30 and is mediated by direct interaction with active Rags heterodimers upon amino acid stimulation.31 Further studies showed that starvation increases lysosomal activity in cultured cells, which requires mTORC1 inhibition and TFEB activation.32 Interestingly, mTORC1 lysosomal localization was found not to be essential for mTORC1 regulation of lysosomal function.32 Collectively, these findings identify a model of crosstalk between lysosomes and the metabolic needs of the cell, in which TFEB cycles between the cytosol and the lysosomal surface. According to this model, the lysosomal localization of TFEB by active Rag GTPases in fully fed cells leads to TFEB phosphorylation by mTORC1, and the subsequent interaction with 14-3-3 proteins keeps TFEB sequestered in the cytosol. Conditions such as amino acid deprivation that inhibit mTORC1 phosphorylation of TFEB leave TFEB free to move to the nucleus and activate its transcriptional program to cope with the catabolic cellular demands. Chemical inhibition of the glycogen synthase kinase 3 (GSK3) also promotes TFEB nuclear translocation33 via TFEB dissociation from 14-3-3 proteins,34 albeit the exact molecular mechanism has not yet been elucidated.
Drugs that act via the PI3K/AKT/mTORC1 pathway, including the nicotinamide phosphoribosyltransferase inhibitor FK86635 and the PI3K inhibitor GDC-0941,36 also promote TFEB nuclear translocation in cultured cells. Additional reports have shown increased TFEB nuclear translocation following administration of 2-hydroxypropyl-β-cyclodextrin37 and the flavonoid genistein,38 two drugs that are currently under investigation for the treatment of Niemann–Pick disease type C and some mucopolysaccharidosis subtypes, respectively. Other flavonoids38 and curcumin39 have also been reported to be able to induce TFEB, but the exact mechanism of action of these drugs still needs to be clarified.
TFEB dephosphorylation requires the phosphatase calcineurin and lysosomal Ca2+ release through mucolipin 1/TRPML1, an ion channel that is under the transcriptional control of TFEB.40 Starvation- and exercise-induced activity of TFEB is indeed suppressed upon calcineurin inhibition.40 Thus, lysosomal Ca2+ release regulates lysosomal processes via calcineurin regulation of TFEB.40
An additional mechanism of TFEB activation has been characterized in osteoclasts, which is based on protein kinase C-β (PKCβ) phosphorylation of TFEB on serines at the protein C-terminus upon signaling of the osteoclast differentiation factor RANKL.41 Enhanced lysosomal biogenesis in this case increases the resorptive function of differentiated osteoclasts, as demonstrated by a diminished ability to resorb the bone matrix observed in mouse osteoclasts with selective deletion of Tfeb.41
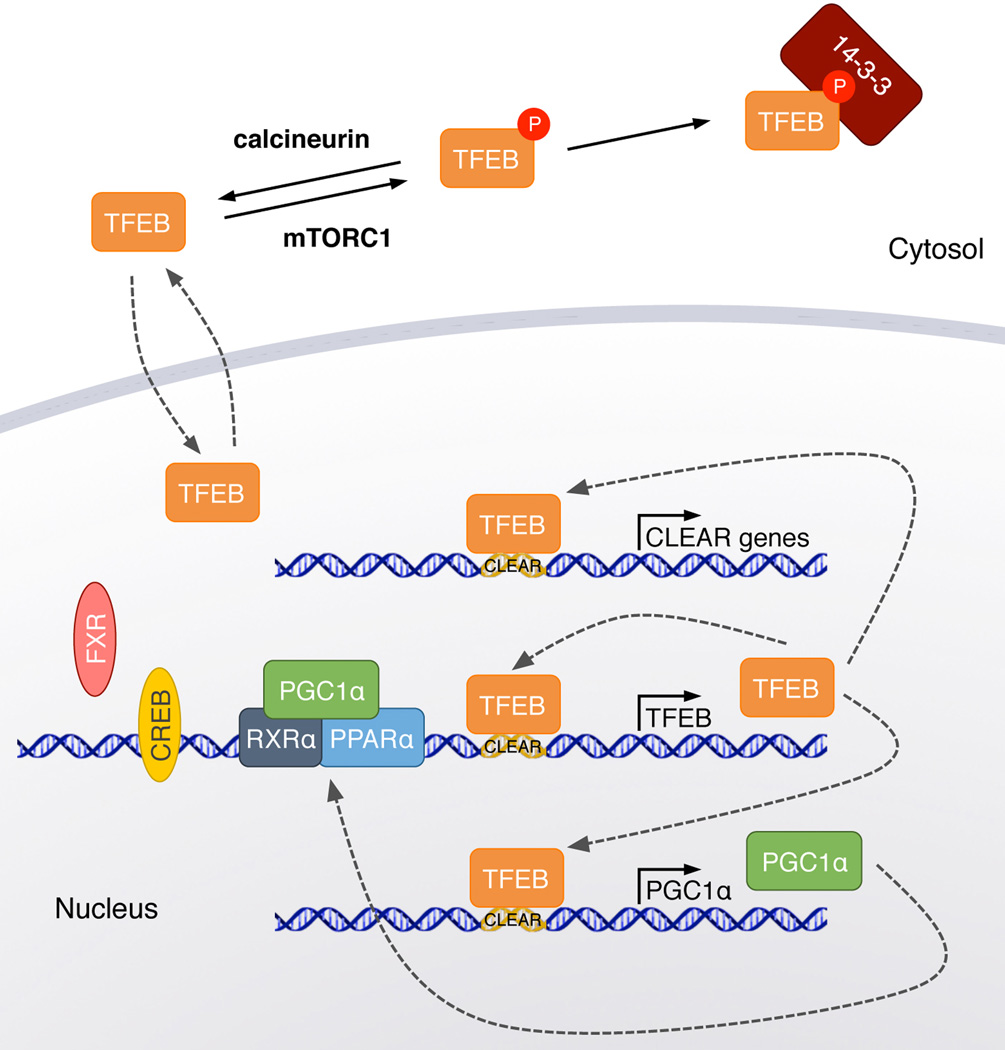
TFEB is a demonstrated target of chaperone-mediated autophagy (CMA), a process of lysosomal degradation of select cytosolic proteins.42 Because blockage of chaperone-mediated autophagy results in increased macroautophagy, the defective degradation of TFEB by CMA may be at least partly responsible for such an increase.42 These findings therefore indicate that CMA-mediated degradation of TFEB may underlie the crosstalk between these two autophagic pathways.42 Together, these studies highlight the complex regulation of TFEB and its tight intertwinement with other main regulatory hubs controlling cell metabolism (Fig. 2).
Figure 2.
Regulation of TFEB. Schematic representation of transcriptional and posttranslational regulation of TFEB. TFEB transcription is reinforced by at least two positive loops, one based on TFEB self-induction, and the other based on TFEB-mediated induction of the transcriptional co-activator PGC1α, which in turn promotes TFEB transcription. TFEB transcription is also positively regulated by CREB in competition with the negative regulator FRX. TFEB phosphorylation by mTORC1 triggers TFEB cytosolic retention by 14-3-3 proteins. Dephosphorylation of TFEB by calcineurin promotes TFEB nuclear translocation and activation of the CLEAR network.
TFEB family members and their TFEB-related functions
TFEB heterodimerizes with TFE3, MITF, and TFEC, three closely related transcription factors that are conserved in vertebrates and which, together with TFEB, constitute the MITF/TFE family.43 Similar to TFEB, TFE3 and MITF are also bound by YWHA/14-3-3 proteins,44, 45 associate with lysosomes in nutrient-replete cells, and translocate to the nucleus upon nutrient starvation or chloroquine-induced lysosomal stress.30, 46 The subcellular localization of TFE3 and MITF also depends on mTORC1 activation status and is regulated via Rag-mediated recruitment to the lysosomal surface.31, 46 TFE3 promotes transcription of lysosomal and autophagy genes, and interestingly, also induces transcription of the mTORC1 activator folliculin and its interacting proteins, FNIP1 and FNIP2, thereby terminating the lysosomal starvation response when nutrients become available.46 Similar to TFE3, TFEB can also induce expression of folliculin and the FNIPs, thus indicating a possible similar feedback mechanism. MITF has been shown to drive endolysosomal biogenesis in melanoma cells.47 TFE3 has also been implicated in the regulation of the Golgi stress response48 and of PGC1α in muscle.49 Furthermore, a role for TFEB and TFE3 has been defined as part of the ER stress response;50 TFEB and TFE3 translocate to the nucleus upon chemically induced ER stress. TFEB activation by ER stress depends on PERK, a well-known effector of the unfolded protein response, and on active calcineurin,50 a PERK target and the TFEB phosphatase.40 The UPR effector, ATF4, has been characterized as the direct TFEB and TFE3 target necessary for the participation of these proteins in the ER stress response.50
The MITF/TFE family has ancient origins that date back at least to the common ancestor of nematodes and other invertebrates. Invertebrates only have one homolog to vertebrate TFEB/MITF/TFE3/TFEC genes—hlh-30 in Caenorhabditis elegans and Mitf in Drosophila, which regulate lysosomal pathways and autophagy with mechanisms similar to the vertebrate genes.8,51,52 C. elegans HLH-30 was found to be required for elongation of lifespan in a subset of conditions, including starvation-induced longevity,8 loss of function of its antagonist transcription factor mxl-3,51 and reduced TOR activity.53 Interestingly, overexpression of HLH-30 itself was able to extend C. elegans lifespan.53
Whereas these studies highlight functional overlap among MITF/TFE proteins and a noticeable conservation of their function throughout evolution, it remains to be established whether factors such as the specific developmental stage, tissue, and metabolic demands dictate the extent of overlapping, complementary, or differential functions of these proteins.
Impaired TFEB signaling as a contributor to disease pathogenesis
Several independent studies support the idea that the function of TFEB may have various layers of intertwinement with the pathogenesis of degenerative disease. Indeed, investigations of human specimens and animal models of neurodegenerative diseases have suggested that impaired TFEB signaling may contribute to disease pathogenesis. Most examples come from diseases in which the primary molecular pathogenic event is the intracellular accumulation of aberrant protein inclusions. For example, Tfeb expression was found to be reduced in the striatum of a mouse model of Huntington disease and in Htt-104Q–expressing Neuro2a cells. The expression of TFEB target enzymes was found to be subsequently diminished, and was rescued by PGC1α-mediated activation of TFEB expression.23
Another example was identified with the observation that lysosomes are depleted in Parkinson’s disease.54 α-Synuclein, a protein pathologically accumulated in Parkinson’s disease, is structurally and functionally similar to 14-3-3 proteins, which are responsible for the cytosolic retention of TFEB.19, 20 TFEB was found to interact with overexpressed α-synuclein—therefore, excess α-synuclein might sequester TFEB and prevent its nuclear translocation.10 This model is supported by an analysis of postmortem tissues from Parkinson’s disease patients showing that TFEB is depleted from the nucleus and accumulates with α-synuclein in Lewy bodies in nigral dopaminergic neurons.10 TFEB sequestration may therefore be responsible for the observed lysosomal depletion in Parkinson’s disease. Indeed, exogenous expression of TFEB restored lysosomal biogenesis and ameliorated disease phenotypes in an animal model of Parkinson’s disease.10
Another intriguing example of impaired TFEB signaling in neurodegenerative disease was provided by the observation that miR-128, a microRNA that reduces TFEB expression,1 is consistently upregulated in the hippocampus,55 monocytes, and lymphocytes56 of Alzheimer’s disease patients. The expression levels of TFEB and the expression and activities of various lysosomal enzymes were also found to be reduced in mononuclear cells from Alzheimer’s disease patients, presumably as a consequence of miR-128 inhibition of TFEB expression.56 Interestingly, these cells displayed a decreased ability to degrade Aβ(1–42), and inhibition of miR-128 expression rescued these cellular phenotypes, including Aβ(1–42) degrading ability.56
Additional reports of impaired TFEB signaling focused on studies of spinobulbar muscular atrophy (SBMA), a disease caused by polyglutamine expansion of the androgen receptor (AR). An analysis of SBMA mice showed that polyQ AR expression chronically enhances autophagy in the skeletal muscle—the major site of polyQ AR toxicity—through inhibition of mTORC1 activity. Indeed, mTORC1 inhibition resulted in activation of TFEB in the skeletal muscle, thereby conferring SBMA mice with elevated sensitivity to autophagy hyperactivation following starvation and exercise and contributing to the detrimental effects observed in the SBMA skeletal muscle.57 Interestingly, other studies reported opposite effects of polyQ AR modulation of TFEB activity in non-muscle tissues. In the spinal cord of SBMA mice, polyQ AR was found to reduce the expression of TFEB via sequestration of the nuclear factor-YA (NF-YA), thus diminishing the activity of the autophagic-lysosomal pathway and subsequently enhancing polyQ toxicity.58 TFEB was also shown to interact physically with both AR and polyQ AR.59 Whereas AR promotes transcription of TFEB, polyQ AR impairs TFEB activation and autophagy in SBMA motor neurons as well as in patient-derived neuronal precursor cells, thus supporting a model of autophagy impairment in the neuronal pathogenesis of SBMA.59 Collectively, these findings suggest a tissue-specific dysregulation of TFEB by polyQ AR and indicate that treatments aimed to enhance autophagy, if not designed to avoid targeting the skeletal muscle, may be detrimental to the health of this tissue.
Finally, it has been reported that presenilin deficiency, a cause of familiar Alzheimer’s disease, diminishes TFEB-mediated activation of starvation-induced autophagy.60 Indeed, presenilin deficiency causes reduced expression of the mTORC1 leucine sensor Sestrin2, resulting in constitutive mTORC1 tethering to the lysosomal membrane and TFEB insensitivity to autophagic stimuli.60
Taken together, these studies support a model in which pathogenic protein accumulation is exacerbated by impaired lysosomal function due to TFEB diminished activity—which, at least in some instances, is caused by the pathogenic protein accumulation itself, thus feeding a negative loop that ultimately inhibits lysosomal degradative pathways.
Examples of an upregulated or unrestrained increase of TFEB function as possible contributors of disease pathogenesis have also been provided. The association of TFEB or TFE3 strong overexpression, resulting from chromosomal translocation events, with the insurgence of renal carcinoma in a subset of patients has long been known.61 A recent study62 has focused on folliculin, a protein whose deficiency causes Birt–Hogg–Dubé syndrome—a condition characterized by hair follicle tumors, an increased risk for the growth of pulmonary cysts, and an elevated incidence of renal carcinoma. Folliculin was found to be required for mTORC1 enrichment on the lysosomal surface. Therefore, in absence of folliculin, TFEB escapes mTORC1-mediated inhibition and displays enhanced activity as shown by increased lysosomal biogenesis.62 In another study, an extensive analysis of human pancreatic ductal adenocarcinoma (PDA) cells revealed that TFEB, TFE3, and MITF are decoupled from mTORC1-mediated regulation of their cytoplasmic retention due to association with importin 8, a nucleocytoplasmic transporter with elevated expression in PDA. Unrestricted activity of the MITF/TFE proteins thus results in high levels of autophagic-lysosomal catabolism, which support PDA growth.63 In a different setting, TFEB overactivation has been implicated in skeletal muscle wasting in congestive heart failure via its angiotensin II–induced upregulation of muscle-enriched E3 ubiquitin ligase muscle RING finger-1 (MuRF1) expression via a complex PKD1/HDAC5/TFEB/MuRF1 cascade.64
Understanding the significance of TFEB dysregulation in the context of the onset and progression of these different diseases merits future studies. A general impression emerges, however, about the necessity of keeping TFEB activity within a certain range. Either too little or too much of TFEB activity, especially when protracted for long periods of time, seems to have unhealthy outcomes that are associated with mechanisms that may vary with the nature of the primary insult and the affected tissue type.
TFEB as a tool to enhance cellular clearance in degenerative storage diseases
Proteinopathies and other degenerative storage diseases—including lysosomal storage disorders—are characterized by the aberrant intracellular accumulation of macromolecules, which, in at least some cases, may contribute to disease pathogenesis. The first demonstration that TFEB could be used as a tool to modulate cellular clearance was provided by monitoring the rate of degradation of glycosaminoglycans (substrates of a series of lysosomal CLEAR enzymes) and of expanded huntingtin protein (an autophagic substrate) upon overexpression of TFEB. In both cases, TFEB promoted degradative pathways and enhanced cellular clearance.1 Similarly, subsequent studies based on heterologous expression of TFEB expanded the range of the potential applications of TFEB modulation to several human diseases.
Parkinson’s disease
Lysosomal depletion and accumulation of autophagic vacuoles have been observed in postmortem samples from Parkinson’s disease patients.54 The parkinsonian neurotoxin 1-methyl-4-phenylpyridinium (MPP+) exerts its toxic effect through inhibition of complex I. This leads to increased mitochondrial ROS production and subsequent lysosomal membrane permeabilization, finally resulting in cytosolic release of cathepsins and cell death.65 TFEB transfection in MPP+–intoxicated human neuroblastoma cells reversed lysosomal depletion and attenuated cell death,65 suggesting that intervention on a secondary but critical pathway in the pathogenic cascade could attenuate disease manifestations. In vivo experiments focused on α-synuclein accumulation significantly extended these results. Adeno-associated viral (AAV)–mediated delivery of TFEB in the brain of a rat model of Parkinson’s disease obtained by α-synuclein overexpression reduced accumulation of α-synuclein oligomers and ameliorated neurodegeneration and behavioral defects.10 Chemical inhibition of mTORC1 by injection of the rapamycin precursor temsirolimus promoted nuclear translocation of TFEB in the brain of Parkinson’s disease rats and provided neuroprotection against α-synuclein toxicity.10 Conversely, AAV-mediated delivery of miR-128 in the brain of this Parkinson’s disease model downregulated TFEB and exacerbated disease features.10
Lysosomal storage disorders
Lysosomal storage disorders are caused by mutations in genes serving the lysosomal system—lysosomal enzymes, their transporters, or other proteins participating in lysosomal biogenesis and homeostasis. TFEB overexpression promoted cellular clearance in cultured cells derived from animal models of multiple sulfatase deficiency (MSD) and mucopolysaccharidosis IIIA (MPSIIIA) and from skin fibroblasts derived from patients with Batten disease (juvenile neuronal ceroid lipofuscinosis, JNCL) and Pompe disease.66 Since lysosomal enzymes are typically non-redundant, mechanisms of macromolecular clearance in these diseases cannot be solely reliant on the upregulation of other lysosomal enzymes. Indeed, it was determined that TFEB promotes lysosomal exocytosis by both stimulating the recruitment of lysosomes to the plasma membrane and increasing the local Ca2+ concentrations—required for the fusion of lysosomal and plasma membranes—through the TRPML1/MCOLN1 channel, a TFEB direct target.66 The injection of AAVs carrying TFEB cDNA in a mouse model of MSD reduced the storage of glycosaminoglycans as well as tissue inflammation and apoptosis,66 demonstrating the potential validity of such an approach. Similarly, AAV-mediated expression of TFEB in cellular and mouse models of Pompe disease, a lysosomal storage disorder characterized by glycogen accumulation in the muscle, resulted in removal of pathologically enlarged lysosomes by virtue of induced lysosomal exocytosis.67 TFEB overexpression resulted in enhanced autophagosomal clearance in these test systems, and experiments conducted in autophagy-defective muscles showed that the overall clearance of accumulated material required a functional autophagic pathway.67 TFE3 was also shown to induce lysosomal exocytosis, and TFE3 overexpression promoted cellular clearance in a model of Pompe disease.46 Finally, one study showed that 2-hydroxypropyl-β-cyclodextrin–mediated activation of TFEB was able to induce clearance of accumulated proteinaceous material in cells defective for the lysosomal protease, tripeptidyl peptidase (TPP1), using a mechanism that requires functional autophagy.37
PolyQ diseases
TFEB overexpression counteracted the aggregation of polyQ-expanded huntingtin in various cellular models of Huntington disease.1,23 In vivo, overexpression of the TFEB inducer PGC1α increased mRNA levels of TFEB and of CLEAR genes and reverted aggregate formation and pathology in a mouse model of Huntington disease.23 Because Huntington disease pathology is characterized by both mitochondrial and autophagic dysfunctions, the possibility of targeting the PGC1α–TFEB axis is therefore particularly attractive for this disease. Treatment of SBMA mice with paeoniflorin, an active component of Paeonia plants, induced nuclear translocation of TFEB and the expression of components of the ubiquitin-proteasome system via increased expression of nuclear factor-YA (NF-YA). Such treatment improved clearance of polyQ-expanded AR, thus mitigating the disease phenotypes.58 Similarly, overexpression of TFEB in SBMA neuronal precursor cells rescued the observed autophagy dysregulation and mitochondrial dysfunction.59 Interestingly, overexpression of Drosophila Mitf in medulloblastoma-derived cells stably expressing polyQ-expanded ataxin 1 (a model of spinocerebellar ataxia type 1, SCA1) also resulted in the reduction of ataxin 1 aggregates, indicating functional conservation of Mitf as a promoter of cellular clearance.52
Tauophaties and Alzheimer’s disease
The Alzheimer’s disease brain is characterized by extracellular accumulation of the β-amyloid peptide (Aβ) and intracellular accumulation of neurofibrillary tangles, insoluble inclusions primarily constituted of hyperphosphorylated and cleaved forms of tau. AAV-mediated TFEB expression in the brain of a mouse model of tauopathy reduced neurofibrillary tangle pathology and rescued synaptic deficits and neurodegeneration.68 TFEB was shown to specifically target hyperphosphorylated and misfolded Tau to degradation, a mechanism that required TFEB activation of its direct transcriptional target, PTEN, which is implicated in Tau clearance by both autophagy-dependent and -independent mechanisms.68 Clearance of the amyloid precursor protein, APP, requires functionally active lysosomes and is enhanced by activation of lysosomal biogenesis induced by GSK3 inhibition.33 TFEB expression was found to be critical for clearance of mutant APP obtained by GSK3 inhibition, showing that TFEB lies downstream of GSK3 in the modulation of autophagy-lysosome pathways.33 Direct investigations of the role of TFEB in the clearance of Aβ demonstrated that overexpression of TFEB promotes Aβ uptake and degradation by astrocytes.69 Exogenous, astrocyte-specific expression of TFEB in amyloid precursor protein (APP)/presenilin 1 (PS1) transgenic mice (a model of familial Alzheimer’s disease) reduced Aβ levels and amyloid plaque load and ameliorated neuropathology and behavior phenotypes.69 Similarly, neuronal expression of TFEB in the hippocampus of APP/PS1 mice promoted lysosomal proliferation and reduced Aβ generation, thereby attenuating plaque deposition.70
Other proteinopathies and conditions characterized by impaired autophagic-lysosomal system
The majority of patients with alpha-1-antitrypsin (AAT) deficiency, a severe liver disorder, are homozygous for a mutation that disrupts normal folding of AAT, thereby causing its toxic aggregation in hepatocytes. AAV-mediated gene transfer of TFEB in a mouse model that recapitulates the human disease enhanced degradation of mutant AAT and ameliorated liver phenotypes, including apoptosis and fibrosis.71 A follow-up study focusing on the mouse lung, which is also affected by AAT aggregation, showed that administration of AAV-TFEB by nasal instillation reduced aggregation of misfolded AAT and ameliorated lung tissue pathology.72 Positive effects of TFEB expression or induction have been reported in additional conditions characterized by lysosomal depletion or impaired autophagy, including BNIP3-induced cardiomyocyte cell death caused by ischemia-reperfusion injury73, 74 and immunoglobulin abnormal light-chain protein (AL-LC) cardiomyopathy.75
Conclusions
The potential therapeutic effect of TFEB-mediated lysosomal enhancement has been shown in several preclinical models of human disease, independent of the presence of impaired TFEB signaling as a possible contributing factor of disease pathogenesis. Additional studies might uncover a broader range of diseases in which modulation of lysosomal function can exert therapeutic effects. Specific studies are also required to establish the limits and possible side effects of life-long treatments based on lysosomal enhancement.
The rapid explosion of studies focused on TFEB function and the pace of acquisition of new information on TFEB regulation and its role in cell metabolism indicate that much potentially remains to be discovered regarding the TFEB/CLEAR network. Future studies will likely expand the number of processes in which TFEB and its close paralogs play critical roles and might refine what has already been learned from the investigation of a wide variety of model systems. The future identification of small drug activators of TFEB with characteristics compatible with long-term treatments will allow investigating the potential clinical translation of TFEB-mediated lysosomal enhancement in degenerative storage diseases.
Acknowledgments
I thank K. Venkatachalam and C. Cummings for helpful discussion and critical reading of the manuscript. Owing to limited space, not all literature in the field could be cited, and I sincerely apologize to those whose articles are not referenced. This paper was supported by NIH Grant NS079618 and grants from the Beyond Batten Disease Foundation.
Footnotes
Conflict of interest
The author declares no conflicts of interest.
References
- 1.Sardiello M, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 2.Sardiello M, Licciulli F, Catalano D, Attimonelli M, Caggese C. MitoDrome: a database of Drosophila melanogaster nuclear genes encoding proteins targeted to the mitochondrion. Nucleic Acids Res. 2003;31:322–324. doi: 10.1093/nar/gkg123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sardiello M, et al. Energy biogenesis: one key for coordinating two genomes. Trends Genet. 2005;21:12–16. doi: 10.1016/j.tig.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Palmieri M, et al. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum Mol Genet. 2011;20:3852–3866. doi: 10.1093/hmg/ddr306. [DOI] [PubMed] [Google Scholar]
- 5.Settembre C, et al. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nezich CL, Wang C, Fogel AI, Youle RJ. MiT/TFE transcription factors are activated during mitophagy downstream of Parkin and Atg5. J Cell Biol. 2015;210:435–450. doi: 10.1083/jcb.201501002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauhan S, et al. ZKSCAN3 is a master transcriptional repressor of autophagy. Mol Cell. 2013;50:16–28. doi: 10.1016/j.molcel.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Settembre C, et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol. 2013;15:647–658. doi: 10.1038/ncb2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song W, et al. TFEB regulates lysosomal proteostasis. Hum Mol Genet. 2013;22:1994–2009. doi: 10.1093/hmg/ddt052. [DOI] [PubMed] [Google Scholar]
- 10.Decressac M, et al. TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein toxicity. Proc Natl Acad Sci U S A. 2013;110:E1817–E1826. doi: 10.1073/pnas.1305623110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castiglioni V, Onorati M, Rochon C, Cattaneo E. Induced pluripotent stem cell lines from Huntington's disease mice undergo neuronal differentiation while showing alterations in the lysosomal pathway. Neurobiol Dis. 2012;46:30–40. doi: 10.1016/j.nbd.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, et al. A role for presenilins in autophagy revisited: normal acidification of lysosomes in cells lacking PSEN1 and PSEN2. J Neurosci. 2012;32:8633–8648. doi: 10.1523/JNEUROSCI.0556-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolt AM, Douglas RM, Klimecki WT. Arsenite exposure in human lymphoblastoid cell lines induces autophagy and coordinated induction of lysosomal genes. Toxicol Lett. 2010;199:153–159. doi: 10.1016/j.toxlet.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logan R, Kong AC, Krise JP. Time-dependent effects of hydrophobic amine-containing drugs on lysosome structure and biogenesis in cultured human fibroblasts. J Pharm Sci. 2014;103:3287–3296. doi: 10.1002/jps.24087. [DOI] [PubMed] [Google Scholar]
- 15.Emanuel R, et al. Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae. Arterioscler Thromb Vasc Biol. 2014;34:1942–1952. doi: 10.1161/ATVBAHA.114.303342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song W, et al. Ceria nanoparticles stabilized by organic surface coatings activate the lysosome-autophagy system and enhance autophagic clearance. ACS Nano. 2014;8:10328–10342. doi: 10.1021/nn505073u. [DOI] [PubMed] [Google Scholar]
- 17.Song W, et al. The autophagic response to polystyrene nanoparticles is mediated by transcription factor EB and depends on surface charge. J Nanobiotechnology. 2015;13:87. doi: 10.1186/s12951-015-0149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porter K, Nallathambi J, Lin Y, Liton PB. Lysosomal basification and decreased autophagic flux in oxidatively stressed trabecular meshwork cells: implications for glaucoma pathogenesis. Autophagy. 2013;9:581–594. doi: 10.4161/auto.23568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unuma K, Aki T, Funakoshi T, Yoshida K, Uemura K. Cobalt protoporphyrin accelerates TFEB activation and lysosome reformation during LPS-induced septic insults in the rat heart. PLoS One. 2013;8:e56526. doi: 10.1371/journal.pone.0056526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pena KA, Kiselyov K. Transition metals activate TFEB in overexpressing cells. Biochem J. 2015;470:65–76. doi: 10.1042/BJ20140645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomes PG, Trambly CS, Fox HS, Tuma DJ, Donohue TM., Jr Acute and Chronic Ethanol Administration Differentially Modulate Hepatic Autophagy and Transcription Factor EB. Alcohol Clin Exp Res. 2015;39:2354–2363. doi: 10.1111/acer.12904. [DOI] [PubMed] [Google Scholar]
- 22.Cho S, Hwang ES. Status of mTOR activity may phenotypically differentiate senescence and quiescence. Mol Cells. 2012;33:597–604. doi: 10.1007/s10059-012-0042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsunemi T, et al. PGC-1alpha rescues Huntington's disease proteotoxicity by preventing oxidative stress and promoting TFEB function. Sci Transl Med. 2012;4:142ra197. doi: 10.1126/scitranslmed.3003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott I, et al. GCN5-like protein 1 (GCN5L1) controls mitochondrial content through coordinated regulation of mitochondrial biogenesis and mitophagy. J Biol Chem. 2014;289:2864–2872. doi: 10.1074/jbc.M113.521641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh A, et al. Activation of peroxisome proliferator-activated receptor alpha induces lysosomal biogenesis in brain cells: implications for lysosomal storage disorders. J Biol Chem. 2015;290:10309–10324. doi: 10.1074/jbc.M114.610659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seok S, et al. Transcriptional regulation of autophagy by an FXR-CREB axis. Nature. 2014;516:108–111. doi: 10.1038/nature13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pena-Llopis S, et al. Regulation of TFEB and V-ATPases by mTORC1. Embo J. 2011;30:3242–3258. doi: 10.1038/emboj.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Settembre C, et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. Embo J. 2012;31:1095–1108. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martina JA, Chen Y, Gucek M, Puertollano R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 2012;8:903–914. doi: 10.4161/auto.19653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roczniak-Ferguson A, et al. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci Signal. 2012;5:ra42. doi: 10.1126/scisignal.2002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martina JA, Puertollano R. Rag GTPases mediate amino acid-dependent recruitment of TFEB and MITF to lysosomes. J Cell Biol. 2013;200:475–491. doi: 10.1083/jcb.201209135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou J, et al. Activation of lysosomal function in the course of autophagy via mTORC1 suppression and autophagosome-lysosome fusion. Cell Res. 2013;23:508–523. doi: 10.1038/cr.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parr C, et al. Glycogen synthase kinase 3 inhibition promotes lysosomal biogenesis and autophagic degradation of the amyloid-beta precursor protein. Mol Cell Biol. 2012;32:4410–4418. doi: 10.1128/MCB.00930-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchand B, Arsenault D, Raymond-Fleury A, Boisvert FM, Boucher MJ. Glycogen synthase kinase-3 (GSK3) inhibition induces prosurvival autophagic signals in human pancreatic cancer cells. J Biol Chem. 2015;290:5592–5605. doi: 10.1074/jbc.M114.616714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cea M, et al. Targeting NAD+ salvage pathway induces autophagy in multiple myeloma cells via mTORC1 and extracellular signal-regulated kinase (ERK1/2) inhibition. Blood. 2012;120:3519–3529. doi: 10.1182/blood-2012-03-416776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enzenmuller S, Gonzalez P, Karpel-Massler G, Debatin KM, Fulda S. GDC-0941 enhances the lysosomal compartment via TFEB and primes glioblastoma cells to lysosomal membrane permeabilization and cell death. Cancer Lett. 2013;329:27–36. doi: 10.1016/j.canlet.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Song W, Wang F, Lotfi P, Sardiello M, Segatori L. 2-Hydroxypropyl-beta-cyclodextrin promotes transcription factor EB-mediated activation of autophagy: implications for therapy. J Biol Chem. 2014;289:10211–10222. doi: 10.1074/jbc.M113.506246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moskot M, et al. The phytoestrogen genistein modulates lysosomal metabolism and transcription factor EB (TFEB) activation. J Biol Chem. 2014;289:17054–17069. doi: 10.1074/jbc.M114.555300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thayyullathil F, Rahman A, Pallichankandy S, Patel M, Galadari S. ROS-dependent prostate apoptosis response-4 (Par-4) up-regulation and ceramide generation are the prime signaling events associated with curcumin-induced autophagic cell death in human malignant glioma. FEBS Open Bio. 2014;4:763–776. doi: 10.1016/j.fob.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medina DL, et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol. 2015;17:288–299. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferron M, et al. A RANKL-PKCbeta-TFEB signaling cascade is necessary for lysosomal biogenesis in osteoclasts. Genes Dev. 2013;27:955–969. doi: 10.1101/gad.213827.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider JL, et al. Loss of hepatic chaperone-mediated autophagy accelerates proteostasis failure in aging. Aging Cell. 2015;14:249–264. doi: 10.1111/acel.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hemesath TJ, et al. microphthalmia, a critical factor in melanocyte development, defines a discrete transcription factor family. Genes Dev. 1994;8:2770–2780. doi: 10.1101/gad.8.22.2770. [DOI] [PubMed] [Google Scholar]
- 44.Bronisz A, et al. Microphthalmia-associated transcription factor interactions with 14-3-3 modulate differentiation of committed myeloid precursors. Mol Biol Cell. 2006;17:3897–3906. doi: 10.1091/mbc.E06-05-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin J, et al. Proteomic, functional, and domain-based analysis of in vivo 14-3-3 binding proteins involved in cytoskeletal regulation and cellular organization. Curr Biol. 2004;14:1436–1450. doi: 10.1016/j.cub.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 46.Martina JA, et al. The nutrient-responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci Signal. 2014;7:ra9. doi: 10.1126/scisignal.2004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ploper D, et al. MITF drives endolysosomal biogenesis and potentiates Wnt signaling in melanoma cells. Proc Natl Acad Sci U S A. 2015;112:E420–E429. doi: 10.1073/pnas.1424576112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taniguchi M, et al. TFE3 is a bHLH-ZIP-type transcription factor that regulates the mammalian Golgi stress response. Cell Struct Funct. 2015;40:13–30. doi: 10.1247/csf.14015. [DOI] [PubMed] [Google Scholar]
- 49.Salma N, Song JS, Arany Z, Fisher DE. Transcription Factor Tfe3 Directly Regulates Pgc-1alpha in Muscle. J Cell Physiol. 2015;230:2330–2336. doi: 10.1002/jcp.24978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martina JA, Diab HI, Brady OA, Puertollano R. TFEB and TFE3 are novel components of the integrated stress response. Embo J. 2016;35:479–495. doi: 10.15252/embj.201593428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Rourke EJ, Ruvkun G. MXL-3 and HLH-30 transcriptionally link lipolysis and autophagy to nutrient availability. Nat Cell Biol. 2013;15:668–676. doi: 10.1038/ncb2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouche V, et al. Drosophila Mitf regulates the V-ATPase and the lysosomal-autophagic pathway. Autophagy. 2016;12:484–498. doi: 10.1080/15548627.2015.1134081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lapierre LR, et al. The TFEB orthologue HLH-30 regulates autophagy and modulates longevity in Caenorhabditis elegans. Nat Commun. 2013;4:2267. doi: 10.1038/ncomms3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH. Alterations in lysosomal and proteasomal markers in Parkinson's disease: relationship to alpha-synuclein inclusions. Neurobiol Dis. 2009;35:385–398. doi: 10.1016/j.nbd.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 55.Lukiw WJ. Micro-RNA speciation in fetal, adult and Alzheimer's disease hippocampus. Neuroreport. 2007;18:297–300. doi: 10.1097/WNR.0b013e3280148e8b. [DOI] [PubMed] [Google Scholar]
- 56.Tiribuzi R, et al. miR128 up-regulation correlates with impaired amyloid beta(1–42) degradation in monocytes from patients with sporadic Alzheimer's disease. Neurobiol Aging. 2014;35:345–356. doi: 10.1016/j.neurobiolaging.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Chua JP, et al. Transcriptional activation of TFEB/ZKSCAN3 target genes underlies enhanced autophagy in spinobulbar muscular atrophy. Hum Mol Genet. 2014;23:1376–1386. doi: 10.1093/hmg/ddt527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tohnai G, et al. Paeoniflorin eliminates a mutant AR via NF-YA-dependent proteolysis in spinal and bulbar muscular atrophy. Hum Mol Genet. 2014;23:3552–3565. doi: 10.1093/hmg/ddu066. [DOI] [PubMed] [Google Scholar]
- 59.Cortes CJ, et al. Polyglutamine-expanded androgen receptor interferes with TFEB to elicit autophagy defects in SBMA. Nat Neurosci. 2014;17:1180–1189. doi: 10.1038/nn.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reddy K, et al. Dysregulation of Nutrient Sensing and CLEARance in Presenilin Deficiency. Cell Rep. 2016;14:2166–2179. doi: 10.1016/j.celrep.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuiper RP, et al. Upregulation of the transcription factor TFEB in t(6;11)(p21;q13)-positive renal cell carcinomas due to promoter substitution. Hum Mol Genet. 2003;12:1661–1669. doi: 10.1093/hmg/ddg178. [DOI] [PubMed] [Google Scholar]
- 62.Petit CS, Roczniak-Ferguson A, Ferguson SM. Recruitment of folliculin to lysosomes supports the amino acid-dependent activation of Rag GTPases. J Cell Biol. 2013;202:1107–1122. doi: 10.1083/jcb.201307084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perera RM, et al. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature. 2015;524:361–365. doi: 10.1038/nature14587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Du Bois P, et al. Angiotensin II Induces Skeletal Muscle Atrophy by Activating TFEB-Mediated MuRF1 Expression. Circ Res. 2015;117:424–436. doi: 10.1161/CIRCRESAHA.114.305393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dehay B, et al. Pathogenic lysosomal depletion in Parkinson's disease. J Neurosci. 2010;30:12535–12544. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Medina DL, et al. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev Cell. 2011;21:421–430. doi: 10.1016/j.devcel.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spampanato C, et al. Transcription factor EB (TFEB) is a new therapeutic target for Pompe disease. EMBO Mol Med. 2013;5:691–706. doi: 10.1002/emmm.201202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Polito VA, et al. Selective clearance of aberrant tau proteins and rescue of neurotoxicity by transcription factor EB. EMBO Mol Med. 2014;6:1142–1160. doi: 10.15252/emmm.201303671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiao Q, et al. Enhancing astrocytic lysosome biogenesis facilitates Abeta clearance and attenuates amyloid plaque pathogenesis. J Neurosci. 2014;34:9607–9620. doi: 10.1523/JNEUROSCI.3788-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiao Q, et al. Neuronal-Targeted TFEB Accelerates Lysosomal Degradation of APP, Reducing Abeta Generation and Amyloid Plaque Pathogenesis. J Neurosci. 2015;35:12137–12151. doi: 10.1523/JNEUROSCI.0705-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pastore N, et al. Gene transfer of master autophagy regulator TFEB results in clearance of toxic protein and correction of hepatic disease in alpha-1-anti-trypsin deficiency. EMBO Mol Med. 2013;5:397–412. doi: 10.1002/emmm.201202046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hidvegi T, et al. Enhancing Autophagy with Drugs or Lung-directed Gene Therapy Reverses the Pathological Effects of Respiratory Epithelial Cell Proteinopathy. J Biol Chem. 2015;290:29742–29757. doi: 10.1074/jbc.M115.691253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma X, Godar RJ, Liu H, Diwan A. Enhancing lysosome biogenesis attenuates BNIP3-induced cardiomyocyte death. Autophagy. 2012;8:297–309. doi: 10.4161/auto.18658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma X, et al. Regulation of the transcription factor EB-PGC1alpha axis by beclin-1 controls mitochondrial quality and cardiomyocyte death under stress. Mol Cell Biol. 2015;35:956–976. doi: 10.1128/MCB.01091-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guan J, et al. Lysosomal dysfunction and impaired autophagy underlie the pathogenesis of amyloidogenic light chain-mediated cardiotoxicity. EMBO Mol Med. 2014;6:1493–1507. doi: 10.15252/emmm.201404190. [DOI] [PMC free article] [PubMed] [Google Scholar]