Abstract
Background
Veterinary literature lacks data about cardiovascular–renal disorders (CvRD) and cardiorenal‐anemia syndrome (CRAS) in dogs.
Hypothesis
A direct correlation exists between ACVIM class and IRIS stage; chronic kidney disease (CKD) complicates chronic mitral valve disease (CMVD) more often than does anemia in dogs.
Animals
One hundred and fifty‐eight client‐owned dogs with CMVD.
Methods
Signalment, physical examination findings, electrocardiography, thoracic radiographs, echocardiography, and blood analysis were retrospectively evaluated to assess the prevalence of CKD and anemia in dogs with CMVD and to investigate the relationships among ACVIM class, IRIS stage, and survival.
Results
The prevalence of CKD and anemia in dogs with CMVD was significantly higher than in the general population of dogs. Dogs being treated for heart failure had a significantly higher prevalence of CKD than did dogs that had not received treatment. A statistically significant direct correlation was found between ACVIM class and IRIS stage. Severe heart disease, severe renal disease or both, furosemide administration, and advanced age at diagnosis of heart disease were associated with shorter survival time. Survival time of dogs affected by CvRD was statistically shorter than survival time of dogs affected by CMVD alone.
Conclusion and Clinical Relevance
Chronic mitral valve disease is associated with increased prevalence of CKD and anemia in dogs. Treatment for medical management of heart failure may play a role in inducing CKD. Class of heart disease and IRIS stage were directly correlated. Cardiovascular–renal disorders decrease survival time compared to the only presence of CMVD alone, whereas anemia does not play a central role in worsening heart function.
Keywords: Anemia, Cardiorenal syndrome, Cardiovascular–renal disorders, Chronic kidney disease, Clinical pathology, Endocardiosis, Heart disease, Hematology
Abbreviations
- AoGmax
aortic peak gradient
- AoVmax
aortic peak velocity
- BSA
body surface area
- BW
body weight
- CBC
complete blood count
- CMVD
chronic mitral valve disease
- CRAS
cardiorenal‐anemia syndrome
- CRS
cardiorenal syndrome
- CRS
cardiorenal syndrome
- CRAS
cardiorenal‐anemia syndrome
- CMVD
chronic mitral valve disease
- CKD
chronic kidney disease
- CvRD
cardiovascular–renal disorders
- d
diastole
- EDVI
end diastolic volume index
- EF%
left ventricular ejection fraction
- ESVI
end systolic volume index
- EVmax
E peak velocity
- FS%
fractional shortening
- Hb
hemoglobin
- HF
heart failure
- HR
heart rate
- Hr
hazard ratio
- Ht
hematocrit
- IVS
interventricular septal thickness
- LA
left atrial diameter
- Ao
aortic root diameter
- LA/Ao
left atrial to aortic root ratio
- LVEDDn
normalized left ventricular end diastolic diameter calculated according to Cornell's method of allometric scaling
- LVID
left ventricular internal diameter
- LVPW
left ventricular posterior wall thickness
- MCH
mean corpuscular hemoglobin
- MCHC
mean corpuscular hemoglobin concentration
- MCV
mean corpuscular volume
- MR
peak velocity of mitral regurgitation
- NTG
no‐treatment group
- RBC
red blood cells
- s
systole
- SBP
systolic blood pressure
- sCr
serum creatinine
- TG
treatment group
- TR
peak velocity of tricuspid regurgitation
- UREA
serum urea
- WBC
white blood cells
Recently, the field of human medicine has been challenged by a dual epidemic of heart failure and renal insufficiency.1 Many hospitalized patients have various degrees of heart and kidney dysfunction. Primary disorders of 1 of these 2 organs often result in secondary dysfunction or injury to the other.2 The presence of the two problems in the same patient is referred as cardiorenal syndrome (CRS), currently defined in dogs and cats as cardiovascular–renal disorders (CvRD) by the CRS Consensus Group.3 Five subtypes of CRS are described in the literature: CRS type 1 (acute CRS), characterized by a rapid worsening of cardiac function, leading to acute kidney injury; CRS type 2 (chronic CRS), characterized by chronic abnormalities in cardiac function causing progressive chronic kidney disease (CKD); CRS type 3 (acute renocardiac syndrome), characterized by an abrupt and primary worsening of kidney function, leading to acute cardiac dysfunction; CRS type 4 (chronic renocardiac syndrome), characterized by a condition of primary CKD contributing to decreased cardiac function, ventricular hypertrophy, diastolic dysfunction, an increased risk of adverse cardiovascular events, or some combination of these; and, CRS type 5 (secondary CRS), characterized by the presence of combined cardiac and renal dysfunction associated with acute or chronic systemic disorders.2 Furthermore, anemia is often associated with heart failure (HF) and renal insufficiency in human medicine.3 Cardiorenal syndrome with anemia forms a triad described as cardiorenal‐anemia syndrome (CRAS). Chronic kidney disease has an estimated prevalence of 45–63% among human patients with chronic heart failure (CHF), whereas little information concerning the prevalence of renal dysfunction in dogs with naturally occurring HF is available.4, 5
The prevalence of azotemia in dogs with heart disease varies, according to the veterinary literature, between 7.4 and 24.1%.1 , 4, 5, 6, 7, 8 The prevalence of anemia in human patients with HF varies between 10 and 49%, as compared to the 8.1–22.8% range reported in dogs with heart disease.1 , 8, 9, 10 The most common heart disease affecting dogs and leading to CHF, is chronic mitral valve disease (CMVD), also known as endocardiosis and myxomatous valve degeneration.11 The aims of our study were to assess the prevalence of CKD associated with azotemia and the prevalence of anemia complicating CMVD in dogs, to evaluate a possible connection between the class of cardiac disease (ACVIM classification) and the class of renal disease (IRIS staging system), to investigate the correlation among the main variable of the IRIS staging system, echocardiographic variables and laboratory variables and evaluate differences in survival time among dogs affected by CMVD, CvRD, and CRAS.
Materials and Methods
Two‐thousand two‐hundred and seventy‐one medical records of dogs presented to the Cardiology Service of the Department of Veterinary Science and Public Health, University of Milan, between January 2003 and December 2012 were evaluated retrospectively.
The inclusion criteria were: dogs with complete clinical examination (including signalment, history, and physical examination), thoracic radiographs, a CMVD diagnosis based on echocardiographic examination (presence of mitral leaflet thickening, prolapse, or both associated with an abnormal mitral regurgitant jet on Doppler color flow imaging) performed by a trained observer, electrocardiogram (ECG) and laboratory test results (CBC and serum biochemistry panel, including serum creatinine [sCr] and serum urea concentrations [UREA]).11, 12 Patients with azotemia must have had complete urine analysis performedto be included. The exclusion criteria were: other heart disease, neoplasm, or systemic diseases such as hyperadrenocorticism and diabetes mellitus. Patients suspected to have prerenal or postrenal azotemia (eg, dehydration supported by CBC and biochemical analysis, concomitant urinary tract obstruction) were excluded. Greyhounds, working dogs, or dogs receiving a high protein diet were excluded.
Dogs were categorized according to ACVIM classification and IRIS staging system and divided into 2 groups: treatment group (TG) and no‐treatment group (NTG) according to the presence or absence of medical management of HF.
Diagnosis of CKD
Diagnosis of CKD was supported by history, clinical examination, imaging, biochemistry, and urinalysis that were performed on the basis of the clinical presentation. Dogs were considered affected by CKD when a history of polyuria and polydipsia was confirmed by low urine specific gravity (<1.020) and at least 1 of the following: fasting sCr, assessed on at least 2 occasions in a stable patient, was above the reference range (sCr <1.4 mg/dL) increased sCr associated with proteinuria and a previous diagnosis of CKD was reported in the history. Fasting ≥8 h was considered adequate.
Echocardiography and ECG
All echocardiographic studies were performed using 2 ultrasound machine, both equipped with 5–7.5 MHz and 2.5–3 MHz, multifrequency phased array transducers.2 , 3 Dogs were consecutively positioned in right and left lateral recumbency. All echocardiographic measurements were made on conscious dogs in accordance with the guidelines of the American Society of Echocardiography using the leading‐edge to leading‐edge method for M‐mode measurements and Hansson's method for the 2‐dimensional (2D) measurements of left atrial (LA) and aortic root (Ao) diameters.13, 14
From the right parasternal short‐axis M‐mode view at the chordae level, the following variables were obtained: interventricular septal thickness (IVS), left ventricular internal diameter (LVID), and left ventricular posterior wall thickness (LVPW) in diastole (d), and systole (s). The following variables were obtained from 2D views: Ao and LA from right parasternal short‐axis view. Mitral valve inflow (E peak velocity – EVmax, E/A ratio), aortic peak velocity (AoVmax) and peak gradient (AoGmax), peak velocity of mitral and tricuspid regurgitations (MR and TR) were evaluated using the color Doppler and the spectral Doppler.15 The following variables were calculated: left atrial‐to‐aortic root ratio (LA/Ao), fractional shortening (FS%), left ventricular ejection fraction (EF%), end systolic volume index (ESVI), end diastolic volume index (EDVI), and normalized left ventricular end diastolic diameter calculated according to Cornell's method of allometric scaling (LVEDDn).16 The EDVI and ESVI were calculated according to the Teichholz formula, normalized to body surface area (BSA). A standard 6‐lead ECG was obtained in awake dogs to assess heart rate and diagnose cardiac arrhythmias.
Hematological and Biochemical Analyses
Data obtained from the CBC (white blood cell count [WBC], red blood cell count [RBC], hemoglobin [Hb], hematocrit [Ht], mean corpuscular volume [MCV], mean corpuscular hemoglobin [MCH], mean corpuscular hemoglobin concentration [MCHC]), UREA, sCr, and blood urea concentration (BUN)/sCr ratio were recorded. Reference intervals for canine UREA and sCr were 20–60 mg/dL and <1.4 mg/dL, respectively. Dogs were grouped according to sCr (www.iris.kidney.com) as azotemic (sCr ≥1.4 mg/dL) and nonazotemic (sCr <1.4 mg/dL); and according to Hb concentration as anemic (Hb <12.9 g/dL) and nonanemic (Hb ≥12.9 g/dL; using laboratory reference intervals).
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics 20 and C5.0. Baseline descriptive statistics were presented as mean and standard deviation for normally distributed continuous variables, whereas non‐normally distributed variables were presented as median and interquartile (IQ) range. Normality of the distribution was tested using nonparametric Kolmogorov‐Smirnov tests. Data were analyzed using Student's t‐tests to investigate differences between sets of data and simple or multiple ordinal regression tests to describe the relationships between dependent and independent variables. Categorical variables were analyzed using Chi‐Square analysis. A P value <.05 was considered significant. The considered dependent variables were ACVIM class, IRIS stage, UREA, and sCr. The independent variables were: all evaluated echocardiographic variables, body weight (BW), body surface area (BSA), heart rate (HR), systolic blood pressure (SBP), murmur grade, treatment versus no‐treatment (based on the administration of furosemide), the total dose of furosemide administered (mg/kg/day), and CBC variables. Using the C5.0 software, we developed decision trees and rules sets with the aim of providing veterinarians clinical guidelines to be followed whenever new patients are presented.4
Investigators or trained senior veterinary students conducted telephone interviews with dog owners or reviewed medical records to determine the clinical outcome of each dog. Information obtained was: was the dog dead or alive, had the dog been euthanized or died spontaneously and reasons for euthanasia. In the case of spontaneous death, the possible causes, including cardiac‐related sudden death, presence of syncope, or progression of HF were determined. Cardiac‐related death was defined as death occurring because of progression of clinical signs of HF. Euthanasia because of refractory HF was scored as cardiac‐related death. Sudden death was regarded as cardiac‐related if no other cause of death was identified.
Survival time was counted from the day of diagnosis of CMVD to either the day of death or closing time of the study (June 1, 2014). The end‐point of the study was cardiac death.
Dogs still alive at the end of the study period and dogs that died of noncardiac disease were right‐censored.
Dogs were sorted into groups (azotemic versus nonazotemic and anemic versus nonanemic) to compare survival times. The Kaplan‐Meier method was used to estimate survival function and plot time to event curves in the different groups; equality of survival distributions was tested by the Log Rank method.
Effects on survival of the following variables at baseline were evaluated: ACVIM class, IRIS stage, presence or absence of anemia, UREA, age at diagnosis, BW, treatment of heart failure (yes/no), LVEDDn, and LA/Ao. Simple and multiple Cox proportional hazard analysis was performed in order to determine the effect of any variable on survival.
Results
Population Characteristics and Descriptive Statistics
Of the 2271 dogs evaluated, 48% had CMVD. Among them, 158 dogs of both sexes (94 males and 64 females) fulfilled the inclusion criteria and were enrolled in the study. Of these, 20% of males and 65% of females were neutered. They were between 5 and 17 years old (median age, 11.56; IQ range, 2.51 years), with BW ranging from 2 to 48 kg (median age, 8.5; IQ range, 8 kg). The most commonly represented breeds were mongrel (42%), miniature Poodle (10%), Yorkshire Terrier (10%), Shih‐Tzu (6%), Doberman Pinscher (5%), and Dachshund (5%). In the group (TG) (including 70.9% of the patients) 64% received furosemide and an angiotensin converting enzyme (ACE) inhibitor (benazepril or enalapril) and 36% received triple treatment with furosemide, an ACE inhibitor, and pimobendan. The ACE inhibitor and pimobendan were administered at standard dosage (benazepril, 0.25–0.5 mg/kg q12h; enalapril, 0.5 mg/kg q12h; pimobendan, 0.25–0.35 mg/kg q12h), whereas furosemide was administered according to the severity of heart disease (3.7 ± 2.5 mg/kg/day). Echocardiographic results are reported in Table 1. Dogs were classified as follow: 20.9% ACVIM B1, 9.5% ACVIM B2, 67.1% ACVIM C, and 2.5% ACVIM D.
Table 1.
Echocardiographic parameters in 158 dogs affected by CMVD
| n | Min | Max | Mean or median | St.dev or IQ range | |
|---|---|---|---|---|---|
| HR (bpm) | 49 | 80 | 240 | 146 | 40 |
| IVSd (mm) | 151 | 3.40 | 18.00 | 7.70 | 2.70 |
| LVIDd (mm) | 151 | 16.60 | 74.70 | 35.70 | 13.10 |
| LVPWd (mm) | 151 | 4.10 | 15.10 | 7.20 | 2.10 |
| IVSs (mm) | 151 | 4.40 | 19.80 | 11.00 | 3.30 |
| LVIDs (mm) | 152 | 7.90 | 53.00 | 19.80 | 8.80 |
| LVPWs (mm) | 151 | 6.50 | 18.60 | 11.20 | 3.90 |
| EF% | 151 | 30.10 | 94.99 | 75.51 | 12.85 |
| FS% | 151 | 14.20 | 68.83 | 42.54 | 8.81 |
| ESVI (mL/m2) | 152 | 3.76 | 182.59 | 28.31 | 20.90 |
| EDVI (mL/m2) | 151 | 24.48 | 549.60 | 119.33 | 69.42 |
| Ao (mm) | 149 | 7.10 | 30.20 | 14.40 | 5.30 |
| LA (mm) | 149 | 11.80 | 66.00 | 29.00 | 12.40 |
| LA/Ao | 149 | 0.75 | 4.85 | 1.87 | 0.75 |
| Evmax (m/s) | 106 | 0.42 | 1.92 | 1.01 | 0.62 |
| E/A | 101 | 0.50 | 2.95 | 1.23 | 0.80 |
| AoVmax (m/s) | 100 | 0.67 | 3.76 | 1.22 | 0.36 |
| AoGmax (mmHg) | 100 | 1.80 | 14.14 | 5.91 | 3.43 |
| MR (m/s) | 123 | 3.08 | 6.86 | 5.38 | 0.69 |
| TR (m/s) | 80 | 1.31 | 5.09 | 2.75 | 0.98 |
Heart Rate (HR), Interventricular septal thickness (IVS), left ventricular internal diameter (LVID), and left ventricular posterior wall thickness (LVPW) in diastole (d) and systole (s). Fractional shortening (FS%), left ventricular ejection fraction (EF%), Endsystolic volume index (ESVI), Enddiastolic volume index (EDVI). Aortic root diameter (Ao), left atrial diameter (LA), left atrial to aortic root ratio (LA/Ao). Mitral valve inflow (E peak velocity—EVmax, E/A ratio), aortic peak velocity (AoVmax) and peak gradient (AoGmax), peak velocity of mitral and tricuspid regurgitations (MR and TR). Data have been ordered as commonly stored during echocardiography.
Among dogs with CHF, 74.7% were nonazotemic and 25.3% were azotemic (12.6% in IRIS stage 2, 11.4% in IRIS stage 3, and 1.3% in IRIS stage 4). The nonazotemic group included: 87.9% of ACVIM B1 dogs, 100% of ACVIM B2 dogs, 67.9% of ACVIM C dogs, and 50% of ACVIM D dogs (Fig 1). The nonazotemic group represented more often than the azotemic group in all ACVIM classes, but the frequency of IRIS stage 2 and 3 was higher in ACVIM classes C and D. The prevalence of CKD associated with azotemia in dogs with CMVD was 25%. The prevalence of CKD in symptomatic dogs (ACVIM classes C and D) was 32%.
Figure 1.
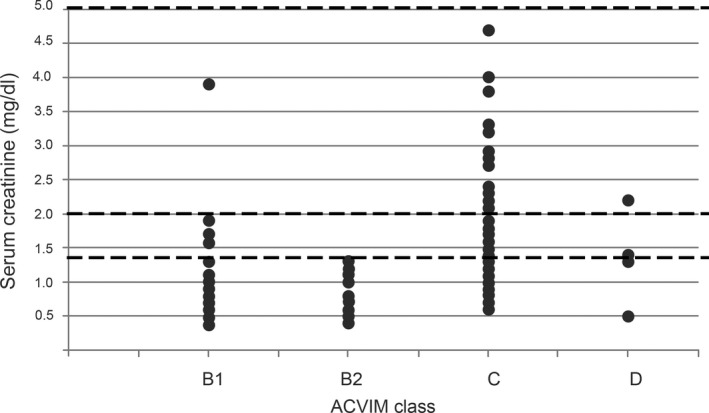
Distribution of serum creatinine concentrations observed in 158 dogs affected by CMVD according to ACVIM classification. The dotted lines represent the IRIS staging system cutoff values of 1.4 mg/dL, 2 mg/dL, and 5 mg/dL. The nonazotemic group (sCr < 1.4 mg/dL) was more represented than the azotemic group (sCr ≥ 1.4 mg/dL) in all the ACVIM classes. Number of dogs in each ACVIM class: 33 dogs in B1, 15 dogs in B2, 104 dogs in C, 4 dogs in D. Dogs in IRIS stage 4 were censored to better display data (2 dogs censored).
The prevalence of CKD was significantly higher in the TG than in the no‐treatment group NTG (32% versus 1%, P < .001). The mean sCr and UREA concentrations were significantly higher in the TG than in the NTG (sCr mean, 1.5 mg/dL versus 1.0 mg/dL, P < .001; UREA mean, 83.8 mg/dL versus 58.7 mg/dL, P = .006). The prevalence of anemia was 15%. There were no differences among the prevalence of anemia in different ACVIM classes and no difference between the TG and the NTG. The prevalence of anemia was significantly higher in the azotemic group than in the nonazotemic group (40% vs 10%, P = .04) and higher in advanced IRIS stage (37% IRIS 3 vs 17% IRIS 2, P = .02).
Inferential Statistics
The statistically significant correlations between the considered variables are reported in Table 2. Simple ordinal regression showed that an increase in IRIS stage led to an increase in the probability of being in a higher ACVIM class (odds ratio [OR], 2.5, 95%; confidence interval [CI], 1.4, 4.5; proportional odds assumption met), and that an increase in ACVIM class led to an increase in the probability of being in a higher IRIS stage.
Table 2.
Statistically significant correlations between renal function indicators and other variables
| Correlation | Spearmann's coefficient | P value |
|---|---|---|
| UREA‐Furosemide (mg/kg/day) | 0.31 | <.001 |
| UREA‐IRIS | 0.55 | <.001 |
| UREA‐sCr | 0.57 | <.001 |
| UREA Treatment versus NO‐Treatment | 0.18 | .02 |
| UREA‐ACVIM | 0.19 | .02 |
| UREA‐SBP | 0.59 | .06 |
| UREA‐WBC | 0.15 | .07 |
| sCr‐ACVIM | 0.29 | <.001 |
| sCr Treatment versus NO‐Treatment | 0.31 | <.001 |
| sCr‐Murmur Grade | 0.23 | .01 |
| sCr‐WBC | 0.18 | .04 |
| sCr‐SBP | 0.52 | .10 |
| sCr‐Hb | −0.11 | .21 |
| sCr‐MCHC | −0.08 | .36 |
| ACVIM‐BW | −0.23 | <.001 |
| ACVIM‐Furosemide (mg/kg/day) | 0.28 | <.001 |
| ACVIM‐ESVI | 0.37 | <.001 |
| ACVIM‐E/A | 0.42 | <.001 |
| ACVIM‐Murmur Grade | 0.46 | <.001 |
| ACVIM‐EDVI | 0.56 | <.001 |
| ACVIM‐ LVEDDn | 0.57 | <.001 |
| ACVIM‐Evmax | 0.62 | <.001 |
| ACVIM‐LA/Ao | 0.66 | <.001 |
| ACVIM Treatment versus NO‐Treatment | 0.95 | <.001 |
| ACVIM‐MR | −0.25 | .01 |
| ACVIM‐HR | 0.37 | .01 |
| ACVIM‐TR | 0.27 | .02 |
| IRIS Treatment versus NO‐Treatment | 0.26 | <.001 |
| IRIS‐LA/Ao | 0.23 | .01 |
| IRIS‐LVEDDn | 0.21 | .01 |
| IRIS‐HR | 0.32 | .02 |
| IRIS‐WBC | 0.15 | .07 |
| IRIS‐Hb | −0.09 | .27 |
Serum urea (UREA), serum creatinine (sCr), body weight (BW), heart rate (HR), systolic blood pressure (SBP), white blood cells (WBC), hemoglobin (Hb), mean corpuscular hemoglobin concentration (MCHC), End systolic volume index (ESVI), End diastolic volume index (EDVI), normalized left ventricular end diastolic diameter (LVEDDn), left atrial to aortic root ratio (LA/Ao), mitral valve inflow (E peak velocity—EVmax, E/A ratio), peak velocity of mitral and tricuspid regurgitations (MR and TR).
Survival Analysis
At the end of the follow‐up period, 45 dogs (28%) died or were euthanized because of refractory cardiac disease, 48 dogs (30%) died of noncardiac causes, 42 dogs (26%) were still alive, and 23 (14%) dogs were lost at follow‐up. Median time to death for dogs with cardiac death was 12 months (IQ range, 23.5 months) and median time to death for noncardiac death was 11 months (IQ range, 24.5 months). Of the 9 variables used as predictors in the univariate analysis, LA/Ao, (Hazard ratio [Hr], 3.26; CI, 1.2–5.3; P = <.001), age at diagnosis (Hr, 1.22; CI, 1.06–1.4; P = .005), IRIS stage (Hr, 1.57; CI, 1.1–2.2; P = .01), LVEDDn (Hr, 3.01; CI, 1.57–5.8; P = .001), furosemide versus no‐treatment (Hr, 2.87; CI, 1.33–6.2; P = .007), ACVIM class (Hr, 2.03; CI, 1.31–3.16; P = .002), and azotemic status (Hr, 2.56; CI, 1.36–4.8; P = .004) were associated with worse outcomes. Results of the univariate Cox proportional hazard regressions are shown in Table 3.
Table 3.
Univariate Cox regressions results
| Sig. | Hazard ratio | 95% CI for Exp(B) | ||
|---|---|---|---|---|
| Lower | Upper | |||
| LA/Ao* | <0.001 | 3.259 | 1.995 | 5.324 |
| LVEDDn | 0.001 | 3.015 | 1.569 | 5.795 |
| Treatment versus NO‐Treatment | 0.007 | 2.87 | 1.328 | 6.203 |
| AZOTEMIC | 0.004 | 2.559 | 1.358 | 4.821 |
| ACVIM | 0.002 | 2.033 | 1.307 | 3.162 |
| IRIS | 0.01 | 1.571 | 1.115 | 2.212 |
| Age at Diagnosis* | 0.005 | 1.223 | 1.064 | 1.405 |
| UREA | 0.073 | 1.004 | 1 | 1.007 |
| ANEMIA | 0.651 | 0.759 | 0.23 | 2.504 |
We checked for the proportionality of hazards assumption by plotting LogMinusLog curves (for ordinal variables) and Schoenfeld residuals (for scalar variables). For those variables (marked with *) which did not meet the assumption, the hazard ratio has to be considered only a pooled value.
The presence of increased LA/Ao (Hr, 3.38; CI, 1.50–7.65; P = .003) and age at diagnosis (Hr, 1.2; CI, 1.02–1.4; P = .024) remained the only significant predictors of poor outcome in the multivariate analysis, even after introducing an interaction term for variables for which the proportional hazard assumption was not met.
Survival time was statistically different between azotemic dogs versus nonazotemic dogs, and between dogs with CvRD and dogs with CMVD, whereas there was no difference in survival time when anemic dogs and nonanemic dogs were compared (Figure 2).
Figure 2.
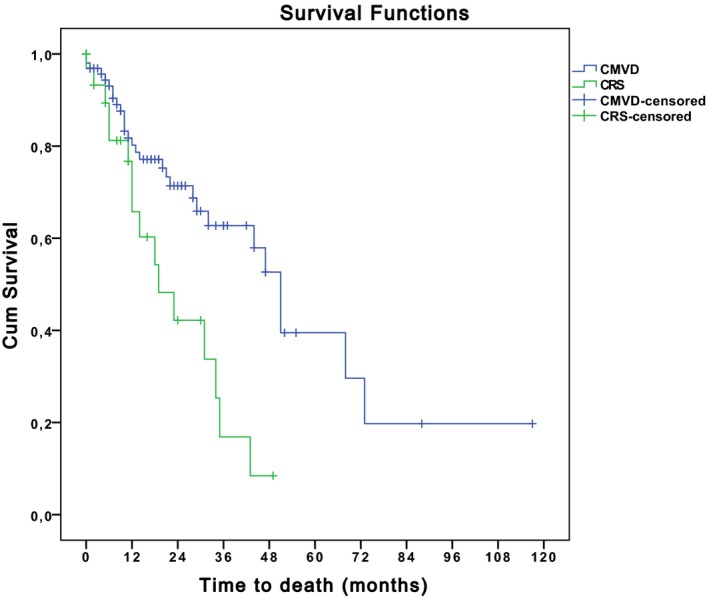
Comparison of survival time between dogs affected by CRS and dogs with CMVD. Blue: dogs affected by CMVD. Green: dogs with CRS. Survival time was statistically different between dogs with CRS versus dogs with CMVD (P = .002). End‐point of the study was cardiac death. Dogs still alive at the end of the study period and dogs that died of noncardiac disease were right‐censored. The Kaplan‐Meier method was used to estimate survival function and plot time to event curves in the different groups and equality of survival distributions was tested by Log Rank.
Discussion
The studied population was representative of the general population of dogs affected by CMVD (older dogs of small and medium size breeds). The prevalence of CKD complicating CMVD in the studied population was significantly higher than the prevalence of CKD in the general population of dogs evaluated at the University of Milan (25% versus 15%, P < .0001; unpublished data) and higher than the prevalence of CKD reported by others (0.05–5.8%).17, 18 The prevalence of CKD in symptomatic dogs was lower than the prevalence reported in human medicine (32% vs 45–63%). The influence of CMVD on the prevalence of CKD suggests an influence of heart disease on renal function. Previously published prevalences of azotemia in dogs with CMVD were 7.4, 24.1, and 14.5%.1 , 7, 19 The differences reported by different investigators could be explained by the different percentages of dogs with decompensated heart failure included in the studies. Unfortunately, differences in the definition of azotemia and HF classifications make it difficult to compare data. The connection between class of cardiac failure and class of kidney disease was confirmed by the statistically significant correlation found between the two variables (P = .003). Advanced ACVIM class could be predictive of advanced IRIS stage and vice versa. This finding, as well as the statistically significant correlation found between ACVIM and both UREA and sCr, is of clinical importance in the management of patients with severe heart disease. Furthermore, the 33% of dogs in congestive HF with concomitant azotemia require specific management.
The IRIS stages directly correlated with the echocardiographic variables of heart enlargement (LA/Ao and LVEDDn) suggesting a direct connection between cardiac remodeling and renal impairment. The ACVIM class showed a statistically significant correlation with the echocardiographic variables of heart enlargement (LA/Ao, EDVI, and LVEDDn), with Doppler variables related to diastolic function (EVmax and E/A), with MR (systolic left ventricle impairment and high LA pressure decrease MR in advanced heart disease), with TR (which is an indirect evaluation of systolic pulmonary arterial pressure that increases with heart failure class), with HR (worsening heart disease induces sympathetic system activation), and with murmur grade, as recently reported in the veterinary literature.20, 21 The direct correlation found between UREA, sCr, and SBP could be explained considering that in dogs affected by CKD, renin‐angiotensin‐aldosterone system (RAAS) activation leads to water and salt retention and increasing SBP.
Despite demographic risk factors previously identified for CKD including small body size, no statistically significant correlation was seen between IRIS stage and BW or BSA.18 This unexpected result suggests that body size does not seem to influence CKD severity, whereas it may influence HF severity.
Treatment seems to influence renal function by increasing UREA, sCr, and IRIS stage even if these results probably are affected by the most severe cardiac condition in dogs requiring medical management of HF (severe heart disease require high furosemide dosage administration, and these 2 conditions are inseparables). The amount of furosemide administered was directly correlated with UREA, but not with IRIS stage and sCr. This finding could be explained by considering that the amount of furosemide administered increases with worsening cardiac function. In situations of compromised renal blood flow, as with advanced cardiac disease, decreased flow rate in the tubules allows more time for urea reabsorption. The increased reabsorption of urea from within the renal tubule leads to UREA increasing disproportionately to sCr.19, 22, 23
Considering that an increase in UREA with a normal sCr is most likely consistent with a prerenal azotemia, the serum BUN/sCr ratio has been suggest to differentiate prerenal and renal azotemia.24 In our study, the BUN/sCr ratio was not correlated with any ACVIM class or IRIS stage. Thus, according to previously published data, the BUN/sCr ratio alone cannot be used to distinguish renal from prerenal azotemia.25 Distinguishing renal from prerenal azotemia still is a challenge in dogs with cardiac disease because of the unreliability, in patients receiving diuretics, of the urine specific gravity.
Hemoglobin was negatively correlated with sCr and IRIS stage, confirming the existence of a link between renal impairment degree and anemia. No correlation was found between anemia and classes of HF, supporting the previous finding of a lack of correlation between anemia and ACVIM classification1. Patients in more advanced ACVIM class were characterized by higher HR, probably because of an increased sympathetic tone. The direct correlation found between HR and IRIS stage could be explained by considering that advanced IRIS stages are correlated with anemia and decreased Hb concentration that induce sympathetic system activation and increase HR.
The presence of azotemic dogs in ACVIM class B1 (12.1%) suggests the existence, in some dogs included in this study, of primary renal damage. This finding highlights the difficulties of distinguishing CRS type 2 from type 4 patients. Furthermore, in some patients renal impairment and CMVD probably coexisted. Regardless of these considerations, the presence of advanced heart disease, advanced renal disease or both, furosemide administration (correlated with advanced ACVIM class) and advanced age at diagnosis of heart disease all decrease survival time. A key point in our results is the difference in life expectancy between the analyzed groups: the survival time of dogs affected by CvRD was statistically lower than the survival time of dogs affected by CMVD alone.
The main limitations of this study relate to its retrospective nature. As commonly reported in human medicine, CRS Type 2 and type 4 patients could not be clearly differentiated. Moreover, to improve the reliability of our study, we applied strict inclusion criteria that decreased sample size. Therefore, small sample size and underrepresentation of ACVIM class B2 could have affected some results. The lack of biomarkers of early renal injury, of urine examination and of abdominal ultrasound examination in all cases probably led to an underestimation of the prevalence of CKD.
Clinical Relevance
The prevalence of CKD and anemia in dogs with CMVD was significantly higher than the prevalence in the general population of dogs. Medical management of HF affects the prevalence of CKD. The ACVIM class and IRIS stage were directly correlated. Cardiovascular–renal disorder decreases survival time compared to the presence of CMVD alone, whereas anemia does not seem to play a central role in worsening heart function. Further investigations are needed to determine if CMVD or administration of drugs for medical management of HF can adversely affect renal function.
Acknowledgments
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Ohad DG, Berkowitz J, Bdolah‐Abram T. Is the cardiorenal‐anemia syndrome prevalent in dogs? (abstr), ACVIM congress 2010. J Vet Intern Med 2010;24:672
Esaote Medical System, Megas Esaote Cvx
Esaotte Medical System, Esaote MyLab50
Release 2.07 GPL Edition
References
- 1. Pokhrel N, Maharjan N, Dhakal B, et al. Cardiorenal syndrome: A literature review. Exp Clin Cardiol 2008;13:165–170. [PMC free article] [PubMed] [Google Scholar]
- 2. Ronco C, Happio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol 2008;52:1527–1539. [DOI] [PubMed] [Google Scholar]
- 3. Pouchelon JL, Atkins CE, Bussadori C, et al. Cardiovascular‐renal axis disorders in the domestic dog and cat: A veterinary consensus statement. J Small Anim Pract 2015;56:537–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nicolle AP, Chetboul V, Allerheiligen T, et al. Azotemia and glomerular filtration rate in dogs with chronic valvular disease. J Vet Intern Med 2007;21:943–949. [DOI] [PubMed] [Google Scholar]
- 5. Ronco C, Maisel A. Volume overload and cardiorenal syndromes. Congest Heart Fail 2010;16(Suppl 1):Si–Siv. [DOI] [PubMed] [Google Scholar]
- 6. Häggström J, Hansson K, Karlberg BE, et al. Effects of long‐term treatment with enalapril or hydralazine on the renin‐angiotensin‐aldosterone system and fluid balance in dogs with naturally acquired mitral valve regurgitation. Am J Vet Res 1996;57:1645–1652. [PubMed] [Google Scholar]
- 7. Atkins CE, Brown WA, Coats JR, et al. Effects of long‐term administration of enalapril on clinical indicators of renal function in dogs with compensated mitral regurgitation. J Am Vet Med Assoc 2002;221:654–658. [DOI] [PubMed] [Google Scholar]
- 8. Guglielmini C, Poser H, Pria AD, et al. Red blood cell distribution width in dogs with chronic degenerative valvular disease. J Am Vet Med Assoc 2013;243:858–862. [DOI] [PubMed] [Google Scholar]
- 9. Palazzuoli A, Antonelli G, Nuti R. Anemia in cardio‐renal syndrome: Clinical impact and pathophysiologic mechanisms. Heart Fail Rev 2011;16:603–607. [DOI] [PubMed] [Google Scholar]
- 10. Moe GW, Ezekowitz JA, Lepage S, et al. The 2014 canadian cardiovascular society heart failure management guidelines focus update: Anemia, biomarkers, and recent therapeutic trial implications. Can J Cardiol 2015;31:3–16. [DOI] [PubMed] [Google Scholar]
- 11. Ettinger SJ, Feldman EC. Textbook of Veterinary Internal Medicine, 6th ed St. Louis, Missouri, USA: Elsevier Saunders; 2005. [Google Scholar]
- 12. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med 2009;23:1142–1150. [DOI] [PubMed] [Google Scholar]
- 13. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. J Vet Intern Med 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 14. Hansson K, Häggström J, Kvart C, et al. Left atrial to aortic root indices using two‐dimensional and m‐mode echocardiography in cavalier king Charles spaniels with and without left atrial enlargement. Vet Rad Ultrasound 2002;43:568–575. [DOI] [PubMed] [Google Scholar]
- 15. Boon JA. Veterinary Echocardiography, 2nd ed Oxford, UK, Wiley‐Blackwell, 2011. [Google Scholar]
- 16. Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med 2004;18:311–321. [DOI] [PubMed] [Google Scholar]
- 17. Bartlett PC, Van Buren JW, Bartlett AD, et al. Case‐control study of risk factors associated with feline and canine chronic kidney disease. Vet Med Int 2010;20:2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Neill DG, Elliott J, Church DB, et al. Chronic kidney disease in dogs in UK veterinary practices: Prevalence, risk factors, and survival. J Vet Int Med 2013;27:814–821. [DOI] [PubMed] [Google Scholar]
- 19. Palazzuoli A, Ronco C. Cardio‐renal syndrome: An entity cardiologists and nephrologists should be dealing with collegially. Heart Fail Rev 2011;16:503–508. [DOI] [PubMed] [Google Scholar]
- 20. Chetboul V, Tissier R. Echocardiographic assessment of canine degenerative mitral valve disease. J Vet Cardiol 2012;14:127–148. [DOI] [PubMed] [Google Scholar]
- 21. Ljungvall I, Rishniw M, Porciello F, et al. Murmur intensity in small‐breed dogs with myxomatous mitral valve disease reflects disease severity. J Small Anim Pract 2014;55:545–550. [DOI] [PubMed] [Google Scholar]
- 22. Médaille C, Trumel C, Concordet D, et al. Comparison of plasma/serum urea and creatinine concentrations in the dog: A 5‐year retrospective study in a commercial veterinary clinical pathology laboratory. J Vet Med A Physiol Pathol Clin Med 2004;51:119–123. [DOI] [PubMed] [Google Scholar]
- 23. Boswood A, Murphy A. The effect of heart disease, heart failure and diuresis on selected laboratory and electrocardiographic parameters in dogs. J Ver Cardiol 2006;8:1–9. [DOI] [PubMed] [Google Scholar]
- 24. Stockham SL, Scott MA. Fundamentals of Veterinary Clinical Pathology, 2nd ed Oxford, UK, Blackwell Publishing; 2008. April. [Google Scholar]
- 25. Finco DR, Duncan JR. Evaluation of blood urea nitrogen and serum creatinine concentrations as indicators of renal dysfunction: A study of 111 cases and a review of related literature. J Am Vet Med Assoc 1976;168:593–601. [PubMed] [Google Scholar]


