Abstract
A susceptible-infectious-susceptible (SIS) epidemic model that describes the coinfection and cotransmission of two infectious diseases spreading through a single population is studied. The host population consists of two subclasses: susceptible and infectious, and the infectious individuals are further divided into three subgroups: those infected by the first agent/pathogen, the second agent/pathogen, and both. The basic reproduction numbers for all cases are derived which completely determine the global stability of the system if the presence of one agent/pathogen does not affect the transmission of the other. When the constraint on the transmissibility of the dually infected hosts is removed, we introduce the invasion reproduction number, compare it with two other types of reproduction number and show the uniform persistence of both diseases under certain conditions. Numerical simulations suggest that the system can display much richer dynamics such as backward bifurcation, bistability and Hopf bifurcation.
Keywords: coinfection, invasion reproduction number, backward bifurcation, bistability, Hopf bifurcation, uniform persistence
1 Introduction
Different infectious agents may infect or colonize a host at the same time [21]. Many examples can be found, these involving HIV [30, 37] (for example, HIV and TB [19], HIV and Hepatitis B [12, 26], HIV and Hepatitis C [23], and HIV and malaria [2]), as well as some not involving HIV (for example, Hepatitis B and C coinfection [11], gonorrhea and Chlamydia [13], and herpes simplex viruses 1 and 2 [41, 59]) Moreover, simultaneous infection may occur with multiple strains or serotypes of the same organism, as is the case for influenza [20, 49], human papilloma virus [9], and HIV [55, 63, 22], for just three of many examples. However, simultaneous colonization or infection may occur even when there appears to be little or no interaction between the two agents, as in the case of infection by ocular strains of chlamydia and nasopharyngeal colonization by pneumococcus [24]. The dynamics of coinfection is important in this case, because antimicrobials used to treat one infection may affect the other (e.g., [51, 24]).
A variety of mathematical models for coinfections with multiple specific diseases, such as HIV/TB [43, 48, 6, 47], HIV/gonorrhea [40], HIV/malaria [1, 39], malaria and meningitis [29], general diseases [5, 7, 35, 28], and microparasites (viruses, bacteria, protozoa, fungi) [54, 10, 3, 4, 61], have been developed and analyzed in the past few years. Ferguson et al. [16] and Kawaguchi et al. [27] presented models to describe the coinfection of two serotypes of dengue virus in a human community. With respect to the interaction between nonspecific agents or pathogens, Blyuss and Kyrychko [7] studied a two-disease SIS model with equal transmission efficiency for both susceptible and singly infected individuals; Allen et al. [5] studied an SI model for a single host population with two viral infections, in which one is vertically transmitted and the other is horizontally transmitted; Zhang et al. [60] proposed an ODEs coinfection model with two strains of parasites and two host types to study the influence of heterogeneities in parasite virulence and host life history on the persistence and spread of parasite strains; Martcheva and Pilyugin [35] considered an epidemic model of two diseases: a primary disease and a secondary disease, structured by time since infection structure (for the primary disease); in the monograph of Keeling and Rohani [28], the interaction of two pathogens spreading through a host population was discussed in four cases: complete cross-immunity, no cross-immunity, enhanced susceptibility and partial cross-immunity. Among these models either the uninfected hosts cannot become infected with both diseases/strains directly [38, 35, 28, 10], or there is no recovery and an infection is lifelong [54, 5, 4], or both [60, 3].
In this paper, we develop and analyze a simple model of multiple infections; this model includes the possibility that the two agents are simultaneously transmitted with aspects of Chlamydia trachomatis and pneumococcus, though we do not restrict the analysis to this setting (see [31, 46] for examples of cotransmission in vector-borne disease ecology and human case report, respectively), thereby inaugurating a dual infection. Our model will also include the possibility that an individual who is currently infected with one agent will become dually infected as a result of an exposure to the second agent. In this paper, the condition of being simultaneously infected by multiple agents will be referred to simply as coinfection. Our model is similar to the model of Blyuss and Kyrychko [7] where the disease induced mortality is included, the doubly infected hosts recover from both diseases simultaneously and strong restrictions on transmission parameters are required, and to the models for coinfection by different species in Tanaka and Feldman [54] and Alizon [4] where disease-induced mortality may occur, but no recovery is possible (and the forces of infection follow a different rule).
We will assess a two-disease SIS model with no immunity or cross-immunity. For simplicity, we will refer to the first and second disease, recognizing that the model applies equally well to colonization or to subclinical infections. In Section 3, we carry out a complete global stability analysis of the model for the case where the force of infection of one disease is not affected by the presence of the other (i.e., no interaction between two infections). In Section 4, when the two infections interact with each other, we calculate the invasion reproduction numbers and obtain their epidemiologically meaningful lower and upper bounds, and show that the interaction outcome could be extinction of one or both diseases or persistence of both diseases. In Section 5, four numerical examples are provided to support the existence of competitive exclusion, backward bifurcation, bistability and Hopf bifurcation, respectively.
2 The model
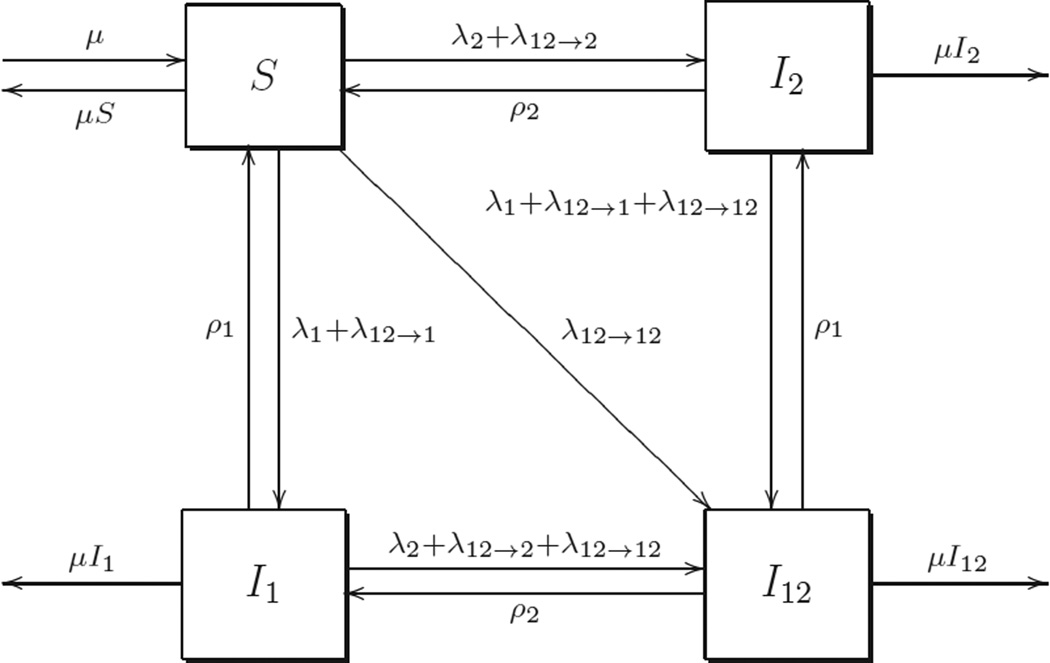
We propose a simple SIS epidemic model with two infectious agents (or strains of the same agent) spreading through one host species. Let S(t), I1(t), I2(t) and I12(t) be the fractions of the population infected with no infectious agent, the first agent, the second agent and both agents at time t, respectively. A susceptible individual who contacts coinfected persons can be infected with either one or both disease agents as a result of a single contact. Using ocular strains of Chlamydia trachomatis and nasopharyngeal pneumococcus as an example, transmission of either or both organisms could occur as a result of a single contact. This process is illustrated in Figure 1. The model is then described by a system of four ordinary differential equations as follows:
| (2.1) |
where the forces of infection are proportional to disease prevalence, i.e.,
Figure 1.
Flowchart of a two-disease coinfection model. S, I1, I2 and I12 represent the fractions of population infected with no infectious agent, the first agent, the second agent and both agents, respectively.
Parameters ρ1 and ρ2 represent the recovery rate of the first and second diseases, respectively. The disease induced death rate is ignored. We assume that the natural birth and death rates are balanced and equal to μ, so that the total population size is constant. All parameters are assumed to be positive, except that β12 ≥ 0. As stated earlier, the possibility of simultaneous transmission from a single contact with a dually infected individual, which we model according to the assumption β12 > 0. An underlying assumption in the model is that individuals in all disease states have the same contact rate; we do not assume that individuals have fewer contacts if they are infected. The rates/probabilities of transmission of the model in Blyuss and Kyrychko [7] satisfy β12 + β10 = β1, β12 + β02 = β2, β12 = β1β2, which is a special case of ours.
Following the method and notations of van den Driessche and Watmough [58] and Diekmann et al. [14, 15], we have
The basic reproduction number associated with the model (2.1) is defined as the spectral radius of the next generation matrix FV−1, i.e., ℛ0 = max{ℛ10, ℛ20, ℛ30}, where
Let . Clearly, the set Ω is positively invariant for system (2.1), so we will always set initial values within Ω. It is immediate that system (2.1) has up to three boundary equilibria as follows.
Proposition 2.1
For system (2.1), we have
the disease-free equilibrium E0 = (1, 0, 0, 0) always exists;
the equilibrium with the presence of only the first disease E1 = (1/ℛ10, 1 − 1/ℛ10, 0, 0) exists if and only if ℛ10 > 1;
the equilibrium with the presence of only the second disease E2 = (1/ℛ20, 0, 1 − 1/ℛ20, 0) exists if and only if ℛ20 > 1.
3 Noninteracting transmission
We begin with the simpler case where the presence of each disease does not affect the transmission of the other; throughout this section, we assume that a doubly infected individual has the same total infectivity as a singly infected person, which translates to
| (H) |
Under the assumption (H), the persistence and extinction of one disease, and the total fraction of people infected by that disease are not affected by the presence of the other disease. The ability of a disease to invade an uninfected population is completely determined by its own basic reproduction number.
Theorem 3.1
Let Ω0 = {E0}, Ω1 = {(S, I1, 0, 0) ∈ Ω : I1 > 0} and Ω2 = {(S, 0, I2, 0) ∈ Ω : I2 > 0}. For system (2.1) under assumption (H), we have
if ℛ0 ≤ 1 then the disease-free equilibrium E0 is globally asymptotically stable in Ω;
if ℛ10 > 1 ≥ ℛ20 (or ℛ20 > 1 ≥ ℛ10), then E1 (or E2) is globally asymptotically stable in Ω\(Ω0 ∪ Ω2) (or Ω\(Ω0 ∪ Ω1)) where Ω0 ∪ Ω2 (or ( Ω0 ∪ Ω1)) is the attractor of E0;
-
if ℛ10 > 1 and ℛ20 > 1, then there exists a unique coexistence equilibrium, denoted by , which is globally asymptotically stable in
Here Ω0, Ω1 and Ω2 are the attractor of E0, E1 and E2, respectively.
Proof
The summations of the second and fourth equations of (2.1) and the third and fourth equations of (2.1) give
and
respectively. It follows from S + I1 + I2 + I12 = 1 that the above two logistic equations satisfy
respectively, if both diseases present initially. If, for example, ℛ10 ≤ 1, then I1(t) + I12(t) → 0 which implies . Thus, the first two arguments are established.
Now we assume that ℛ10 ≥ ℛ20 > 1 and the symmetric case ℛ20 ≥ ℛ10 > 1 can be proved similarly. If exists, then it must satisfy
| (3.1) |
and substituting them into the first equation of (2.1) yields
| (3.2) |
Meanwhile, solving in terms of S* from the first equation of (2.1) gives
Claim 1
If β12 = 0, then and there exists a unique endemic equilibrium
If β12 > 0, then (3.2) has two roots, labeled by and , satisfying . Here ℜ(z) is the real part of a complex number z. Dividing both sides of (3.2) by β12 yields
where and . Clearly, υ1 ≤ υ2 < 1, g > 2 and g > 2h.
Claim 2
. It follows from and
that both and are positive real numbers and . In addition,
When , the positivities of and follow from and is equivalent to , which is guaranteed by
Consequently, if ℛ10 > 1 and ℛ20 > 1, then (2.1) has a unique endemic equilibrium
where S* ∈ (0, 1) is the unique feasible solution to (3.2).
The spectrum of the Jacobian matrix of system (2.1) at E12 is
which means the endemic equilibrium is locally asymptotically stable. It follows from the result on asymptotically autonomous differential equations [56] that E12 is globally attractive. Hence the endemic equilibrium is globally asymptotically stable when it exists.
Remark 3.2
When coinfection is impossible, the competitive exclusion principle holds and the disease with a larger reproduction number must exclude the other [8]. Thus coinfection is a mechanism for the coexistence of multiple agents or pathogens [35].
Remark 3.3
It follows from S* + (υ1 − S*) + (υ2 − S*) + (υ1 − S*)(υ2 − S*)/S* ≤ 1 and (3.1) that min{υ1, υ2} > S* ≥ υ1υ2,, and . Both the uninfected population and the coinfected population are increasing in the cotransmission rate. Indeed, differentiating F(S*) = 0 with respect to β12 gives
and implies that .
In the setting of multiple diseases, we may treat the entire population (mass treatment, ρi → ρi + θ for any i), or only a fraction of people infected by a specific disease (targeted treatment, ρi → ρi + θi for some i). Under both treatment strategies, it is shown that for model (2.1) with the restriction (H) the uninfected population is always increased by choosing a higher treatment rate; this differs from the two-disease model we studied in [17].
Corollary 3.4
For system (2.1) under assumption (H), the fraction of susceptible, S*, is always increasing in the mass treatment rate θ (or targeted treatment rate θi).
Proof
If β12 = 0, then under the mass treatment and targeted treatment we have
respectively. Now assume that β12 > 0 and υ1 ≥ υ2, it follows from the differentiation of F(S*) = 0 with respect to targeted treatment rate θi or mass treatment rate θ that
and
The last inequality holds because of S* − gυ1 + h < 0 and 2S* − (υ1 + υ2 + g − 1 − h) < 0.
4 Interacting transmission
In the rest of the paper, we remove the requirement (H), namely, β12 + β10 ≠ β1 or β12 + β02 ≠ β2. Biologically, this means that a dually-infected individual may transmit each agent either more or less efficiently than a person infected with each agent singly. Three possible scenarios are listed as follows:
Mutual enhancement: β12 + β10 > β1 and β12 + β02 > β2;
Enhancement and inhibition: β12 + β10 > β1 and β12 + β02 < β2, or β12 + β10 < β1 and β12 + β02 > β2;
Mutual inhibition: β12 + β10 < β1 and β12 + β02 < β2.
The dynamics are simple equilibrium if the double infection has mild impact on the transmission of one of the two infections. The following proof utilizes the theory of monotone dynamical systems [52], simplfying the proof of Theorem 3.1.
Proposition 4.1
Assume that dual infection has no impact on the transmission of the first disease, i.e., β12 + β10 = β1. For system (2.1), every orbit with initial value in Ω converges to an equilibrium.
Proof
The assumption β12 + β10 = β1 implies that I1(t) + I12(t) → max{0, 1 − 1/ℛ10} as t → ∞ for I1(0) + I12(0) > 0. If ℛ10 ≤ 1, then either E0 or E2 is globally stable. If ℛ10 > 1, then I1(t) + I12(t) → 1 − 1/ℛ10 and S(t) + I2(t) → 1/ℛ10 as t → ∞. Thus, the 4-dimensional system (2.1) can be reduced to a 2-dimensional system in I1 and I2. Denote the Jacobian matrix of this two-dimensional system by J2 = (aij)2×2, where
Thus, the reduced 2-dimensional system is competitive. By Theorem 3.2.2 in Smith [52], every orbit converges to an equilibrium.
The stability analysis for system (2.1) now becomes more complicated, and we need to introduce a new threshold parameter—the invasion reproduction number, which is used to measure the ability of one disease to invade an equilibrium of the other disease [42, 35, 60]. We now assume that the equilibrium E1 exists, or equivalently, ℛ10 > 1. Using the next generation matrix method [14, 15, 58], the vectors for the rate of the appearance of new infections by disease two and the rate of transfer of individuals are, respectively,
where (S̄, Ī1) = (1/ℛ10, 1 − 1/ℛ10). The derivatives of ℱ2 and 𝒱2 at (I2, I12) = (0, 0) are, respectively,
The characteristic polynomial of the matrix is A2λ2 + A1λ + A0 = 0, where
The invasion reproduction number of disease 2, denoted by , is given by the spectral radius of the non-negative matrix , i.e.,
It follows from A0 > 0 that the characteristic equation has two positive roots as β12 > 0. If β12 = 0 then A0 = 0 and .
Similarly, we can derive a formula for the invasion reproduction number of disease 1, denoted by . By Theorem 2 in van den Driessche and Watmough [58], disease 1 can invade disease 2 if and disease 2 can invade disease 1 if (see Appendix A for a direct proof).
Proposition 4.2
For system (2.1), when the equilibrium E1 exists (i.e., ℛ10 > 1), it is locally asymptotically stable if and unstable if . Symmetrically, the equilibrium E2 is locally asymptotically stable if and unstable if .
Define ℛ̃20 = (β12 + β02)/(ρ2 + μ); this quantity, analogous to a basic reproduction number, is the number of cases of disease 2 resulting from the introduction of a dually infected person into a wholly susceptible population. We can establish the lower and upper bounds for the invasion reproduction number .
Proposition 4.3
Assume that E1 exists, or equivalently, ℛ10 > 1. Then the following statements are valid:
if and only if β2 > β12 + β02;
if and only if β2 < β12 + β02;
if and only if β2 = β12 + β02.
Proof
has the same sign as β2 − β12 − β02. In fact, we can rewrite as
If G ≡ 2β2A2 + (ρ2 + μ)A1 ≥ 0, then is equivalent to
If G < 0, then G = (ρ2 + μ)(G0 + (β2 − β12 − β02)G1) where
Note that G0 > 0 and G1 > 0 when ℛ10 > 1. If β2 ≥ β12 + β02 then G > 0, a contradiction.
On the other hand, is equivalent to
This completes the proof.
Therefore, it is possible that as β2 < β12 + β02. Moreover, we will show that the second disease may be able to invade in the presence of the first disease even if it cannot persist alone. That is, the presence of the first disease promotes persistence of the second disease. Similar to the model in Martcheva and Pilyugin [35], we can obtain the following result about the consequence of competition.
Proposition 4.4
For system (2.1), if ℛ̃10 ≤ 1 or ℛ̃20 ≤ 1 and ℛ10 ≤ 1, ℛ20 ≤ 1, then both diseases go extinct and the disease-free equilibrium is globally stable; if max{ℛ10, ℛ̃10} ≤ 1 (or max{ℛ20, ℛ̃20} ≤ 1) then disease 1 (or 2) goes extinct; if min{ℛ10, ℛ̃10} > 1 (or min{ℛ20, ℛ̃20} > 1) then disease 1 (or 2) persists.
Proof
It follows from the second and fourth equations of system (2.1) that
and hence
By a simple comparison principle [53], we get
if I1(0) + I12(0) > 0.
Remark 4.5
A necessary condition for the existence of a coexistence equilibrium is that max{ℛi0, ℛ̃i0} > 1 for i = 1, 2. When E* exists, it satisfies
The above result indicates that the two diseases coexist whenever min{ℛi0, ℛ̃i0} > 1, i = 1, 2. By a similar argument to that of Theorem 2.5 in Gao and Ruan [18], we will show that it remains true under a weaker condition: , i ≠ j, i, j = 1, 2.
Theorem 4.6
For model (2.1), if
| (4.1) |
then (2.1) admits at least one coexistence equilibrium and both diseases are uniformly persistent, i.e., there is a constant κ > 0 such that each solution φt(x0) ≡ (S(t), I1(t), I2(t), I12(t)) of system (2.1) with x0 ≡ (S(0), I1(0), I2(0), I12(0)) ∈ Ω0 ≡ {(S, I1, I2, I12) ∈ Ω : I1I2 > 0 or I12 > 0} satisfies
Proof
Denote ∂Ω0 = Ω\Ω0 = {(S, I1, I2, I12) ∈ Ω : I1 = 0 or I2 = 0, I12 = 0}. It is sufficient to show that system (2.1) is uniformly persistent with respect to (Ω0, ∂Ω0). Obviously, ∂Ω0 is relatively closed in Ω. It is clear that Ω and Ω0 are positively invariant and system (2.1) is point dissipative.
Let M∂ = {x0 ∈ ∂Ω0 : φt(x0) ∈ ∂Ω0 for t ≥ 0}. Therefore, M∂ = ∂Ω0. The boundary equilibria E0, E1 and E2 are in M∂. Let Ws(Ei) be the stable manifold of Ei for i = 0, 1, 2. We will show that Ws(Ei) ∩ Ω0 = ∅ whenever (4.1) holds.
Define
It follows from ℛ10 > 1 that there is an ε0 > 0 such that for ε ∈ [0, ε0]. Select η0 small enough such that
We claim that for x0 ∈ Ω0, where ‖ · ‖ is the Euclidean norm. Supposing not, then by translation, we have ‖φt(x0) − E0‖ ≤ η0 for all t ≥ 0 and hence
By a comparison theorem, I1(t) → ∞ as t → ∞; the contradiction establishes the result.
To show Ws(E1) ∩ Ω0 = ∅, we define
Since s(F2 − V2) > 0 if and only if , there is an ε1 > 0 such that s(Mε) > 0 for ε ∈ [0, ε1]. Recall that (S̄, Ī1) = (1/ℛ10, 1 − 1/ℛ10). Choose η1 small enough such that
We claim that for x0 ∈ Ω0. Supposing not, then again by translation, we have ‖φt(x0) − E1‖ ≤ η1 for all t ≥ 0 and hence
Notice that Mε1 has a positive eigenvalue s(Mε1) associated to a positive eigenvector. It follows from a comparison theorem that I2(t) → ∞ and I12(t) → ∞ as t → ∞, a contradiction.
Since Ws(E0) = {E0}, Ws(E1) = {(S, I1, I2, I12) ∈ Ω : I1 > 0, I2 = I12 = 0}, Ws(E2) = {(S, I1, I2, I12) ∈ Ω : I2 > 0, I1 = I12 = 0} and M∂ = Ws(E0)∪Ws(E1)∪Ws(E2), {E0}, {E1} and {E2} are isolated invariant sets and acyclic in M∂. By Theorem 4.6 in Thieme [57], system (2.1) is uniformly persistent with respect to (Ω0, ∂Ω0). Moreover, by Theorem 2.4 in Zhao [62], we know that system (2.1) has an equilibrium . It is easy to check that E* is a positive equilibrium of system (2.1).
Remark 4.7
Similarly, we can show the uniform persistence of both diseases and the existence of a coexistence equilibrium under the assumption ℛ10 > 1 and , or ℛ20 > 1 and . In this case, one disease goes extinct in the absence of the other and the presence of the other disease mediates the coexistence. Control strategies toward a reduction of both infections are favored.
A more detailed classification for the transmission dynamics of the coinfection model (2.1) based on its basic reproduction numbers and invasion reproduction numbers [35] is beyond the scope of current paper. For example, we are particularly interested in the local/global stability of the coexistence equilibrium under conditions in Theorem 4.6, but numerical examples in the next section show that an unstable coexistence equilibrium could present in case of backward bifurcation, bistability or a Hopf bifurcation. This suggests that it is hard to use the well-known Routh-Hurwitz criterion to determine the local stability of the coexistence equilibrium.
5 Numerical Simulations
In this section, we illustrate, by numerical examples, possible phenomena the model of coinfection may exhibit.
Example 5.1. Competitive exclusion
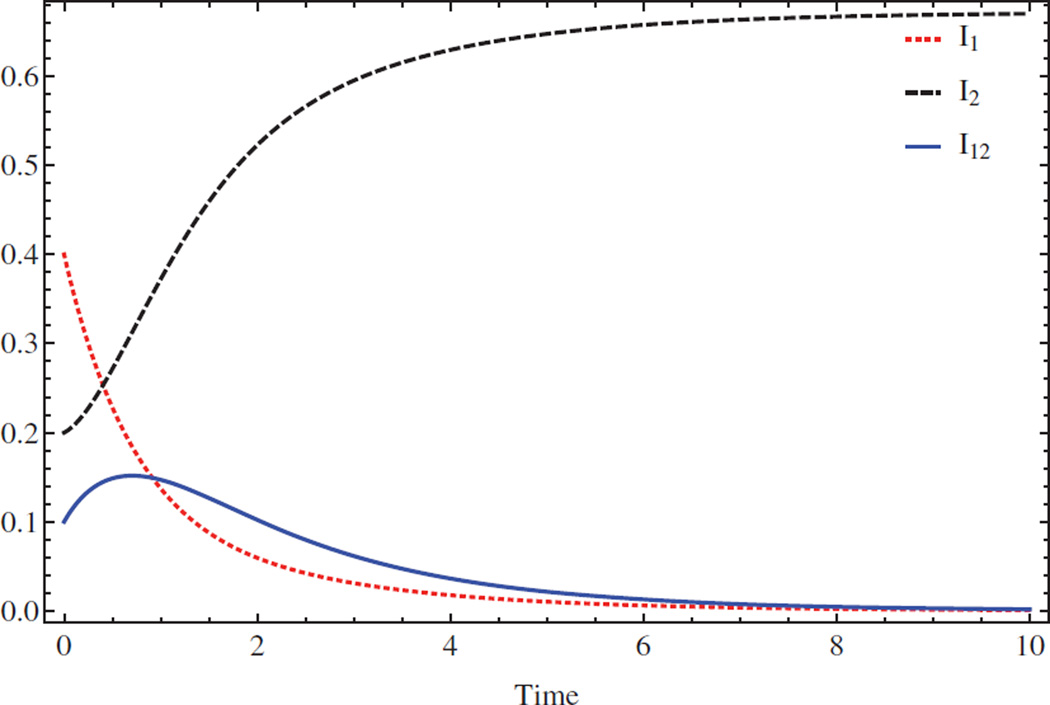
Two diseases cannot coexist even if each one can persist independently. Parameter values: β1 = 1.05, β2 = 2.5, ρ1 = 1, ρ2 = 0.8, μ = 0.02, β12 = 0.05, β10 = 0.2, β02 = 2. The respective basic reproduction numbers are ℛ10 = 1.0294 > 1 and ℛ20 = 3.0488 > 1. Figure 2 shows that the first disease goes extinct while the second persists. It provides a theoretically plausible treatment strategy for some pathogens: suppose that 1 < ℛ10 < ℛ20, β12 + β10 < β1 and β12 + β02 < β2; there is an effective way to treat pathogen 2, but not for pathogen 1. Here, one could in principle introduce pathogen 2 to eradicate pathogen 1.
Figure 2.
Numerical solution of system (2.1) with initial condition (S(0), I1(0), I2(0), I12(0)) = (0.3, 0.4, 0.2, 0.1). Red dotted line-I1, black dashed line-I2 and solid blue line-I12. Two infections cannot coexist even if each one can survive independently.
Moreover, consider a scenario in which β1 = 2.9, β2 = 3, ρ1 = ρ2 = μ = 1, β12 = 0.5, β10 = 2, β02 = 0.1; we have ℛ10 = 1.45, ℛ20 = 1.5, and . Thus, E1 is locally stable but E2 is unstable and there is no coexistence equilibrium. Interestingly, in this case the competitive exclusion principle still holds, but the disease with a higher reproduction number dies out while the other one persists (in contrast with [8]).
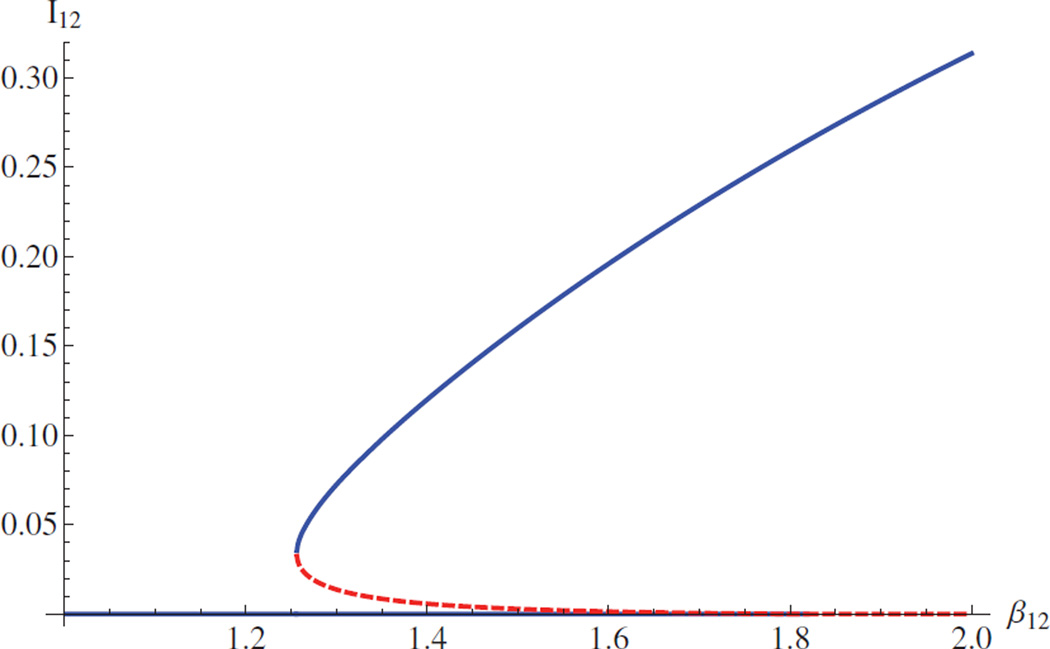
Example 5.2. Backward bifurcation
Assume that ℛ10 < 1 and ℛ20 < 1. If β12 + β10 > β1 and β12 + β02 > β2, then it is possible that both diseases become persistent. Parameter values: β1 = 0.9, β2 = 0.7, ρ1 = 1, ρ2 = 0.8, μ = 0.02, β10 = β02 = 0.6. A bifurcation diagram for model (2.1) shows that the endemic equilibrium value of I12 with respect to β12 is presented in Figure 3. In this setting, there is one stable coexistence equilibrium and one unstable coexistence equilibrium for β12 ∈ (1.256, 1.82). Biologically, this means that a small perturbation in model parameters or initial conditions may lead to a large difference in the dynamic behavior of the disease. The occurrence of backward bifurcation precludes, in general, the global stability of the disease-free equilibrium as ℛ0 < 1. Note that backward bifurcation still exists even if there is no cotransmission (β12 = 0).
Figure 3.
Backward bifurcation arising from the change of β12. Blue solid line-stable, red dashed line-unstable. Two diseases can coexist even if each one dies out independently.
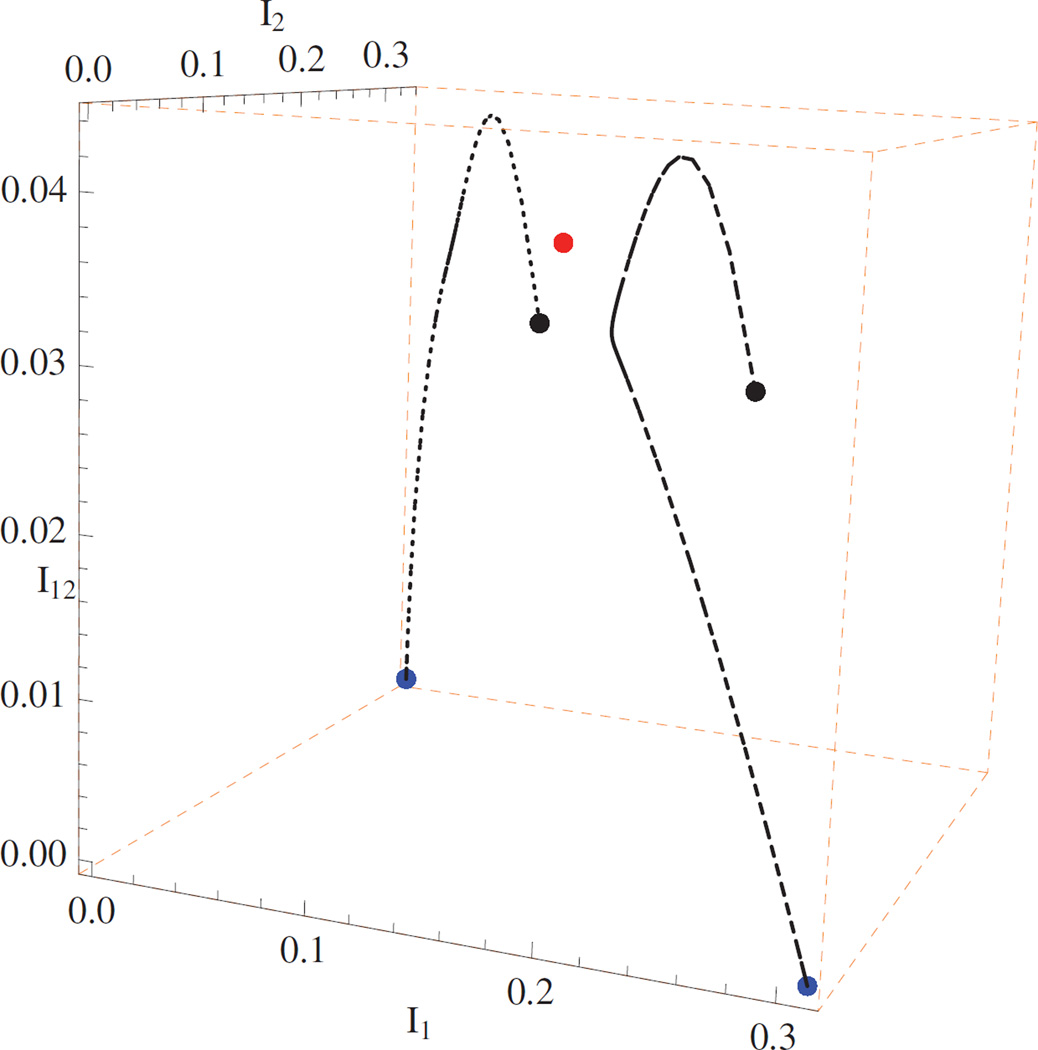
Example 5.3. Bistability (ℛ10 > 1, ℛ20 > 1, and )
The two boundary equilibria E1 and E2 are locally stable, while the coexistence equilibrium E* is unstable. Parameter setting: β1 = 2.9, β2 = 3, ρ1 = ρ2 = μ = 1, β12 = 0.5, β10 = 0.3, β02 = 0.05. Direct calculations yield the basic reproduction numbers ℛ10 = 1.45, ℛ20 = 1.5 and the invasion reproduction numbers . A bistability phenomenon is observed in Figure 4. The disease outcome depends on initial conditions and there exists a smooth surface separating the feasible region into two domains.
Figure 4.
Numerical solutions of system (2.1) with initial conditions: (0.62, 0.25, 0.1, 0.03) and (0.62, 0.1, 0.25, 0.03) (black dots). Bistability: the two boundary equilibria E1 = (20/29, 9/29, 0, 0) and E2 = (2/3, 0, 1/3, 0) (blue dots) are locally stable, and the coexistence equilibrium E* ≈ (0.701, 0.165, 0.096, 0.038) (red dot) is unstable.
The occurrence of bistability also implies that the infection with a higher reproduction number is not necessarily the winner, so to speak, of the competition. In addition, there is no bistability phenomenon when the mortality rate μ is small enough (see Appendix B for a proof). Namely, if ℛ20 > max{ℛ10, 1}, then the second disease always persists as μ → 0.
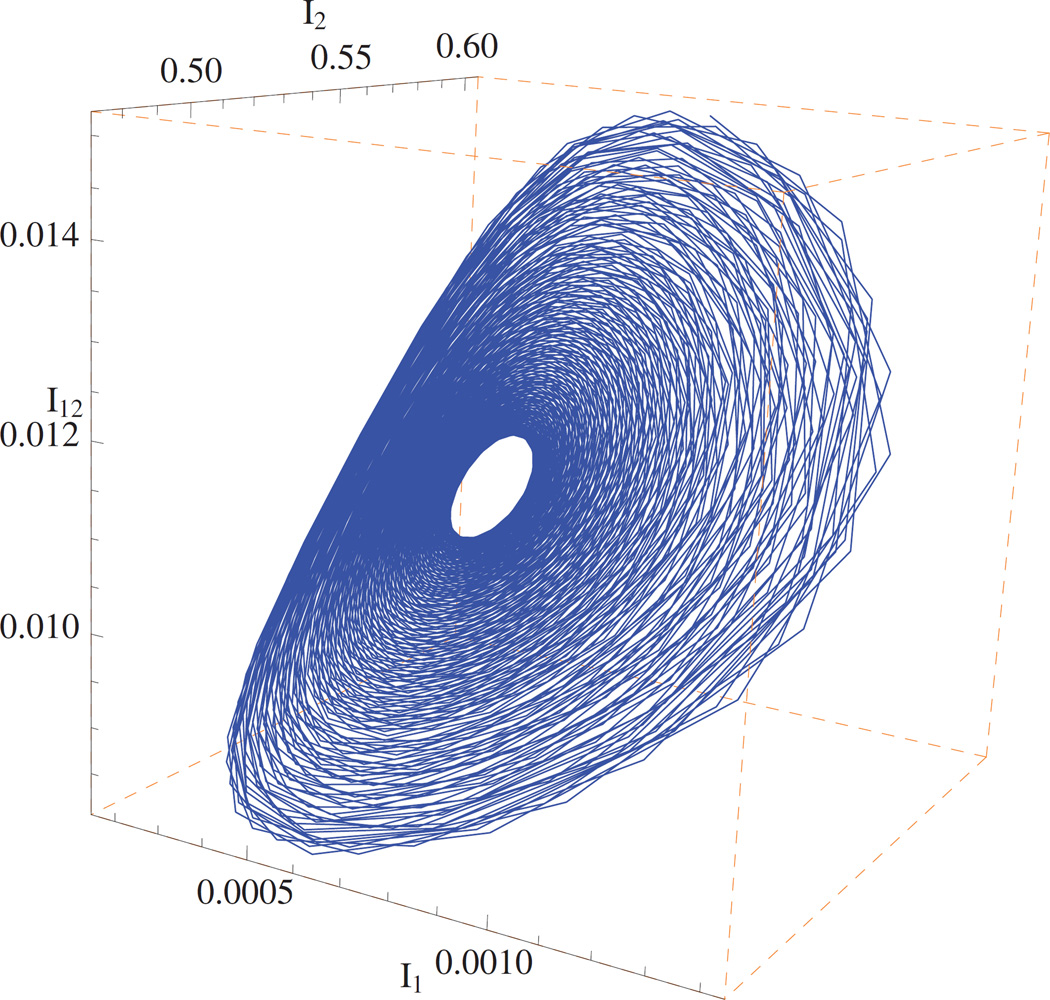
Example 5.4. Hopf bifurcation
The model can exhibit non-equilibrium dynamical behavior—periodic oscillations under certain conditions. Set β1 = 5, β2 = 0.2, ρ1 = ρ2 = 0, μ = 1, β12 = 0.805, β10 = 0 and β02 ∈ [70, 100]. It follows from ℛ10 = 5 > 1 > ℛ20 = 0.2 and that both diseases are uniformly persistent and there is a coexistence equilibrium (see Remark 4.7). The Jacobian matrix at the coexistence equilibrium has a pair of complex eigenvalues with negative real parts as β02 ∈ [70, 84.74), a pair of purely imaginary eigenvalues as β02 ≈ 84.74, and a pair of complex eigenvalues with positive real parts as β02 ∈ (84.74, 100]. Therefore, the coexistence equilibrium loses its stability and a Hopf bifurcation appears (see Figure 5). For sufficiently small but nonzero ρ1, ρ2 and β10, the system still has a Hopf bifurcation.
Figure 5.
Hopf bifurcation-two diseases coexist in an oscillatory mode.
6 Discussion
In this paper, we proposed a simple two-disease SIS coinfection model featuring simultaneous transmission of infection due to contacts with dually infected individuals, as well as superinfection leading to dual infection. In our model, there are four epidemiological classes: susceptible to both diseases, susceptible to disease 2 but infectious for disease 1, susceptible to disease 1 but infectious for disease 2, and infectious for both diseases. It is a simplified version of the two-disease three-strain model in Gao et al. [17] in which the first disease exhibits both drug sensitive and resistant strains. The cotransmission dynamics of two diseases modeled as a SIS process is of interest as a simplified model of, for instance, ocular chlamydia [33, 32, 34] and respiratory pneumococcal colonization [36]. In hyperendemic trachoma regions, both organisms are common and both are affected by trachoma control programs [24].
We considered the case for which the transmission of the first disease is completely unaffected by the presence of the second; that is, when coinfected people transmit infection to the same degree as singly infected people, disease dynamics are completely determined by the basic reproduction numbers of each. The two diseases coexist at an endemic level as long as they can persist independently. However, if the assumption does not hold, then we calculated the invasion reproduction number to measure the ability of one disease to invade the other at its steady state. The relation between the basic reproduction number and the invasion reproduction number was investigated, and sufficient conditions for the persistence of both diseases were obtained. Using numerical methods, more complicated dynamics including backward bifurcation, bistability and Hopf bifurcation were found. The disease with smaller reproduction number may be able to invade and competitively exclude the other disease even if there is no coexistence steady state. It is noteworthy that some of these dynamical behaviors have been observed in previous studies based on different models [7, 35, 60, 61]. Our study provides some more advanced theoretical results on the stability and persistence of a reasonable coinfected model. The results throughout this paper for one disease also holds for the other due to the symmetry of the model. Note that some of our analytical results are applicable to models of coinfection by different parasites (e.g. [54, 4, 3]) where there is no recovery (ρ1 = ρ2 = 0).
It is well known that, for a simple SIS epidemic model, the disease goes extinct if the basic reproduction number is less than unity and persists at a unique endemic equilibrium otherwise. Our analysis shows that the disease dynamics become more complicated in the presence of a second disease. For instance, the presence of coinfection could mediate coexistence despite that fact that one or both diseases could not survive independently. Different types of interaction in dually infected hosts can yield different outcomes. These findings emphasize the importance of understanding the interactions among pathogens and developing a multi-disease approach in the treatment of coinfected patients.
A complete classification of the model studied here is not yet available. For example, we would like to know how to rigorously prove the existence of Hopf bifurcations and whether a Hopf bifurcation is impossible for small death rate. It will be interesting to analyze our model from the perspective of evolutionary epidemiology [45, 10, 3, 4]. The current model may be extended to other natural history models, such as the SEIR assumption, or include three or more agents/strains [61]. There may be partial cross-immunity or enhanced susceptibility to either of the two infections [28]. Coinfected patients may have more serious illness and take a longer time to recover. In general, treatment of one infection in a doubly infected person may affect the other infection directly or indirectly (e.g. [50, 25, 44, 51]). We will leave these for future consideration.
Acknowledgments
We thank Drs. Thomas M. Lietman, Donald L. DeAngelis, Sergei Pilyugin, and Linda J. S. Allen for helpful comments and suggestions. This study was partially supported by the Models of Infectious Disease Agent Study (MIDAS) (NIH/NIGMS U01GM087728), NSF grant (DMS-1412454), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (No. TP2015050), and Shanghai Gaofeng Project for University Academic Program Development.
Appendix
A. The equivalence between s(F2 − V2) < 0 and
As we know, the boundary equilibrium E1 is locally asymptotically stable if and only if all eigenvalues of the Jacobian matrix F2 − V2 have negative real parts. This is also equivalent to by Theorem 2 in van den Driessche and Watmough [58]. Here we provide a direct proof. For simplicity, we consider two matrices
satisfying a, b, c, d, h, i, j, k ≥ 0 and hk − ij > 0. The characteristic polynomial of the matrix F2 − V2 is
By the Routh-Hurwitz criterion, the spectral bound s(F2 − V2) < 0 if and only if
| (6.1a) |
| (6.1b) |
Meanwhile, the characteristic polynomial of the matrix is A2λ2 + A1λ + A0 = 0, where
Then if and only if
| (6.2a) |
| (6.2b) |
Note that (6.1b) is the same as (6.2b). It suffices to show that (6.1) implies (6.2a) and (6.2) implies (6.1a), respectively.
It follows from (6.1) that h > a and k > d. Thus
On the other hand, suppose that h − a + k − d ≤ 0, then (6.1b) or (6.2b) implies that h < a and k < d. We obtain
a contradiction. The proof is complete.
B. The nonexistence of bistability for small μ
If ℛ20 > max{ℛ10, 1}, then the second disease always persists for sufficiently small μ. We only need to show the instability of the equilibrium E1 when it exists. In fact, E1 is locally asymptotically stable if and only if s(F2 −V2) < 0. The characteristic equation of F2 − V2 is β1λ2 + C1(β1)λ + C0(β1) = 0, where
Here a2 = ρ2 + μ − β12 − β02, , and a0 = −(ρ1 + μ)(β2(ρ2 + μ − β12) − β02(ρ2 + μ)).
Thus, by the Routh-Hurwitz stability criterion, E1 is locally stable if C1 > 0 and C0 > 0. In particular, if β12 = 0 then E1 is locally stable if C0 > 0 (by Appendix A). For the instability of the equilibrium E1, It suffices to show that C0 < 0 in case of ℛ10 > 1 and β12 + β02 < ρ2 + μ by Remark 4.5. This follows from a2 > 0, a0 < 0, β1 < (ρ1 + μ)ℛ20 and
for small enough μ > 0.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abu-Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science. 2006;314(5805):1603–1606. doi: 10.1126/science.1132338. [DOI] [PubMed] [Google Scholar]
- 2.Alemu A, Shiferaw Y, Addis Z, Mathewos B, Birhan W. Effect of malaria on HIV/AIDS transmission and progression. Parasit. Vectors. 2013;6:18. doi: 10.1186/1756-3305-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alizon S. Co-infection and super-infection models in evolutionary epidemiology. Interface Focus. 2013;3(6):20130031. doi: 10.1098/rsfs.2013.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alizon S. Parasite co-transmission and the evolutionary epidemiology of virulence. Evolution. 2013;67(4):921–933. doi: 10.1111/j.1558-5646.2012.01827.x. [DOI] [PubMed] [Google Scholar]
- 5.Allen LJ, Langlais M, Phillips CJ. The dynamics of two viral infections in a single host population with applications to hantavirus. Math. Biosci. 2003;186(2):191–217. doi: 10.1016/j.mbs.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Bhunu CP, Garira W, Mukandavire Z. Modeling HIV/AIDS and tuberculosis coinfection. Bull. Math. Biol. 2009;71(7):1745–1780. doi: 10.1007/s11538-009-9423-9. [DOI] [PubMed] [Google Scholar]
- 7.Blyuss KB, Kyrychko YN. On a basic model of a two-disease epidemic. Appl. Math. Comput. 2005;160(1):177–187. [Google Scholar]
- 8.Bremermann HJ, Thieme HR. A competitive exclusion principle for pathogen virulence. J Math. Biol. 1989;27(2):179–190. doi: 10.1007/BF00276102. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi AK, Katki HA, Hildesheim A, Rodríguez AC, Quint W, Schiffman M, Van Doorn LJ, Porras C, Wacholder S, Gonzalez P, Sherman ME, Herrero R CVT Group. Human papillomavirus infection with multiple types: pattern of coinfection and risk of cervical disease. J Infect. Dis. 2011;203(7):910–920. doi: 10.1093/infdis/jiq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choisy M, de Roode JC. Mixed infections and the evolution of virulence: effects of resource competition, parasite plasticity, and impaired host immunity. Am. Nat. 2010;175(5):E105–E118. doi: 10.1086/651587. [DOI] [PubMed] [Google Scholar]
- 11.Chu C-J, Lee S-D. Hepatitis B virus/hepatitis C virus coinfection: epidemiology, clinical features, viral interactions and treatment. J Gastroenterol. Hepatol. 2008;23(4):512–520. doi: 10.1111/j.1440-1746.2008.05384.x. [DOI] [PubMed] [Google Scholar]
- 12.Chun HM, Mesner O, Thio CL, Bebu I, Macalino G, Agan BK, Bradley WP, Malia J, Peel SA, Jagodzinski LL, Weintrob AC, Ganesan A, Bavaro M, Maguire JD, Landrum ML Infectious Disease Clinical Research Program HIV Working Group. HIV outcomes in Hepatitis B virus coinfected individuals on HAART. J Acquir. Immune. Defic. Syndr. 2014;66(2):197–205. doi: 10.1097/QAI.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creighton S, Tenant-Flowers M, Taylor CB, Miller R, Low N. Co-infection with gonorrhoea and chlamydia: how much is there and what does it mean? Int. J. STD & AIDS. 2003;14(2):109–113. doi: 10.1258/095646203321156872. [DOI] [PubMed] [Google Scholar]
- 14.Diekmann O, Heesterbeek JA, Metz JA. On the definition and the computation of the basic reproduction ratio r 0 in models for infectious diseases in heterogeneous populations. J Math. Biol. 1990;28(4):365–382. doi: 10.1007/BF00178324. [DOI] [PubMed] [Google Scholar]
- 15.Diekmann O, Heesterbeek JA, Roberts MG. The construction of next-generation matrices for compartmental epidemic models. JR Soc. Interface. 2010;7(47):873–885. doi: 10.1098/rsif.2009.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson R, Anderson N, Gupta S. The effect of antibody-dependent enhancement on the transmission dynamics and persistence of multiple-strain pathogens. Proc. Natl. Acad. Sci. U.S.A. 1999;96(2):790–794. doi: 10.1073/pnas.96.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao D, Lietman TM, Porco TC. Antibiotic resistance as collateral damage: the tragedy of the commons in a two-disease setting. Math. Biosci. 2015;263:121–132. doi: 10.1016/j.mbs.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao D, Ruan S. An SIS patch model with variable transmission coefficients. Math. Biosci. 2011;232(2):110–115. doi: 10.1016/j.mbs.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Getahun H, Gunneberg C, Granich R, Nunnl P. HIV infection—associated tuberculosis: the epidemiology and the response. Clin. Infect. Dis. 2010;50(Suppl 3):S201–S207. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 20.Greenbaum BD, Li OTW, Poon LLM, Levine AJ, Rabadan R. Viral reassortment as an information exchange between viral segments. Proc. Natl. Acad. Sci. U.S.A. 2012;109(9):3341–3346. doi: 10.1073/pnas.1113300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffiths EC, Pedersen AB, Fenton A, Petchey OL. The nature and consequences of coinfection in humans. J Infection. 2011;63(3):200–206. doi: 10.1016/j.jinf.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross KL, Porco TC, Grant RM. HIV-1 superinfection and viral diversity. AIDS. 2004;18(11):1513–1520. doi: 10.1097/01.aids.0000131361.75328.47. [DOI] [PubMed] [Google Scholar]
- 23.Gupta P. Hepatitis C virus and HIV type 1 co-infection. Infect. Dis. Rep. 2013;5(Suppl 1):e7. doi: 10.4081/idr.2013.s1.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haug S, Lakew T, Habtemariam G, Alemayehu W, Cevallos V, Zhou Z, House J, Ray K, Porco T, Rutar T, Keenan J, Lietman TM, Gaynor BD. The decline of pneumococcal resistance after cessation of mass antibiotic distributions for trachoma. Clin. Infect. Dis. 2010;51(5):571–574. doi: 10.1086/655697. [DOI] [PubMed] [Google Scholar]
- 25.Jarvis B, Faulds D. Lamivudine. A review of its therapeutic potential in chronic hepatitis B. Drugs. 1999;58(1):101–141. doi: 10.2165/00003495-199958010-00015. [DOI] [PubMed] [Google Scholar]
- 26.Kang M, Hollabaugh K, Pham V, Koletar SL, Wu K, Smurzynski M, Aberg JA. Virologic and serologic outcomes of mono versus dual HBV therapy and characterization of HIV/HBV coinfection in a US cohort. J Acquir. Immune. Defic. Syndr. 2014 Jun;66(2):172–180. doi: 10.1097/QAI.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawaguchi I, Sasaki A, Boots M. Why are dengue virus serotypes so distantly related? Enhancement and limiting serotype similarity between dengue virus strains. Proc. Roy. Soc. Lond. B. Biol. Sci. 2003;270(1530):2241–2247. doi: 10.1098/rspb.2003.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keeling MJ, Rohani P. Modeling Infectious Diseases in Humans and Animals. Princeton: Princeton University Press; 2008. [Google Scholar]
- 29.Lawi G, Mugisha J, Omolo-Ongati N. Mathematical model for malaria and meningitis co-infection among children. Appl. Math. Sci. 2011;5(47):2337–2359. [Google Scholar]
- 30.Lawn SD. AIDS in Africa: the impact of coinfections on the pathogenesis of HIV-1 infection. J Infection. 2004;48(1):1–12. doi: 10.1016/j.jinf.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Levin ML, Fish D. Acquisition of coinfection and simultaneous transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis ticks. Infect. Immun. 2000;68(4):2183–2186. doi: 10.1128/iai.68.4.2183-2186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lietman TM, Gebre T, Ayele B, Ray KJ, Maher MC, See CW, Emerson PM, Porco TC TANA Study Group. The epidemiological dynamics of infectious trachoma may facilitate elimination. Epidemics. 2011;3(2):119–124. doi: 10.1016/j.epidem.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lietman TM, Porco TC, Dawson C, Blower S. Global elimination of trachoma: how frequently should we administer mass chemotherapy? Nat. Med. 1999;5(5):572–576. doi: 10.1038/8451. [DOI] [PubMed] [Google Scholar]
- 34.Liu F, Porco TC, Mkocha HA, Muñoz B, Ray KJ, Bailey RL, Lietman TM, West SK. The efficacy of oral azithromycin in clearing ocular chlamydia: mathematical modeling from a community-randomized trachoma trial. Epidemics. 2014;6:10–17. doi: 10.1016/j.epidem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martcheva M, Pilyugin S. The role of coinfection in multidisease dynamics. SIAM J. Appl. Math. 2006;66(3):843–872. [Google Scholar]
- 36.McCormick AW, Whitney CG, Farley MM, Lynfield R, Harrison LH, Bennett NM, Schaffner W, Reingold A, Hadler J, Cieslak P, Samore MH, Lipsitch M. Geographic diversity and temporal trends of antimicrobial resistance in Streptococcus pneumoniae in the United States. Nat. Med. 2003;9(4):424–430. doi: 10.1038/nm839. [DOI] [PubMed] [Google Scholar]
- 37.Modjarrad K, Vermund SH. Effect of treating co-infections on HIV-1 viral load: a systematic review. Lancet Infect. Dis. 2010;10(7):455–463. doi: 10.1016/S1473-3099(10)70093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosquera J, Adler FR. Evolution of virulence: a unified framework for coinfection and superinfection. J Theor. Biol. 1998;195(3):293–313. doi: 10.1006/jtbi.1998.0793. [DOI] [PubMed] [Google Scholar]
- 39.Mukandavire Z, Gumel A, Garira W, Tchuenche J. Mathematical analysis of a model for HIV-malaria co-infection. Math. Biosci. Eng. 2009;6(2):333–359. doi: 10.3934/mbe.2009.6.333. [DOI] [PubMed] [Google Scholar]
- 40.Mushayabasa S, Tchuenche J, Bhunu C, Ngarakana-Gwasira E. Modeling gonorrhea and HIV co-interaction. BioSystems. 2011;103(1):27–37. doi: 10.1016/j.biosystems.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Perkins D, Chong H, Irvine B, Domagalski J. Genital co-infection with herpes simplex viruses type 1 and 2: comparison of real-time PCR assay and traditional viral isolation methods. J Cell. Mol. Med. 2007;11(3):581–584. doi: 10.1111/j.1582-4934.2007.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porco TC, Blower SM. HIV vaccines: the effect of the mode of action on the coexistence of HIV subtypes. Math. Popul. Stud. 2000;8(2):205–229. [Google Scholar]
- 43.Porco TC, Small PM, Blower SM. Amplification dynamics: predicting the effect of HIV on tuberculosis outbreaks. J Acquir. Immune. Defic. Syndr. 2001;28(5):437–444. doi: 10.1097/00042560-200112150-00005. [DOI] [PubMed] [Google Scholar]
- 44.Price H, Dunn D, Pillay D, Bani-Sadr F, de Vries-Sluijs T, Jain MK, Kuzushita N, Mauss S, úñez MN, Nüesch R, Peters M, Reiberger T, Stephan C, Tan L, Gilson R. Suppression of HBV by tenofovir in HBV/HIV coinfected patients: a systematic review and meta-analysis. PLoS ONE. 2013;8(7):e68152. doi: 10.1371/journal.pone.0068152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Restif O. Evolutionary epidemiology 20 years on: challenges and prospects. Infect. Genet. Evol. 2009;9(1):108–123. doi: 10.1016/j.meegid.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Ridzon R, Gallagher K, Ciesielski C, Mast EE, Ginsberg MB, Robertson BJ, Luo C-C, DeMaria A., Jr Simultaneous transmission of human immunodeficiency virus and hepatitis C virus from a needle-stick injury. N Engl. J. Med. 1997;336(13):919–922. doi: 10.1056/NEJM199703273361304. [DOI] [PubMed] [Google Scholar]
- 47.Roeger L, Feng Z, Castillo-Chavez C. Modeling TB and HIV co-infections. Math. Biosci. Eng. 2009;6(4):815–837. doi: 10.3934/mbe.2009.6.815. [DOI] [PubMed] [Google Scholar]
- 48.Sharomi O, Podder C, Gumel A, Song B. Mathematical analysis of the transmission dynamics of HIV/TB coinfection in the presence of treatment. Math. Biosci. Eng. 2008;5(1):145–174. doi: 10.3934/mbe.2008.5.145. [DOI] [PubMed] [Google Scholar]
- 49.Sharp GB, Kawaoka Y, Jones DJ, Bean WJ, Pryor SP, Hinshaw V, Webster RG. Coinfection of wild ducks by influenza A viruses: distribution patterns and biological significance. J Virol. 1997;71(8):6128–6135. doi: 10.1128/jvi.71.8.6128-6135.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shelburne SA, Visnegarwala F, Darcourt J, Graviss EA, Giordano TP, White AC, Hamill RJ. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. AIDS. 2005;19(4):399–406. doi: 10.1097/01.aids.0000161769.06158.8a. [DOI] [PubMed] [Google Scholar]
- 51.Skalet AH, Cevallos V, Ayele B, Gebre T, Zhou Z, Jorgensen JH, Zerihun M, Habte D, Assefa Y, Emerson PM, Gaynor BD, Porco TC, Lietman TM, Keenan JD. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7(12):e1000377. doi: 10.1371/journal.pmed.1000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith HL. Monotone Dynamical Systems: An Introduction to the Theory of Competitive and Cooperative Systems. Vol. 41. Providence, RI: American Mathematical Society; 1995. [Google Scholar]
- 53.Smith HL, Waltman P. The Theory of the Chemostat. Cambridge University Press; 1995. [Google Scholar]
- 54.Tanaka MM, Feldman MW. Theoretical considerations of cross-immunity, recombination and the evolution of new parasitic strains. Journal of Theoretical Biology. 1999;198(2):145–163. doi: 10.1006/jtbi.1999.0906. [DOI] [PubMed] [Google Scholar]
- 55.Templeton AR, Kramer MG, Jarvis J, Kowalski J, Gange S, Schneider MF, Shao Q, Zhang GW, Yeh M-F, Tsai H-L, Zhang H, Markham RB. Multiple-infection and recombination in HIV-1 within a longitudinal cohort of women. Retrovirology. 2009;6:54. doi: 10.1186/1742-4690-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thieme HR. Convergence results and a poincaré-bendixson trichotomy for asymptotically autonomous differential equations. J Math. Biol. 1992;30(7):755–763. [Google Scholar]
- 57.Thieme HR. Persistence under relaxed point-dissipativity (with application to an endemic model) SIAM J. Math. Anal. 1993;24(2):407–435. [Google Scholar]
- 58.van den Driessche P, Watmough J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002;180(2):29–48. doi: 10.1016/s0025-5564(02)00108-6. [DOI] [PubMed] [Google Scholar]
- 59.Xu F, Schillinger JA, Sternberg MR, Johnson RE, Lee FK, Nahmias AJ, Markowitz LE. Seroprevalence and coinfection with herpes simplex virus type 1 and type 2 in the United States, 1988–1994. J Infect. Dis. 2002;185(8):1019–1024. doi: 10.1086/340041. [DOI] [PubMed] [Google Scholar]
- 60.Zhang P, Sandland GJ, Feng Z, Xu D, Minchella DJ. Evolutionary implications for interactions between multiple strains of host and parasite. J Theor. Biol. 2007;248(2):225–240. doi: 10.1016/j.jtbi.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 61.Zhang X, Cao K. The impact of coinfections and their simultaneous transmission on antigenic diversity and epidemic cycling of infectious diseases. BioMed Res. Int. 2014;2014:375862. doi: 10.1155/2014/375862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao X-Q. Uniform persistence and periodic coexistence states in infinite-dimensional periodic semiflows with applications. Canad. Appl. Math. Quart. 1995;3:473–495. [Google Scholar]
- 63.Zhu T, Wang N, Carr A, Wolinsky S, Ho DD. Evidence for coinfection by multiple strains of human immunodeficiency virus type 1 subtype B in an acute seroconvertor. J Virol. 1995;69(2):1324–1327. doi: 10.1128/jvi.69.2.1324-1327.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]