Abstract
Background
Echocardiography is a cost‐efficient method to screen cats for presence of heart disease. Current reference intervals for feline cardiac dimensions do not account for body weight (BW).
Objective
To study the effect of BW on heart rate (HR), aortic (Ao), left atrial (LA) and ventricular (LV) linear dimensions in cats, and to calculate 95% prediction intervals for these variables in normal adult pure‐bred cats.
Animals
19 866 pure‐bred cats.
Methods
Clinical data from heart screens conducted between 1999 and 2014 were included. Associations between BW, HR, and cardiac dimensions were assessed using univariate linear models and allometric scaling, including all cats, and only those considered normal, respectively. Prediction intervals were created using 95% confidence intervals obtained from regression curves.
Results
Associations between BW and echocardiographic dimensions were best described by allometric scaling, and all dimensions increased with increasing BW (all P<0.001). Strongest associations were found between BW and Ao, LV end diastolic, LA dimensions, and thickness of LV free wall. Weak linear associations were found between BW and HR and left atrial to aortic ratio (LA:Ao), for which HR decreased with increasing BW (P<0.001), and LA:Ao increased with increasing BW (P<0.001). Marginal differences were found for prediction formulas and prediction intervals when the dataset included all cats versus only those considered normal.
Conclusions and Importance
BW had a clinically relevant effect on echocardiographic dimensions in cats, and BW based 95% prediction intervals may help in screening cats for heart disease.
Keywords: Heart dimensions, M‐mode, Prediction intervals, Screening
Abbreviations
- Ao
aorta
- BW
body weight
- CI
confidence interval
- FS%
fractional shortening
- HCM
hypertrophic cardiomyopathy
- HR
heart rate
- IVSd
interventricular septum diastole
- IVSs
interventricular septum systole
- LA
left atrium
- LA:Ao
left atrial‐to‐aortic root diameter ratio
- LV
left ventricle
- LVFWd
left ventricular free wall diastole
- LVFWs
left ventricular free wall systole
- LVIDd
left ventricular internal diameter diastole
- LVIDs
left ventricular internal diameter systole
- RCM
restrictive cardiomyopathy
Pure‐bred cats are commonly screened by echocardiography for breeding purposes for the presence of heart disease, in particular hypertrophic cardiomyopathy (HCM). Currently used reference intervals for cardiac dimensions in cats are mostly based on studies using first‐ or second‐generation ultrasound systems, often with unguided M‐mode imaging.1, 2, 3, 4 Furthermore, these studies included a limited number of presumably normal cats, or cats of 1 breed examined at 1 center by 1 or a few observers.1, 2, 3, 4, 5, 6, 7, 8 Accordingly, critics have questioned the usefulness of some of these published reference ranges because of the ultrasound systems used, small sample sizes, lack of data points for the extremes of body size, wide prediction intervals, and the use of inappropriate statistical methods.9 The association between body weight (BW) and echocardiographic measurements has been examined in a few studies, and the results suggested a small effect of BW.1, 4, 8, 10 Recently, a study in Bengal cats utilized allometric scaling in creating normal reference ranges for echocardiographic measurements in this particular breed.11 However, fixed reference ranges, ignoring the possible effect of BW, are most commonly used. For instance, upper limits for diastolic left ventricular (LV) wall thickness of 5.512 to 6 mm13, 14 and a left atrial (LA) diameter of 16 mm15, 16 have been proposed. This approach likely applies to cats of a certain body size, but would underdiagnose LV hypertrophy or LA dilatation in smaller cats and overdiagnose it in larger cats. Given that, in the absence of obesity, adult pure‐bred cats range in body size from 2.5 kg to >10 kg,17 it is important to identify equations that accurately describe the associations between body size and cardiac dimensions in cats.
In dogs, the effect of body size on cardiac dimensions is well known and measurements of cardiac dimensions often are normalized to BW using the principle of allometric scaling.18, 19, 20 In brief, the principle of allometric scaling relates a measurement, such as echocardiographic dimensions and physiological variables, with BW (mass or volume) based on the following relationship, where Y represents the measurement and a and b are constants:
The constant b is often referred to as the scaling exponent, and its numerical value differs depending on exactly which variables are related to BW.18, 19, 20 For 1‐dimensional measurements, such as echocardiographic dimensions, it has a theoretical value of 0.33, and for 2‐dimensional measurements, it has a theoretical value of 0.67. For metabolic rate, it has a theoretical value of 0.75.18, 19, 20 Studies in dogs, people, and Rhesus monkeys have shown that this principle is comparably accurate in predicting the association between echocardiographic measurements and BW.18, 21, 22, 23 However, cats have a narrower range of BW, and this association might not be clinically relevant over the range of cat weights.
Heart rate (HR) is routinely assessed during physical examination. It is described to be lower when cats are not restrained, as well as when the cats are in their home environment.24, 25 An inverse association between BW and HR has been described in cats, but in a comparably small and homogenous population.1
Therefore, the aims of our study were to investigate the independent effect of BW on HR, aortic (Ao), LA, and LV linear dimensions in cats using a statistical approach in a large cohort of cats, and to calculate 95% prediction intervals for these variables in normal adult pure‐bred cats.
Materials and Methods
Clinical data from records of heart screens conducted in Europe, Australia, New Zealand, and North America between 1999 and 2014 within the PawPeds screening program (www.pawpeds.com) were entered into a database. In this program, owners and screeners are instructed to only screen cats that are apparently healthy, nonpregnant, and nonlactating. Cat characteristics, including BW and results from physical and echocardiographic examinations (including use of a sedative), were included in the database. There was no specification of type of scale used for measuring BW. Heart rate was obtained during the physical examination by cardiac auscultation. The final classification of the cat was entered, including the following classes: normal, equivocal for LV hypertrophy as defined below, hypertrophic cardiomyopathy (HCM), restrictive cardiomyopathy (RCM), and other cardiac diagnosis.
Cats were classified at the discretion of each examiner into diagnostic groups. As general recommendations to examiners in the program, cats could be classified as normal when subjective assessment of cardiac morphology was judged to be within normal variation and supported by a diastolic LV thickness of <5.0 mm in a cat that had a BW that fell in the typical range (2.5–6 kg). Cats outside of this weight range were recommended to be evaluated on a case‐by‐case basis.7, 8 Equivocal classification could be considered when the subjective impression from the echocardiogram could not allow normal phenotypes to be distinguished from mild forms of HCM, the LV wall thickness was between 5.0 and 5.5 mm in a cat that had a BW that fell in the typical range (2.5–6 kg) or both,26, 27 or the clinical correlate of a specific finding was undefined. Cats with normal LV wall thickness and systolic anterior motion (SAM) also could be classified as equivocal. A diagnosis of HCM could be considered when a subjective impression of hypertrophy (regional, global or papillary muscle hypertrophy) was supported by a M‐mode diastolic LV wall thicknesses of the interventricular septum (IVSd), left ventricular free wall (LVFWd), or both that measured ≥5.5 mm in a cat that has a BW that fell in the typical range (2.5–6 kg).12 Restrictive cardiomyopathy (RCM) classification could be considered in cats with normal LV wall thickness and LA or biatrial enlargement (LA enlargement defined as LA:Ao >1.5,28 right atrial enlargement does not have a measurement definition).29 In cases in which a cat had been subjected to repeated screens, only the most recent screen report was retained in the database. Thus, data from only 1 screening report per cat was included in the database. For the purpose of this study, we divided cats into 2 groups: those with normal and those with abnormal echocardiograms (including equivocal, HCM, RCM, or other cardiac diagnosis).
Echocardiography
The instructions to screeners in the PawPeds program state that cats must be scanned from beneath while in right lateral recumbency. The LV should be examined in 3 different 2D echocardiographic views: a right parasternal long‐axis LV outflow view; a right parasternal LV inflow view; and a right parasternal LV short‐axis view at the level of the papillary muscles. Left ventricular dimensions should be measured from M‐mode images recorded from a right parasternal short‐axis view at the level of the papillary muscles according to guidelines.30 End‐diastolic measurements of LV diameter should be made at the end of diastole, just before the onset of systole, and end‐systolic LV diameter measured at the nadir of septal motion, from leading edge to leading edge.30 Simultaneous electrocardiographic (ECG) monitoring is recommended, but not mandatory. The left atrial‐to‐aortic root diameter ratio (LA:Ao) should be obtained from a right parasternal short‐axis view in early diastole at the first frame after aortic valve closure.31, 32 Two‐dimensional images of the LV outflow tract should be used to identify the presence of systolic anterior motion of the mitral valve, color Doppler echocardiography should be used to interrogate for LV outflow tract obstruction, and spectral Doppler should be used to assess maximal velocities of the left and right ventricular outflow tracts.
Statistical Methods
All statistical calculations were made using a commercially available computerized program.1 A value of P < .05 was considered significant. Median and interquartile range (IQR) were used to provide group‐wise descriptive statistics for continuous variables. Differences between groups for continuous variables were tested using the Mann–Whitney U‐test. The chi‐squared test was used for testing the distribution of categorical variables.
Univariate linear regression and allometric scaling were used to evaluate associations among BW, HR, and echocardiographic measurements. Associations examined by allometric scaling were performed as previously described, using double‐logarithmic transformations of the variables.18 For both the linear regression analyses and the allometric scaling method, 2 datasets were used for each outcome variable: 1 including all cats and 1 including only cats classified as normal. For each analysis, the distributions of residuals were tested for normality using the Shapiro–Wilk W test. The adjusted R 2 is defined as the percentage of the total sum of squares that can be explained by the prediction formula, and it also considers the degrees of freedom for variables added. Using the regression analyses, the 95% individual prediction intervals that appear in Figures 1 and 2 and Table 3 were calculated according to the following formula33:
Figure 1.
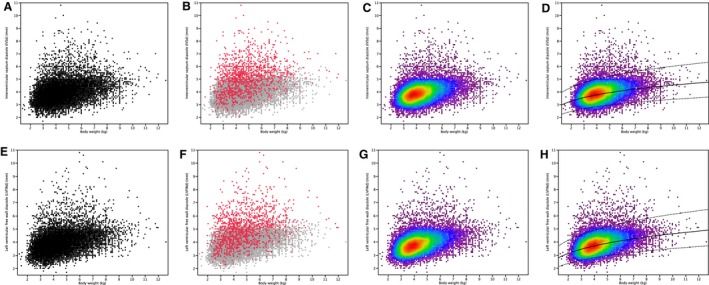
Scatter plots and heat maps of diastolic left ventricular wall thicknesses plotted against body weight in 19,866 cats. Figures A–D show plots of diastolic interventricular septal wall thickness; Figures E–H show plots of diastolic free wall thickness. Figures A and E: all cats (n = 19,866). Figures B and F: cats with abnormal echocardiograms (n = 1,406, red dots) and cats with normal echocardiograms (n = 18,460, gray dots). Figures C and G: heat maps of all cats (n = 19,866). Figures D and H: heat maps as in Figures C and G, with superimposed regression line (solid line) and 95% prediction intervals (dotted lines) (n = 19,866). In Figures C, D, G and H, dots are coded according to their density at each point in the plot, from purple (<5% of the observations below) to dark red (95% of the observations below). Each color represents 5% intervals.
Figure 2.
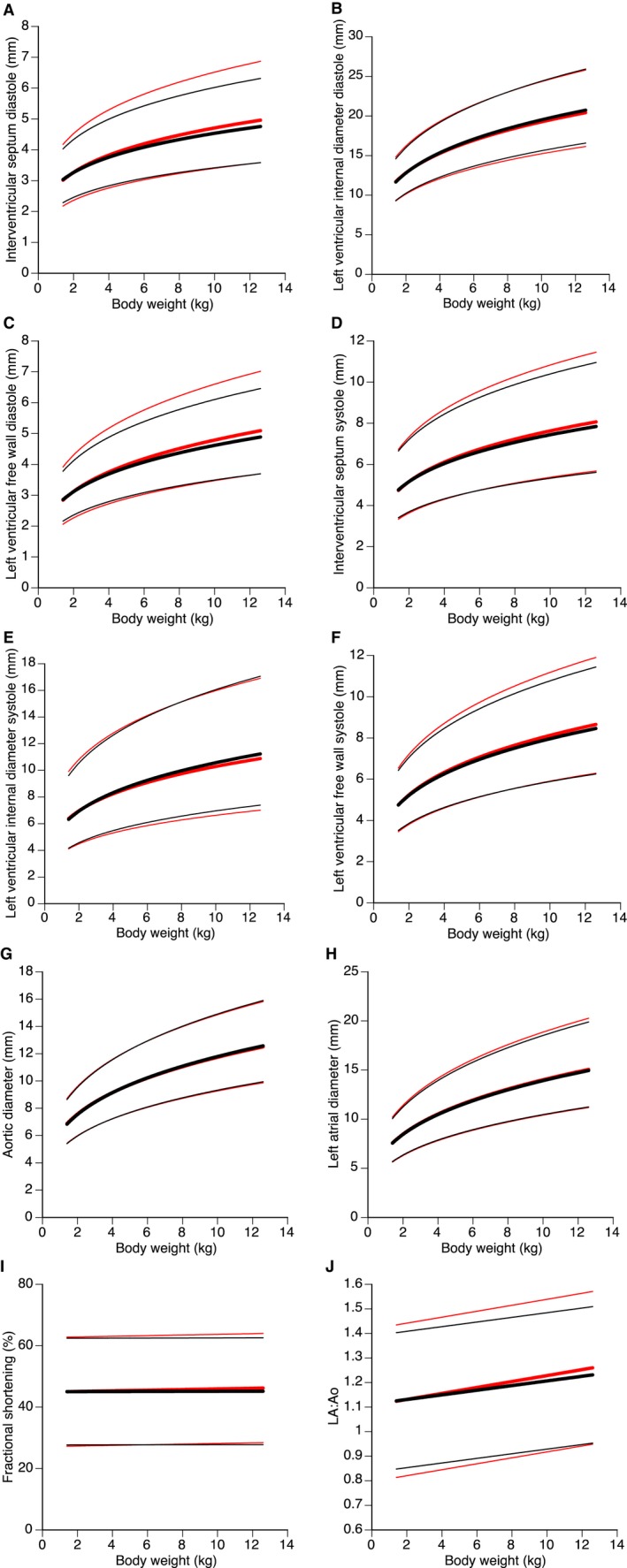
Regression lines (bold lines) and 95% prediction intervals (thin lines) for body weight plotted against diastolic interventricular septum in diastole (A), left ventricular internal diameter in diastole (B), left ventricular free wall in diastole (C), interventricular septum in systole (D), left ventricular internal diameter in systole (E), left ventricular free wall in systole (F), aortic (G) and left atrial (H) diameters, fractional shortening (I), and left atrial‐to‐aortic root ratio (J). Red lines indicate regression lines and 95% prediction intervals for all cats (n = 19,866). Black lines indicate regression lines and 95% prediction intervals including only cats with normal echocardiograms (n = 18,460).
Table 3.
Predicted cardiac dimensions and 95% prediction intervals for 18460 cats with normal echocardiograms according to specific, stated criteria (see Materials and Methods)
| Weight (kg) | IVSd (mm) | LVIDd (mm) | LVFWd (mm) | IVSs (mm) | LVIDs (mm) | LVFWs (mm) | FS (%) | LA (mm) | Ao (mm) | LA:Ao |
|---|---|---|---|---|---|---|---|---|---|---|
| 1.5 | 3.1 (2.3–4.0) | 11.9 (9.5–15.0) | 2.9 (2.2–3.8) | 4.8 (3.5–6.7) | 6.4 (4.2–9.6) | 4.8 (3.6–6.5) | 45 (28–62) | 7.7 (5.8–10.2) | 7.0 (5.5–8.8) | 1.13 (0.85–1.40) |
| 2.0 | 3.3 (2.5–4.3) | 12.8 (10.2–16.0) | 3.1 (2.4–4.1) | 5.2 (3.7–7.2) | 6.9 (4.6–10.5) | 5.2 (3.9–7.1) | 45 (28–62) | 8.5 (6.3–11.2) | 7.5 (6.0–9.5) | 1.13 (0.85–1.40) |
| 2.5 | 3.4 (2.6–4.5) | 13.6 (10.9–17.0) | 3.2 (2.5–4.4) | 5.4 (3.9–7.6) | 7.4 (4.8–11.2) | 5.5 (4.1–7.5) | 45 (28–62) | 9.1 (6.8–12.0) | 8.0 (6.3–10.1) | 1.14 (0.86–1.41) |
| 3.0 | 3.5 (2.7–4.7) | 14.2 (11.4–17.8) | 3.4 (2.6–4.5) | 5.7 (4.1–7.9) | 7.7 (5.1–11.7) | 5.8 (4.3–7.9) | 45 (28–62) | 9.6 (7.2–12.7) | 8.4 (6.7–10.7) | 1.14 (0.86–1.42) |
| 3.5 | 3.7 (2.8–4.9) | 14.8 (11.9–18.5) | 3.6 (2.7–4.7) | 5.9 (4.2–8.2) | 8.0 (5.3–12.2) | 6.0 (4.5–8.2) | 45 (28–62) | 10.0 (7.6–13.4) | 8.8 (7.0–11.1) | 1.15 (0.87–1.42) |
| 4.0 | 3.8 (2.8–4.9) | 15.4 (12.2–19.2) | 3.7 (2.8–4.8) | 6.0 (4.3–8.4) | 8.3 (5.5–12.6) | 6.3 (4.6–8.5) | 45 (28–62) | 10.5 (7.9–13.9) | 9.1 (7.2–11.6) | 1.15 (0.88–1.43) |
| 4.5 | 3.9 (2.9–5.1) | 15.8 (12.7–19.8) | 3.8 (2.9–5.0) | 6.2 (4.4–8.7) | 8.6 (5.7–13.0) | 6.5 (4.8–8.7) | 45 (28–62) | 10.9 (8.2–14.5) | 9.4 (7.5–11.9) | 1.15 (0.88–1.43) |
| 5.0 | 3.9 (3.0–5.2) | 16.3 (13.0–20.3) | 3.9 (3.0–5.1) | 6.4 (4.6–8.9) | 8.8 (5.8–13.4) | 6.6 (4.9–9.0) | 45 (28–62) | 11.2 (8.4–14.9) | 9.7 (7.7–12.3) | 1.16 (0.88–1.43) |
| 5.5 | 4.0 (3.0–5.3) | 16.7 (13.4–20.9) | 4.0 (3.0–5.3) | 6.5 (4.7–9.1) | 9.0 (6.0–13.7) | 6.8 (5.0–9.2) | 45 (28–62) | 11.6 (8.7–15.4) | 10.0 (7.9–12.6) | 1.16 (0.89–1.44) |
| 6.0 | 4.1 (3.1–5.4) | 17.1 (13.7–21.4) | 4.1 (3.1–5.4) | 6.6 (4.7–9.3) | 9.3 (6.1–14.1) | 7.0 (5.1–9.4) | 45 (28–62) | 11.9 (8.9–15.8) | 10.2 (8.1–12.9) | 1.16 (0.89–1.44) |
| 6.5 | 4.2 (3.1–5.5) | 17.4 (14.0–21.8) | 4.2 (3.1–5.5) | 6.7 (4.8–9.4) | 9.4 (6.2–14.3) | 7.1 (5.3–9.6) | 45 (28–62) | 12.2 (9.2–16.2) | 10.5 (8.3–13.2) | 1.17 (0.90–1.45) |
| 7.0 | 4.2 (3.2–5.6) | 17.8 (14.2–22.2) | 4.3 (3.2–5.6) | 6.9 (4.9–9.6) | 9.6 (6.3–14.6) | 7.3 (5.4–9.8) | 45 (28–62) | 12.5 (9.4–16.6) | 10.7 (8.4–13.5) | 1.18 (0.90–1.46) |
| 7.5 | 4.3 (3.2–5.7) | 18.1 (14.5–22.6) | 4.3 (3.3–5.7) | 7.0 (5.0–9.7) | 9.8 (6.5–14.9) | 7.4 (5.5–10.0) | 45 (28–62) | 12.7 (9.6–16.9) | 10.9 (8.6–13.8) | 1.18 (0.91–1.46) |
| 8.0 | 4.3 (3.3–5.8) | 18.4 (14.7–23.0) | 4.4 (3.3–5.8) | 7.1 (5.1–9.9) | 10.0 (6,6–15.1) | 7.5 (5.6–10.2) | 45 (28–62) | 13.0 (9.8–17.3) | 11.1 (8.8–14.0) | 1.19 (0.91–1.47) |
| 8.5 | 4.4 (3.3–5.8) | 18.7 (15.0–23.4) | 4.4 (3.4–5.9) | 7.2 (5.1–10.0) | 10.1 (6.7–15.4) | 7.6 (5.6–10.3) | 45 (28–62) | 13.2 (10.0–17.6) | 11.3 (8.9–14.3) | 1.19 (0.92–1.47) |
| 9.0 | 4.4 (3.3–5.9) | 19.0 (15.2–23.7) | 4.5 (3.4–5.9) | 7.3 (5.2–10.2) | 10.3 (6.8–15.6) | 7.7 (5.7–10.5) | 45 (28–62) | 13.5 (10.1–17.9) | 11.5 (9.1–14.5) | 1.20 (0.92–1.47) |
| 9.5 | 4.5 (3.4–6.0) | 19.3 (15.4–24.0) | 4.6 (3.4–5.9) | 7.4 (5.3–10.3) | 10.4 (6.9–15.8) | 7.9 (5.8–10.6) | 45 (28–63) | 13.7 (10.3–18.2) | 11.6 (9.1–14.7) | 1.20 (0.92–1.48) |
| 10.0 | 4.5 (3.4–6.0) | 19.5 (15.6–24.4) | 4.6 (3.5–6.1) | 7.4 (5.3–10.4) | 10.5 (6.9–16.0) | 8.0 (5.9–10.8) | 45 (28–63) | 13.9 (10.5–18.5) | 11.8 (9.3–14.9) | 1.21 (0.92–1.48) |
| 10.5 | 4.6 (3.5–6.1) | 19.8 (15.8–24.7) | 4.7 (3.5–6.2) | 7.5 (5.4–10.5) | 10.7 (7.1–16.3) | 8.1 (6.0–10.9) | 45 (28–63) | 14.1 (10.6–18.8) | 11.9 (9.5–15.1) | 1.22 (0.94–1.49) |
| 11.0 | 4.6 (3.5–6.1) | 20.0 (16.0–25.0) | 4.7 (3.5–6.2) | 7.6 (5.4–10.6) | 10.8 (7.2–16.5) | 8.1 (6.0–11.0) | 45 (28–63) | 14.3 (10.8–19.1) | 12.1 (9.6–15.3) | 1.22 (0.94–1.50) |
HR, heart rate; IVSd, interventricular septum diastole; LVIDd, left ventricular internal diameter diastole; LVFWd, left ventricular free wall diastole; IVSs, interventricular septum systole; LVIDs, left ventricular internal diameter systole; LVFWs, left ventricular free wall systole; FS%, fractional shortening; Ao, aortic diameter; LA, left atrial diameter; and LA:Ao, left atrial‐to‐aortic ratio.
This article was published online on 30 Aug 2016. An error was subsequently identified by the author. This notice is included in the online version to indicate that both have been corrected on 1 Sep 2016.
where Y t is the calculated value of Y for a given value of x, t is the Student's t value for n‐2 degrees of freedom, n is the number of data points, S xy is the standard error of the estimate, X is the mean of the individual x values, ∑ (x i − X)2 is the sum of the squared deviations of the sample mean.
Results
Cats
Screen reports from 19,866 cats were included in the database. The cats had a median age of 1.8 years (IQR, 1.1–3.2 years) and a median BW of 4.2 kg (IQR, 3.5–5.1 kg). Female cats were more frequently represented than male (12,699 versus. 7,167 cats, P < .001). Information concerning sexual status was available for 67% of cats, and intact cats were more common than neutered (11,496 versus 1,872 cats, P < .001). With regard to breed, the database consisted of 5,274 Maine Coon, 3,301 Norwegian Forest, 2,663 British Shorthair, 1,832 Siberian, 1,809 Ragdoll, 1,258 Sphynx, 914 Birman, 745 Cornish Rex, 584 Bengal, 526 Devon Rex, 204 Persian, and 756 cats of other breeds. The latter group consisted of 38 different breeds of which British Longhair, European, and Ocicat were the most common (>50 cats per breed). Information concerning sedation for the echocardiographic examination was available in 93% of the cats, of which the majority (95%) of cats had been unsedated (913 sedated versus 17,468 unsedated). Echocardiograms were considered normal in 18,460 cats, equivocal in 529 cats, indicative of HCM in 686 cats, indicative of other cardiac diagnosis in 183 cats, and RCM in 8 cats. Cats with remarks on the echocardiogram (equivocal, HCM, RCM, and other diagnosis) were significantly older and had a higher BW compared to cats with unremarkable echocardiograms (all P < .001, Table 1).
Table 1.
Medians and interquartile ranges (IQR) for age, body weight, heart rate, and echocardiographic measurements in all cats, in cats classified as normal, according to stipulated criteria (see Materials and Methods), and in cats with abnormal findings
| Variable | All (n = 19,866) | Normal (n = 18,460) | Abnormal (n = 1,406) | P‐value |
|---|---|---|---|---|
| Age (years) | 1.8 (1.1–3.2) | 1.8 (1.1–3.1) | 4.5 (1.5–4.5) | <.0001 |
| Body weight (kg) | 4.2 (3.5–5.1) | 4.2 (3.5–5.1) | 4.6 (3.7–5.9) | <.0001 |
| Heart rate (beats/min) | 180 (160–196) | 180 (160–195) | 180 (160–200) | <.0001 |
| IVSd (mm) | 3.9 (3.5–4.3) | 3.9 (3.5–4.3) | 5.2 (4.3–5.9) | <.0001 |
| LVIDd (mm) | 15.6 (14.3–17.0) | 15.7 (14.3–17.1) | 15.0 (13.4–16.7) | <.0001 |
| LVFWd (mm) | 3.8 (3.4–4.3) | 3.8 (3.4–4.2) | 5.1 (4.2–5.8) | <.0001 |
| IVSs (mm) | 6.2 (5.5–7.0) | 6.2 (5.5–7.0) | 7.6 (6.4–8.6) | <.0001 |
| LVIDs (mm) | 8.5 (7.3–9.8) | 8.5 (7.4–9.8) | 7.5 (6.1–9) | <.0001 |
| LVFWs (mm) | 6.5 (5.8–7.2) | 6.4 (5.7–7.1) | 7.7 (6.7–8.8) | <.0001 |
| FS% | 45 (39–51) | 45 (39–51) | 49 (42–56) | <.0001 |
| Ao (mm) | 9.3 (8.5–10.2) | 9.3 (8.5–10.2) | 9.5 (8.8–10.5) | <.0001 |
| LA (mm) | 10.8 (9.6–12.0) | 10.8 (9.6–12.0) | 11.7 (10.0–13.4) | <.0001 |
| LA:Ao | 1.14 (1.06–1.24) | 1.13 (1.06–1.23) | 1.2 (1.10–1.35) | <.0001 |
IVSd, interventricular septum diastole; LVIDd, left ventricular internal diameter diastole; LVFWd, left ventricular free wall diastole; IVSs, interventricular septum systole; LVIDs, left ventricular internal diameter systole; LVFWs, left ventricular free wall systole; FS%, fractional shortening; Ao, aortic diameter; LA, left atrial diameter; and LA:Ao, left atrial‐to‐aortic ratio.
Echocardiography
Heart rate and echocardiographic measurements are summarized in Table 1. Interventricular septum in diastole (IVSd) was thicker than the LVFWd by 0.06 mm when comparing all cats or only normal cats (both P < .0001). Differences between cats with remarks on the echocardiograms compared to those with unremarkable findings were found significant for all variables (all P < .0001).
Effect of Body Weight
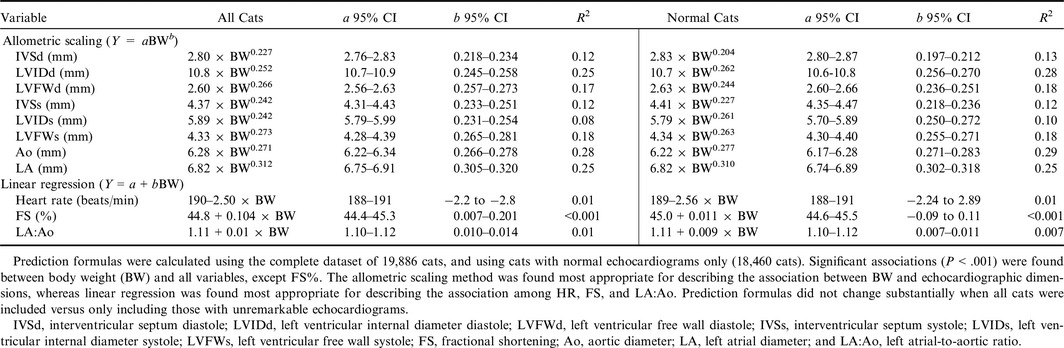
As a consequence of the residual distribution in the regression analyses, associations between BW and echocardiographic dimensions were most accurately described by allometric scaling, while linear regression most accurately described associations between BW and HR, fractional shortening (FS%), and LA:Ao. All echocardiographic measurements, except FS%, increased with increasing BW (all P < .001, Table 2). The highest associations, as indicated by highest R 2, were found for Ao, LVIDd, LA, LVFWd, and LVFWs. For most of these variables, BW accounted for <25% of the variability in the echocardiographic variable, whether examined by allometric scaling or linear regression. Weak associations were found among BW and HR and LA:Ao, where HR decreased with increasing BW (P < .001) and LA:Ao increased with increasing BW (P < .001, Table 2). Marginal differences were found in prediction formulas when the dataset included all cats versus only those considered normal (Table 2). For all echocardiographic variables, the scaling exponent was smaller than the biologically predicted exponent of 0.333 (Table 2).
Table 2.
Prediction formulas and 95% prediction intervals for constants concerning the associations between body weight and heart rate and echocardiographic measurements using the allometric scaling method and unilinear regression models
95% Prediction Intervals
For each echocardiographic variable, 95% prediction intervals were calculated from the regression analyses (Fig 1). As mentioned previously, small differences were found in estimates of constants in the prediction formulas when all cats versus only those with normal echocardiograms were included in the analyses. Thus, small differences in 95% prediction intervals were found for LV wall thickness in both systole and diastole, as well as for LVIDs, LA, and LA:Ao, whereas almost complete overlaps were found for LVIDd, Ao, and FS% (Fig 2). Numerical values of estimates and 95% prediction intervals by BW are presented in Table 3. These values were based only on cats with normal echocardiograms according to stipulated criteria (see Materials and Methods).
Discussion
This is the largest published study of standard echocardiographic measurements in cats. The results showed that BW has a clinically relevant impact on most standard echocardiographic measurements in cats, which suggests that a fixed reference interval is not appropriate for all cats. We also found that, for a given BW, the 95% prediction intervals for end‐diastolic wall thickness in pure‐bred cats are lower than found in many previously published studies in cats.1, 2, 3, 4, 5, 6, 10, 12, 16, 34 The present study suggests that, like dogs and other mammalian species, allometric scaling produces more accurate predictions of echocardiographic dimensions than simple linear regression. Furthermore, BW had a clinically inconsequential (albeit statistically significant) effect on HR and LA:Ao. The only examined variable that BW did not have an impact on was FS%. The prediction formulas and 95% individual prediction intervals did not change substantially when all cats, including those with abnormal echocardiograms, were analyzed compared to when only those with normal echocardiograms were included, indicating that our prediction estimates were robust.
Our estimates for the exponentials in the allometric scaling were, for most variables, between 0.2 and 0.3, which is less than previously reported in dogs and lower than the theoretical value of 0.33,18, 19, 20 indicating that these variables might scale differently in cats than in dogs. However, the majority of cats (95%) in the present study had BW between 2.6 and 7.7 kg, which is a smaller range compared to previously published studies in dogs, in which BW ranged between 3 and 70 kg.18 The comparably narrow weight range means that it is more difficult to identify the exact mathematical formula that is most effective in predicting echocardiographic values. Furthermore, the R 2 for the prediction formulas also were smaller compared to results in dogs, in which R 2 values were reported to range between 0.77 and 0.88.18 Likewise, the R 2 values of the present study generally were lower than previously reported in normal cats, in which linear regression was used to describe the association between echocardiographic dimensions and BW.4, 8, 10 The lower R 2 in the present study could again be, in comparison with studies in dogs, an effect of a comparably narrow weight range, but also the small dimensions that are being measured in cats (down to fractions of a millimeter), which can be regarded as the lower limit of most ultrasound systems in the clinical setting. In comparison with previous studies in cats, the present study included a much larger number of cats with larger variation in size and body composition, examined by multiple observers and using different ultrasound systems, factors that are likely to affect the variation.
Normal 95% prediction intervals can be established in many different ways. The most common way in veterinary cardiovascular medicine is to study a population of presumably normal individuals and calculate a reference interval based on the standard deviation of the (presumably normally distributed) study population. However, this approach ignores some of the variation that exists in the population because all presumably abnormal individuals, and potentially a small proportion of normal individuals that are deemed abnormal before analysis, are excluded. Furthermore, it necessarily classifies some of the included normal individuals (usually 2.5% at each end of the distribution) as abnormal, even though they are healthy. It also requires accurate phenotypic assignation of individuals as normal or diseased and ignores the possibility of occult disease. That is, subjects are classified as normal on criteria other than those being evaluated (such as history of disease and physical examination findings), which might not be present in subjects with occult disease. Another approach is to perform discriminant or receiver operating characteristic (ROC) analyses to establish optimal cutoff values between normal and abnormal findings,35, 36 but these methods might be of limited use because they require a classification of true‐positive and true‐negative individuals (with regard to disease status) and doing so often is not possible in the clinical setting. A third method is to study the entire population and compare the estimates to those obtained in a similar analysis including a subset of presumably normal individuals. This method has the benefit of being independent of the classification of the cat, because all cats, regardless of clinical status, are included in the analysis. Because of the difficulty in accurately classifying cats as normal or abnormal, and because these differentiations often are based on the very echocardiographic criteria being examined (creating a circular argument), we chose to use this last approach. Our results indicate that estimates of prediction formulas and 95% individual prediction intervals were not substantially different between the entire population and the group of cats with normal echocardiograms. Only slightly higher values were obtained for IVSd, IVSs, LVFWd, LVFWs, and LA:Ao when all cats were included compared to only those with normal echocardiograms. This finding indicates that the prediction formulas and 95% prediction intervals were comparably robust.
Identification of cats with echocardiographic measurements that fall outside of the normal 95% prediction interval may help the clinician establish a diagnosis, but it is generally recommended that the diagnosis of HCM not be made based on 1 or a few measurements that fall slightly outside of the normal reference interval.26 Furthermore, investigators have demonstrated that the diagnosis of LV hypertrophy is method dependent, resulting in substantial differences of prevalence of LV hypertrophy depending on the method chosen.16 Our study was not aimed at characterizing features of HCM or any other cardiac disease. Rather, it was aimed at investigating the impact of BW on echocardiographic measurements and establishing prediction formulas and 95% prediction intervals for these variables. The results might help clinicians identify cats with abnormal measurements, but clinicians should recognize that a cat might have ≥1 abnormal echocardiographic measurements and be free of cardiac disease, because substantial anatomic variation exists in normal cats, and data acquisition may have been suboptimal.26 Furthermore, there is a distinct difference between an echocardiographic measurement outside of the reference interval and a clinically relevant abnormal measurement. For example, in dogs, it has been suggested in different studies investigating prognostic variables in myxomatous mitral valve disease that a LA:Ao >1.7 is prognostic for survival.37 The reference threshold for LA:Ao in dogs is generally considered to be <1.5. Thus, a dog might have an abnormal LA:Ao, but the abnormal value might be inconsequential. In cats diagnosed with HCM, presence of extreme hypertrophy (LV diastolic wall thickness >9 mm) has been shown to have prognostic value.38 This degree of LV hypertrophy is well above 6‐mm wall thickness, a value that has been suggested to identify cats with LV hypertrophy and HCM.13 This cutoff of 6 mm is higher than the upper 95% prediction interval of 5.4 mm in our study for cats weighing <6 kg, which in the present study population would include approximately 85% of the cats. Furthermore, the 6‐mm cutoff would only apply to cats weighing >8 kg. All cats <8 kg had upper limits <5.5 mm, and cats weighing <5 kg had upper limits <5 mm.
The present study identified a small but significant effect of BW on HR and LA:Ao. With large sample populations, statistically significant associations can be found, but they can be so weak that they lack clinical relevance. The R 2 and the estimates of the constants in prediction formulas are more informative than the P‐values in this setting because they provide information on strength of association and the impact of 1 variable on another. With the prediction formulas, BW explained 1% of the change in HR. Similarly, BW explained ≤1% of changes in LA:Ao (Table 2). The prediction formulas only suggest a decrease of 2.56 beats/min per kilogram or an increase in LA:Ao of 0.01 per kilogram increase in BW—changes that clearly lack clinical relevance. Thus, clinicians can consider LA:Ao a weight‐independent measure of LA size and an LA:Ao <1.5 to be normal for all cats (Table 3). Conversely, absolute LA dimensions vary sufficiently with BW that weight‐based thresholds should be considered (Table 3). An inverse relationship to BW previously has been described for HR in cats, but in a comparably small and homogenous population.1 Such an association also has been shown in a large population of dogs,39 in which the HR decreased by 0.23 beats/min per kilogram increase in BW, which is considerably lower than that found in the included cats of the present study. To the authors’ knowledge, the the LA:Ao has not previously been shown to be influenced by BW in cats.
The present study has some limitations. Body condition score was not included in the screen protocol, which means that degree of obesity could not be assessed. Furthermore, the examinations and echocardiograms were obtained by many examiners using different scales (for measuring BW) and many different ultrasound systems in pure‐bred cats of many different breeds. Like any other method, echocardiography is associated with inter‐ and intra‐observer variation. Several other studies have included only 1 or a few examiners at 1 center using 1 or a few different ultrasound systems, which is likely to produce smaller variation of the constants in the prediction formula and a more narrow 95% individual prediction interval. The variation and the prediction intervals are likely to be larger in our study, but at the same time, our study has a higher overall validity in the general pure‐bred cat population, because the estimates are based on measurements made by many examiners in cats of many different breeds. Thus, it takes into account many sources of variation. Therefore, the estimates in the prediction formulas and 95% individual prediction intervals are likely to be more conservative (i.e, wider) in the present study.
Although the age range was comparably wide in our study, the age distribution was skewed toward comparably young cats, approximately 40% were between 2 and 3 years and 18% were between 3 and 4 years old, which may have influenced the results of our study. Older cats also are more likely to be affected by primary noncardiac disease that may influence echocardiographic measurements, which in turn might have increased the overall variation.
The associations between BW and echocardiographic variables were modeled by linear regression (HR, LA:Ao, FS%) and allometric scaling (all other echocardiographic variables). Other methods of modeling may have led to slightly different estimates and 95% CI,40, 41 although the heat plots in Figure 1 indicate that the distribution of measurements fall inside the 95% CI and that the line of fit crosses in the middle of the area with highest density of observations. Furthermore, the diagnostic criteria were based on an assumed association between body size and echocardiographic dimensions, and because of this, the criteria for identification of hypertrophy varied depending on body size. The use of a priori definitions of hypertrophy may have constrained prediction intervals, but the difference in estimates and CI were small when including all versus including only normal cats. Given the number of persons responsible for entering information into the screening reports and database, some inaccuracy of recording is inevitable. However, any error in data entry was likely random rather than systematic and therefore not likely to result in bias. It is also possible that some apparently healthy cats were in fact suffering from undiagnosed noncardiac disease. However, this misclassification would have decreased the probability of detecting associations or differences between groups and thus does not diminish the clinical relevance of the findings of our study.
Conclusions
Body weight has a clinically relevant effect on echocardiographic dimensions in cats, and this effect must be taken into account when determining normal prediction intervals. The association between BW and echocardiographic dimensions was best described by allometric scaling. Body weight‐based 95% prediction intervals determined in the present study may help in screening cats for heart disease.
Acknowledgments
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnote
JMP Pro v 11.2 (SAS Inc, Cary NC)
References
- 1. Jacobs G, Knight DH. M‐mode echocardiographic measurements in nonanesthetized healthy cats: Effects of body weight, heart rate, and other variables. Am J Vet Res 1985;46:1705–1711. [PubMed] [Google Scholar]
- 2. Moise NS, Dietze AE, Mezza LE, et al. Echocardiography, electrocardiography, and radiography of cats with dilatation cardiomyopathy, hypertrophic cardiomyopathy, and hyperthyroidism. Am J Vet Res 1986;47:1476–1486. [PubMed] [Google Scholar]
- 3. Pipers FS, Reef V, Hamlin RL. Echocardiography in the domestic cat. Am J Vet Res 1979;40:882–886. [PubMed] [Google Scholar]
- 4. Sisson DD, Knight DH, Helinski C, et al. Plasma taurine concentrations and M‐mode echocardiographic measures in healthy cats and in cats with dilated cardiomyopathy. J Vet Intern Med 1991;5:232–238. [DOI] [PubMed] [Google Scholar]
- 5. Chetboul V, Petit A, Gouni V, et al. Prospective echocardiographic and tissue Doppler screening of a large Sphynx cat population: Reference ranges, heart disease prevalence and genetic aspects. J Vet Cardiol 2012;14:497–509. [DOI] [PubMed] [Google Scholar]
- 6. Drourr L, Lefbom BK, Rosenthal SL, et al. Measurement of M‐mode echocardiographic parameters in healthy adult Maine Coon cats. J Am Vet Med Assoc 2005;226:734–737. [DOI] [PubMed] [Google Scholar]
- 7. Granström S, Godiksen MT, Christiansen M, et al. Prevalence of hypertrophic cardiomyopathy in a cohort of British Shorthair cats in Denmark. J Vet Intern Med 2011;25:866–871. [DOI] [PubMed] [Google Scholar]
- 8. Gundler S, Tidholm A, Häggström J. Prevalence of myocardial hypertrophy in a population of asymptomatic Swedish Maine coon cats. Acta Vet Scand 2008;50:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abbott JA. Feline hypertrophic cardiomyopathy: An update. Vet Clin North Am Small Anim Pract 2010;40:685–700. [DOI] [PubMed] [Google Scholar]
- 10. Petric AD, Rishniw M, Thomas WP. Two‐dimensionally‐guided M‐mode and pulsed wave Doppler echocardiographic evaluation of the ventricles of apparently healthy cats. J Vet Cardiol 2012;14:423–430. [DOI] [PubMed] [Google Scholar]
- 11. Scansen BA, Morgan KL. Reference intervals and allometric scaling of echocardiographic measurements in Bengal cats. J Vet Cardiol 2015;17:S282–S295. [DOI] [PubMed] [Google Scholar]
- 12. Stepien RL. Specific feline cardiopulmonary conditions In: Fuentes VL, Swift S, eds. Manual of Small Animal Cardiorespiratory Medicine and Surgery. Cheltenham: British Small Animal Veterinary Association; 1998:254–257. [Google Scholar]
- 13. Fox PR, Liu SK, Maron BJ. Echocardiographic assessment of spontaneously occurring feline hypertrophic cardiomyopathy. An animal model of human disease. Circulation 1995;92:2645–2651. [DOI] [PubMed] [Google Scholar]
- 14. Paige CF, Abbott JA, Elvinger F, et al. Prevalence of cardiomyopathy in apparently healthy cats. J Am Vet Med Assoc 2009;234:1398–1403. [DOI] [PubMed] [Google Scholar]
- 15. Schober KE, Maerz I, Ludewig E, et al. Diagnostic accuracy of electrocardiography and thoracic radiography in the assessment of left atrial size in cats: Comparison with transthoracic 2‐dimensional echocardiography. J Vet Intern Med 2007;21:709–718. [DOI] [PubMed] [Google Scholar]
- 16. Wagner T, Fuentes V, Payne J, et al. Comparison of auscultatory and echocardiographic findings in healthy adult cats. J Vet Cardiol 2010;12:171–182. [DOI] [PubMed] [Google Scholar]
- 17. Kienzle E, Moik K. A pilot study of the body weight of pure‐bred client‐owned adult cats. British J Nutrit 2011;106(Suppl 1):S113–S115. [DOI] [PubMed] [Google Scholar]
- 18. Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med 2004;18:311–321. [DOI] [PubMed] [Google Scholar]
- 19. Lambert R, Tessier G. Theorie de la similitude biologique. Ann Physicochim Biol 1927;3:212–246. [Google Scholar]
- 20. Li JKJ. Scaling and invariants in cardiovascular biology In: Brown JH, West GB, eds. Scaling in Biology. West Oxford: Oxford University Press; 2000:113–128. [Google Scholar]
- 21. Sisson D, Schaeffer D. Changes in linear dimensions of the heart, relative to body weight, as measured by M‐mode echocardiography in growing dogs. Am J Vet Res 1991;52:1591–1596. [PubMed] [Google Scholar]
- 22. Voogd PJ, Rijsterborgh H, Van Zwieten G, et al. Percentiles of echocardiographic dimensions in healthy children and young adolescents In: Lancee CT, ed. Echocardiology. Proceedings of the Third Symposium on Echocardiology. The Hague, The Netherlands: Martinus Nijhoff Publishers; 1979:299–307. [Google Scholar]
- 23. Korcarz CE, Padrid PA, Shroff SG, et al. Doppler echocardiographic reference values for healthy rhesus monkeys under ketamine hydrochloride sedation. J Med Primatol 1997;26:287–298. [DOI] [PubMed] [Google Scholar]
- 24. Abbott JA. Heart rate and heart rate variability of healthy cats in home and hospital environments. J Feline Med Surg 2005;7:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hanås S, Tidholm A, Egenvall A, et al. Twenty‐four hour Holter monitoring of unsedated healthy cats in the home environment. J Vet Cardiol 2009;11:17–22. [DOI] [PubMed] [Google Scholar]
- 26. Häggström J, Fuentes VL, Wess G. Screening for hypertrophic cardiomyopathy in cats. J Vet Cardiol. 2015;17 Suppl 1:S134–49. In press. [DOI] [PubMed] [Google Scholar]
- 27. Marz I, Wilkie LJ, Harrington N, et al. Familial cardiomyopathy in Norwegian Forest cats. J Feline Med Surg 2014; J Vet Cardiol 2016; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abbott JA, MacLean HN. Two‐dimensional echocardiographic assessment of the feline left atrium. J Vet Intern Med 2006;20:111–119. [DOI] [PubMed] [Google Scholar]
- 29. Fox PR, Basso C, Thiene G, et al. Spontaneously occurring restrictive nonhypertrophied cardiomyopathy in domestic cats: A new animal model of human disease. Cardiovasc Pathol 2014;23:28–34. [DOI] [PubMed] [Google Scholar]
- 30. Sahn DJ, DeMaria AN, Kisslo JA, et al. Recommendations regarding quantitation in M‐mode echocardiography: Results of a survey of echocardiographic measurements. Circulation 1978;58:1072–1083. [DOI] [PubMed] [Google Scholar]
- 31. Hansson K, Haggstrom J, Kvart C, et al. Left atrial to aortic root indices using two‐dimensional and M‐mode echocardiography in cavalier King Charles spaniels with and without left atrial enlargement. Vet Radiol Ultrasound 2002;43:568–575. [DOI] [PubMed] [Google Scholar]
- 32. Schober KE, Maerz I. Assessment of left atrial appendage flow velocity and its relation to spontaneous echocardiographic contrast in 89 cats with myocardial disease. J Vet Intern Med 2006;20:120–130. [DOI] [PubMed] [Google Scholar]
- 33. Snedecor GW, Cochran WG. Statistical Methods, 6th ed Ames, Iowa: Iowa State University Press; 1967. [Google Scholar]
- 34. Kayar A, Ozkan C, Iskefli O, et al. Measurement of M‐mode echocardiographic parameters in healthy adult Van cats. Japan J Vet Res 2014;62:5–15. [PubMed] [Google Scholar]
- 35. Friedman JH. Regularized discriminant analysis. J Am Stat Assoc 1989;84:165–175. [Google Scholar]
- 36. Akobeng AK. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr 2007;96:644–647. [DOI] [PubMed] [Google Scholar]
- 37. Borgarelli M, Savarino P, Crosara S, et al. Survival characteristics and prognostic variables in canine mitral regurgitation attributable to myxomatous valve disease in dogs. Vet Intern Med 2008;22:120–128. [DOI] [PubMed] [Google Scholar]
- 38. Payne JR, Borgeat K, Connolly DJ, et al. Prognostic indicators in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2013;27:1427–1436. [DOI] [PubMed] [Google Scholar]
- 39. Hezzell MJ, Dennis SG, Humm K, et al. Relationships between heart rate and age, bodyweight and breed in 10,849 dogs. J Small Anim Pract 2013;54:318–324. [DOI] [PubMed] [Google Scholar]
- 40. Ebert TA, Russell MP. Allometry and Model II non‐linear regression. J Theor Biol 1994;168:367–372. [Google Scholar]
- 41. Motulsky HJ, Ransnas LA. Fitting curves to data using nonlinear regression: A practical and nonmathematical review. FASEB J 1987;1:365–374. [PubMed] [Google Scholar]


