Abstract
Background
Quantification of brain herniation on MRI and its immediate clinical implications are poorly described.
Objectives
Define the normal position of caudal fossa structures on brain MRIs in dogs and cats utilizing morphometry, compare this to dogs and cats with caudal transtentorial herniation (CTH), foramen magnum herniation (FMH) or both identified on MRI, and investigate associations between herniation severity, clinical signs, and 24‐hour outcome.
Animals
Ninety‐two controls (66 dogs, 26 cats), 119 cases with herniation (88 dogs, 31 cats).
Methods
Retrospective case series. The MRI database was searched for controls with normal brain anatomy and cases with brain herniation. Morphometry in controls established TTX (transtentorial to rostroventral cerebellum) to quantify CTH and FMX (caudoventral cerebellum to foramen magnum) to quantify FMH. Measurements were compared between cases and controls. Correlations with specific clinical variables and outcome were investigated.
Results
Measurements in medium/large control dogs versus small dog and cat controls were significantly different (P < .001, TTX: −0.46, −0.305, −0.3, FMX: 0.695, 0.27, 0.25, respectively). 119/1564 (7.6%) cases that underwent brain imaging had brain herniation. TTX and FMX were significantly different between controls and cases with CTH or FMH (P < .001). 67/89 (75%) cases with supratentorial lesions had no signs directly attributable to herniation. 71/119 (60%) had a normal anesthetic recovery. TTX was significantly associated with 24‐hour survival (P < .001).
Conclusions and clinical importance
Brain herniation can be quantified on MRI. Clinical signs directly attributable to brain herniation commonly are absent, and more severe CTH based on TTX is associated with a worse short‐term outcome.
Keywords: Caudal transtentorial, Foramen magnum herniation, Morphometry, Magnetic resonance Imaging
Abbreviations
- CTH
caudal transtentorial herniation
- FMH
foramen magnum herniation
- TTX
transtentorial line to the rostroventral aspect of the cerebellum
- FMX
caudoventral aspect of the cerebellum to foramen magnum line
- ICP
intracranial pressure
- MRI
magnetic resonance imaging
- CKCS
Cavalier King Charles Spaniels
- ICC
intra‐class correlation coefficient
- IQR
intraquartile range
Brain herniation refers to shifting of brain structures relative to their normal position within the calvarium.1, 2 There are five recognized types of brain herniation with caudal transtentorial (CTH) and foramen magnum (FMH) herniation being most clinically relevant.2 Herniation occurs secondary to various intracranial lesions and historically, diagnosis relied on clinical signs and evidence of herniation on post‐mortem examination.2 Relying on necropsy is problematic in that removal of the skull might disrupt the ability to discern herniation, and a poor prognosis is inevitable. Clinical signs classically associated with brain herniation include altered consciousness accompanied by changes in pupil size and responsiveness to light (CTH), and altered cardiorespiratory patterns (FMH).2, 3 Clinical signs supportive of herniation, however, might not consistently be present or be difficult to differentiate from primary brainstem disease.4, 5
The widespread use of magnetic resonance imaging (MRI) for detection of intracranial disease has led to increased frequency of diagnosis of brain herniation in dogs and cats, and a changing view of its prognosis, but data on the immediate and long term clinical implications are lacking. Indeed, in humans and dogs, presence of herniation on MRI does not always correlate with presence, type or severity of clinical signs.5, 6, 7 When acute conditions are considered, presence of herniation has been associated with a lower Glasgow Coma Scale (GCS) score and higher fatality rate in humans with subdural hematomas8 and dogs with traumatic brain injury.9 Similarly, presence of FMH in dogs with meningoencephalitis of unknown origin was associated with increased risk of fatality.10 In a preliminary study, two‐thirds of cats with rostrotentorial lesions and herniation had signs of brainstem dysfunction but prognosis has not been reported.1 Based on the current literature, the immediate clinical implications of the presence and severity of brain herniation identified on MRI in dogs and cats are unclear. An objective means of quantifying the severity of brain herniation that can be used across breeds could provide a tool to investigate the clinical significance of brain herniation, and the pathophysiology of intracranial disease.
In humans, landmarks and measurements defining herniation have been established on brain MRIs but such measurements have not been reported in dogs and cats.6 Morphometric analysis of the caudal fossa using MRI in Chiari‐like malformation in Cavalier King Charles Spaniels (CKCS) and Brussels Griffons is reported but the definition of FMH was variable.11, 12, 13, 14 The aims of this study were to define the normal position of cerebellum and brainstem structures within the calvarium utilizing morphometric analysis of normal dog and cat brain MRIs, to compare these measurements to those obtained from dogs and cats with CTH, FMH or both identified on MRI by a radiologist, and, in this affected population, to determine if there is an association between severity of brain herniation defined using these MRI‐based measurements, presence of clinical signs and short‐term outcome.
Materials and Methods
Case Selection
This is a retrospective case series. The imaging database of the NC State Veterinary Hospital was searched between 2005 and 2013 for dogs and cats that underwent brain MRI for any reason. “Controls” consisted of dogs and cats with normal brain MRIs determined by a board certified radiologist. Imaging and medical records of control animals were reviewed to confirm lack of identifiable intracranial lesions (Data S1). “Cases” were identified from radiology reports indicating overt or suspected CTH or FMH. Search terms included: “transtentorial herniation,” “foramen magnum herniation,” “brain herniation,” “evidence of increased intracranial pressure,” “suspected or borderline herniation,” “caudal fossa crowding,” or “cerebellar vermis malpositioning.” All MRIs were performed using a 1.5T scanner2 and all animals were positioned with the head and neck extended. Images were reviewed using standard transverse and sagittal sequences (T1W pre and post‐contrast, T2W, GRE/T2*, PD, FLAIR) to confirm the presence of caudal shifting of intracranial structures. CTH was defined as unilateral or bilateral herniation of the parahippocampal gyri of the cerebral cortex ventral to the tentorium cerebelli, and FMH was characterized as caudal displacement of the cerebellar vermis into or through the foramen magnum. Only animals with CTH, FMH or both with a complete medical record were included. Animals with a diagnosis of Chiari‐like malformation and FMH indicated in the radiology report were excluded due to the relatively high incidence of cerebellar herniation in the absence of additional intracranial lesions in this population.
Clinical Information
Data collection in cases consisted of clinical, pathologic and imaging information obtained from the medical records. Clinical data included signalment, presenting signs, duration of signs, initial neurolocalization, presence and character of signs of brainstem disease, treatments administered for presumed increased ICP, diagnosis, location of primary lesion, recovery from general anesthesia and outcome. Duration of signs of neurologic disease was categorized as acute (<1 week) or chronic (>1 week). Neurolocalization was defined as forebrain, brainstem, cerebellum, multifocal or cervical based on initial neurologic examination findings performed by a board certified neurologist or neurology resident. Signs of brainstem disease were designated as present or absent and defined as tetraparesis/ataxia with cranial nerve deficits excluding cranial nerves I and II, or severely altered level of consciousness (stupor, coma) suggestive of ascending reticular activating system (ARAS) involvement. Cases with severely altered mental status that presented in status epilepticus or with severe cluster seizures with or without concurrent administration of anti‐convulsants were classified as forebrain; they were not included in the group with signs of brainstem disease due to inability to eliminate the influence of seizure activity and associated medications on consciousness.
Treatments administered included corticosteroids or diuretics (mannitol, hypertonic saline) or emergency decompressive surgery that might have impacted clinical status or outcome. There was no standardized protocol regarding dose or timing of administration of drugs. For the purposes of this study, only medications given during or immediately after general anesthesia for brain imaging were considered. All animals underwent general anesthesia using a standard protocol of fentanyl3 premedication, propofol4 induction and isoflurane5 for maintenance. Anesthetic recovery was determined to be normal or abnormal, with abnormal recoveries categorized as prolonged (>25 minutes from discontinuing anesthetic gas to extubation or failure to regain pre‐anesthetic level of mentation), failure to regain consciousness (purposeful response to stimulation), or failure to regain spontaneous respiration requiring mechanical ventilation.
The location of primary intracranial pathology was categorized as rostrotentorial, caudotentorial or multifocal when lesions encompassed both compartments. Diagnoses were grouped into neoplasia (suspected or confirmed), inflammatory (infectious or non‐infectious, suspected or confirmed) and other (including all other underlying causes identified). Location of lesions and diagnosis were confirmed histopathologically when possible. If no necropsy was performed, a suspected diagnosis was based on available clinical and imaging data. In order to capture the effect of herniation on immediate survival amongst this diverse population of cases with regard to diagnoses and treatment interventions, outcome was categorized into animals that died or were euthanized within 24 hours or animals that survived greater than 24 hours from the time of imaging.
Morphometry
Morphometric analysis was performed on midsagittal T2‐weighted MR images utilizing specific bony landmarks. All measurements were made by one of the authors (ML) utilizing commercially available software.6 Published bony landmarks were used and included: dorsal aspect of the cribriform plate, dorsal aspect of the dorsal sella turcica, rostral aspect of the osseous tentorium cerebelli, caudoventral aspect of the occipital bone (dorsal margin of the foramen magnum) and caudal aspect of the basioccipital bone (ventral aspect of the foramen magnum).11 Three lines were created utilizing these landmarks (Fig 1). Three linear measurements were included in the analysis: skull length, the transtentorial line to the rostral‐most point along the ventral aspect of the cerebellum (approximately at the level of the junction between the lingula and central lobules) (TTX) and the most caudal point on the ventral aspect of the cerebellum to the foramen magnum line (FMX) (Fig 1). TTX was utilized to quantify CTH and FMX was used for FMH. All measurements were made in case and control animals. A ratio was created by dividing TTX and FMX by skull length to determine if this would normalize measurements between animals with heads of varying sizes and shapes (corrected TTX and corrected FMX). Measurements were repeated on a subset (n = 25) of control and case animals to assess repeatability.
Figure 1.
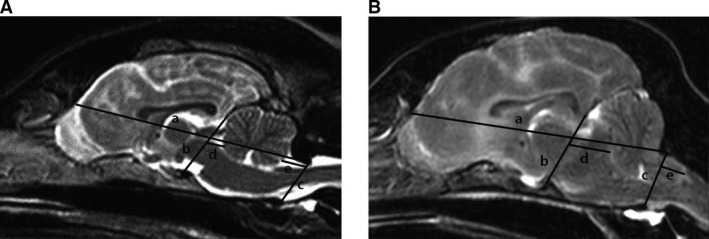
Mid‐sagittal T2‐weighted magnetic resonance images depicting bony landmarks and measurements utilized in morphometric analysis in a normal brain (A) and a brain with CTH and FMH (B). a: skull length line, b: transtentorial line, c: foramen magnum line, d: transtentorial line to rostroventral cerebellum (TTX), e: caudoventral cerebellum to foramen magnum line (FMX). For TTX (line d), all measurements were to the right of b and were designated as negative numbers. For FMX (line e), measurements to the left of c were positive, to the right of c were negative and those where the caudoventral aspect of the cerebellum was even with c were designated as zero. CTH: caudal transtentorial herniation, FMH: foramen magnum herniation.
Statistical Analysis
Analysis was performed using SAS Version 9.47. Breeds in the study population were compared to the neurology service and entire hospital populations as well as to animals that underwent brain MRI by testing for differences in proportion for each breed of interest. Intra‐rater repeatability of measurement data was measured using the Intra‐class Correlation Coefficient (ICC).8 In order to determine whether correction by skull length or division of raw data into body weight categories provided the most effective means of controlling for differing body sizes/species, correlation coefficients were calculated between skull length, FMX and TTX, and scatterplots of corrected versus raw measurements were created.
Summary data were established for TTX and FMX (raw and corrected) in case and control animals. Measurement data (raw only) were further subdivided by species and size into small breed dogs (</=15 kg), medium to large breed dogs (>15 kg) and cats, with the same variables calculated for each subgroup. The decision to use 15 kgs as a cutoff was based on inspection of raw measurements in control dogs that demonstrated a natural separation roughly dividing the population into two body weight categories. There were insufficient numbers to investigate measurements by breed or breed category (brachycephalic, mesaticephalic, dolichocephalic).
Cases were grouped according to their type of herniation. CTH refers to all cases with CTH (CTH only + CTH/FMH) and FMH refers to all cases with FMH (FMH only + CTH/FMH) throughout unless otherwise specified. A One‐way ANOVA was used to compare TTX and FMX (raw and corrected) in cases (CTH or FMH, respectively) to controls. For raw data only, comparisons between cases and controls for each measurement were then repeated after subdividing by species (cat vs dog) and size (cat vs small dog vs medium/large dog).
Logistic regression was performed to investigate whether severity of herniation based on the measurement data (both raw and corrected) for each type of brain herniation correlated with short‐term survival (</=24 hours, >24 hours), anesthetic complications (yes/no), presence of signs of brainstem disease (yes/no), or duration of signs of neurologic disease (acute/chronic). Analyses were performed using raw measurements with size (medium/large dog vs small dog/cat) incorporated into the model and with corrected measurements. Duration of signs and species were added as modifying factors when examining the relationship between each measurement and survival, anesthetic outcome and brainstem dysfunction. Raw and adjusted P‐values were generated with multiple comparisons corrected using the Sidak correction. In all analyses, P < 0.05 was considered statistically significant and p a refers to the adjusted P‐value.
Results
Control Population
Animals
There were 26 cats and 66 dogs, with 9 mixed breed dogs, 8 Labrador retrievers and 35 other breeds represented by 3 or fewer dogs. There were 36 medium to large breeds (>15 kg, 17–56.5 kg) and 30 small breeds (</=15 kg, 2.6–15 kg). Cat breeds included 20 domestic shorthairs, 2 domestic longhairs, 1 Ragdoll, 1 Maine Coon and 2 Siamese. Labrador retrievers and domestic shorthair cats were comparable to the distribution of the overall hospital population (P = 0.70 and P = 0.23, respectively), animals that were presented to the Neurology Service (P = 0.66 and P = 0.27, respectively) and those that underwent brain MRI during the study period (P = 0.81 and P = 0.52, respectively). There were 44 mesaticephalic dogs, 9 brachycephalic dogs, 13 dolichocephalic dogs and 26 mesaticephalic cats.
Clinical Findings
The most common presenting signs were confirmed or suspected seizures (n = 66) followed by vestibular or facial nerve dysfunction (n = 12). Diagnoses included epilepsy (n = 56) followed by no neurologic cause identified (n = 14), and idiopathic peripheral vestibular dysfunction or facial nerve paralysis (n = 11). None had identifiable intracranial disease (Data S1).
Morphometry
Bony landmarks were easily identified and measurements were repeatable in both control and cases (Skull length ICC = 0.99, TTX ICC = 0.98, FMX ICC = 0.84). TTX (median: −0.375 cm, range: −0.2 to −0.57) and FMX (median: 0.34 cm, range: 0.15–1.08) for all control animals are presented in Figure 2. Both TTX and FMX were significantly different when considering all controls as a group compared to each subgroup (medium/large dogs, small dogs, cats) and between medium/large dogs and the other two groups (small dogs and cats). There was no difference in measurements between small dogs and cats and data from these 2 groups were collapsed into one group for further analysis. TTX showed a moderate correlation to skull length (R 2 = 0.407) and FMX showed a strong correlation to skull length (R 2 = 0.795) (Fig 3A). While normalizing for skull length produced a more uniform population, corrected measurements still varied by size (Fig 3b) suggesting this was not an ideal correction factor. Given this, data are presented as raw measurements with incorporation of body size (cats/small dogs and medium/large dogs) and whole population corrected measurements when making comparisons in animals with herniation as alternative means to account for variations in head morphology.
Figure 2.
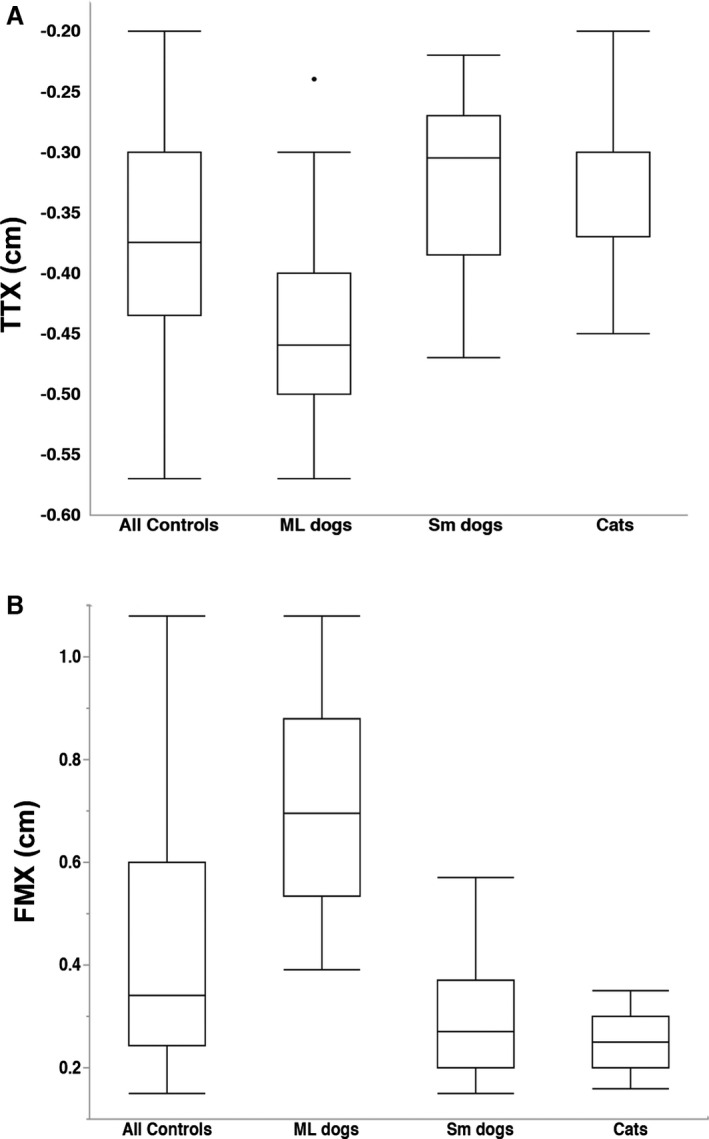
Measurements in control animals and subdivided by dog size and species. (A) TTX for all controls was significantly different from ML dogs (P < 0.001), Sm dogs (P = 0.009) and cats (P = 0.005) and ML dogs were significantly different from Sm dogs and cats (both P < 0.001). (B) FMX for all controls was significantly different from ML dogs (P < 0.001), Sm dogs (P = 0.002) and cats (P < 0.001) and ML dogs were significantly different from Sm dogs and cats (both P < 0.001). Tukey boxplots depicting median, minimum and maximum values within 1.5 times the Interquartile Range (IQR) and outliers for each group. ML: medium/large, Sm: small, TTX: transtentorial to rostroventral cerebellum line. FMX: caudoventral cerebellum to foramen magnum line.
Figure 3.
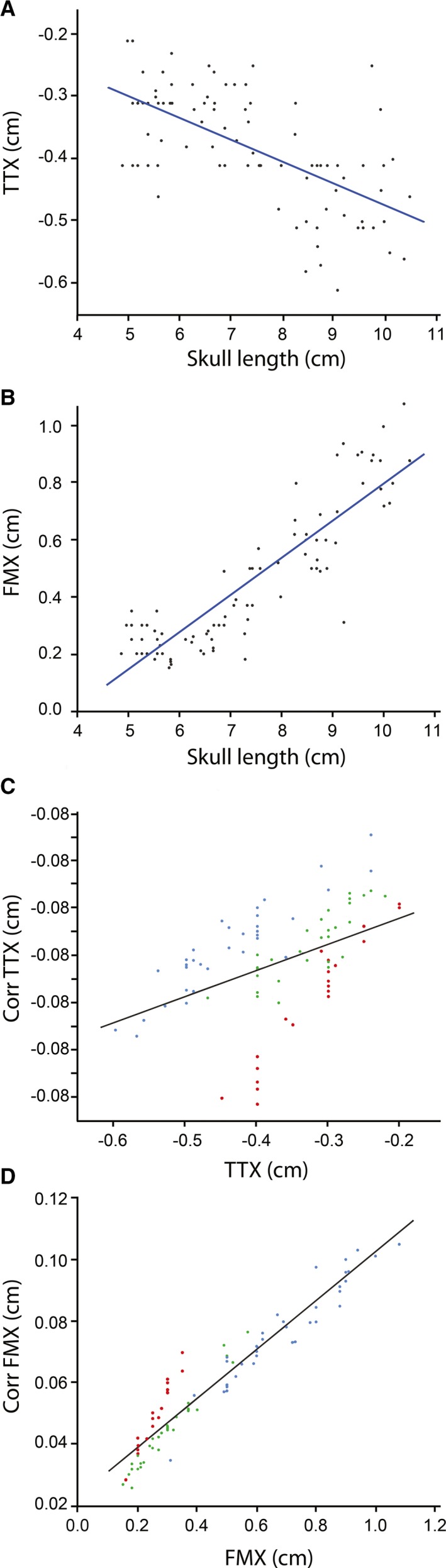
Comparison of raw versus corrected measurements in control animals. (A) Raw TTX vs Skull length. R 2 = 0.407. (B) Raw FMX vs Skull length. R 2 = 0.795. (C) Corrected TTX vs raw TTX and, (D) Corrected FMX vs raw FMX showing persistent grouping by size despite accounting for skull length. Blue: Medium/large dogs, Green: Small dogs, Red: Cats, Corr: corrected, TTX: transtentorial to rostroventral cerebellum line, FMX: caudoventral cerebellum to foramen magnum line.
Case Population
Animals
One hundred and nineteen cases with brain herniation were identified that met inclusion criteria, 40 with CTH only, 19 with FMH only, and 60 with a combination of CTH and FMH. There were 31 cats (4 CTH only, 2 FMH only, 25 CTH/FMH) and 88 dogs (36 CTH only, 17 FMH only, 35 CTH/FMH). All cats were either domestic short or longhaired breeds. The most common dog breeds included mixed breed dogs (11), Boxer (11), Boston Terrier (6), and Golden Retriever (5) with an additional 39 breeds comprising three or fewer cases. Golden Retriever prevalence was similar to that of the overall hospital population (P = 0.974), the Neurology Service (P = 0.445) and dogs undergoing brain MRI during the study period (P = 0.928). Boxers and Boston Terriers were overrepresented compared to the overall hospital population (P = 0.014 and P = 0.040, respectively). Boxers were also overrepresented compared to dogs that presented to the neurology service (P = 0.015) and dogs that underwent brain MRI during the study period (P = 0.044). Forty‐five dogs were considered medium to large breeds (>15 kg, 16–45.6 kg) and 43 were small breeds (</=15 kg, 1.5–12 kg).
Clinical Findings
Clinical findings of herniation cases are summarized in Table 1. The majority of cases (n = 77) presented with signs of forebrain disease with no evidence of signs caused by herniation. There were 38 cases (32%) including 30 dogs and 8 cats with a neuroanatomic localization of brainstem or multifocal exhibiting signs of brainstem dysfunction of which 16 had primary brainstem pathology on MRI. The remaining 4 cases had cerebellar (n = 3) or cervical (n = 1) localization. Rostrotentorial lesions were identified on MRI in 89 cases whereas multifocal (n = 18) and caudotentorial lesions (n = 12) were less common. Twenty‐two of the 89 (25%) cases with rostrotentorial lesions had signs of brainstem dysfunction, of which vestibular signs were the most common (Table 2).
Table 1.
Clinical information in 119 animals with brain herniation
| Dogs | Cats | |
|---|---|---|
| N = 88 | N = 31 | |
| Presenting complaint | ||
| Behavior Change | 60 (68%) | 16 (52%) |
| Seizures | 46 (52%) | 8 (26%) |
| Circling | 15 (17%) | 8 (26%) |
| Ataxia | 17 (19%) | 2 (6%) |
| Duration | ||
| Acute (<1 week) | 35 (40%) | 15 (49%) |
| Chronic (>1 week) | 53 (60%) | 16 (51%) |
| Neurolocalization | ||
| Forebrain | 55 (63%) | 22 (71%) |
| Brainstem | 10 (11%) | 4 (13%) |
| Cerebellum | 1 (1%) | 0 (0%) |
| Cervical | 2 (2%) | 1 (3%) |
| Multifocal | 20 (23%) | 4 (13%) |
| Primary intracranial lesion location | ||
| Supratentorial | 65 (74%) | 24 (78%) |
| Infratentorial | 11 (12%) | 1 (3%) |
| Diffuse/Multifocal | 12 (14%) | 6 (19%) |
| Diagnosis | ||
| Neoplasia (suspected or confirmed) | 72 (82%) | 22 (71%) |
| Inflammatory (suspected or confirmed) | 11 (12%) | 7 (23%) |
| Other | 5 (6%) | 2 (6%) |
Table 2.
Characteristics of 22 cases with herniation with supratentorial lesions and signs of brainstem disease
| CTH | FMH | CTH/FMH | |
|---|---|---|---|
| Dogs | 6 | 1 | 10 |
| Cats | 0 | 0 | 5 |
| Specific signs | |||
| Cranial nerve deficits | 5 | 1 | 12 |
| Pupillary abnormalities | 2 | 1 | 2 |
| Vestibular dysfunction | 2 | 1 | 10 |
| Absent gag reflex | 2 | 0 | 5 |
| Severely altered mentation | 4 | 1 | 7 |
Eighty‐eight cases received one or more medications for suspected increased ICP during or immediately after general anesthesia (Table 3). Two cases (1 dog, 1 cat) were taken directly for decompressive surgery under the same anesthetic episode and recovered uneventfully. 71/119 (60%) of cases had a normal anesthetic recovery and 78 cases (65.5%) survived at least 24 hours after diagnosis (Table 3). Prolonged anesthetic recovery, the need for mechanical ventilation, and death or euthanasia within 24 hours of diagnosis were more common in cases with combined CTH/FMH than either CTH only or FMH only (Table 3). Of the 41 cases that failed to survive to 24 hours, 38 were euthanized including 7 (18%) in which no attempt was made to allow for recovery from general anesthesia.
Table 3.
Treatment interventions, anesthetic recovery and short‐term outcome in cases with herniation
| CTH | FMH | CTH/FMH | |
|---|---|---|---|
| Treatments | |||
| None | 14 | 2 | 13 |
| Mannitol | 18 | 15 | 43 |
| Dexamethasone SP | 23 | 11 | 41 |
| Both | 15 | 10 | 37 |
| Decompressive surgery | 1 | 0 | 1 |
| Anesthetic recovery | |||
| Normal | 27 | 13 | 31 |
| Abnormal | 10 | 5 | 22 |
| Dogs | 10 | 4 | 16 |
| Cats | 0 | 1 | 6 |
| Not attempted | 2 | 0 | 5 |
| Unknown | 1 | 1 | 2 |
| Type of abnormal recovery | |||
| Prolonged | 7 | 4 | 13 |
| Remained comatose | 3 | 0 | 2 |
| Needed mechanical ventilation | 0 | 1 | 7 |
| Survival | |||
| <24 hours | 12 | 3 | 26 |
| Dogs | 12 | 2 | 17 |
| Cats | 0 | 1 | 9 |
| With brainstem abnormalities | 4 | 3 | 18 |
| With acute onset of signs | 8 | 2 | 10 |
| With abnormal anesthetic recovery | 6 | 2 | 14 |
| 24 hours | 28 | 16 | 34 |
| Dogs | 24 | 15 | 18 |
| Cats | 4 | 1 | 16 |
| With brainstem abnormalities | 3 | 7 | 3 |
| With acute onset of signs | 13 | 7 | 10 |
| With abnormal anesthetic recovery | 4 | 3 | 8 |
MRI Findings
Brain herniation was detected in 119 out of a total of 1564 brain MRIs performed during the study period resulting in a prevalence of brain herniation of 7.6%. There were three cases (one dog with combined CTH/FMH and two dogs with FMH only) for which the intracranial lesion obscured identification of some of the landmarks and resulted in the inability to accurately perform TTX. The dog with CTH/FMH was eliminated from the analysis of this measurement; the two dogs with FMH only would never have been included in the group of cases in which TTX was analyzed as CTH was not present.
Morphometry
TTX was significantly different between controls (median: −0.375 cm, range: range: −0.2 to −0.57) and cases with CTH (median: −0.82, range: −0.42 to −1.4) (Fig 4A, P < 0.001). This was also true when controls and cases were subdivided according to size (P < 0.001) and species (P < 0.001). There was no significant difference in TTX between cases with CTH only versus cases with CTH/FMH (P = 0.67), or between control animals and those with FMH only (P = 0.27). Corrected TTX was also significantly different between controls and cases with CTH (P < 0.001).
Figure 4.
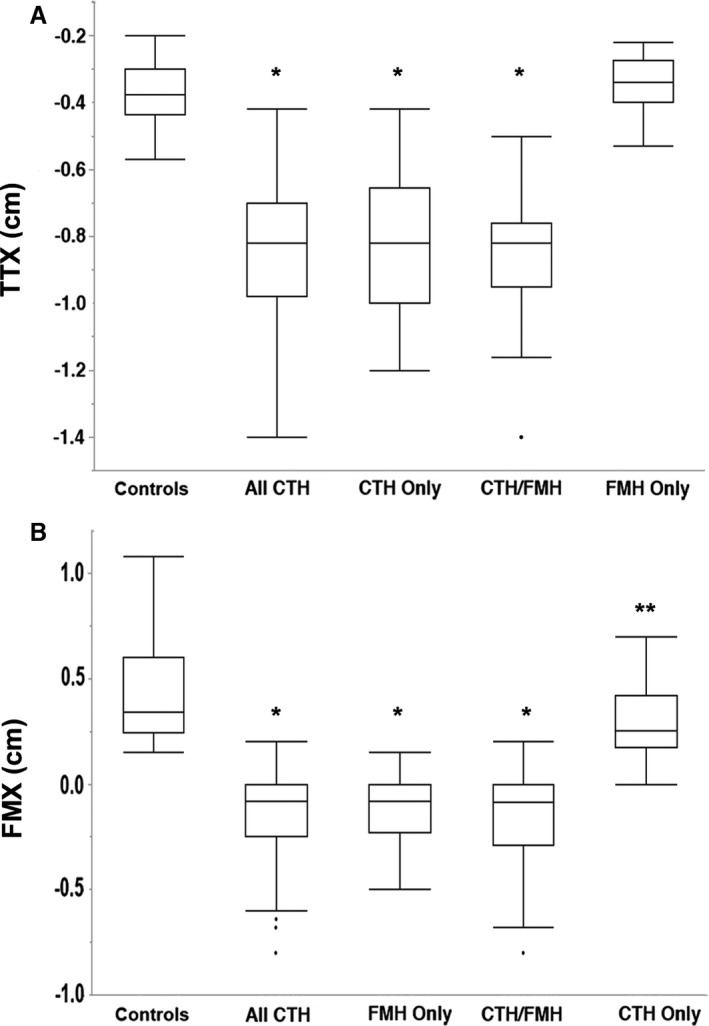
Measurements in control animals compared to herniation cases. (A) TTX in all controls was significantly different from All CTH, CTH Only and CTH/FMH cases (*P < 0.001). (B) FMX in all controls was significantly different from All FMH, FMH Only and CTH/FMH cases (*P < 0.001). All controls were significantly different from CTH Only cases (**P = 0.0026). Tukey boxplots depicting median, minimum and maximum values within 1.5 times the Interquartile Range and outliers for each group. CTH: Caudal transtentorial herniation, FMH: foramen magnum herniation, TTX: transtentorial to rostroventral cerebellum line. FMX: caudoventral cerebellum to foramen magnum line.
FMX was significantly different between controls (median: 0.34 cm, range: 0.15–1.08) and cases with FMH (median: −0.08, range: −0.8 to 0.2) (Fig 4B, P < 0.001). It was also different among controls and cases matched for size (P < 0.001) and for species (P < 0.001). There was no significant difference in FMX between cases with FMH only versus cases with CTH/FMH (P = 0.79), but there was a significant difference when control animals were compared with cases with CTH only (P = 0.0026). Corrected FMX was also significantly different between controls and cases with FMH (P < 0.001).
Associations between Morphometry, Clinical Signs and Outcome
In cases with CTH (n = 100), a longer TTX was associated with worse short‐term survival (P < 0.001, p a = 0.028) but not presence of signs of brainstem disease (P = 0.0065, p a = 0.17) or anesthetic complications (P = 0.12, p a = 0.93). Similarly, corrected TTX was associated with survival (P = 0.001, p a = 0.03) but not signs of brainstem disease (P = 0.016, p a = 0.35) or anesthetic complications (P = 0.28, p a = 0.96). The probability of surviving 24 hours in cases with CTH stratified by size is shown in Figure 5A. TTX was not associated with duration of signs of neurologic disease (P = 0.57, p a = 0.99) or species (P = 0.15, pa = 0.92). When duration of signs and species were considered as modifiers, neither factor influenced outcome for a given TTX [duration of signs (P = 0.090, p a = 0.87); species (P = 0.39, p a = 0.95)]. Similarly, the frequency of anesthetic complications or signs of brainstem disease was not significantly associated with duration (P = 0.32, p a = 0.92, P = 0.15, p a = 0.93 respectively), or species (P = 0.15, p a = 0.94, P = 0.99, p a = 0.99, respectively) for a given TTX.
Figure 5.
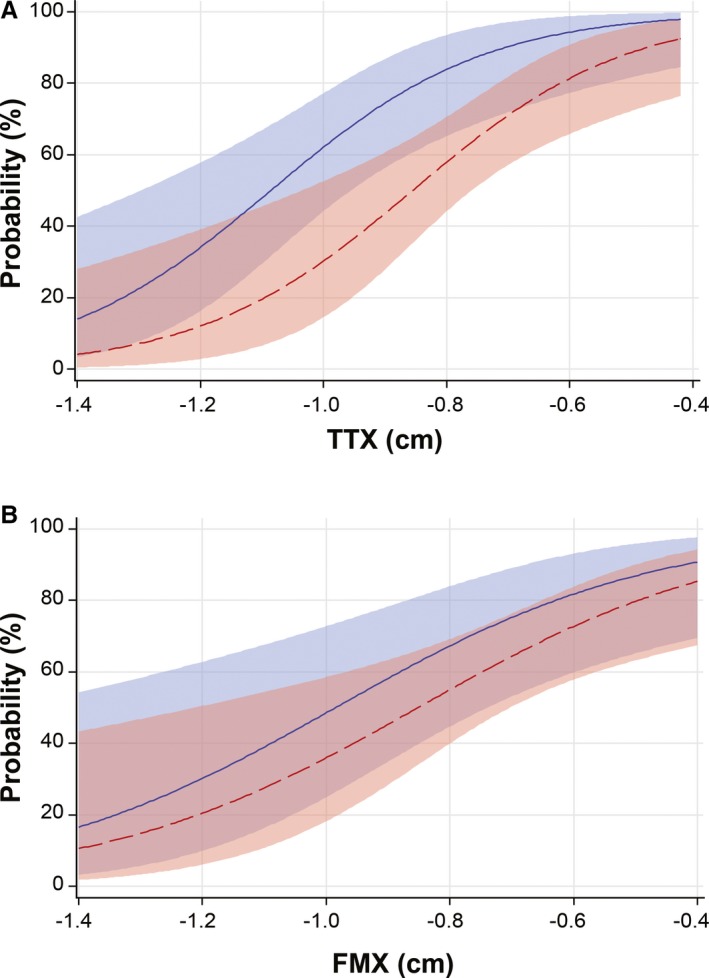
Probability curves depicting short‐term survival in herniation cases. (A). Probability of survival to 24 hours in cases with CTH based on TTX with 95% confidence limits. (B). Probability of survival to 24 hours in cases with FMH based on FMX with 95% confidence limits. CTH: Caudal transtentorial herniation, FMH: foramen magnum herniation, TTX: transtentorial to rostroventral cerebellum line, FMX: caudoventral cerebellum to foramen magnum line. Blue: Medium/large dogs, Red: Small dogs/Cats
In cases with FMH (n = 79), there was no association between FMX and short‐term survival (P = 0.0038, p a = 0.11), signs of brainstem disease (P = 0.14, p a = 0.94) or anesthetic complications (P = 0.060, p a = 0.77). Using corrected FMX, there was also no significant correlation with survival (P = 0.0050, p a = 0.14), anesthetic complications (P = 0.042, p a = 0.65) or signs of brainstem disease (P = 0.13, p a = 0.93). The probability of surviving 24 hours in cases with FMH stratified by size is demonstrated in Figure 5B. For a given FMX, there was no association between short‐term survival and duration of signs or species (P = 0.31, p a = 0.97, P = 0.85, p a = 0.99, respectively). Similarly, there was no association between the frequency of anesthetic complications or signs of brainstem disease and duration (P = 0.92, p a = 0.99, P = 0.19, p a = 0.94, respectively) or species (P = 0.25, p a = 0.97, P = 0.27, p a = 0.97, respectively) for a given severity of FMH.
Discussion
We developed simple measurements performed on standard MRI sequences in dogs and cats with normal brains and those with a variety of intracranial lesions providing a more objective means by which to complement the evaluation of dogs and cats with brain herniation. Body size and skull length significantly impacted these measurements and should be considered when making clinical correlations or treatment related decisions. In cases with CTH, our results showed that more severe herniation based on TTX length was associated with decreased short‐term survival. Clinical signs directly attributable to brain herniation were usually absent with only one quarter of cases with rostrotentorial lesions demonstrating signs supportive of herniation.
Measurements were performed in dogs and cats with structurally normal MRIs to establish the normal position of the cerebellum and brainstem structures relative to the foramen magnum and the tentorium cerebelli. While no hand drawn measurements are truly objective, especially given anatomic variations between breeds and species, this study defined specific landmarks. It is possible the foramen magnum line in particular was not placed precisely at the level of the foramen magnum due to difficulties differentiating bone from ligamentous tissue, however, the same point for the dorsal aspect was utilized for all animals allowing comparison to each other. It is also important to note that the control population was not clinically normal; they were imaged because of neurologic dysfunction but none had detectable lesions on MRI or a diagnosis that would suggest gross intracranial changes (see Data S1). To account for the importance of head size and morphology, we attempted corrections with various variables (not reported here) in these control animals, of which skull length provided the best results. However, while variability in TTX and FMX between species and dogs of differing body sizes and head morphologies was reduced after adjusting for skull length, it did not produce the desired uniformity. Categorization by body weight was therefore incorporated into the statistical model when analyzing raw measurements in controls and cases with herniation which generated results qualitatively the same as using values corrected for skull length.
Prior studies have investigated relationships between breed, head shape and caudal fossa anatomy, most extensively in CKCS.11, 12, 13, 14, 15, 16 A positive correlation between increasing body weight and caudal fossa size has been consistently demonstrated. However, there have been conflicting reports regarding the relative volume of hindbrain parenchyma between various brachycephalic and mesaticephalic breeds.11, 13, 14, 16 Both brachycephalic dogs and CKCS have been reported to have larger total brain volumes relative to their weights compared to mesaticephalic dogs.14 Furthermore, brachycephalic dogs and CKCS demonstrated a smaller increase in subarachnoid space compared to mesaticephalic dogs as body weight increased.14 These results suggest that not only is body weight important but breed and head shape further influence the caudal fossa anatomy.
While we did not calculate the volume of the calvarium or hindbrain parenchyma, our findings of significantly longer TTX and FMX in medium to large breed controls, the majority of which were mesaticephalic breeds, cannot be explained by larger skull size alone since correcting for skull length did not completely normalize our data. Our results could be explained by a disproportionately larger subarachnoid space and smaller hindbrain parenchymal volume in medium to large dogs relative to smaller breed dogs.14 Our control group had insufficient numbers of any individual breed to allow investigation of the influence of head shape/breed type (brachycephalic, mesaticephalic or dolichocephalic) on the normal anatomy but this might be an important consideration in future studies. Morphometric studies that examine a single breed or group of similar breeds are likely to yield more uniform measurements and allow detection of smaller changes in position of brain structures. Similarly, while the head shape and size of cats is generally more uniform, there are no prior studies investigating the influence of head shape on calvarial and intracranial anatomy amongst cats of different size or breeds or in comparison to dogs. Our results, which included predominantly domestic shorthairs and no brachycephalic breeds, suggest minimal variation and that caudal fossa morphometry overlaps with small breed dogs.
We demonstrated that this morphometric analysis had high intra‐observer repeatability and was easily applied to animals with intracranial disease. Our measurements were significantly different between control animals and those with herniation supporting their potential utility as a quantitative assessment that could enhance the traditional anatomical definition of CTH and FMH. There is a general paucity of information in the literature regarding brain herniation in dogs and cats and prior definitions have been variable making comparisons between studies difficult.2, 5 , 1 While more severe herniation is generally accepted as easy to recognize on MRI, these measurements could assist in assessment of borderline or mild herniation. For example, we found that FMX in cases with CTH only was significantly different when compared to control animals, suggesting the position of the cerebellum is more caudal amongst animals with identifiable CTH, even if overt FMH is not diagnosed.
Despite the widespread use of MRI to diagnose intracranial disease and the general suggestion that brain herniation carries a poor prognosis, the clinical implications of finding CTH or FMH on advanced imaging have not been investigated extensively.4, 5, 9 Indeed, lack of specific survival information makes it difficult to advise owners at the time of identifying herniation on MRI. Using TTX and FMX to quantify herniation severity, we attempted to uncover relationships between brain herniation on imaging and presence of signs of brainstem disease, delayed recovery from anesthesia and 24‐hour survival, in order to improve prediction of disease severity and prognosis. Sixty‐five percent of cases presented with signs of forebrain disease, with no signs directly attributable to herniation, consistent with prior studies.5 However, signs of brainstem dysfunction were more likely with increasing severity of herniation (CTH) although associations between brainstem signs and raw and corrected TTX failed to reach significance after adjustment for multiple comparisons. It is possible that, given our definition of signs of brainstem disease, cases with mild dysfunction might have been excluded limiting our ability to uncover significant relationships. When signs of brainstem disease were present, vestibular abnormalities were most common similar to other studies suggesting that vestibular signs might be most consistently apparent in dogs and cats with herniation and should alert the clinician to the possibility of herniation.5 , 1
TTX and FMX (raw and corrected) were not associated with greater frequency of anesthetic complications and 60% of cases overall had an unremarkable anesthetic recovery. However, additional factors might have impacted anesthetic recovery in our population including the fact that many cases received medications that can reduce ICP preemptively in the absence of clinical signs overtly suggestive of elevated ICP or difficulty with general anesthesia. Our population included animals with a variety of lesions with no standardized treatment protocol for their primary disease process or the herniation, and 18% of euthanized cases were euthanized while still under general anesthesia. Larger numbers of cases with each type of herniation and more uniform therapeutic intervention would be necessary to further evaluate these relationships.
Acute encephalopathies in humans and dogs, including acute traumatic brain injury, stroke and encephalitis, are associated with a worse outcome when brain herniation is present. While the impact of the presence or absence of herniation has been reported, we extended this by investigating the impact of the severity of brain herniation (in a population in which herniation was present in all).8, 9, 10 We chose to focus on immediate survival since there was no standardized treatment regimen and cases included animals with a variety of intracranial diseases and variable prognoses. TTX but not FMX, was significantly associated with survival to 24 hours. Thus, in cases with combined CTH/FMH, TTX could provide the most useful information for clinicians. However, the influence of treatment and the decision to euthanize complicates interpretation of results. Indeed, 38 of the 41 cases that survived less than 24 hours were euthanized, reflecting the clinical presumption of poor prognosis associated with brain herniation especially in conjunction with other factors such as age, diagnosis and severity of signs of neurologic disease. While euthanasia likely reduced the survival rate in our cases with brain herniation and could have hindered our ability to determine if these measurements are useful to predict short‐term survival, it is also possible that some animals with more severe herniation were not captured by our study having died due to that complication prior to undergoing advanced brain imaging.
We also investigated whether, for a given severity of herniation, duration of signs (acute versus chronic) or species had an influence on short‐term survival but we were unable to demonstrate a significant association. This might reflect our population in which brain herniation likely developed relatively slowly, given that mass lesions were by far the most common imaging diagnosis. Herniation that occurs secondary to chronic disease would allow greater time for brain parenchyma to compensate and tolerate the herniation regardless of when neurologic abnormalities indicative of intracranial disease first became clinically apparent. This assertion is comparable to other studies in humans.6 Nevertheless, our findings suggest that historical and signalment information might be less helpful in interpreting severity of MRI‐identified brain herniation.
Limitations of this study include the retrospective nature, effect of owner‐elected euthanasia, lack of standardized treatment protocols, and inability to determine a simple method to reliably account for the influence of size and skull morphology on morphometry in dogs and cats. Additionally, the landmarks used to define our measurements inherently vary between species and breeds, which could have impacted the objectivity and widespread applicability of our results. Prospective studies are indicated to more fully determine the reliability of these measurements as well as more precisely target the influence of brain herniation in specific populations of animals with intracranial disease. Since the present study was just aimed at the immediate effects of herniation, investigation of the long‐term impact also warrants further study.
In conclusion, we have provided a means by which to quantify the two most common and relevant types of brain herniation, CTH and FMH, and more objectively determine their impact on prognosis and clinical status. We demonstrated the potential utility of these measurements to identify animals with imminent herniation for example, but additional study in more uniform populations is needed to determine their ultimate clinical utility. Our data demonstrate that, even though clinical signs directly attributable to brain herniation were commonly absent, more severe CTH decreases survival beyond 24 hours although short‐term outcome was heavily influenced by euthanasia at the time of diagnosis.
Supporting information
Data S1. Clinical summary of control population.
Acknowledgments
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
All work for this study was completed at the North Carolina State University College of Veterinary Medicine.
This work was not supported by any grant or funding agency.
This work was partially previously presented as an oral abstract at the 2013 ACVIM Forum in Seattle, WA
Footnotes
de Freitas MI, Liebel FX, Goncalves R, Volk H, Rudorf H, Guilherme S, et al. Brain Herniation in 113 Cats: A Descriptive Study. Proceedings of the ACVIM Forum; 2015 Jun 3–6; Indianapolis, IN
Siemens Medical Solutions USA, Inc., Malvern, PA
Fentanyl Citrate Injection USP (50 mcg/mL), West Ward Pharmaceutical, Eatontown, NJ 07724
Propoflo™ (10 mg/mL), product of Sweden, Abbott Laboratories, North Chicago, IL 60064
VET ONE® Fluriso™, Product of United Kingdom, Distributed by MWI, Boise, ID 83705
E‐film version 3.3VET, Osirix version 6.0
SAS Version 9.4, SAS Institute, Cary, NC
Lu, Li and Nawar Shara (2007), “Reliability analysis: Calculate and Compare Intra‐class Correlation Coefficients (ICC) in SAS,” NorthEast SAS Users Group: Statistics and Data Analysis
References
- 1. Meyer A. Herniation of the brain. Arch Neurol Psychol 1920;4:387–400. [Google Scholar]
- 2. Kornegay JN, Oliver JE, Gorgacz EJ. Clinicopathologic features of brain herniation in animals. J Am Vet Med Assoc 1983;182:1111–1116. [PubMed] [Google Scholar]
- 3. Bagley RS. Pathophysiologic sequelae of intracranial disease. Vet Clin North Am Small Anim Pract 1996;26:711–733. [PubMed] [Google Scholar]
- 4. Bitterman S, Lang J, Henke D, et al. Magnetic resonance imaging signs of presumed elevated intracranial pressure in dogs. Vet J 2014;201:101–108. [DOI] [PubMed] [Google Scholar]
- 5. Walmsley GL, Herrtage ME, Dennis R, et al. The relationship between clinical signs and brain herniation associated with rostrotentorial mass lesions in the dog. Vet J 2006;172:258–264. [DOI] [PubMed] [Google Scholar]
- 6. Reich JB, Sierra J, Camp W, et al. Magnetic resonance imaging measurements and clinical changes accompanying transtentorial and foramen magnum brain herniation. Ann Neurol 1993;33:159–170. [DOI] [PubMed] [Google Scholar]
- 7. Feldmann E, Gandy SE, Becker R, et al. MRI demonstrates descending transtentorial herniation. Neurology 1988;38:697–701. [DOI] [PubMed] [Google Scholar]
- 8. Kalita J, Misra UK, Vajpeyee A, et al. Brain herniations in patients with intracerebral hemorrhage. Acta Neurol Scand 2009;119:254–260. [DOI] [PubMed] [Google Scholar]
- 9. Beltran E, Platt SR, McConnell JF, et al. Prognostic value of early magnetic resonance imaging in dogs after traumatic brain injury: 50 cases. J Vet Int Med 2014;28:1256–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lowrie M, Smith PM, Garosi L. Meningoencephalitis of unknown origin: Investigation of prognostic factors and outcome using a standard treatment protocol. Vet Rec 2013;172:527. [DOI] [PubMed] [Google Scholar]
- 11. Carrera I, Dennis R, Mellor DJ, et al. Use of magnetic resonance imaging for morphometric analysis of the caudal cranial fossa in Cavalier King Charles Spaniels. Am J Vet Res 2009;70:340–345. [DOI] [PubMed] [Google Scholar]
- 12. Cerda‐Gonzalez S, Olby NJ, McCullough S, et al. Morphology of the caudal fossa in Cavalier King Charles Spaniels. Vet Radiol Ultrasound 2009;50:37–46. [DOI] [PubMed] [Google Scholar]
- 13. Cross HR, Capello R, Rusbridge C. Comparison of cerebral cranium volumes between cavalier King Charles spaniels with Chiari‐like malformation, small breed dogs and Labradors. J Small Pract 2009;50:399–405. [DOI] [PubMed] [Google Scholar]
- 14. Schmidt MJ, Amort KH, Failing K, et al. Comparison of the endocranial and brain volumes in brachycephalic dogs, mesaticephalic dogs, and Cavalier King Charles spaniels in relation to their body weight. Acta Vet Scand 2014;56:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knowler SP, McFadyen AK, Freeman C, et al. Quantitative analysis of chiari‐like malformation and syringomyelia in the griffon bruxellois dog. PLoS One 2014;9:e88120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thames RA, Roberston ID, Flegel T, et al. Development of a morphometric magnetic resonance image parameter suitable for distinguishing between normal dogs and dogs with cerebellar atrophy. Vet Radiol Ultrasound 2010;51:246–253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Clinical summary of control population.


