Abstract
Background
Cervical cancer is one of the major causes of cancer death of females worldwide. Radiotherapy is considered effective for cervical cancer treatment, but the low radiosensitivity found in some cases severely affects therapeutic outcomes. This study aimed to reveal the role of CD146, an important adhesion molecule facilitating tumor angiogenesis, in regulating radiosensitivity of cervical cancer cells.
Material/Methods
CD146 protein expression was compared in normal cells, cervical cancer cells with lower radiosensitivity, and cervical cancer cells with higher sensitivity from cervical squamous cell carcinoma patients. Anti-CD146 monoclonal antibody AA98 was used to inhibit CD146 in human cervical cancer SiHa cells with relatively low radiosensitivity, and then the cell survival and apoptosis changes after radiation were detected by colony formation assay and flow cytometry.
Results
CD146 protein was significantly up-regulated in cervical cancer cells (P<0.001), especially in cancer cells with lower radiosensitivity. The SiHa cells treated with AA98 showed more obvious inhibition in cell survival (P<0.05) and promotion in cell apoptosis (P<0.01) after radiation, compared to the untreated cells. More dramatic changes in apoptotic factors Caspase 3 and Bcl-XL were also detected in AA98-treated cells.
Conclusions
These results indicate that inhibiting CD146 improves the effect of radiation in suppressing SiHa cells. This study shows the potential of CD146 as a target for increasing radiosensitivity of cervical cancer cells, which might allow improvement in treatment outcome in cervical cancer. Further studies are necessary for understanding the detailed mechanism of CD146 in regulating radiosensitivity.
MeSH Keywords: Antigens, CD146; Radiation Tolerance; Uterine Cervical Neoplasms
Background
Cervical cancer is a malignant cervix disease with high incidence and has become the third leading cause of cancer death of females in less developed countries after liver and stomach cancers. In 2012, cervical cancer led to an estimated 265 700 deaths worldwide [1]. Risk factors include weak immune system and unhealthy living habits, but the vast majority of cervical cancer cases are associated with human papillomavirus (HPV) infection. Persistent infection with HPV in the cervix is considered necessary for the development of cervical cancer; therefore, certain HPV proteins are effective markers for this disease [2]. Molecular tools, such as high-risk viral strains DNA HPV 16 and 18, are being used as an effective screening strategy with obvious advantages over cytology methods [3,4]. Other biomolecular markers, such as high-risk mRNAs and DNA methylation features, have been investigated [5, 6], offering new methods for the diagnosis of cervical cancer.
The treatment of cervical cancer includes surgical treatments, such as hysterectomy, trachelectomy and loop electrical excision procedure, as well as adjuvant radiotherapy and cisplatin-based chemotherapy. Radiotherapy shows great therapeutic effectiveness in reducing the risk of cancer relapse [7]. However, some cases have local reoccurrence of cervical cancer cells after radiotherapy, due to the low radiosensitivity that may be caused by enhanced DNA repair mechanism [8], hypoxia [9], cellular glutathione [10], or other factors, like HPV oncoproteins [11]. Researchers have detected lower radiosensitivity in cancer-initiating cells (CICs) of human cervical cancer cell lines HeLa, SiHa, Ca Ski, and C-4 I [12], showing that CICs are likely to be responsible for the low radiosensitivity of cervical cancer cells. Among the frequently-used cell lines in cervical cancer studies, SiHa cells are less sensitive to radiation, and cell lines ME180 and C33A are relatively sensitive to radiation [13], providing appropriate materials for radiosensitivity research in cervical cancer. Still, efficient strategies avoiding the resistance of radiotherapy are of great value for reducing the recurrence rate and improving the outcome of cervical cancer treatment.
CD146, also known as melanoma cell adhesion molecule (MCAM), plays key roles in cell adhesion, as well as the pathological progression of inflammation and even cancers [14]. It is defined as a marker of melanoma progression, and its expression allows the interaction between melanoma cells and cellular elements of the vascular system, thus accelerating melanoma growth and metastasis [15]. Overexpression of CD146 is also indicated to be a promising marker for cervical cancer, and it is positively correlation with the pathological subtype in this disease [16]. However, no association has been reported between CD146 and radiosensitivity of cervical cancer cells. The purpose of this study was to investigate the potential role of CD146 in modulating radiosensitivity of cervical cancer cells. In the present study, we compared the expression of CD146 protein in cancer tissues sensitive or resistant to radiotherapy and normal tissues in cervical squamous cell carcinoma patients. AA98, an anti-CD146 monoclonal antibody, was used to inhibit CD146 in SiHa cells, and the role of CD146 in cell radiosensitivity was reflected in changing degree of cell survival and apoptosis after radiation. These experiments provide new evidence of the effects of inhibiting CD146 on increasing radiosensitivity of cervical cancer cells, and lay the foundation for targeting CD146 in cervical cancer treatment.
Material and Methods
Tissue sampling
Cervical cancer tissues and adjacent normal tissues were sampled from 50 cervical squamous cell carcinoma patients ages 29 to 73 years (49.9±11.3) hospitalized in our institute from December 2013 to November 2014. No patient had received any treatment for cervical cancer before. According to the staging standard proposed by the International Federation of Gynecology and Obstetrics, the cancer conditions of these patients fell into IIB (27 cases), IIIA (16 cases), and IIIB (7 cases). No metastasis to lymph nodes or organs outside the pelvic cavity was found. The tissue samples were quickly frozen in liquid nitrogen and then stored at −80°C for protein extraction. The sampling process was approved by the Ethics Committee of our institute each patient and we obtained consent from all patients. After sampling, the patients received radical radiotherapy once a week for 6 weeks. A follow-up examination was carried out during the 4 months after radiotherapy. If the cancer remained or relapsed, the corresponding samples were characterized into the lower radiosensitivity group, and the other cancer samples were considered sensitive to the radiotherapy (higher radiosensitivity group).
SiHa cell culture
Human cervical squamous cell carcinoma SiHa cells (ATCC, Manassas, VA) were cultured in Dulbecco’s modified Eagle’s medium/F12 supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (Gibco, Carlsbad, CA). The cells were cultured in a humidified atmosphere with 5% CO2 at 37°C, and divided into 2 groups: the AA98-treated group (AA98) and the control group (Control). For the AA98 group, 10 μg/mL anti-CD146 monoclonal antibody AA98 (IM1010, Merck Millipore, Boston, MA) was added in the medium before the following analysis.
Cell survival assay
For cell survival assay after radiation, SiHa cells in logarithmic phase were digested into single-cell suspension by Trypsin (Gibco) and 100 cells were seeded in culture plates with culture medium for incubation overnight, after which irradiation was performed using a medical electron linear accelerator (Shinva, Zibo, China) at a dose of 0.3 Gy/min for 30 min at room temperature. After irradiation, the plates were incubated in a humidified atmosphere with 5% CO2 at 37°C for 2 weeks. For each group (Control or AA98), cells without the radiation treatment were designated as the blank control. The experiment in each sample was performed in triplicate. After the incubation, the medium was removed and the cells were washed twice in phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde (Vetec, Shanghai, China) for 15 min, incubated in Giemsa stain (HarveyBio, Beijing, China) for 15 min, washed with water, and air-dried. The cells were then counted under an optical microscope (Leica Microsystems, Wetzlar, Germany) and the surviving fraction (SF) was calculated as (the colony formation number of Control or AA98 group after radiation/the colony formation number of the corresponding blank control) ×100%.
Cell apoptosis assay
Cell apoptosis was analyzed at 0 h, 72 h, and 7 days after radiation using flow cytometry and Annexin V-FITC Apoptosis Detection Kit I (Univ-bio, Shanghai, China) according to the manufacturer’s instructions. Briefly, SiHa cells that underwent radiation treatment as described above were collected by centrifugation. After washing in PBS twice, the cells were resuspended in binding buffer and transferred to 96-well plates (1×105 cells per well). Annexin V-FITC and propidium iodide (PI) were added to each well and the cells were incubated at room temperature in the dark for 15 min, after which the binding buffer was added, and the cells were detected by flow cytometry (BD Biosciences, San Jose, CA). The FITC-positive and PI-negative cells were considered to be the apoptotic cells. The experiment in each sample was performed in triplicate.
Western blot
The SiHa cells with or without AA98 treatment received radiation as described above, after which they were collected and lysed in lysis buffer (Beyotime, Shanghai, China). The same amount of protein samples (20 μg) was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then the protein bands on the gel were transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was first incubated in 5% skim milk at room temperature for 2 h for blockage, and then incubated in AA98, anti-Caspase 3, anti-Bcl-XL, or anti-β-actin (ab2302, ab178844, ab129348, Abcam, Cambridge, UK). β-actin was used as an internal reference. After washing in Tris-buffered saline with Tween (TBST), the membrane was incubated in the horseradish peroxidase-conjugated secondary antibody at room temperature for 1 h. Signals were developed by ECL Plus Western Blotting Substrate (Pierce, Carlsbad, CA). The grey-scale value of each band was compared to that of the β-actin bands, and analyzed by ChemiDoc XRS System (Bio-Rad, Hercules, CA). The experiment was repeated 3 times.
Statistical analysis
Results are indicated as the mean ± standard deviation. Data were first analyzed by F test for homogeneity of variance and then the t test for significant difference in SPSS 20 (IBM, NY, USA). Differences between groups were considered statistically significant at P<0.05.
Results
CD146 is up-regulated in cervical cancer with lower radiosensitivity
The protein expression level of CD146 was compared in cervical cancer cells and the adjacent normal cells from patients. Results showed that CD146 protein was significantly increased in cervical cancer cells with higher radiosensitivity compared to normal cells (P<0.001, Figure 1A, 1B), and the cancer cells with lower radiosensitivity possessed even higher CD146 protein level than the cancer cells sensitive to radiotherapy (P<0.01). These results indicate that CD146 was overexpressed in cervical cancer cells, especially in cells with lower radiosensitivity, suggesting its association with radiosensitivity of cervical cancer cells.
Figure 1.
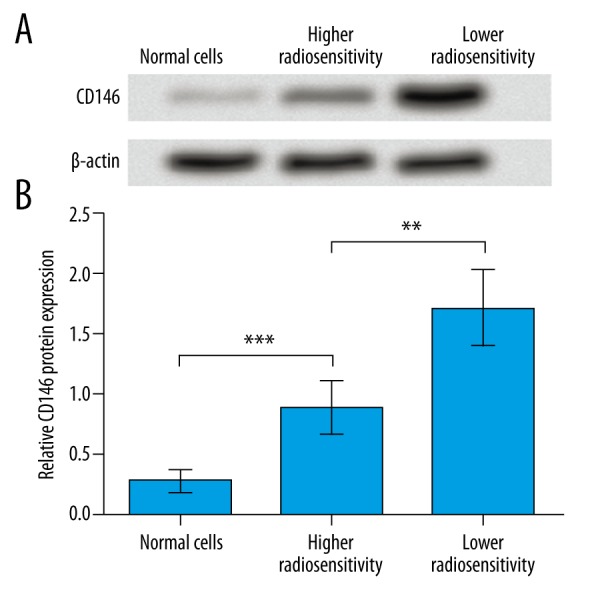
CD146 is up-regulated in cervical cancer cells, especially those with lower radiosensitivity. (A) Western blot results show the relative protein expression patterns of CD146 in cervical cancer cells and the adjacent normal cells of cervical squamous cell carcinoma patients. β-actin was used as an internal reference. (B) Histogram shows the relative grey-scale value of CD146 bands compared to β-actin bands (n=3). ** P<0.01. *** P<0.001.
Inhibiting CD146 improves the effects of radiation on cell survival and apoptosis
The expression pattern of CD146 led us to speculate about the relationship between CD146 expression and the radiosensitivity of cervical cancer cells. The anti-CD146 monoclonal antibody AA98 was applied to cultured SiHa cells, a human cervical squamous carcinoma cell line with relatively high resistance to radiotherapy [13,17], to investigate the effects of CD146 on cell radiosensitivity. The SiHa cells were seeded in culture medium and treated with radiation. Colony formation assay showed that SF of cells treated with AA98 was much lower than in cells without AA98 treatment (P<0.05, Figure 2), indicating that inhibiting CD146 could improve the effects of radiation on suppressing cell proliferation and survival.
Figure 2.
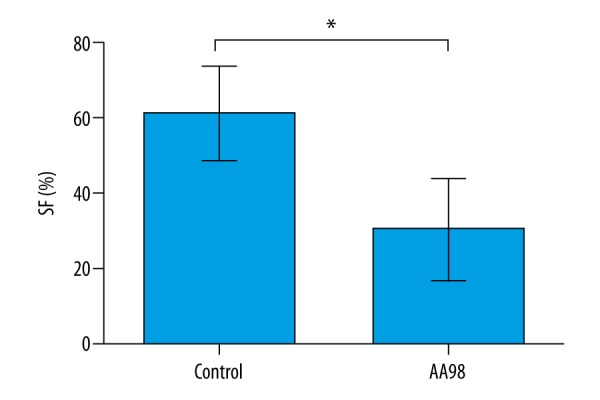
Inhibiting CD146 improves the effects of radiation on suppressing SiHa cell survival. Cell survival was detected by colony formation assay (n=3) and indicated as SF (surviving fraction). Control, SiHa cells without AA98 treatment. AA98, SiHa cells with AA98 treatment. * P<0.05.
Further, cell apoptosis assay was performed using flow cytometry and results showed that at 72 h and 7 d after radiation, the cell apoptosis of SiHa cells treated by AA98 was promoted compared to the untreated cells, and significant differences were found at these 2 time points (P<0.01, Figure 3), indicating that inhibiting CD146 improved the effects of radiation on promoting cell apoptosis.
Figure 3.
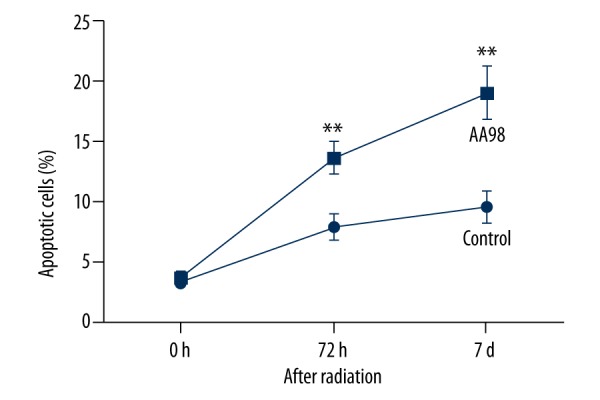
Inhibiting CD146 improves the effects of radiation on promoting SiHa cell apoptosis. Cell apoptosis assay by flow cytometry was conducted at 0 h, 72 h, and 7 days after irradiation. Control, SiHa cells without AA98 treatment. AA98, SiHa cells with AA98 treatment. Histogram shows the flow cytometry results of triple experiments. ** P<0.01.
To confirm the cell apoptosis change detected by flow cytometry, 2 apoptosis indicators, caspase 3 and Bcl-XL, were analyzed by Western blot. Results showed that after radiation, caspase 3 protein was up-regulated and Bcl-XL protein was down-regulated in AA98-treated SiHa cells (Figure 4A), with significant difference compared to the untreated cells (P<0.001, Figure 4B), indicating that inhibiting CD146 by AA98 could further promote caspase 3 and inhibit Bcl-XL. Because caspase 3 is a pro-apoptotic factor and Bcl-XL is anti-apoptotic in various cancers, including cervical cancer [18,19], the changing patterns of them was consistent with the promoted cell apoptosis in AA98-treated cells, further confirming that inhibiting CD146 can improve the effects of radiation on SiHa cell apoptosis.
Figure 4.
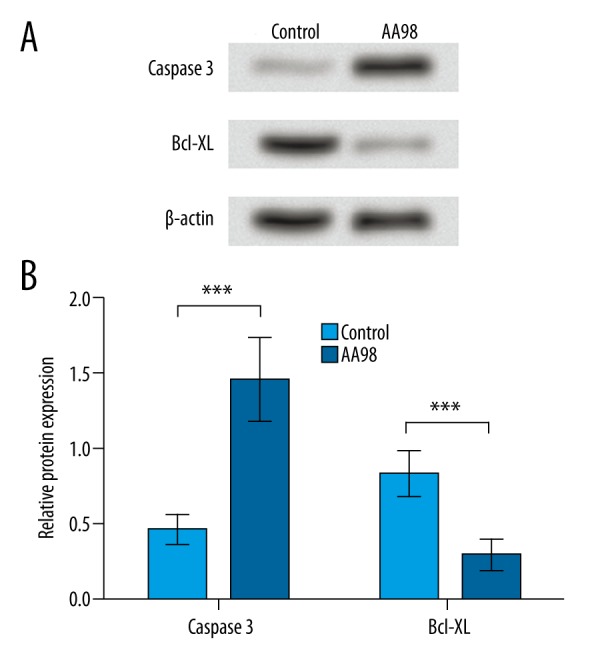
Inhibiting CD146 promotes caspase 3 and inhibits Bcl-XL protein expression compared to untreated cells. Control, SiHa cells without AA98 treatment. AA98, SiHa cells with AA98 treatment. (A) Western blot shows the protein expression patterns of caspase 3 and Bcl-XL in SiHa cells with or without AA98 treatment. β-actin was used as an internal reference. (B) Histogram shows the relative expression level of caspase 3 and Bcl-XL by comparing the grey-scale value to β-actin bands (n=3). *** P<0.001. Bcl-XL, B-cell lymphoma (extra-large).
Discussion
This study found up-regulated expression of CD146 protein in cervical cancer cells of cervical squamous cell carcinoma patients, especially in the cancer cells with lower sensitivity to radiotherapy. AA98, an anti-CD146 monoclonal antibody, was used to investigate the role of CD146 in the radiosensitivity of SiHa cells. We found that inhibiting CD146 in SiHa cells aggravates the cell survival inhibition and cell apoptosis promotion by radiation, indicating improved radiosensitivity of SiHa cells.
CD146 is a potential therapeutic target for a variety of diseases, including gastric cancer and breast cancer, where it functions as an epithelial-mesenchymal transition inducer [20–22]. A previous study has shown that CD146 mRNA expression can be induced by cisplatin in cisplatin-sensitive cancer cell lines, suggesting its involvement in resistance to cytotoxic chemotherapeutic agents [23]. CD146 protein was up-regulated in cervical cancer cells compared to normal cells, which is consistent with its elevation in cervical squamous cell carcinoma, as reported previously [24], and was further increased in the cervical cancer cells with lower radiosensitivity, indicating the association of CD146 with radiosensitivity of cervical cancer cells, as verified in this study in AA98-treated SiHa cells. Significant disparities were detected between the AA98-treated and untreated SiHa cells in cell survival and apoptosis after radiation. Cells treated with AA98 possessed higher sensitivity to radiation based on the more obvious changes in the reduced cell SF and increased cell apoptosis, which show that inhibiting CD146 by AA98 improved SiHa cell radiosensitivity, and that CD146 is an inducer of radioresistance in cervical cancer cells.
The monoclonal antibody AA98 is identified as anti-CD146 and capable of inhibiting proliferation and migration of human umbilical vein endothelial cells, thereby suppressing angiogenesis and tumor growth, possibly via regulating nuclear factor-κB (NF-κB) [25,26]. Numerous studies used AA98 for the inhibition of CD146 to reveal roles of CD146 in tumor angiogenesis. For example, CD146 dimerization, which is required for tumor angiogenesis [27], is dramatically decreased by AA98, thus helping to reduce the risk of malignancy in living cells [28]. Knockdown of CD146 allows the suppression in migration and proliferation of vascular endothelial cells, suggesting the possibility of using CD146 as a target in anti-angiogenesis therapy [29]. Targeting CD146 in endothelial cells alleviates inflammation responses and prevents colitis and carcinogenesis [30]. In addition to these findings, this study established the connections between CD146 and radiosensitivity in cervical cancer cells using AA98 to inhibit CD146. When the CD146 molecules on SiHa cell surface were blocked, the cells became more sensitive to radiation, reflecting the anti-radiation functions of CD146 and the possibility of targeting CD146 to increase radiosensitivity of cervical cancer cells and to improve the outcome of radiotherapy.
It has been reported that the mechanism involving ubiquitin protein ligase E3A and p53 is close related to the radiosensitivity of cervical cancer [31]. The mechanism of CD146 in affecting cell radiosensitivity has not been defined in previous studies, although its roles in facilitating tumor angiogenesis are likely to include the regulation of NF-κB, the direct interaction with vascular endothelial growth factor receptor 2 [32], or the up-regulation of p53 in breast cancer [33]. In this study, inhibiting CD146 was shown to affect the changing degrees of 2 apoptotic factors, Caspase 3 and Bcl-XL, after irradiation, showing that CD146 might regulate cell apoptosis pathways. It is also possible that CD146 influenced cell apoptosis via modulating other cellular processes, since suppressed DNA repair is closely related to enhanced radiosensitivity of cancer cells [34,35]. Further studies will concentrate on the association between CD146 and radiation resistance mechanisms, such as DNA repair, for a better understanding of the mechanisms.
Conclusions
This study shows the relationship between CD146 and cervical cancer radiosensitivity. Inhibiting CD146 with its antibody AA98 obviously improves the radiosensitivity of SiHa cells. These results suggest that CD146 is an anti-radiation factor and a potential target for cervical cancer treatment. Detailed mechanism studies are still necessary for a comprehensive understanding of the role of CD146 in radiosensitivity.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Source of support: Departmental sources
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Combes JD, Pawlita M, Waterboer T, et al. Antibodies against high-risk human papillomavirus proteins as markers for invasive cervical cancer. Int J Cancer. 2014;135:2453–61. doi: 10.1002/ijc.28888. [DOI] [PubMed] [Google Scholar]
- 3.Origoni M, Prendiville W, Paraskevaidis E. Cervical cancer prevention: New frontiers of diagnostic strategies. Biomed Res Int. 2015;2015:250917. doi: 10.1155/2015/250917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dijkstra MG, Snijders PJ, Arbyn M, et al. Cervical cancer screening: On the way to a shift from cytology to full molecular screening. Ann Oncol. 2014;25:927–35. doi: 10.1093/annonc/mdt538. [DOI] [PubMed] [Google Scholar]
- 5.Wentzensen N, Sherman ME, Schiffman M, Wang SS. Utility of methylation markers in cervical cancer early detection: Appraisal of the state-of-the-science. Gynecol Oncol. 2009;112:293–99. doi: 10.1016/j.ygyno.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nye MD, Hoyo C, Huang Z, et al. Associations between methylation of paternally expressed gene 3 (PEG3), cervical intraepithelial neoplasia and invasive cervical cancer. PLoS One. 2013;8:e56325. doi: 10.1371/journal.pone.0056325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barney BM, Petersen IA, Dowdy SC, et al. Intraoperative Electron Beam Radiotherapy (IOERT) in the management of locally advanced or recurrent cervical cancer. Radiat Oncol. 2013;8:80–88. doi: 10.1186/1748-717X-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuhrman CB, Kilgore J, LaCoursiere YD, et al. Radiosensitization of cervical cancer cells via double-strand DNA break repair inhibition. Gynecol Oncol. 2008;110:93–98. doi: 10.1016/j.ygyno.2007.08.073. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Zhang J, Wang X, et al. HIF-1 and NDRG2 contribute to hypoxia-induced radioresistance of cervical cancer Hela cells. Exp Cell Res. 2010;316:1985–93. doi: 10.1016/j.yexcr.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 10.Xiang L, Xie G, Liu C, et al. Knock-down of glutaminase 2 expression decreases glutathione, NADH, and sensitizes cervical cancer to ionizing radiation. Biochim Biophys Acta. 2013;1833:2996–3005. doi: 10.1016/j.bbamcr.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Aungsumart S, Vaeteewoottacharn K, Chamutpong S, Ponglikitmongkol M. Chemo-radio resistance in cervical cancer induced by HPV16 E7. ScienceAsia. 2007;33:5–11. [Google Scholar]
- 12.López J, Poitevin A, Mendoza-Martínez V, et al. Cancer-initiating cells derived from established cervical cell lines exhibit stem-cell markers and increased radioresistance. BMC Cancer. 2012;12:48–61. doi: 10.1186/1471-2407-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bose MV, Gopisetty G, Selvaluxmy G, Rajkumar T. Dominant negative Ubiquitin-conjugating enzyme E2C sensitizes cervical cancer cells to radiation. Int J Radiat Biol. 2012;88:629–34. doi: 10.3109/09553002.2012.702299. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Yan X. CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 2013;330:150–62. doi: 10.1016/j.canlet.2012.11.049. [DOI] [PubMed] [Google Scholar]
- 15.Schlagbauer-Wadl H, Jansen B, Müller M, et al. Influence of MUC18/MCAM/CD146 expression on human melanoma growth and metastasis in SCID mice. Int J Cancer. 1999;81:951–55. doi: 10.1002/(sici)1097-0215(19990611)81:6<951::aid-ijc18>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Zhang J, Wang Z, et al. CD146 is a potential marker for the diagnosis of malignancy in cervical and endometrial cancer. Oncol Lett. 2013;5:1189–94. doi: 10.3892/ol.2013.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ke G, Liang L, Yang JM, et al. MiR-181a confers resistance of cervical cancer to radiation therapy through targeting the pro-apoptotic PRKCD gene. Oncogene. 2013;32:3019–27. doi: 10.1038/onc.2012.323. [DOI] [PubMed] [Google Scholar]
- 18.Guo F, Li Y, Liu Y, et al. ARL6IP1 mediates cisplatin-induced apoptosis in CaSki cervical cancer cells. Oncol Rep. 2010;23:1449–55. doi: 10.3892/or_00000783. [DOI] [PubMed] [Google Scholar]
- 19.Kim KY, Seol JY, Jeon GA, Nam MJ. The combined treatment of aspirin and radiation induces apoptosis by the regulation of bcl-2 and caspase-3 in human cervical cancer cell. Cancer Lett. 2003;189:157–66. doi: 10.1016/s0304-3835(02)00519-0. [DOI] [PubMed] [Google Scholar]
- 20.Liu W-F, Ji S-R, Sun J-J, et al. CD146 expression correlates with epithelial-mesenchymal transition markers and a poor prognosis in gastric cancer. Int J Mol Sci. 2012;13:6399–406. doi: 10.3390/ijms13056399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng Q, Li W, Lu D, et al. CD146, an epithelial-mesenchymal transition inducer, is associated with triple-negative breast cancer. Proc Natl Acad Sci USA. 2011;109:1127–32. doi: 10.1073/pnas.1111053108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bardin N. Identification of CD146 as a component of the endothelial junction involved in the control of cell-cell cohesion. Blood. 2001;98:3677–84. doi: 10.1182/blood.v98.13.3677. [DOI] [PubMed] [Google Scholar]
- 23.Chang X, Monitto CL, Demokan S, et al. Identification of hypermethylated genes associated with cisplatin resistance in human cancers. Cancer Res. 2010;70:2870–79. doi: 10.1158/0008-5472.CAN-09-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Y, Yang Q, Liu H, et al. [The expression and correlation of CD146, MMP-14 in cervical squamous carcinoma]. The Practical Journal of Cancer. 2011;26:16–19. [in Chinese] [Google Scholar]
- 25.Yan X, Lin Y, Yang D, et al. A novel anti-CD146 monoclonal antibody, AA98, inhibits angiogenesis and tumor growth. Blood. 2003;102:184–91. doi: 10.1182/blood-2002-04-1004. [DOI] [PubMed] [Google Scholar]
- 26.Bu P, Gao L, Zhuang J, et al. Anti-CD146 monoclonal antibody AA98 inhibits angiogenesis via suppression of nuclear factor-kappaB activation. Mol Cancer Ther. 2006;5:2872–78. doi: 10.1158/1535-7163.MCT-06-0260. [DOI] [PubMed] [Google Scholar]
- 27.Zheng C, Qiu Y, Zeng Q, et al. Endothelial CD146 is required for in vitro tumor-induced angiogenesis: The role of a disulfide bond in signaling and dimerization. Int J Biochem Cell Biol. 2009;41:2163–72. doi: 10.1016/j.biocel.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Bu P, Zhuang J, Feng J, et al. Visualization of CD146 dimerization and its regulation in living cells. Biochim Biophys Acta. 2007;1773:513–20. doi: 10.1016/j.bbamcr.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Kang Y, Wang F, Feng J, et al. Knockdown of CD146 reduces the migration and proliferation of human endothelial cells. Cell Res. 2006;16:313–18. doi: 10.1038/sj.cr.7310039. [DOI] [PubMed] [Google Scholar]
- 30.Xing S, Luo Y, Liu Z, et al. Targeting endothelial CD146 attenuates colitis and prevents colitis-associated carcinogenesis. Am J Pathol. 2014;184:1604–16. doi: 10.1016/j.ajpath.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 31.Li X. miR-375 modulates radiosensitivity of HR-HPV-positive cervical cancer cells by targeting UBE3A through the p53 pathway. Med Sci Monit. 2015;21:2210–17. doi: 10.12659/MSM.893859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang T, Zhuang J, Duan H, et al. CD146 is a coreceptor for VEGFR-2 in tumor angiogenesis. Blood. 2012;120:2330–39. doi: 10.1182/blood-2012-01-406108. [DOI] [PubMed] [Google Scholar]
- 33.Li W, Yang D, Wang S, et al. Increased expression of CD146 and microvessel density (MVD) in invasive micropapillary carcinoma of the breast: Comparative study with invasive ductal carcinoma-not otherwise specified. Pathol Res Pract. 2011;207:739–46. doi: 10.1016/j.prp.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Amorino GP, Mikkelsen RB, Valerie K, Schmidt-Ullrich RK. Dominant-negative cAMP-responsive element-binding protein inhibits proliferating cell nuclear antigen and DNA repair, leading to increased cellular radiosensitivity. J Biol Chem. 2003;278:29394–99. doi: 10.1074/jbc.M304012200. [DOI] [PubMed] [Google Scholar]
- 35.Enns L, Rasouli-Nia A, Hendzel M, et al. Association of ATM activation and DNA repair with induced radioresistance after low-dose irradiation. Radiat Prot Dosimetry. 2015;166:131–36. doi: 10.1093/rpd/ncv203. [DOI] [PMC free article] [PubMed] [Google Scholar]


