Abstract
Background
Posaconazole is the most active available azole antifungal drug, but absorption and pharmacokinetics are not available to guide dosing regimens in cats.
Objective
To determine the pharmacokinetics of posaconazole in cats given an IV solution and PO suspension.
Animals
Six healthy, adult research cats.
Methods
After a 12‐hour fast, each cat received 15 mg/kg of posaconazole PO suspension with food. Four cats also received 3 mg/kg IV posaconazole after a 7‐day washout period. Plasma was collected at predetermined intervals for analysis using high‐pressure liquid chromatography (HPLC). Concentration data were analyzed using a 2‐compartment pharmacokinetic analysis for IV administration data and a 1‐compartment analysis with first‐order input for PO administration data using Phoenix® software.5
Results
After IV dosing, volume of distribution (V SS) was 1.9 (0.3) L/kg (mean, standard deviation), terminal half‐life (T ½) was 57.7 (28.4) hours, and clearance was 28.1 (17.3) mL/kg/h. After PO dosing, peak concentration (C MAX) was 1.2 (0.5) μg/mL and T ½ was 38.1 (15.0) hours. Bioavailability of PO suspension was 15.9% (8.6). No adverse effects were observed with either route of administration.
Conclusion and Clinical Importance
Despite low PO absorption, these data allow for simulation of PO dosage regimens that could be explored in clinical studies. Two regimens can be considered to maintain targeted trough concentrations of 0.5–0.7 μg/mL as extrapolated from studies in humans: (1) 30 mg/kg PO loading dose followed by 15 mg/kg q48h, or (2) 15 mg/kg PO loading dose followed by 7.5 mg/kg q24h.
Keywords: Bioavailability, Blastomyces, Fungal infection, Histoplasma
Abbreviations
- AUC
area under the concentration‐time curve
- CMAX
peak concentration
- F
fraction of the drug absorbed
- HPLC
high‐pressure liquid chromatography
- LOC
limit of quantification
- QC
quality control
- T1/2
terminal half‐life
- TMAX
time to maximum concentration
- VSS
apparent volume of distribution at steady state
Systemic fungal infections can be life‐threatening in cats, and treatment can be difficult because of frequent dosing and owner compliance.1, 2 Use of other antifungal agents PO in cats is limited by ocular and neurological adverse events (voriconazole), liver injury (itraconazole), and variable PO absorption (itraconazole).3, 4, 5 Posaconazole, a triazole antifungal, represents a new azole with promising activity and pharmacokinetics. It has been shown previously to have the widest spectrum of action of any currently available antifungal agent.6 In people, posaconazole has been shown to have a higher safety margin than other antifungal drugs.7 In dogs, the half‐life of posaconazole, when given with food, was 24 hours for the PO suspension and 42 hours for the delayed‐release tablet, which would allow maintenance of therapeutic blood concentrations with every‐other‐day dosing.8
The purpose of our study, therefore, was to determine the pharmacokinetics of posaconazole in cats after receiving single doses of PO suspension and IV formulation and to use this information in dosing simulations to develop PO dosage regimens for exploration in future clinical studies.
Materials and Methods
Study Population
The research protocol was approved by the University of Tennessee Institutional Animal Care and Use Committee. Six research cats, aged 7–10 years (mean, 8.6 years) with body weights between 3.3 and 6.5 kg (mean, 4.7 kg), were studied. There were 4 castrated males and 2 spayed females. Complete physical examination and routine laboratory testing including a CBC and serum biochemistries were performed on each cat to ensure health before the study.
Experimental Protocol
Food was withheld from all cats for 12 hours before posaconazole administration. A pilot study that involved 2 of the cats was done to optimize starting PO dosage and timing of sample collection. The initial PO posaconazole dosage was 6 mg/kg, based on dosing information in people and dogs. Plasma samples were collected at predetermined intervals for 144 hours. A 30‐day washout period occurred between the initial and the subsequent trials.
After analysis of results from the initial 2 cats, the final PO dosage selected for investigation was 15 mg/kg of posaconazole1 suspension (40 mg/mL). This dose was given with a syringe immediately after a small meal2 (approximately 30 mL canned food) of the cats' regular diet. Food was reintroduced to all cats 4 hours after drug administration.
Four cats also received 3 mg/kg posaconazole3 IV after a 7‐day washout period. The IV dose was prepared according to the manufacturer's label instructions by diluting posaconazole solution (18 mg/mL) in saline (0.9% NaCl) at a ratio of 1 : 9. This solution was immediately infused over a 5‐minute period using a separate cephalic catheter that was not used for blood collection.
Blood Collection
Twelve to 18 hours before administration of the PO formulation of posaconazole, IV jugular, or femoral catheters were aseptically placed after sedation with dexmedetomidine (40 μg/kg IM), ketamine (5 mg/kg IM), and butorphanol (0.4 mg/kg IM). Peripheral cephalic catheters also were placed in cats receiving the IV formulation. Catheters were secured with bandages and irrigated with sterile saline solution to maintain catheter patency. For the higher dosage PO study and the IV administration study, catheters were maintained for 11 days to allow both studies to be completed without catheter replacement. Catheters were irrigated with heparinized saline solution for the 3 days between studies. Blood (1–2 mL) was collected at predetermined intervals for assessment of posaconazole concentrations after PO (0, 0.5, 1, 2, 4, 8, 10, 12, 16, 24, 32, 48, and 72 hours) and IV (0, 0.5, 1, 2, 4, 6, 10, 12, 16, 24, 48, and 56 hours) administration. Samples were transferred into glass tubes containing lithium heparin, immediately placed on ice, and subsequently centrifuged in batches. The plasma was separated and stored in plastic cryovials at −70 to −80°C. Plasma samples were shipped frozen on ice to the North Carolina State University Clinical Pharmacology Laboratory for posaconazole concentration determinations.
Posaconazole Assay
Quantitative determination of posaconazole in feline plasma samples was performed by high‐pressure liquid chromatography (HPLC) as previously described, with slight modifications.8 All experimental plasma samples, quality control (QC) samples, calibration samples, and blank (control) plasma samples were prepared identically. A reference standard of posaconazole4 (99.9% pure) was dissolved in 100% methanol to prepare a stock solution. From this stock solution, further dilutions were made to use as fortifying solutions for plasma in order to generate calibration curves and QC standards in feline plasma. The stock solution was kept at 4°C in a tightly sealed, dark vial, which was determined to be stable throughout the duration of the study. The calibration curve for posaconazole consisted of 8 standard solutions that ranged between 0.05 and 10 μg/mL, including a blank (0 μg/mL) sample. Blank (control) plasma samples also were analyzed with each day's run to check for interfering peaks and estimate background noise. All calibration curves were linear with an R 2 value ≥0.99. Limit of quantification (LOQ) for posaconazole in feline plasma was 0.05 μg/mL, which was determined from the lowest point on a linear calibration curve that produced an acceptable signal‐to‐noise ratio and met acceptance criteria of our previously validated assay. Quality control samples were analyzed each day and compared against the calibration curve. The laboratory used guidelines published by the United States Pharmacopeial Convention.9
Pharmacokinetic Analysis
Plasma drug concentrations were plotted on linear and semilogarithmic graphs for analysis and to allow visual assessment of the best model for pharmacokinetic analysis. Analysis of the curves and pharmacokinetic models then was performed by use of a commercial pharmacokinetics program.5 Compartmental analysis of results for both the IV solution and PO suspension was performed by weighting the values by 1/y 2, where y is the predicted concentration at each time. Specific models (eg, 1‐ or 2‐compartment) were determined for best fit on the basis of visual analysis for goodness‐of‐fit and by visual inspection of residual plots. For the IV data, a 2‐compartment model was used with a corresponding equation of:
where A is the y‐axis intercept for the distribution phase of the curve, α is the slope of the distribution phase of the curve, B is the y‐axis intercept for the elimination phase of the curve, and β is the slope of the elimination phase of the curve. The value for T ½ was estimated by use of the following equation: T 1/2 = (ln 0.5)/β. Other compartmental pharmacokinetic parameters were calculated in accordance with equations provided elsewhere.8
For the dose of the PO suspension, a 1‐compartment model with first‐order absorption was used with a corresponding equation of:
where k 01 is the non‐IV absorption rate, assuming first‐order absorption; D is the non‐IV dose; V is the apparent volume of distribution; and, k 10 is the elimination rate constant. Secondary parameters calculated from the model included C MAX, T MAX, area under the curve (AUC), and the respective absorption and terminal half‐lives (T ½). Percent systemic availability for a non‐IV dose was calculated by use of the following equation:
Statistical Analysis
Geometric means and corresponding coefficients of variation (CVs) were calculated and presented in tables.
Results
The PO suspension of posaconazole was readily consumed by all cats, and no adverse effects were noted with either PO or IV administration. Only 4 cats were available for the IV study. One cat removed the saphenous catheter after the PO study and could not be recatheterized, and 1 cat had a PCV of 24% after completion of the PO study and was removed from the study for humane reasons. Because not all cats could be sampled at the same time points, sampling times were staggered to optimize a complete plasma concentration versus time profile.
Mean plasma concentrations (± standard deviation) for all 6 cats given the PO suspension, as well as the 4 cats given the IV solution, were calculated (Fig 1). Pharmacokinetic parameters were calculated for both the PO suspension and the IV solution (Table 1). After the administration of the IV solution, the volume of distribution (Vss) was 1.9 (0.3) L/kg (mean, standard deviation), terminal half‐life was 57.7 (28.4) hours, and clearance was 28.1 (17.3) mL/kg/h. After the administration of the PO suspension, the peak (C MAX) was 1.2 (0.5) μg/mL, T MAX was 18.7 hours, and T ½ was 38.1 (15.0) hours. Bioavailability from the PO dose was 15.9% (8.6).
Figure 1.
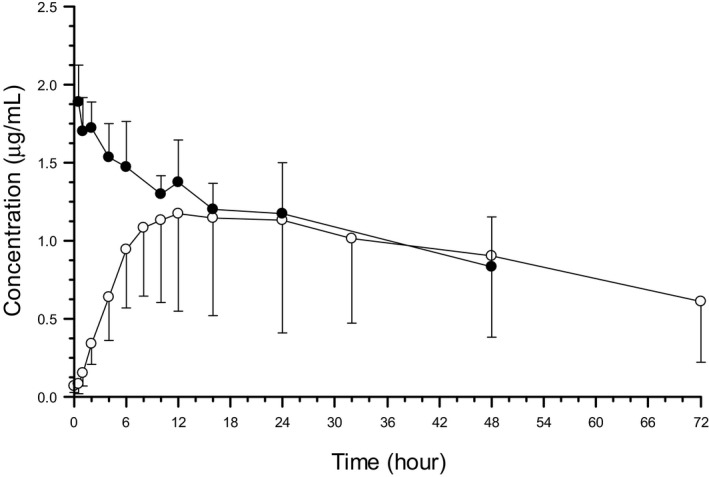
Posaconazole plasma concentrations in cats after administration of an IV solution (3 mg/kg [black circles]; n = 4) and an oral suspension (15 mg/kg [open circles]; n = 6). Time 0 = Time of administration. Values reported are mean + SD concentration after administration of the IV solution and mean – SD concentration after administration of the oral suspensions.
Table 1.
Oral pharmacokinetic values for posaconazole in cats (n = 6) after a dose of 15 mg/kg
| Parameter | Units | Mean | Std. Dev. | CV% |
|---|---|---|---|---|
| AUC | h*μg/mL | 90.05 | 36.64 | 40.69 |
| C MAX | μg/mL | 1.19 | 0.52 | 44.02 |
| k 01 | 1/h | 0.15 | 0.13 | 83.09 |
| k 01 T ½ | hour | 7.30 | 4.80 | 65.85 |
| k 10 | 1/h | 0.02 | 0.01 | 60.04 |
| k 10 T ½ | hour | 38.07 | 15.02 | 39.47 |
| T MAX | hour | 18.70 | 7.32 | 39.13 |
| F% oral | % | 15.91 | 8.64 | 54.33 |
Std. Dev., standard deviation; CV%, coefficient of variation; AUC, area‐under‐the‐curve for plasma concentration versus time profile; C MAX, peak concentration; k 01, absorption rate constant and corresponding half‐life (T ½); k 10, terminal rate constant and corresponding half‐life (T ½); T MAX, time to peak concentration; F, absolute oral absorption (fraction absorbed).
Table 2.
Pharmacokinetic values for posaconazole in cats (n = 4) after an IV dose of 3 mg/kg
| Parameter | Units | Mean | Std. Dev. | CV% |
|---|---|---|---|---|
| A | μg/mL | 0.67 | 0.37 | 54.70 |
| α | 1/h | 2.17 | 1.99 | 91.31 |
| α T ½ | hour | 0.61 | 0.47 | 76.90 |
| AUC | h*μg/mL | 134.43 | 64.68 | 48.12 |
| B | μg/mL | 1.64 | 0.27 | 16.21 |
| β | 1/h | 0.02 | 0.01 | 63.90 |
| β T ½ | hour | 57.74 | 28.42 | 49.22 |
| CL | mL/h/kg | 28.08 | 17.29 | 61.58 |
| k 10 | 1/h | 0.02 | 0.01 | 42.63 |
| k 12 | 1/h | 0.68 | 0.73 | 107.64 |
| k 21 | 1/h | 1.49 | 1.27 | 84.83 |
| MRT | hour | 82.97 | 40.93 | 49.34 |
| V SS | mL/kg | 1855.83 | 298.55 | 16.09 |
Std. Dev., standard deviation; CV%, coefficient of variation; α, distribution rate and accompanying half‐life (T ½); β, elimination rate constant and accompanying half‐life (T ½); k 10, k 12, k 21, microdistribution rate constants; A and B, intercepts for distribution and elimination phases, respectively; CL, systemic clearance; AUC, area‐under‐the‐curve for plasma concentration versus time profile; MRT, mean residence time; V SS, apparent volume of distribution at steady state.
Despite low absorption after PO administration, these data allowed for simulation of PO dosage regimens that could be explored in clinical studies. To maintain targeted trough concentrations of 0.5–0.7 μg/mL as recommended for humans, 2 regimens can be considered for the PO suspension: (1) 30 mg/kg loading dose followed by 15 mg/kg q48h, or (2) a 15 mg/kg loading dose followed by 7.5 mg/kg q24h.10
Discussion
No adverse effects were observed in these healthy cats. The long half‐life (38 hours for the PO suspension) allows achievement of therapeutic concentrations with q24h or q48h administration. Absorption of posaconazole PO suspension was variable in cats, but the variation was within the limits usually observed with PO dose studies in experimental animals (CV% was approximately 40% for AUC, clearance, T ½, and C MAX). Oral absorption of the liquid suspension was only 16%, but still high enough that plasma concentrations could reach therapeutic targets for susceptible fungi. The most effective therapeutic target concentration for cats is undetermined, but administration of posaconazole suspension q48h can be used to attain therapeutic targets that have been recommended for people.10 To maintain targeted trough concentrations of 0.5–0.7 μg/mL, as noted above, 2 regimens can be considered for the PO suspension: (1) a 30 mg/kg loading dose followed by 15 mg/kg q48h, or (2) a 15 mg/kg loading dose followed by 7.5 mg/kg q24h. The ability to maintain target concentrations using every‐other‐day dosing might increase compliance with antifungal treatment for some cats. Whether a once‐daily or every‐other‐day regimen is preferred will depend on patient characteristics and owner preferences. We noted that cats tolerated the taste of this drug much better than other PO antifungal suspensions formulated for people.
Posaconazole is a triazole antifungal that is structurally similar to itraconazole. It is approved for use in human medicine and has been shown to be a potent antifungal agent against clinically important yeast and filamentous fungi.6 Topical forms (otic preparations) are approved for use in dogs. As with itraconazole, posaconazole is best absorbed with food in an acidic environment in people.11 , 6 We assumed that it would also be best absorbed with food in cats and administered with a meal.
Successful treatment with posaconazole has been reported in 2 cats with fungal disease in which other antifungal treatment had failed.12, 13 Those cases involved a Mucor subcutis infection of the nose and invasive orbital aspergillosis. For the cat with aspergillosis, antifungal susceptibility testing for the organism was completed and showed that posaconazole was the most effective treatment. The dosage that was used for both of those cats was 5 mg/kg q24h and treatment duration was 4–5 months.
In human medicine, therapeutic drug monitoring is used to ensure adequate absorption of many antifungal agents.10 For posaconazole, the plasma trough concentration for prophylaxis varies between 0.35 and 0.7 μg/mL and for salvage treatment the concentration is >1 μg/mL.10 Therapeutic plasma drug concentrations of posaconazole have not been determined for cats. We have provided a suggested dosage from this study to consider for future clinical studies, but the safety and efficacy of the dosage regimens described are undetermined in a feline population with naturally occurring disease. Sick cats may absorb the drug differently or have changes in drug clearance not identified by our study of healthy cats. Therefore, clinicians should consider monitoring plasma or serum concentrations of posaconazole. No information is available on the repeated administration of this drug in cats and documenting plasma or serum therapeutic drug concentrations also would provide information on the accumulation, clearance during chronic administration, and adverse effects of this drug in cats.
A delayed release tablet of posaconazole is available and is more bioavailable than the PO suspension in dogs.8 The calculated dosing simulation in that study resulted in a recommended dosage of 5 mg/kg q48h using the extended release tablet in dogs to maintain therapeutic trough plasma concentrations.8 Extended release tablets have not been evaluated in cats, but development of a pediatric PO suspension of extended release granules is underway and should be evaluated in cats. An extended release suspension would be preferred to an extended release tablet in cats because the extended release tablet size is 100 mg and the tablets cannot be divided.
Our study has some limitations. Only 6 cats were involved in the experiment and might not be representative of cats with naturally occurring fungal infections. Only 4 cats were available for the IV phase of the study. Timing of blood collection for the IV phase of the study was staggered among cats to limit sampling‐associated anemia. The study did not identify any immediately apparent clinical adverse effects of posaconazole in these cats. In people, long‐term PO posaconazole use has been associated with gastrointestinal adverse effects (23%) but other treatment‐related adverse events have been reported at a lower rate with posaconazole compared to other azole antifungal agents.14 In our study, serum biochemistry tests were not performed after drug administration to monitor serum liver enzymes activities. With chronic use in people, increased serum alanine transaminase activities only occurred in 3% of cases.14 Because our study only involved a single administration, potential adverse effects from repeated administration of posaconazole require further study.
In conclusion, posaconazole was well‐tolerated by all cats in the study. Despite low oral absorption of posaconazole in cats, plasma drug concentrations that reach therapeutic targets identified for people can be attained.10 These trough concentrations can be maintained in cats using either of 2 regimens for the PO suspension: (1) 30 mg/kg loading dose followed by 15 mg/kg q48h, or (2) a 15 mg/kg loading dose followed by 7.5 mg/kg q24h. The option of every‐other‐day treatment could aid with owner compliance and successful completion of prolonged treatment courses. Evaluation of the proposed dosing regimens using therapeutic drug monitoring in cats with naturally occurring disease is warranted.
Acknowledgments
The authors thank Delta R. Dise, North Carolina State University for performing the drug analysis. The posaconazole oral suspension (40 mg/mL) was a gift from Merck & Co, Inc.
Conflict of Interest Declaration: One of the authors (MGP) has been a consultant for Merck & Co, Inc. and research in his laboratory has been supported by Merck & Co, Inc.
Off‐label Antimicrobial Declaration: This study investigated the pharmacodynamics of posaconazole in cats, which constitutes an off‐label usage of an antimicrobial drug.
All work for this project was performed at the University of Tennessee Veterinary Medical Center, Knoxville, TN and North Carolina State University, Raleigh NC.
Supported in part by the Clinical Pharmacology Laboratory at North Carolina State University and the University of Tennessee, College of Veterinary Medicine Center of Excellence in Livestock Diseases and Human Health Summer Research Program.
Footnotes
Noxafil® oral suspension (40 mg/mL), Patheon Inc, Whitby, ON, Canada
Hill's® Science Diet®. Feline Maintenance, Hill's Pet Nutrition Inc, Topeka, Kansas
Noxafil® injectable (18 mg/mL), Schering‐Plough (Brinny) Co, Brinny, Innishannon, Count Cork, Ireland
Posaconazole analytical reference standard, Sigma‐aldrich Corp, St. Louis, MO
Phoenix® and WinNonlin® software, version 6.3, Pharsight Certara Co, St. Louis, MO
Posaconazole, Noxafil® [package insert]. Whitehouse Station, NJ: Merck & Co Inc, 2014
References
- 1. Legendre AM. Blastomycosis In: Greene CE, ed. Infectious Disease of the Dog and Cat, 4th ed St. Louis: Elsevier Saunders; 2012:606–614. [Google Scholar]
- 2. Brömel C, Greene CE. Histoplasmosis In: Greene CE, ed. Infectious Disease of the Dog and Cat, 4th ed St. Louis: Elsevier Saunders; 2012:614–621. [Google Scholar]
- 3. Vishkautsan P, Papich MG, Thompson G, et al. Pharmacokinetics of voriconazole in healthy cats following intravenous and oral administration. Am J Vet Res 2016;29:1257–1283. [DOI] [PubMed] [Google Scholar]
- 4. Middleton SM, Kubier A, Dirikolu L, et al. Alternate‐day dosing of itraconazole in healthy adult cats. J Vet Pharmacol Therap 2015;39:27–31. [DOI] [PubMed] [Google Scholar]
- 5. Mawby DI, Whittemore JC, Genger S, et al. Bioequivalence of orally administered generic, compounded, and innovator‐formulated itraconazole in healthy dogs. J Vet Intern Med 2014;28:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sabatelli F, Patel R, Mann PA, et al. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob Agents Chemother 2006;50:2009–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Katragkou A, Tsikopoulou F, Roilides E, et al. Posaconazole: When and how? The clinicians view Mycoses 2011;55:110–122. [DOI] [PubMed] [Google Scholar]
- 8. Kendall J, Papich MG. Posaconazole pharmacokinetics after administration of an intravenous solution, oral suspension, and delayed‐release tablet to dogs. Am J Vet Res 2015;76:454–459. [DOI] [PubMed] [Google Scholar]
- 9. United States Pharmacopeia (USP‐NF) . USP 38‐NF 33. General chapter on validation of compendial procedures <1225>. Rockville, Md: United States Pharmacopeial Convention, 2015. [Google Scholar]
- 10. Ashbee HR, Barnes RA, Johnson EM, et al. Therapeutic drug monitoring (TDM) of antifungal agents: Guidelines from the British Society for Medical Mycology. J Antimicrob Chemother 2014;69:1162–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Courtney R, Pai S, Laughlin M, et al. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob Agents Chemother 2003;47:2788–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wray JD, Sparkes AH, Johnson EM. Infection of the subcutis of the nose in a cat caused by Mucor species: Successful treatment using posaconazole. J Fel Med Surg 2008;10:523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McLellan GJ, Aquino SM, Mason DR, et al. Use of posaconazole in the management of invasive orbital aspergillosis in a cat. J Amer Anim Hosp Ass 2006;42:302–307. [DOI] [PubMed] [Google Scholar]
- 14. Raad II, Graybill JR, Bustamante AB, et al. Safety of long‐term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin Infect Dis 2006;42:1726–1734. [DOI] [PubMed] [Google Scholar]


