Abstract
Background
Clopidogrel is commonly prescribed to cats with perceived increased risk of thromboembolic events, but little information exists regarding its antiplatelet effects.
Objective
To determine effects of clopidogrel on platelet responsiveness in cats with or without the A31P mutation in the MYBPC3 gene. A secondary aim was to characterize variability in feline platelet responses to clopidogrel.
Animals
Fourteen healthy cats from a Maine Coon/outbred mixed Domestic cat colony: 8 cats homozygous for A31P mutation in the MYPBC3 gene and 6 wild‐type cats without the A31P mutation.
Methods
Ex vivo study. All cats received clopidogrel (18.75 mg PO q24h) for 14 days. Before and after clopidogrel treatment, adenosine diphosphate (ADP)‐induced P‐selectin expression was evaluated. ADP‐ and thrombin‐induced platelet aggregation was measured by optical aggregometry (OA). Platelet pVASP and ADP receptor response index (ARRI) were measured by Western blot analysis.
Results
Platelet activation from cats with the A31P mutation was significantly (P = .0095) increased [35.55% (18.58–48.55) to 58.90% (24.85–69.90)], in response to ADP. Clopidogrel treatment attenuated ADP‐induced P‐selectin expression and platelet aggregation. ADP‐ and PGE 1‐treated platelets had a similar level of pVASP as PGE 1‐treated platelets after clopidogrel treatment. Clopidogrel administration resulted in significantly lower ARRI [24.13% (12.46–35.50) to 11.30% (−7.383 to 23.27)] (P = .017). Two of 13 cats were nonresponders based on OA and flow cytometry.
Conclusion and Clinical Importance
Clopidogrel is effective at attenuating platelet activation and aggregation in some cats. Cats with A31P mutation had increased platelet activation relative to the variable response seen in wild‐type cats.
Keywords: Cat, Hypertrophic cardiomyopathy, Platelet hyper‐reactivity, Thromboembolism
Abbreviations
- ADP
adenosine diphosphate
- ADP‐Ag
ADP‐induced platelet aggregation
- ARRI
ADP receptor response index
- cAMP
cyclic adenosine monophosphate
- HO
homozygous for the A31P mutation
- MA
maximum amplitude
- MYBPC3
myosin‐binding protein C gene
- OA
optical aggregometry
- pVASP
phosphorylated vasodilator‐stimulated phosphoprotein
- PGE1
prostaglandin E1
- PMV
platelet‐derived microvesicles
- PRP
platelet‐rich plasma
- Throm‐Ag
thrombin‐induced platelet aggregation
- VASP
vasodilator‐stimulated phosphoprotein
- WT
wild type with respect to the A31P mutation
ATE is a life‐threatening complication associated with HCM in cats. Thrombi in left atrial appendages (LAA) of cats can embolize to peripheral and central arteries causing tissue ischemia, ischemic reperfusion injury, and sudden death.1, 2, 3 Despite recent advances in thrombosis research, the underlying role of platelets in the pathophysiology of ATE in cats remains poorly understood.
In people, platelet activation and increased P‐selectin expression are associated with myocardial inflammation, myocardial infarction, and cardiomyopathy.4, 5, 6 Platelet reactivity predicts thromboembolic events associated with myocardial infarct and coronary stent implantation.7, 8, 9 Altered platelet function occurs in cats with HCM.10, 11 Maine Coon cats with severe HCM because of a mutation (A31P) in the myosin‐binding protein C (MYBPC3) gene have increased expression of P‐selectin, platelet‐derived microvesicles (PMVs), and platelet–endothelial cell adhesion molecule‐1.11 This suggested that platelets might play a prominent role in the development of the hypercoagulable state in feline HCM.
Clopidogrel, an antiplatelet drug, effectively inhibits ADP‐induced platelet aggregation in healthy cats.12, 13 Here, we sought to determine whether clopidogrel would be a logical treatment option in cats with perceived risk of ATE.3, 14 In human patients, clopidogrel is a key component of antithrombotic treatment in patients with acute ischemic stroke and coronary stent implantation.15, 16, 17 However, substantial interindividual variability exists and resistance to clopidogrel is associated with recurrent thrombosis and increased morbidity.18 Although clopidogrel is commonly prescribed to cats with ATE, little is known regarding its effects on platelet activation and response variability.
The primary aim of this study was to evaluate the response of feline platelets to clopidogrel and to characterize variability in response to clopidogrel. The secondary aim of this study was to characterize platelet activation and aggregation in cats homozygous for the MYBPC3 A31P mutation before development of the recognized phenotype of HCM. We hypothesized that cats homozygous (HO) for the MYBPC3 A31P mutation would have hyper‐reactive platelets compared to wild‐type (WT) cats without the A31P mutation. We also hypothesized that clopidogrel would attenuate platelet sensitivity to ADP and that cats would exhibit a highly variable response to clopidogrel treatment.
Materials and Method
Animals
The study protocol was approved by the Institutional Animal Care and Use Committee at the University of California, Davis. Fourteen cats were selected from a newly established colony of Maine Coon/outbred mixed domestic cats. Eight cats homozygous (HO) for A31P mutation in the MYPBC3 gene and 6 wild‐type (WT) cats without the A31P mutation were studied. Cats were between 12 and 44 months (median 18.5 months) of age. As part of a separate and ongoing longitudinal study, cardiac echocardiography was assessed for all 14 cats within 2 months before the start of this study. None of the cats had echocardiographic evidence of HCM at the time of the study, and all were considered clinically healthy. Cats were observed for adverse reactions to clopidogrel such as vomiting, inappetence, diarrhea, weight loss, and bleeding diathesis. On rare occasions, blood samples from cats were not included in portions of the study, as sample clotting and inadequate volume of platelet rich plasma (PRP) prevented evaluation. If either blood samples taken before or after clopidogrel were clotted, data from that animal were not included in this portion of the study.
Each cat received 18.75 mg clopidogrel PO for 14 days. Blood was collected on day 0 (1 day before clopidogrel administration) and day 15 (approximately 12 hours after the last clopidogrel dose was administered). Complete blood counts were obtained using an automated analyzer.1 All cats were sedated with a combination of acepromazine (0.05 mg/kg IM) and butorphanol (0.2 mg/kg IM) before venipuncture. Additional doses of acepromazine, butorphanol, or both were administered if required. Blood was drawn from the medial saphenous vein using a 21‐gauge butterfly needle set, and 8 ml of blood was collected into 3.2% trisodium citrate tubes.
Response to clopidogrel was characterized based on the percentage of change of ADP‐induced platelet aggregation (ADP‐Ag) before and after the 14‐day clopidogrel treatment.16 Subjects with ≤10% inhibition of ADP‐Ag after clopidogrel treatment were classified as nonresponders. Cats with >10% inhibition of ADP‐Ag after clopidogrel treatment were considered responders.
Generation of Platelet‐Rich Plasma
Citrated whole blood was transferred to polypropylene tubes and diluted (1 : 5) with Tyrodes–HEPES buffer lacking divalent cations but containing 5 mM dextrose (37°C).19 PRP was generated by centrifugation at 200 × g for 5 minutes at 25–27°C.
Flow Cytometry
PRP platelet count was adjusted to 1 × 107/mL with Tyrodes–HEPES buffer. Platelets were either unstimulated (resting) or stimulated (activated) with 20 μM ADP2 and incubated for 15 minutes (37°C) before the addition of antibodies. Samples were incubated with monoclonal antibodies to CD62P3 and biotinylated monoclonal antibodies to CD41b4 at a final dilution of 1 : 100, respectively, for 45 minutes at 37°C. Samples were then labeled with streptavidin conjugated to Alexa 6335 (30 minutes) and fixed in 1% paraformaldehyde6 in Tyrodes–HEPES buffer.
Flow cytometry was performed using a 5‐color flow cytometer.7 Anti‐mouse compensation beads8 and monoclonal mouse immunoglobulin G1 kappa conjugated to matched experimental fluorochromes9 were used for compensation controls. Platelets were identified by forward and side scatter properties and by 0.9 μm and 3 μm calibration beads. For the identification of CD62P (P‐selectin) and CD41b (α2b subunit of the major platelet integrin, α2bβ3a)‐positive events within the platelet gate, gating boundaries were identified by the use of fluorescence‐minus‐one controls.
Platelet‐derived microvesicles (PMVs) were identified as previously described by Robert et al.20 and Tablin et al.11 Briefly, the microvesicle gate was determined by the use of 0.5 μm and 3 μm calibration beads.10 PMVs were quantified by dividing the number of CD41b‐positive events by the total number of events within the microvesicle gate and expressed as percentages. Flow cytometry data were analyzed by commercially available software.11
Western Blot Analyses of Intracellular Phosphorylation of Vasodilator‐Stimulated Phosphoprotein (VASP)
Phosphorylation of the intracellular protein, VASP, was quantified by Western blot analysis using affinity‐purified polyclonal antibodies. PRP from homozygous and wild‐type cats was generated as described above and diluted with Tyrodes–HEPES buffer to a final platelet concentration of 1 × 108/mL. Samples were incubated with ADP (20 μM), prostaglandin E1 (PGE1) (10 μM), or a combination of ADP (20 μM) and PGE1 (10 μM) [all incubations for 10 minutes, 37°C]. Unstimulated samples served as the resting control population whereas PGE1‐induced VASP phosphorylation served as the positive control population. Samples were then lysed in Laëmmli buffer21 with antiproteases and antiphosphatases (leupeptin, pepstatin, trypsin inhibitor soybean, AESBSF, sodium orthovanadate, EDTA)12 (aproptinin and sodium fluoride)13 and stored at −80°C until further analysis.
Samples were evaluated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred to nitrocellulose membranes. Membranes were stained with Ponceau S (0.1%) to confirm equal and adequate protein transfer. Membranes were blocked with 3% gelatin at 37°C. Affinity‐purified antibodies were used to detect phosphorylation of serine 239 (pVASP)14 and total VASP.15 These antibodies were chosen based upon high sequence homology when evaluated against the published feline VASP protein (XP_006941169). Amino acid sequences of pVASP and VASP antibodies were highly homologous (100% positive with 0 gaps, respectively) when compared to the published feline VASP protein sequence as determined by the Basic Local Alignment Search Tool, BLAST.22 Blots were washed 4 times and probed with anti‐goat antibodies conjugated to horseradish peroxidase (1 : 50,000 dilution in 1% gelatin) (1 hour 37°C) and treated with a chemiluminescent substrate. Blots were imaged with a commercial imaging system16 and densitometry quantified using commercially available software.17 Immunoblotting and imaging for pVASP were performed first followed by stripping of primary and secondary antibodies using commercially available stripping buffer.18 To confirm specificity of antibody binding after stripping, blots were incubated with secondary antibodies against host animal (goat) in which the primary antibody was generated. Blots that showed no residual antibody, as judged by the lack of secondary antibody labeling in the absence of primary antibody, were probed for total VASP on the same membrane. Results were expressed as average optical density (ODu/mm2) within chemiluminescent bands. Relative phosphorylation of VASP was determined by the ratio of average optical densities of pVASP to VASP (pVASP:VASP). The nonphosphorylated VASP served as an internal loading control.
The ability of ADP to inhibit PGE1‐induced phosphorylation of VASP was evaluated by ADP receptor response index (ARRI) using the following calculation as previously described by Hezard et al.23:
Optical Aggregometry
PRP from citrated whole blood was prepared as described above and diluted with Tyrodes–HEPES to a final platelet count of 1.5 × 108/mL, and aggregation measured by optical aggregometry.19 Testing was performed within 60 minutes after venipuncture. Aggregation was recorded for 1 minute before the addition of ADP (40 μM) or thrombin20 (bovine‐derived, 1 Unit/mL), and the reaction runs for 5 additional minutes. Thrombin‐induced aggregation (Throm‐Ag) served as positive control. Percentage of maximal aggregation (amplitude) was calculated using commercially available software.21
Response to Clopidogrel
The response of each cat to clopidogrel treatment was categorized based on previously established criteria for human patients.16 Percent inhibition was calculated by the following formula:
Statistical Analysis
Normality was tested using the Shapiro–Wilk normality test. Normally distributed and paired data were analyzed using t‐test. Nonparametric and paired data were analyzed using the Wilcoxon signed‐rank test. Pearson correlation coefficients were calculated to describe the agreement between different platelet function assessments. Interindividual variability was calculated as the ratio between the standard deviation of a group and its mean (coefficient of variation). Parametric data were presented as mean ± standard deviation, and nonparametric data were presented as median and interquartile range (IQR). Statistical analysis was performed using commercially available software.22 An a priori alpha of P < .05 was considered statistically significant.
Results
Flow Cytometry
Before clopidogrel treatment, ADP stimulation resulted in a significantly higher percentage of P‐selectin‐positive platelets and P‐selectin mean fluorescence intensity (MFI) as compared to unstimulated (resting) platelets (Fig 1). Mean percentages of P‐selectin‐positive platelets were 35.53 ± 21.42% in resting platelets and 46.75 ± 6.27% in ADP‐activated platelets. ADP stimulation resulted in a significant increase in P‐selectin MFI (from 4791 ± 2598 to 7412 ± 4702) (P = .0067). However, after 14 days of clopidogrel treatment, no significant differences in percentage of P‐selectin‐positive platelets and P‐selectin MFI were identified between ADP‐stimulated activation and resting platelets (P = .87, P = .52, respectively) (Fig 2). Mean percentages of P‐selectin‐positive platelets were 50.49 ± 21.85% in resting platelets and 51.55 ± 20.39% in ADP‐activated platelets. Mean P‐selectin MFIs were 453 ± 1272 (resting) and 4350 ± 1519 (activated).
Figure 1.
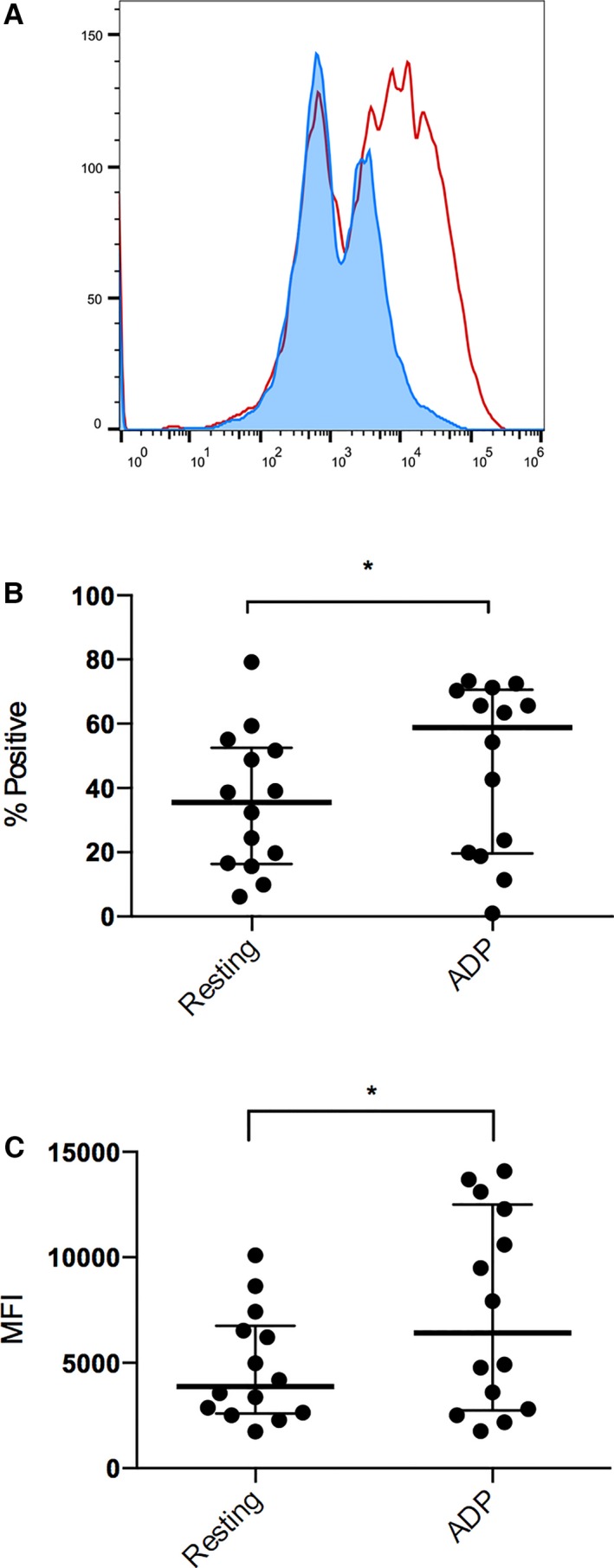
Flow cytometric analysis of P‐selectin expression in 14 cats before clopidogrel treatment. (A) Representative histogram of resting platelets (blue) and ADP‐activated platelets (red) indicating the significant increase in platelet P‐selectin expression before clopidogrel treatment. ADP‐induced stimulation resulted in significant increase of percentage (%) in P‐selectin‐positive platelets (B) and P‐selectin MFI (C) as compared to resting platelets. The bolded line represents the mean, and the lower and upper lines represent the standard deviations.
Figure 2.
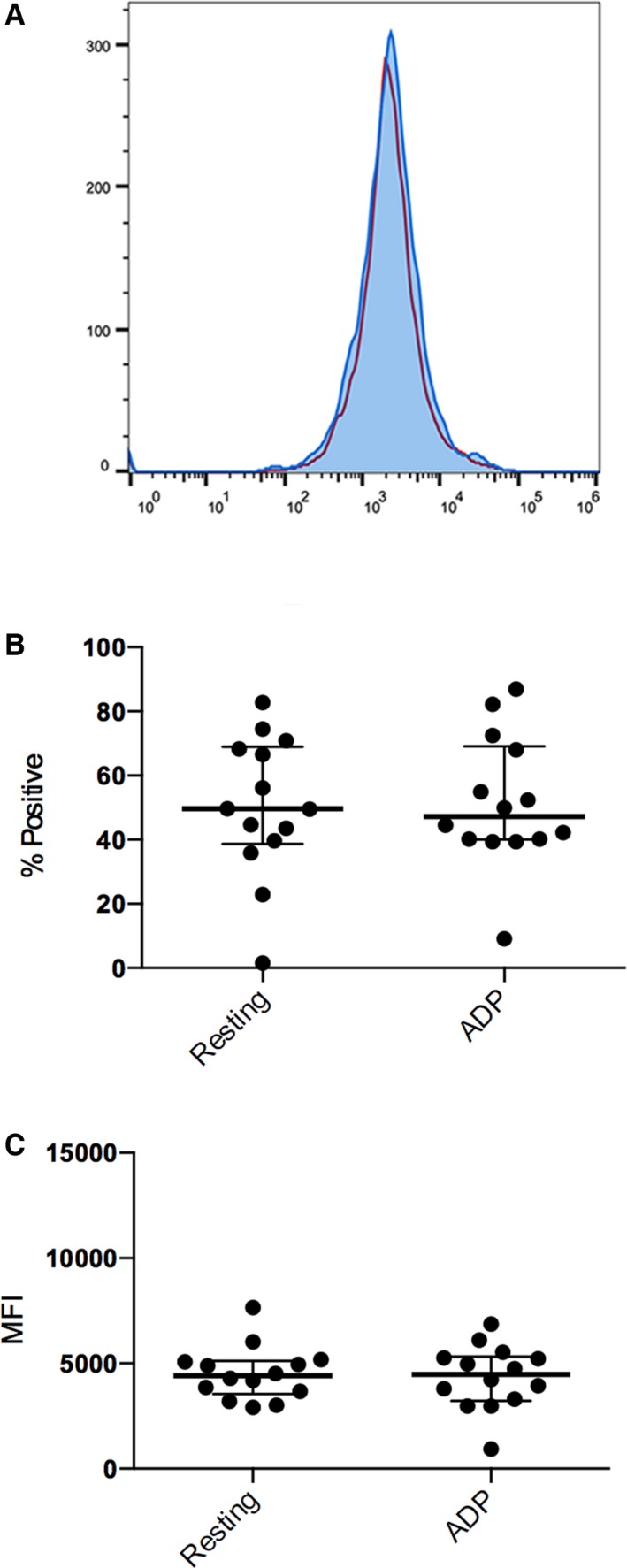
Flow cytometric analysis of P‐selectin expression in 14 cats after clopidogrel treatment (day 15). (A) Representative histogram of resting platelets (blue) and ADP‐activated platelets (red) after clopidogrel treatment. After 14 days of clopidogrel treatment, no significant changes in P‐selectin expression were seen in resting (blue) and ADP‐activated platelets (red). ADP‐induced stimulation did not result in a significant increase of percentage (%) of P‐selectin‐positive platelets (B) and P‐selectin MFI (C) as compared to resting platelets. The bolded line represents the mean and the upper and lower lines represent the standard deviations.
Overall, in cats, HO for A31P mutation of the MYPBC3 gene, ADP‐induced P‐selectin expression (P‐selectin MFI and percentage of P‐selectin‐positive platelets) was significantly higher as compared to resting platelets (P = .0095, P = .016). Before clopidogrel treatment, all cats HO for A31P mutation, but one, had an increase in P‐selectin‐positive platelets after ADP stimulation. Median percentages of P‐selectin‐positive platelets in resting and activated platelets were 35.55% (IQR: 18.58–48.55) and 58.90% (IQR: 24.85–69.90), as shown next to the data points (Fig 3A). Not all HO cats had elevated P‐selectin MFI after ADP stimulation but 4 of 8 cats had substantial increase. When compared to resting platelets, ADP activation resulted in a significant increase in median platelet MFI [from 3215 (IQR: 2347–5904) to 5758 (IQR: 2590–12197)] in HO cats (P = .035) (Fig 3B). The response to ADP was variable in WT cats, with platelets from 2 of 6 cats showing minimal to no response to ADP (Fig 3C and D). Median percentages of P‐selectin‐positive platelets were 34.30% (IQR: 14.27–64.35) and 44.70% (IQR: 15.20–71.08) in resting and activated platelets, respectively. No significant increase in P‐selectin‐positive platelets was found when compared to resting platelets (P = .69). All WT cats, except one, showed increase in P‐selectin MFI after ADP stimulation. No significant increase in median P‐selectin MFI [resting: 5362 (IQR: 3195–8997), activated: 7755 (IQR: 4127–12,650)] was found after ADP‐induced activation (P = .16).
Figure 3.
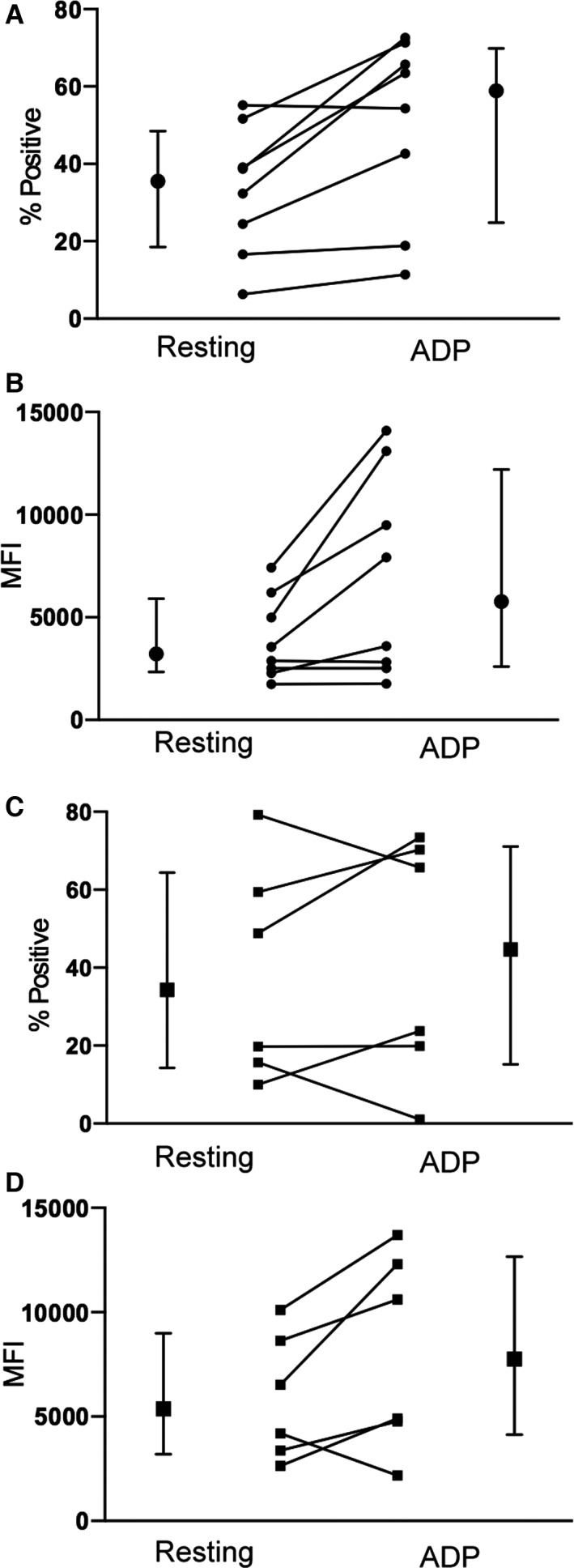
Line plots illustrating the changes in platelet P‐selectin expression in resting and ADP‐stimulated platelets from HO (A, B) and WT (C, D) cats before 14 days of clopidogrel treatment. Medians and interquartile ranges are shown beside the line plots.
After 14 days of clopidogrel treatment, no significant differences in P‐selectin‐positive platelets and P‐selectin MFI were found in resting and activated platelets in either HO or WT cats (Fig 4). ADP stimulation did not result in significant increase in percentage (%) of P‐selectin platelets in either HO (P = .84) or WT (P = .44) cats compared to resting platelets (Fig 4A). In HO cats, median P‐selectin‐positive platelets were 47.10% (IQR: 37.83–65.28) and 43.35% (IQR: 39.60–64.08) in resting and activated platelets, respectively. In WT cats, median P‐selectin‐positive platelets were 58.10% (IQR: 30.18–71.80) (resting) platelets and 52.40% (IQR: 32.42–76.10) (activated). ADP stimulation did not result in significant increase in P‐selectin MFI compared to resting platelets in either HO (P = .73) or WT (P = .44) cats. In HO cats, median P‐selectin MFIs were 4353 (IQR: 3061–5039) (resting) and 4026 (IQR: 2976–5449) (activated). In WT cats, median P‐selectin MFIs were 4633 (IQR: 3820–5805) (resting) and 4600 (IQR: 3915–5480) (activated).
Figure 4.
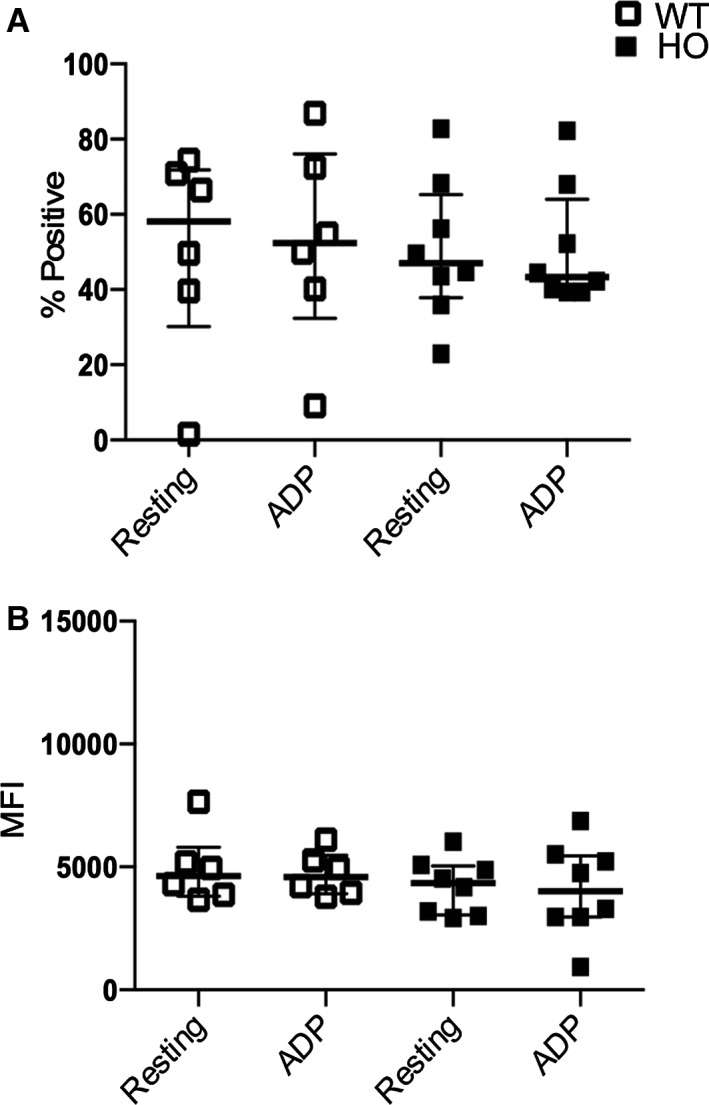
Flow cytometric analysis of platelet P‐selectin expression from HO and WT cats after 14 days of clopidogrel treatment. ADP‐induced stimulation did not result in significant increase in percentage (%) of P‐selectin‐positive platelets (A) and P‐selectin MFI (B).
Before clopidogrel treatment, mean PMVs were 25.12 ± 16.07% (resting) and 20.42 ± 17.46% (activated) in all cats. After clopidogrel treatment, mean PMVs were 15.34 ± 18.12% (resting) and 17.42 ± 21.40% (activated). ADP stimulation did not result in significant differences in the number of PMVs compared to resting platelets (P = .39), nor were there any differences compared to postclopidogrel resting samples (P = .54).
When examining PMVs based on genotype, ADP stimulation resulted in an increase in PMVs [5.67% (IQR: 1.47–36.90) to 10.67% (IQR: 2.28–44.75)] before clopidogrel treatment in cats HO for A31P mutation, but the difference was not statistically significant (P = .25). In WT cats, PMVs did not significantly increase after stimulation with ADP [resting: 6.45% (IQR: 3.37–19.48), activated: 3.43% (IQR: 2.25–22.80) before treatment (P = .44). After clopidogrel treatment, no significant differences in PMVs were noted between resting (27.45%, IQR: 8.14–47.65) and activated (23.70%, IQR: 11.19–32.45) in HO cats. Similarly, no differences in PMVs were found in WT cats [resting: 23.75% (IQR: 15.47–29.43), activated: 13.35% (IQR: 4.96–20.48)] (P = .43).
Optical Aggregometry
The amplitude of ADP‐Ag was not significantly different from Throm‐Ag on day 0 (P = .093). The mean maximum amplitudes (MA) of ADP‐Ag and Throm‐Ag were 51 ± 24.25% and 61.92 ± 19.48%, respectively. However, after 14 days of clopidogrel treatment, the mean MA of ADP‐Ag (8.54 ± 4.72%) was significantly lower than Throm‐Ag (56.25 ± 28.33%) in all cats (P = .0001). ADP‐Ag was significantly higher on day 0 than on day 15 (P = .0002). Throm‐Ag on days 0 and 15 was not significantly different (P = .68) (Fig 5).
Figure 5.
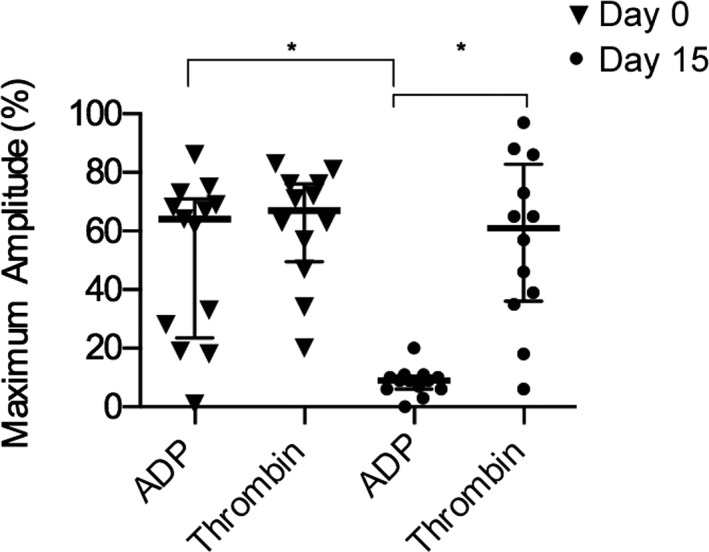
Percentages (%) of maximum aggregation measured by optical aggregometry in 13 cats before (day 0) and after clopidogrel (day 15). The bolded line represents the mean, and lower and upper lines represent the standard deviations.
Before clopidogrel treatment, ADP‐Ag [median MA: 47.50% (IQR: 21.25–73)] was not significantly different from Throm‐Ag [median MA: 63% (IQR: 34.00–76)] in HO cats (P = .45). Similarly in WT cats, ADP‐Ag [median MA: 68% (IQR: 41–71)] was not different from Throm‐Ag [median MA: 71% (IQR: 60–82)] in WT cats (P = .31). On day 15, significant reduction in ADP‐Ag [median MA: 9.50% (IQR: 6.25–10.75)] was found in HO cats when compared to Throm‐Ag [median MA: 47.50% (IQR: 34.00–76)] in HO cats (P = .016). In WT cats, ADP‐Ag [median MA: 9.00% (3.00–14.50)] was significantly lower than Throm‐Ag [median MA: 65% (IQR: 37.00–79.50)] (P = .040). As expected, clopidogrel treatment did not lead to a significant decrease in the MA of Throm‐Ag in either HO or WT cats (P = .750, P = .438, respectively). ADP‐Ag amplitude in HO cats was not significantly different from ADP‐Ag in WT cats on day 15 (P = .65). Because of inadequate volume of PRP on day 0 and sample clotting on day 15 in a single cat (WT), no optical aggregometry data were available from that subject.
Platelet VASP Phosphorylation and ADP Receptor Response Index
Two cats (1 WT and 1 HO) were excluded from Western blot analyses because of inadequate PRP volume on day 0.
Representative Western blots are shown in Figure 6. Before clopidogrel treatment, the mean relative pVASP intensities of PGE1‐treated platelets (0.54 ± 0.24) were not significantly different from either resting (0.58 ± 0.32) or ADP‐treated (0.46 ± 0.21) platelets (P = .44, P = .21, respectively). However, before clopidogrel treatment, relative pVASP was significantly lower in PGE1+ADP‐treated platelets (0.43 ± 0.20) than in platelets treated with PGE1 alone (P = .049) (Fig 7A).
Figure 6.
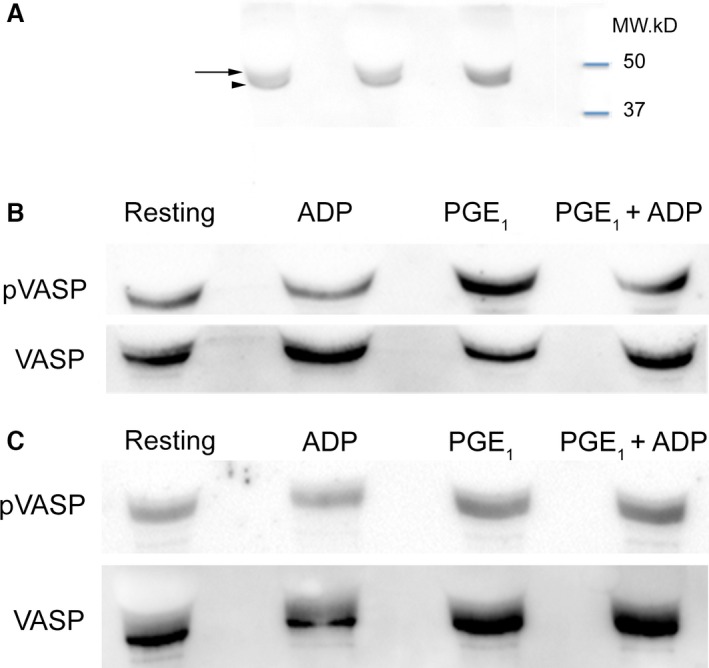
Representative Western blot analysis of platelet vasodilator‐stimulated phosphoprotein (VASP) phosphorylation in 3 different cats. (A) The molecular weight of total VASP is approximately at 46 kDa (Arrow head). Phosphorylation of serine 239 of VASP (pVASP) caused the protein to migrate to 50 kDa (black arrow). Immunoblots were shown to demonstrate the differences in molecular weight. (B) PGE 1 serves as a positive control, as the rise in cAMP after PGE 1 treatment results in strong pVASP expression. Platelets were either unstimulated/resting or treated with 20 μM ADP, 10 μM PGE 1 (positive control) or a combination of 20 μM ADP and 10 μM PGE 1. Before clopidogrel treatment, ADP inhibited pVASP and partially inhibited PGE 1‐induced pVASP. (C) After clopidogrel treatment, irreversible antagonism of P2Y12 abolished the effect of ADP on PGE 1‐induced pVASP. The degree of VASP phosphorylation in resting and ADP‐treated platelets also was similar to positive control.
Figure 7.
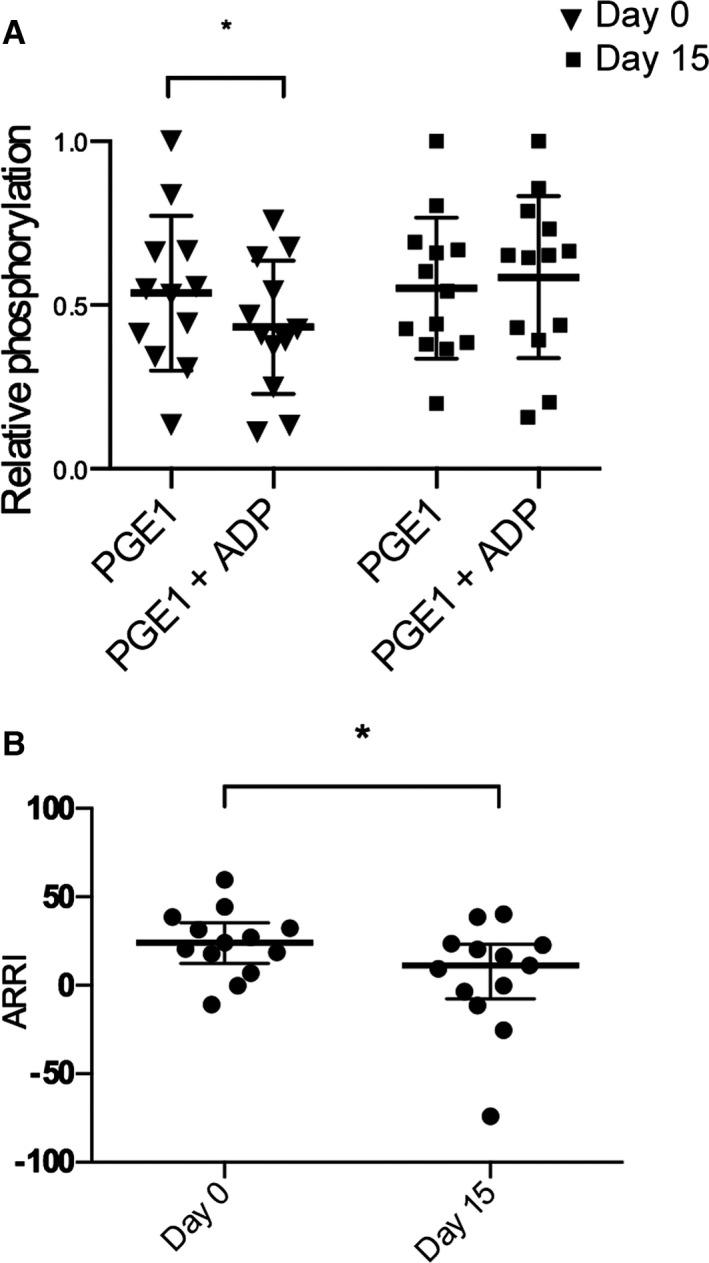
(A) Relative phosphorylation of vasodilator‐stimulated phosphoprotein (VASP) quantified by Western blot analysis in platelets from 12 cats before (day 0) and after 14 days (day 15) of clopidogrel treatment. (B) ADP receptor response index (ARRI) in 12 cats before (day 0) and after 14 days (day 15) of clopidogrel treatment. The bolded line represents the mean, and the upper and lower lines represent the interquartile range.
After 14 days of clopidogrel treatment, relative pVASP in platelets treated with PGE1 and ADP was not significantly different from platelets treated with PGE1 alone (P = .62) (Fig 7A). Mean relative pVASP was not significantly different among PGE1‐treated (0.55 ± 0.22), resting (0.37 ± 0.25), and ADP‐treated (0.37 ± 0.18) platelets (P = .21, P = .21, respectively). ARRI after clopidogrel treatment was significantly lower than ARRI before treatment (P = .017) (Fig 7B). The median ARRIs were 24.13% (IQR: 12.46–35.50) and 11.30% (IQR: −7.383 to 23.27) on day 0 and day 15, respectively.
Response to Clopidogrel
None of the cats displayed clinical signs suggestive of adverse reactions to clopidogrel. Of 13 samples available for optical aggregometry, 2 nonresponders and 11 responders to clopidogrel treatment were identified based on platelet response to ADP‐Ag. Both nonresponders had low ADP‐Ag before and after clopidogrel treatment (1–3 and 18–20%). The median percent inhibition among responders was 86.21% (IQR: 81.82–88.71). ADP stimulation also did not result in a significant increase in percent‐positive P‐selectin when compared to resting platelets of responders. The two nonresponders showed variable increases in P‐selectin MFI despite clopidogrel treatment whereas virtually all responders, with one exception, had decreased P‐selectin expression (Fig 8). ARRI was also significantly decreased in responders after treatment (P = .020). Nonresponders also were observed to have reduced ARRI after clopidogrel treatment. Within the population of responders, 7 cats were HO and 4 cats were WT.
Figure 8.
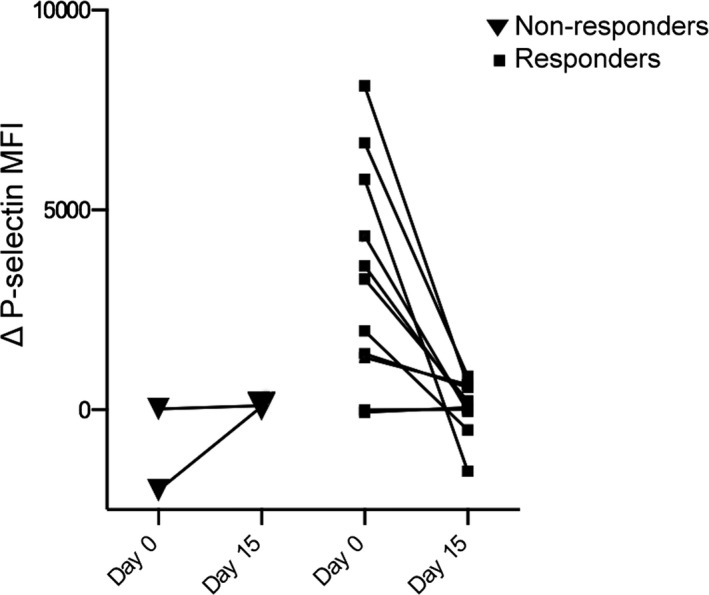
Line plots illustrating the changes in P‐selectin expression before and after 14 days of clopidogrel treatment in 11 responders and 2 nonresponders. On day 15, the differences in P‐selectin mean MFI between resting and ADP‐stimulated platelets in nonresponders were higher than those on day 0. All responders but one had an attenuated response to ADP stimulation.
Overall, interindividual variability in response to clopidogrel treatment was high, ranging from 34.92 to 100% depending on the tests used to assess clopidogrel response. Measurement of ARRI using Western blot analysis had the highest interindividual variability (CVARRI = 26.9) whereas flow cytometric detection of P‐selectin expression had the lowest interindividual variability (CVMFI = 0.35, CV% = 0.40). ADP‐Ag using OA had a CV of 0.55.
Discussion
In the absence of HCM, cats HO for the A31P mutation of the MYPBC3 gene had reactive platelets as measured by increased ADP‐induced P‐selectin expression relative to the more variable response seen in WT cats. Here, we showed that 14 days of clopidogrel treatment attenuated ADP‐mediated P‐selectin expression on platelets from either HO or WT cats, illustrating the potent antiplatelet effects of clopidogrel in apparently healthy cats with or without the A31P mutation. A dosage of 18.75 mg, PO, given every 24 hours for 14 days also resulted in significant inhibition of ADP‐induced aggregation and increase in pVASP.
Once metabolized in the liver, the active metabolite of clopidogrel covalently binds with P2Y12, one of two platelet ADP receptors. P2Y12 is a purinergic class of G‐protein‐coupled receptor and clopidogrel abolishes ADP‐mediated downstream signaling by inhibiting inside‐out activation of the platelet integrin, α2bβ3, which is critical for platelet aggregation.24, 25 The profound inhibition of platelet aggregation after clopidogrel treatment observed in this study indicates that a similar mechanism of action likely occurs in feline platelets.
P‐selectin, an integral protein of the α‐granule membrane, is exposed on the platelet surface by fusion of the α‐granule membrane with plasma membrane. Because fusion is necessary for platelet granule secretion, surface expression of P‐selectin serves as a useful marker of ADP‐induced platelet activation and α‐granule secretion.26 Upon ADP stimulation, platelet P‐selectin expression in cats HO for the A31P mutation was significantly upregulated. This finding further suggests the conclusion that cats with genetic predisposition to HCM have procoagulant platelets that predispose them to intracardiac thrombi formation and increased risk for ATE. Platelet P‐selectin expression in this population of cats was generally higher than previously reported.11 The differences in results were likely because of the use of different CD62P antibodies, in vitro activation from sample handing and centrifugation, age and genetic differences between the studied populations. In humans, platelet reactivity is associated with mortality because of myocardial infarction.27, 28 Platelet reactivity can be the result of platelet priming, a regulatory mechanism that amplifies the platelet response to agonists and, thus, contributes to thrombus formation and growth.29 For that reason, neutralization of platelet priming presents a novel treatment paradigm for thrombus prevention, and thus, further studies in HCM cats are warranted.
The attenuated ADP‐induced P‐selectin expression by clopidogrel treatment suggests additional benefits of this drug in ATE cats. Because the binding of P‐selectin to its ligand, P‐selectin glycoprotein ligand‐1, facilitates recruitment of leukocytes to thrombi and enhances platelet–leukocyte interaction and tissue factor expression by capturing leukocyte‐derived microvesicles, inhibition of this interaction may further reduce the progression of thrombosis and inflammation.30, 31, 32 Spontaneous echocardiographic contrast, a swirling blood flow pattern associated with blood stasis in the LAA, is a common finding and a suggested predictor of thromboembolic outcomes in feline HCM.33, 34, 35 A study utilizing direct analysis of left atrial blood showed that human patients with spontaneous echocardiographic contrast had increased platelet P‐selectin expression and platelet–leukocyte aggregates.36 However, it remains unknown whether elevated P‐selectin expression in cats predisposed to HCM could be associated with increased platelet–neutrophil interaction and spontaneous echocardiographic contrast in the LAA. Considering that several ex vivo studies have demonstrated the effectiveness of clopidogrel in attenuating platelet–leukocyte adhesion and platelet‐dependent leukocyte activation, further studies are required to investigate the effects of clopidogrel on platelet–leukocyte interactions in cats.32, 37, 38
Clopidogrel did not affect ADP‐mediated expression of PMVs in the present study. Studies on human platelets have shown that P2Y12 receptors are involved in PMV formation, loss of phospholipid asymmetry and externalization of phophatidylserine.39, 40 There are several reasons that may explain the negative results found in this study. Firstly, although P2Y12 potentiates PMV formation, ADP, when used alone, is considered a weak agonist and may lack the ability to induce PMV formation in vitro.41 Furthermore, the use of citrate as an anticoagulant might have inhibited PMV formation because low extracellular calcium concentration prevents activation of calcium‐dependent calpain, which facilitates PMV formation by degrading structural proteins such as actin‐binding protein.37 Because cats with severe HCM were demonstrated to have a significant increase in PMV, it is also possible that PMV formation could be associated with disease severity and that the lack of clinical HCM precluded any significant changes in PMV formation.11
In human platelets, the binding of ADP to P2Y12 activates the G‐protein, Gi2α, which inhibits adenyl cyclase and reduces cyclic AMP (cAMP). cAMP, an important mediator of platelet reactivity, stimulates cAMP‐dependent protein kinase A, which phosphorylates a number of substrate proteins responsible for platelet inhibition.42 Activation of platelets by ADP, therefore, decreases the activation of protein kinase A resulting in decreased phosphorylation of VASP. The active metabolite of clopidogrel irreversibly binds to P2Y12 and abolishes the downstream signaling causing increases in cAMP and pVASP. Because PGE1 increases intracellular cAMP and causes subsequent VASP phosphorylation, PGE1‐treated platelets served as the positive controls in this study. By treating platelets with both ADP and PGE1, the difference in pVASP measured as ARRI served as an indirect marker of P2Y12 inhibition. In agreement with human studies, cats had significantly lower ARRI after 14 days of clopidogrel treatment. In addition, relative pVASP was similar in PGE1‐treated and resting platelets after clopidogrel treatment. Although the platelet ADP receptors and G proteins have not been characterized in feline platelets, the results of the current study suggest that there is a similar intracellular signaling mechanism of action.
In the present study, no differences in relative pVASP were found in resting, ADP‐treated and PGE1‐treated platelets. A possible explanation for this finding is that other pathways may have influenced VASP phosphorylation in feline platelets. For instance, phosphorylation of VASP is influenced by cyclic GMP‐dependent protein kinase (PKG) that is independent of P2Y12 inhibition in human platelets.43 In addition, processing of samples and preparation of PRP could exert mechanical stress on platelets and facilitate platelet–platelet interaction causing in vitro activation and release of endogenous ADP, which could influence pVASP.44, 45
The present study showed an important degree of interindividual variability in response to a defined dose of clopidogrel suggesting that some cats may not be adequately protected from ATE. This heterogeneous response to clopidogrel is well described in human beings.18, 46, 47 Genetic polymorphisms of CYP2C19, a key enzyme in the biotransformation of clopidogrel, are known to affect pharmacodynamics and pharmacokinetics of clopidogrel as low responders often carry low‐function CYP2C19 alleles.48, 49 Other mechanisms of variation include drug interaction with proton pump inhibitors and P2Y12 receptor gene polymorphisms that influence not only the ADP responsiveness but also the extent of platelet inhibition by clopidogrel.50, 51 Interindividual variability may also reflect the sensitivity and variability of the techniques used to assess platelet activation. The large variations and ranges of ARRIs likely reflected the semiquantitative nature of the data generated by Western Blot analysis.
Similar to previously published human studies, a proportion of cats (2/13) did not respond to clopidogrel. Interestingly, platelets from both nonresponder cats had diminished response to ADP on OA and flow cytometry but maintained normal response to thrombin before and after clopidogrel treatment. Although both flow cytometry and OA identified nonresponders to ADP, they do not examine the same cellular events that occur after ADP stimulation and, therefore, may have different specificities in assessing clopidogrel responsiveness.52 Nonresponders in this case would be more appropriately defined as nonresponders to ADP because no residual platelet activation to ADP was found after clopidogrel treatment. This suggests that cats in this population may have P2Y12 receptor polymorphisms, which will require genetic studies for confirmation. Because clopidogrel resistance is associated with increased morbidities in human patients such as stent thrombosis, recurrent ischemic cardiovascular events and myocardial infarction, further research into the mechanism and clinical significance of clopidogrel resistance in HCM cats is needed.46, 53
The present study has several limitations. Firstly, the definition of clopidogrel responsiveness in this study is empiric. Because no information on this subject exists in veterinary medicine, we used guidelines previously established by Gurbel et al.18 who used a similar concentration of ADP in OA to assess clopidogrel responsiveness in patients undergoing percutaneous coronary intervention. In addition, cut‐offs generated in most large‐scale clinical trials are highly dependent on the subset of patients studied. It should be noted that the cut‐offs used in this study were solely for categorizing the degree of clopidogrel response and should not be used to guide clinical decisions. Future clinical trials examining clopidogrel responses in a large population of HCM or ATE cats are needed. It is unknown at this stage whether the progression of HCM may affect platelet reactivity in cats. Additionally, the higher concentration of ADP (40 μM) used in OA versus the 20 μM concentrations used for flow cytometry and Western blots is a limitation. Although there is evidence suggesting that acepromazine may decrease platelet aggregation in dogs, its effects on platelet function in cats are poorly understood.54, 55 Lastly, exclusion of subjects because of sample clotting or inadequate volume of PRP may have led to type I error.
In conclusion, our study shows that platelet activation and aggregation are significantly inhibited by 14 days of clopidogrel treatment. Platelets from cats HO for the A31P mutation were more reactive than control platelets which had a variable response to ADP. Clopidogrel response variability was demonstrated in cats by ADP‐Ag, P‐selectin expression and VASP phosphorylation suggesting that some cats are pharmacologically resistant to clopidogrel. ADP‐Ag and flow cytometry could be useful diagnostic tests in assessing clopidogrel response in cats and should be further investigated to optimize patient outcomes.
Acknowledgment
This study was funded in part by NIH HL093603 (Harris). The corresponding author is funded by the Morris Animal Foundation (D15CA‐907).
Conflicts of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
All work was performed at the University of California, Davis.
The study was presented at the International Veterinary Emergency and Critical Care Symposium, Washington, DC, September 2015.
These two individuals contributed equally as senior authors.
Footnotes
Coulter ACT diff, Beckman‐Coulter Inc, Miami, FL
Sigma‐Aldrich, St. Louis, MO
Catalogue: 12‐626‐80 eBioscience, San Diego, CA
Catalogue: 13‐0411‐85, eBioscience, San Diego, CA
Invitrogen, Carlsbad, CA
Electron Microscopy Sciences, Hatfield, PA
Beckman‐Coulter FC500 Flow Cytometer, Beckman‐Coulter Inc
BD Biosciences, San Diego, CA
eBioscience, San Diego, CA
Polysciences Inc, Warrington, PA
FlowJo, Tree Str Inc, Ashland, OR
Calbiochem, La Jolla, CA
Sigma‐Aldrich, St. Louis, MO
Catalogue Number: Sc‐23507, Santa Cruz Biotechnology, INC., Dallas, Texas
Catalogue Number: Sc‐1853, Santa Cruz Biotechnology, INC., Dallas, Texas
FluroChem E chemilumiescence, ProteinSimple, San Jose, CA
AlphaView Software, ProteinSimple, San Jose, CA
Thermo Fisher Scientific, Rockford, IL
490‐2D Optical Aggregometer, Chrono‐Log Corporation, Havertown, PA
Sigma, St Louis, MO
Chrono‐Log Corporation, Havertown, PA
Prism 6.0e, GraphPad Software, La Jolla, CA
References
- 1. Rush JE, Freeman LM, Fenollosa NK, Brown DJ. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990–1999). J Am Vet Med Assoc 2002;220:202–207. [DOI] [PubMed] [Google Scholar]
- 2. Smith SA, Tobias AH, Jacob KA, et al. Arterial thromboembolism in cats: Acute crisis in 127 cases (1992–2001) and Long‐term management with low‐dose aspirin in 24 cases. J Vet Intern Med 2003;17:73–83. [DOI] [PubMed] [Google Scholar]
- 3. Borgeat K, Wright J, Garrod O, et al. Arterial thromboembolism in 250 cats in general practice: 2004‐2012. J Vet Intern Med 2014;28:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weikert U, Kuhl U, Schultheiss HP, Rauch U. Platelet activation is increased in patients with cardiomyopathy: Myocardial inflammation and platelet reactivity. Platelets 2002;13:487–491. [DOI] [PubMed] [Google Scholar]
- 5. Cherian P, Hankey GJ, Eikelboom JW, et al. Endothelial and platelet activation in acute ischemic stroke and its etiological subtypes. Stroke 2003;34:2132–2137. [DOI] [PubMed] [Google Scholar]
- 6. Schmalbach B, Stepanow O, Jochens A, et al. Determinants of platelet‐leukocyte aggregation and platelet activation in stroke. Cerebrovasc Dis 2015;39:176–180. [DOI] [PubMed] [Google Scholar]
- 7. Lev EI, Alviar CL, Arikan ME, et al. Platelet reactivity in patients with subacute stent thrombosis compared with non‐stent‐related acute myocardial infarction. Am Heart J 2007;153:41.e1–6. [DOI] [PubMed] [Google Scholar]
- 8. Migliorini A, Valenti R, Marcucci R, et al. High residual platelet reactivity after clopidogrel loading and long‐term clinical outcome after drug‐eluting stenting for unprotected left main coronary disease. Circulation 2009;120:2214–2221. [DOI] [PubMed] [Google Scholar]
- 9. Valenti R, Marcucci R, Capodanno D, et al. Residual platelet reactivity to predict long‐term clinical outcomes after clopidogrel loading in patients with acute coronary syndromes: Comparison of different cutoff values by light transmission aggregometry from the responsiveness to clopidogrel and stent thrombosis 2‐acute coronary syndrome (RECLOSE 2‐ACS) study. J Thromb Thrombolysis 2015;40:76–82. [DOI] [PubMed] [Google Scholar]
- 10. Helenski CA, Ross JN Jr. Platelet aggregation in feline cardiomyopathy. J Vet Intern Med 1987;1:24–28. [DOI] [PubMed] [Google Scholar]
- 11. Tablin F, Schumacher T, Pombo M, et al. Platelet activation in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2014;28:411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hogan DF, Andrews DA, Green HW, et al. Antiplatelet effects and pharmacodynamics of clopidogrel in cats. J Am Vet Med Assoc 2004;225:1406–1411. [DOI] [PubMed] [Google Scholar]
- 13. Hamel‐Jolette A, Dunn M, Bedard C. Plateletworks: A screening assay for clopidogrel therapy monitoring in healthy cats. Can J Vet Res 2009;73:73–76. [PMC free article] [PubMed] [Google Scholar]
- 14. Fuentes VL. Arterial thromboembolism: Risks, realities and a rational first‐line approach. J Feline Med Surg 2012;14:459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yi X, Chi W, Wang C, et al. Low‐molecular‐weight heparin or dual antiplatelet therapy is more effective than aspirin alone in preventing early neurological deterioration and improving the 6‐month outcome in ischemic stroke patients. J Clin Neurol 2015;11:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Serebruany VL, Malinin AI, Jerome SD, et al. Effects of clopidogrel and aspirin combination versus aspirin alone on platelet aggregation and major receptor expression in patients with heart failure: The Plavix Use for Treatment Of Congestive Heart Failure (PLUTO‐CHF) trial. Am Heart J 2003;146:713–720. [DOI] [PubMed] [Google Scholar]
- 17. Eisenstein EL, Anstrom KJ, Kong DF, et al. Clopidogrel use and long‐term clinical outcomes after drug‐eluting stent implantation. JAMA 2007;297:159–168. [DOI] [PubMed] [Google Scholar]
- 18. Gurbel PA, Bliden KP, Hiatt BL, O'Connor CM. Clopidogrel for coronary stenting: Response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 2003;107:2908–2913. [DOI] [PubMed] [Google Scholar]
- 19. Norris JW, Pratt SM, Auh J‐H, et al. Investigation of a novel, heritable bleeding diathesis of thoroughbred horses and development of a screening assay. J Vet Intern Med 2006;20:1450–1456. [DOI] [PubMed] [Google Scholar]
- 20. Robert S, Poncelet P, Lacroix R, et al. Standardization of platelet‐derived microparticle counting using calibrated beads and a Cytomics FC500 routine flow cytometer: A first step towards multicenter studies? J Thromb Haemost 2009;7:190–197. [DOI] [PubMed] [Google Scholar]
- 21. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685. [DOI] [PubMed] [Google Scholar]
- 22. Altschul SF, Gish W, Miller W, et al. Basic local alignment search tool. J Mol Biol 1990;215:403–410. [DOI] [PubMed] [Google Scholar]
- 23. Hezard N, Metz D, Garnotel R, et al. Platelet VASP phosphorylation assessment in clopidogrel‐treated patients: Lack of agreement between Western blot and flow cytometry. Platelets 2005;16:474–481. [DOI] [PubMed] [Google Scholar]
- 24. Hollopeter G, Jantzen HM, Vincent D, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 2001;409:202–207. [DOI] [PubMed] [Google Scholar]
- 25. Zhang FL, Luo L, Gustafson E, et al. ADP is the cognate ligand for the orphan G protein‐coupled receptor SP1999. J Biol Chem 2001;276:8608–8615. [DOI] [PubMed] [Google Scholar]
- 26. Klinkhardt U. Clopidogrel but not aspirin reduces P‐selectin expression and formation of platelet‐leukocyte aggregates in patients with atherosclerotic vascular disease. Clin Pharmacol Ther 2003;73:232–241. [DOI] [PubMed] [Google Scholar]
- 27. Trip MD, Cats VM, van Capelle FJL, Vreeken J. Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N Engl J Med 1990;322:1549–1554. [DOI] [PubMed] [Google Scholar]
- 28. Marcucci R, Valente S, Gori AM, et al. Global platelet hyperreactivity and elevated C‐reactive protein levels predict long term mortality in STEMI patients. Thromb Res 2014;134:884–888. [DOI] [PubMed] [Google Scholar]
- 29. Falcinelli E, Giannini S, Boschetti E, et al. Platelets release active matrix metalloproteinase‐2 in vivo in humans at a site of vascular injury: Lack of inhibition by aspirin. Br J Haematol 2007;138:221–230. [DOI] [PubMed] [Google Scholar]
- 30. Martinod K, Wagner DD. Thrombosis: Tangled up in NETs. Blood 2014;123:2768–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sreeramkumar V, Adrover JM, Ballesteros I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science 2014;346:1234–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gould TJ, Vu TT, Swystun LL, et al. Neutrophil extracellular traps promote thrombin generation through platelet‐dependent and platelet‐independent mechanisms. Arterioscler Thromb Vasc Biol 2014. Sep;34:1977–1984. [DOI] [PubMed] [Google Scholar]
- 33. Schober KE, Maerz I. Assessment of left atrial appendage flow velocity and its relation to spontaneous echocardiographic contrast in 89 cats with myocardial disease. J Vet Intern Med 2006;20:120–130. [DOI] [PubMed] [Google Scholar]
- 34. Stokol T, Brooks M, Rush JE, et al. Hypercoagulability in cats with cardiomyopathy. J Vet Intern Med 2008;22:546–552. [DOI] [PubMed] [Google Scholar]
- 35. Payne JR, Borgeat K, Connolly DJ, et al. Prognostic indicators in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2013;27:1427–1436. [DOI] [PubMed] [Google Scholar]
- 36. Zotz RJ, Müller M, Genth‐Zotz S, Darius H. Spontaneous echo contrast caused by platelet and leukocyte aggregates? Stroke 2001;32:1127–1233. [DOI] [PubMed] [Google Scholar]
- 37. Evangelista V, Manarini S, Dell'Elba G, et al. Clopidogrel inhibits platelet‐leukocyte adhesion and platelet‐dependent leukocyte activation. Thromb Haemost 2005;94:568–577. [PubMed] [Google Scholar]
- 38. Akinosoglou K, Alexopoulos D. Use of antiplatelet agents in sepsis: A glimpse into the future. Thromb Res 2014;133:131–138. [DOI] [PubMed] [Google Scholar]
- 39. Takano K, Asazuma N, Satoh K, et al. Collagen‐induced generation of platelet‐derived microparticles in whole blood is dependent on ADP released from red blood cells and calcium ions. Platelets 2004;15:223–229. [DOI] [PubMed] [Google Scholar]
- 40. Berny‐Lang MA, Jakubowski JA, et al. P2Y(12) receptor blockade augments glycoprotein IIb‐IIIa antagonist inhibition of platelet activation, aggregation, and procoagulant activity. J Am Heart Assoc 2013;2:e000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Storey RF, Sanderson HM, White AE, et al. The central role of the P‐2T receptor in amplification of human platelet activation, aggregation, secretion and procoagulant activity. Br J Haematol 2000;110:925–934. [DOI] [PubMed] [Google Scholar]
- 42. Smolenski A. Novel roles of cAMP/cGMP‐dependent signaling in platelets. J Thromb Haemost 2012;10:167–176. [DOI] [PubMed] [Google Scholar]
- 43. Smolenski A, Bachmann C, Reinhard K, et al. Analysis and regulation of vasodilator‐stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phosphospecific monoclonal antibody. J Bio Chem 1998;273:20029–20035. [DOI] [PubMed] [Google Scholar]
- 44. Veeraputhiran M, Ware J, Dent J, et al. A comparison of washed and volume‐reduced platelets with respect to platelet activation, aggregation, and plasma protein removal. Transfusion 2011;51:1030–1036. [DOI] [PubMed] [Google Scholar]
- 45. Gharehbaghian A, Salimian M, Taherian AA, et al. Variations in intraplatelet phospho‐VASP expression due to pre‐analytical sample preparations, illustration of a quality control issue in platelet pharmacology. Iran J Pharm Res 2015;14:321–328. [PMC free article] [PubMed] [Google Scholar]
- 46. De Miguel A, Ibanez B, Badimon JJ. Clinical implications of clopidogrel resistance. Thromb Haemost 2008;100:196–203. [PubMed] [Google Scholar]
- 47. Jaremo P, Lindahl TL, Fransson SG, Richter A. Individual variations of platelet inhibition after loading doses of clopidogrel. J Intern Med 2002;252:233–238. [DOI] [PubMed] [Google Scholar]
- 48. Mega JL, Close SL, Wiviott SD, et al. Cytochrome P‐450 polymorphisms and response to clopidogrel. New Engl J Med 2009;360:354–362. [DOI] [PubMed] [Google Scholar]
- 49. Jinnai T, Horiuchi H, Makiyama T, et al. Impact of CYP2C19 polymorphisms on the antiplatelet effect of clopidogrel in an actual clinical setting in Japan. Circ J 2009;73:1498–1503. [DOI] [PubMed] [Google Scholar]
- 50. Zahno A, Brecht K, Bodmer M, et al. Effects of drug interactions on biotransformation and antiplatelet effect of clopidogrel in vitro. Br J Pharmaco 2010;161:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee S‐J, Jung I‐S, Jung E‐J, et al. Identification of P2Y12 single‐nucleotide polymorphisms and their influences on the variation in ADP‐induced platelet aggregation. Thromb Res 2011;127:220–227. [DOI] [PubMed] [Google Scholar]
- 52. Lesmesle G, Landel JB, Bauters A, et al. Poor agreement between light transmission aggregometry, Verify Now P2Y12 and vasodilatator‐stimulated phosphoprotein for clopidogrel low‐response assessment: A potential explanation of negative results of recent randomized trials. Platelets 2014;25:499–505. [DOI] [PubMed] [Google Scholar]
- 53. Vlachojannis GJ, Dimitropoulos G, Alexopoulos D. Clopidogrel resistance: Current aspects and future directions. Hellenic J Cardiol 2011;52:236–245. [PubMed] [Google Scholar]
- 54. Barr SC, Ludders JW, Looney AL, et al. Platelet aggregation in dogs after sedation with acepromazine and atropine and during subsequent general anesthesia and surgery. Am J Vet Res 1992;53:2067–2070. [PubMed] [Google Scholar]
- 55. Conner BJ, Hanel RM, Hansen BD, et al. Effects of acepromazine maleate on platelet function assessed by use of adenosine diphosphate activated‐ and arachidonic acid‐ activated modified thromboelastography in healthy dogs. Am J Vet Res 2012;73:595–601. [DOI] [PubMed] [Google Scholar]


