Abstract
Background
Obesity in horses is increasing in prevalence and can be associated with insulin insensitivity and laminitis. Current treatment strategies for obesity include dietary restriction and exercise. However, whether exercise alone is effective for decreasing body fat is uncertain.
Hypothesis
Our hypothesis was that twice daily use of a dynamic feeding system for 3 months would induce sustained, low‐intensity exercise thereby decreasing adiposity and improving insulin sensitivity (SI).
Animals
Eight, university‐owned, mixed‐breed, adult ponies with body condition scores (BCS) ≥5/9 were used.
Methods
Two treatments (“feeder on” or “feeder off”) were administered for a 3‐month period by a randomized, crossover design (n = 4/treatment). An interim equilibration period of 6 weeks at pasture separated the 2 study phases. Measurements of body mass (body weight, BCS, cresty neck score [CrNS], and morphometry), body fat (determined before and after the “feeder on” treatment only), triglycerides, and insulin sensitivity (SI; combined glucose‐insulin test) were undertaken before and after treatments.
Results
The dynamic feeding system induced a 3.7‐fold increase in the daily distance travelled (n = 6), compared to with a stationary feeder, which significantly decreased mean BCS (6.53 ± 0.94 to 5.38 ± 1.71), CrNS (2.56 ± 1.12 to 1.63 ± 1.06) and body fat (by 4.95%). An improvement in SI did not occur in all ponies.
Conclusions and Clinical Importance
A dynamic feeding system can be used to induce sustained (daily), low‐intensity exercise that promotes weight loss in ponies. However, this exercise may not be sufficient to substantially improve SI.
Keywords: Equine metabolic syndrome, Horse, Insulin, Obesity
Abbreviations
- AUC
area under the curve
- BCS
body condition score
- BW
body weight
- Cmax
maximum concentration
- CGIT
combined glucose‐insulin test
- CrNS
cresty neck score
- D2O
deuterium oxide
- EMS
equine metabolic syndrome
- NSC
nonstructural carbohydrate
- PP
positive phase
- SI
insulin sensitivity
- TBW
total body water
The prevalence of obesity in horses has been reported to be as high as 50%.1, 2, 3 Pasture improvement and the abundance of well‐marketed complete horse feeds have the potential to increase caloric intake, whereas overbreeding (overstocking) and smaller property sizes have decreased the space available for unstructured exercise.4, 5 As is the case in other species, increased adiposity has led to a rise in conditions that can be associated with obesity or regional adiposity in horses, including equine metabolic syndrome (EMS), reproductive abnormalities, and osteochondrosis.6, 7, 8, 9
Refractoriness to insulin by insulin‐sensitive tissues has been associated with EMS and can lead to persistent hyperinsulinemia.9 In addition, the consumption of diets containing large amounts of nonstructural carbohydrates (NSC) can result in higher postprandial insulin secretion in insulin‐dysregulated ponies, compared to normal ponies.10 Although the complex relationship among regional adiposity, obesity, and insulin dysregulation requires further investigation, hyperinsulinemia has been shown to be associated with an increased laminitis risk.11, 12, 13 Preventing development of this incapacitating foot disease is a key concern in the management of insulin‐dysregulated horses.
The current strategies for managing obesity and EMS and preventing insulin‐associated, pasture‐associated laminitis, or both are limited and principally rely on caloric restriction and exercise.14, 15 Although dietary restriction has been shown to be efficacious for weight loss,16, 17 available data on whether or not exercise is effective in decreasing obesity and improving insulin sensitivity (SI) are relatively limited.18, 19, 20, 21, 22 The few available reports are somewhat contradictory because of the different nature of the exercise programs used (or observed), but a strong positive association between consistent exercise and improvement in metabolic function was not apparent.18, 21
We have demonstrated previously that horses will use a dynamic feeding system designed to induce prolonged, low‐intensity exercise in an unsupervised manner (i.e, requiring regular walking in order to continually access a forage ration).23 Thus, our hypotheses were that twice daily use of a dynamic feeding system for 3 months would induce sustained, low‐intensity exercise, which would decrease adiposity and improve SI in ponies.
Materials and Methods
Dynamic Feeder
During operation, the custom‐made dynamic feeder is only accessible from 1 side at a time, with 2 sliding doors that open alternately on each side of the feeder to allow access to an internal hayrack for a predetermined period of time (5 minutes cycle for the current study). At the end of this period, an electronic timer triggers closure of 1 sliding door while simultaneously opening the door on the opposite side (Fig 1A). Thus, the animal is required to walk to the other side of the feeder in order to continue to access the forage. A dividing fence can be used to increase the distance walked by the animal in between feeding periods (Fig 1B).
Figure 1.
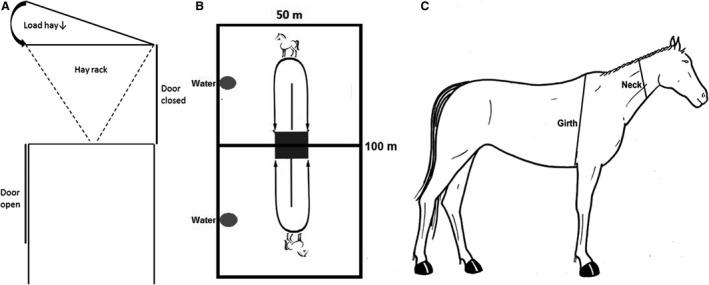
(A) The dynamic feeder allows access to a hayrack from alternate sides via an automatic sliding door system. (B) The distance that ponies walk to access alternating sides of the feeder during operation can be increased with the use of a fence. (C) Circumferential measurements of the neck and heart girth were measured by 2 operators at a standardized location (Artwork courtesy of M Schutze).
Animals
Eight, university‐owned, mixed‐breed ponies (12.1 ± 3.7 years; 7 male, 1 female) with body condition scores (BCS) ≥ 5/924 were used. None of the ponies had used a dynamic feeding system before and all were normal (except for BCS) on physical examination. Ponies received a forage‐based diet of lucerne hay (2% body weight [BW] as fed; NSC content, 10.1% dry matter) and a vitamin and mineral supplement.1 The daily ration was divided into 2 equal meals and administered at 0700 and 1530 each day. Water intake was unrestricted. Ponies were housed in flat, pasture‐free paddocks with access to shade and shelter for the duration of the study. Ponies could contact each other over the fences for mutual grooming, except for the section of fence where the feeder was placed, because this fence was higher to avoid contact during feeding. Routine prophylactic care including vaccinations, parasite control, farriery, and dental work was maintained for each pony as part of a herd health program. Ethical approval for the study was granted by the University Animal Ethics Committees (SVS/043/14/MORRIS; 1400000217).
Study Design
Ponies received 2 treatments (“feeder on” or “feeder off”) administered by a randomized, crossover design for a 3‐month period (i.e, 4 ponies [2 pairs] in each group). The treatment periods were separated by a 6‐week rest period designed to allow ponies to return to their prestudy BW and BCS. During this equilibration phase, the ponies were fed the same diet as during the treatment periods, but also had free access to pasture. The space allocation for each pony was similar and consistent across treatment phases. All ponies were subject to assessments of body mass, adiposity, and SI (as outlined below) before and after each treatment. Total percent body fat was determined before and after the “feeder on” treatment phase only because of financial constraints.
The distance travelled each day by the ponies was assessed with a lightweight, global positioning system tracking device.2 The distance travelled in a 12‐hour period (0600–1800) was measured 3 times at consistent intervals during each treatment phase for 6 ponies (the mare and gelding pairing was not tracked because of behavioral issues), and the mean daytime distance calculated. Behavioral responses to the feeding system were assessed by use of remote cameras3 (with real‐time data transfer) and live observations.
Before each phase, ponies were acclimated to the dynamic feeding system for a period of 2 weeks and taught to use the feeder by the investigators during this period. During acclimation, the investigators monitored behavioral responses to the feeder and ensured appropriate pairs of ponies were selected. Ponies were matched for size and behavior to ensure optimal, safe use of the feeding system and minimize aggressive behavior in relation to feeding. The ponies were graded on feeder use as either A‐ or B‐rated users, based on behavioral observations. Qualitative assessment of feeder use enabled a more accurate assessment in relation to the effect of the dynamic feeder (given that the distance travelled was related to willingness to use the feeder, pony size, and fitness). Ponies rated an A (n = 5) exercised consistently when there was food in the feeder with committed traversing of the fence line, deviated minimally from the travelled path, adopted a faster pace when exercising, did not wait on 1 side of the feeder and were difficult to distract from the task. Ponies rated a B (n = 3) made good use of the feeder in the first hour but periodically waited on 1 side afterward, occasionally deviated from the walking path (to roll or water), appeared to forget how to use the feeder and could be distracted from feeder use by human or more dominant pony interference (e.g, squealing, ears back).
Morphometric Measurements
Body weight was determined with a large animal weighing scale (Ruddweigh4). Body condition24 and cresty neck (CrNS)25 scores were assessed independently by 1 experienced operator (blinded) and by 1 study investigator (MdL; nonblinded) and the average scores calculated.24, 25 Heart girth and neck circumference were measured at a set anatomical location (Fig 1C) by 2 operators (as above), according to a standardized protocol and the mean measurement obtained. Concordance between the 2 operators was excellent for all morphometric measurements (Lin's ρc > 0.9).
Hormone Analysis
Fasting triglyceride concentration was determined by enzymatic determination (AU6805) in serum samples collected at 0800 immediately before and immediately after each phase. A combined glucose‐insulin tolerance test (CGIT) also was performed immediately before and after each treatment phase at 0800 after an overnight fast according to standard protocols.26 Briefly, the evening before each test an indwelling catheter was placed aseptically in a jugular vein and covered, with patency maintained with heparinized saline. At 0800 the next day, glucose (150 mg/kg BW) was administered as a bolus followed by insulin (0.1 IU/kg BW). Blood glucose concentration was measured immediately on whole blood with a glucometer6 validated for use in horses by the investigators (data not shown) at 0, 1, 5, 15, 25, 35, and 45 minutes and then every 15 minutes up to 2.5 h. Plasma samples were placed immediately on ice for 10 minutes before centrifugation (10 minutes, 1500 × g), separated into 1 mL aliquots, and rapidly frozen and stored at −80°C. Serum samples were allowed to clot for 30 minutes at ambient temperature before processing and storage as above. Serum insulin concentration was determined retrospectively by a commercial diagnostic laboratory with a validated chemiluminescent assay (Immulite 20007) in samples (5 mL) obtained at 0, 45, and 75 minutes of the CGIT.27
Body Fat Determination
The percentage body fat was determined by a deuterium oxide (D2O) dilution method according to a protocol adapted for horses.28 Briefly, syringes containing D2O (0.12 g/kg BW) were weighed, the D2O was administered via an indwelling catheter, and then the syringes were reweighed to calculate the exact mass of D2O administered. Plasma samples (10 mL; chilled sodium heparin tubes) were obtained before and 4 hours after D2O administration by venipuncture from the opposite jugular vein and the sample placed immediately on ice. Food and water were withheld during the test.
Deuterium enrichment in the plasma samples obtained pre‐ and post‐D2O administration was determined by gas isotope ratio mass spectrometry. The dilution space (D) was determined according to the following equation: where Pb is the baseline plasma, a is the molar mass of the stock deuterium solution, and Ea, Eb, and Ep are the isotopic enrichments (units) of the dose, baseline, and postdose plasma, respectively.28 Total body water (TBW) was calculated by means of a 4% correction factor: and the final fat‐free mass determined by incorporating a hydration factor of 73%.28
Statistical Analyses
All data were normally distributed (Shapiro‐Wilk test) and analyzed parametrically. Before and after treatment phase measurements of BW, morphometry, triglyceride concentration, and CGIT parameters were compared with 2‐way, repeated measures ANOVA. One‐way measures (percentage BW and fat reduction and distance travelled) were compared by a paired t‐test. The relationship between triglyceride concentration and body condition score fitted a single rectangular, 2‐parameter hyperbola by the equation f = a*x/(b + x). Concordance between operator measurements was assessed by Lin's concordance coefficient. Analyses of the CGIT data included: area under the insulin and glucose‐time concentration curves (AUC); maximum glucose concentration (Cmax); positive phase duration for glucose (PP; i.e, the time taken for blood glucose concentration to return to baseline); and glucose clearance (Cl) during the PP which was calculated as follows: . The AUC was calculated by the trapezoidal rule. Data were analyzed by SigmaPlot8 and are reported as mean ± SD. Significance was set at P < .05.
Results
Use of the Dynamic Feeder
All ponies remained healthy and free from lameness for the duration of the study and no change to pony pairs was required. The ponies learned to use the dynamic feeding system very quickly (within 48 hour), although 2 ponies took up to 5 days to use it consistently. Behavioral issues arose between the mare and gelding pairing (e.g, squealing, ears back), but impediment to feeder use was minimized by an individual housing configuration and a higher fence alongside the feeder (Fig 1B). Ponies using the dynamic feeder walked (some trotted initially) for approximately 2 hour twice daily. Overall, the average daily distance travelled by the ponies was 3.7‐fold greater (P = .01) when fed from the dynamic feeder, compared to when they were fed from the stationary feeder (Fig 2A).
Figure 2.
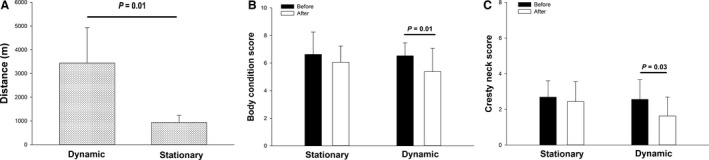
(A) The ponies travelled farther when using the dynamic feeder, compared to a stationary feeder (n = 6). Body condition (B) and cresty neck (C) scores were significantly decreased after 3 months of sustained, low‐intensity exercise in ponies (n = 8). However, use of a standard, stationary hay feed for 3 months did not decrease either body condition (B) or cresty neck (C) scores.
Body Morphometrics and Adiposity
Using the dynamic feeder for 3 months decreased mean BCS (6.53 ± 0.94 to 5.38 ± 1.71; P = .01), and CrNS (2.56 ± 1.12 to 1.63 ± 1.06, P = .03), compared to no change after using the stationary feeder (Fig 2B, C). The dynamic feeding system also caused a decrease (P = .01) in body fat percentage (4.95 ± 3.82%; range 1.1–10.8%). Serum triglyceride concentration demonstrated a significant hyperbolic relationship with BCS before the study (Fig 3A). This curvilinear relationship is consistent with the potential for saturable triglyceride removal processes at the extremes of adiposity.29 Triglyceride concentration was not altered by either the stationary or dynamic feeder (Fig 3B).
Figure 3.
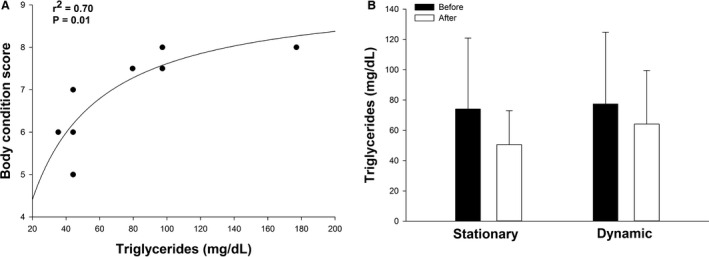
(A) Basal serum triglyceride concentration had a significant hyperbolic relationship with body condition score in ponies (n = 8). (B) Sustained low‐intensity exercise did not result in a decrease in mean (±SD) serum triglyceride concentration, despite a decrease in body condition score. Feeding from a stationary feeder that did not induce exercise also did not alter serum triglyceride concentration.
Neither feeder had an effect on heart girth or neck circumference (Table 1). The mean decrease in BW was not significant after dynamic feeder use (Table 1). Similarly, the change in BW (Fig 4) after use of the dynamic feeder did not differ compared to by a stationary feeder (−3.1 ± 5.1% and −0.62 ± 2.15%, respectively, P = .07).
Table 1.
Mean ± SD for BW and circumferential measurements of the heart girth and neck taken in 8 ponies before and after use of a dynamic or stationary feeder
| BW (kg) | Girth (cm) | Neck (cm) | |
|---|---|---|---|
| Stationary | |||
| Before | 255 ± 88.3 | 147 ± 16.4 | 83.6 ± 9.33 |
| After | 254 ± 90.5 | 146 ± 17.6 | 80.8 ± 8.27 |
| Dynamic | |||
| Before | 254 ± 89.7 | 148 ± 19.6 | 81.4 ± 8.32 |
| After | 246 ± 87.7 | 146 ± 18.3 | 82.1 ± 8.2 |
BW, Body weight.
Figure 4.
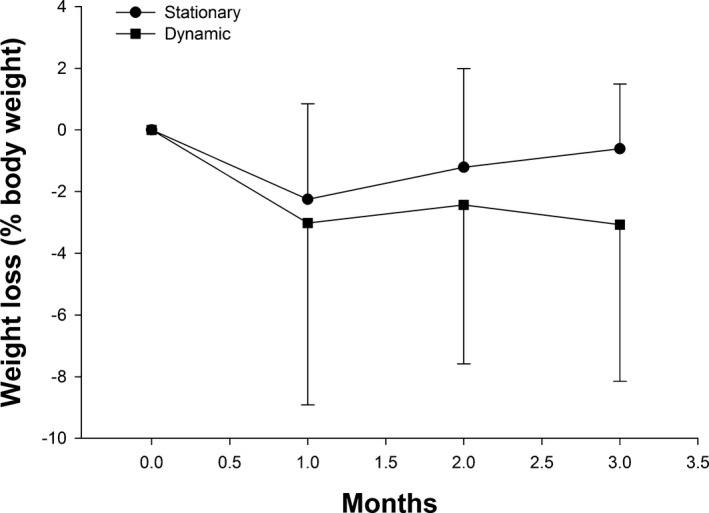
The mean (±SD) percentage change in body weight was recorded after use of a dynamic feeder that induced sustained low‐intensity exercise for 3 months and compared to use of a stationary feeder.
Insulin Sensitivity
No markers of SI (G Cmax, PP duration, Clearance, AUCGlucose, or AUCInsulin) were improved by the dynamic feeder for 3 months, compared to a stationary feeder. However, if SI parameters were examined for the A‐rated users only, PP duration (P = .046) and AUCinsulin (P = .045) were marginally improved after using the dynamic feeder (Table 2). By comparison, there was no difference before and after either dynamic or stationary feeder use for the B‐rated users (Table 2).
Table 2.
Combined glucose‐insulin test results (mean ± SD) for 8 ponies before and after using a dynamic or stationary feeder. Ponies were rated on feeder use as either A (n = 5) or B (n = 3)
| AUCG | G Cmax (mM) | PP (min) | Clearance (PP) | AUCI | |
|---|---|---|---|---|---|
| Stationary | |||||
| A before | 749 ± 185 | 12.7 ± 1.3 | 62.2 ± 38 | 0.21 ± 0.16 | 4285 ± 4753 |
| A after | 796 ± 143 | 13.8 ± 1.1 | 58.8 ± 29 | 0.21 ± 0.18 | 5600 ± 6059 |
| B before | 776 ± 49 | 13.2 ± 1.7 | 49.8 ± 9.2 | 0.17 ± 0.05 | 2278 ± 1060 |
| B after | 898 ± 52 | 14.3 ± 2.7 | 76.8 ± 35 | 0.14 ± 0.07 | 5854 ± 7105 |
| Dynamic | |||||
| A before | 803 ± 167 | 13.2 ± 1.4 | [Link]69.8 ± 36 | 0.17 ± 0.14 | b6791 ± 5716 |
| A after | 746 ± 104 | 12.9 ± 3.1 | 55.8 ± 34 | 0.19 ± 0.11 | 4279 ± 4357 |
| B before | 873 ± 84 | 14.5 ± 2.7 | 72.2 ± 39 | 0.16 ± 0.09 | 5959 ± 7009 |
| B after | 996 ± 108 | 14.6 ± 0.9 | 101 ± 19 | 0.1 ± 0.03 | 7501 ± 7976 |
AUC, area under curve; G, glucose; Cmax, maximum glucose concentration; PP, positive phase; before and after comparisons within treatment and group: a P = .05; b P = .04; comparisons of before values between and within treatments (i.e, A vs A before and A vs B before) were all P > .05.
Discussion
Persistent low‐intensity exercise (walking) was achieved in the current study by use of a novel, dynamic feeding system that significantly increased the daily distance travelled by ponies, compared to using a standard hay feeder. This consistent exercise over a 3‐month period decreased fat percentage and improved body condition, consistent with the hypothesis that increased exercise decreases adiposity. However, the dynamic feeding system did not result in an improvement in SI (i.e, AUCinsulin and PP duration) in all ponies.
The ponies had no difficulty learning to use the feeding system after some initial training. However, behavioral responses to the feeding system were not consistent. The side‐by‐side design of the feeder ensured sufficient competition between paired ponies to promote consistent use of the feeder, and their physical separation at the feeder largely prevented dominance aggression from interfering with feeding. Despite this arrangement, 3 ponies were not sufficiently appetite‐driven to use the feeder consistently for the entire 2‐hour period. As a result, improvements in SI were either not apparent, or were less marked, in these individuals. Subjectively, willingness to use the feeder improved over the 3‐month period in 2 of the poorer responding ponies. The reason for this increased compliance is unknown although an improvement in exercise tolerance may have contributed. Alterations to the feeder design may be able to address these behaviors, for example, by modifying the time allowed for feeding or using a movement sensor to trigger alternate door opening. The use of the feeder also can be adapted to suit individual requirements, such as increasing the amount of time spent accessing a forage ration (with smaller, more frequent meals). The ponies foraged for approximately 4 hours per day (2 × 2 hours) and although no stereotypies were noted in the current study, adverse effects, such as gastric ulceration or behavioral abnormalities, could develop with restricted foraging. To address this concern, the time spent foraging (i.e, using the feeder) each day could be increased by altering the feeding regime.
The decrease in body fat and improvement in BCS observed in our study support the concept that exercise can be used to decrease obesity in horses. Previous studies on the impact of exercise on obesity have yielded conflicting results.18, 21 One prior study found no association between exercise and risk of obesity.21 However, the horses and ponies in that survey completed only 4–6 hours of low‐intensity exercise on average per week, compared with the 3–4 hours per day in the current study. A different study found that exercise of low then high intensity for 8 weeks decreased BW and fat mass.18 The decrease in fat mass in that study was substantially higher than in our study (21–34% vs 5%), yet as with our study there was no apparent improvement in heart girth and neck measurements. The reason why the morphometric measurements failed to reflect the decrease in fat percentage is unknown, but may be related to the time course of the study, given that a decrease in neck and heart girth circumference occurred after a 6‐month weight loss program in client‐owned horses,17 or may indicate that initial fat loss occurs from specific sites.16
Despite using different methodologies for assessing insulin dynamics, a previous study also found no improvements in SI,18 which may indicate that exercise is not a good approach to improving SI in horses, as it is in other species.30, 31 However, a different study demonstrated that postexercise spikes in plasma insulin concentration were less marked after 14 days of low‐intensity exercise.32 In our study, the improved insulin parameters recorded in ponies that used the feeder consistently appear to indicate that exercise has some beneficial effect on SI. In humans, it is not the intensity of the exercise that is most important for improving SI, but the overall amount of exercise undertaken,33 which is consistent with the improved SI in ponies that were more willing to exercise consistently until the feeder was empty.
Exercise may improve skeletal muscle insulin signaling in horses, at least in the immediate postexercise period.19, 34 In addition, studies in humans have indicated that the persistence of improved glucose disposal and insulin sensitivity after exercise is variable and often wanes within 16–18 hours.30, 35 The CGITs were performed approximately 24 hours after the last feeding session in our study, which means that any transient improvements in SI in the immediate postexercise period would not have been detected. However, longer term improvements in SI have been identified after physical activity36, 37 and potentially, different types or longer periods of exercise or both may be required to substantially improve markers of insulin and glucose metabolism in horses in the longer term. In addition, CGIT data showed some inherent variability in our study, which is likely a function of small sample size, but also may be due to the labile nature of insulin and previous reports that the glucose parameters of the CGIT lack repeatability in horses.26 Alternatively, tests used for measuring SI in our study (CGIT) and a previous study18 (frequently sampled IV glucose tolerance test) may not have been optimal for detecting early changes in insulin responsiveness, given that they do not include an assessment of the enteroinsular axis.10
The persistence of any beneficial effects of feeder use was not assessed in our study. The ponies returned to prestudy BW within 6 weeks of ceasing feeder use after a return to pasture access and continued hay supplementation. This outcome suggests that maintaining weight loss induced by the feeding system would require its continuous use. Conversely, the effect of continuous feeder use on BW and fat mass is unknown, and studies to determine whether longer term use of the dynamic feeder can induce additional benefit, or alternatively lead to adaptation and a plateau in BW, are required.
Previous studies have demonstrated that dietary restriction is effective at decreasing body mass and improving insulin parameters.16, 38, 39 Although our study did not restrict feed intake below 2% of BW (as may be recommended to achieve weight loss17, 27), the ponies did not have the ability to increase their feed intake, which may have contributed to their decrease in body fat and body condition during dynamic feeder use. In humans, a reduction in energy intake was superior to 12 weeks of aerobic exercise for improving SI.40 Taken together, these data may indicate that a combination of dietary restriction and sustained low‐intensity exercise is the optimal approach to weight loss in horses.
The feeding pattern of horses in the wild is based on natural roaming behavior.41 This process of “browse and move” while seeking forage results in frequent consumption of small amounts of roughage while traveling considerable distances.42 Domestic horses frequently lack enough space to emulate this feeding pattern and may not have any access to natural pasture. Alterations in the feeding and housing of domestic horses may contribute to behavioral, digestive, and metabolic diseases.43, 44, 45, 46 Whether the use of a dynamic feeding system that induces structured, unsupervised exercise in ponies housed in small paddocks would be beneficial for other applications beyond weight loss and EMS is unknown.
Overall, we determined that a dynamic feeding system can be used to induce sustained, low‐intensity exercise that promotes weight loss in ponies. Furthermore, for ponies that commit to using the feeder and are willing to travel >3 km per day, some improvement in SI also may occur. However, it appears that exercise alone may not be sufficient to achieve substantial metabolic reform in animals with increased adiposity and that a combination of dietary restriction and exercise may constitute the optimal approach to weight loss.
Funding Sources
The study was funded by the Morris Animal Foundation, USA, and Queensland University of Technology (QUT), Australia. The funding bodies had no role in the conception or execution of the study.
Acknowledgments
The authors thank Jessica van Haeften, Lisa Clancy, Alexandra Meier and Natashja Burton for technical assistance.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This study was presented as a research abstract at the Bain Fallon Conference in NSW, Australia, in July, 2015.
Footnotes
Barastoc horse block, Ridley Corporation, Melbourne Victoria, Australia
Wintec G‐Rays 2, Berlin, Germany
Hero, Go Pro Inc, San Mateo, CA
Gallagher, Melbourne, Vic, Australia
Beckman Coulter, Lane Cove, NSW, Australia
Accu‐Check, Roche Diagnostics, Castle Hills, NSW, Australia
Siemens, Bayswater, Vic, Australia
SigmaPlot v. 12.5, Systat Software Inc, San Jose, CA
References
- 1. Thatcher CD, Pleasant RS, Geor RJ, et al. Prevalence of overconditioning in mature horses in southwest Virginia during the summer. J Vet Intern Med 2012;26:1413–1418. [DOI] [PubMed] [Google Scholar]
- 2. Wyse CA, McNie KA, Tannahill VJ, et al. Prevalence of obesity in riding horses in Scotland. Vet Record 2008;162:590–591. [DOI] [PubMed] [Google Scholar]
- 3. Robin CA, Ireland JL, Wylie CE, et al. Prevalence of and risk factors for equine obesity in Great Britain based on owner‐reported body condition scores. Equine Vet J 2015;47:196–201. [DOI] [PubMed] [Google Scholar]
- 4. RSPCA, Blue Cross, World Horse Welfare, Horse World and The British Horse Society . Left on the verge: The approaching equine crisis in England and Wales. 2012;1–8.
- 5. Watts K. Pasture management to minimize the risk of equine laminitis. Vet Clin North Am‐Equine Pract 2010;26:361–369. [DOI] [PubMed] [Google Scholar]
- 6. Vick MM, Sessions DR, Murphy BA, et al. Obesity is associated with altered metabolic and reproductive activity in the mare: Effects of metformin on insulin sensitivity and reproductive cyclicity. Reprod Fertil Dev 2006;18:609–617. [DOI] [PubMed] [Google Scholar]
- 7. Raubenheimer D, Machovsky‐Capuska GE, Gosby AK, et al. Nutritional ecology of obesity: From humans to companion animals. Br J Nutr 2015;113(Suppl):S26–S39. [DOI] [PubMed] [Google Scholar]
- 8. Johnson PJ, Wiedmeyer CE, Messer NT, et al. Medical implications of obesity in horses‐lessons for human obesity. J Diabetes Sci Technol 2009;3:163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frank N, Geor RJ, Bailey SR, et al. Equine metabolic syndrome. J Vet Intern Med 2010;24:467–475. [DOI] [PubMed] [Google Scholar]
- 10. de Laat MA, McGree JM, Sillence MN. Equine hyperinsulinemia: Investigation of the enteroinsular axis during insulin dysregulation. Am J Physiol – Endocrinol Metabol 2016;310:E61–E72. [DOI] [PubMed] [Google Scholar]
- 11. McGowan CM, Frost R, Pfeiffer DU, et al. Serum insulin concentrations in horses with equine Cushing's syndrome: Response to a cortisol inhibitor and prognostic value. Equine Vet J 2004;36:295–298. [DOI] [PubMed] [Google Scholar]
- 12. Treiber K, Hess T, Kronfeld D, et al. Insulin resistance and compensation in laminitis‐predisposed ponies characterized by the minimal model. Pferdeheilkunde 2007;23:237–240. [Google Scholar]
- 13. Menzies‐Gow NJ, Harris PA, Elliott J. Prospective cohort study evaluating risk factors for the development of pasture‐associated laminitis in the UK. Equine Vet J 2016. doi: 10.1111/evj.12606. [DOI] [PubMed] [Google Scholar]
- 14. Becvarova I, Pleasant RS. Managing obesity in pasture‐based horses. Compendium: Continuing Educat Vet 2012;34:E1–E4. [PubMed] [Google Scholar]
- 15. Harris P, Bailey SR, Elliott J, et al. Countermeasures for pasture‐associated laminitis in ponies and horses. J Nutr 2006;136:2114S–2121S. [DOI] [PubMed] [Google Scholar]
- 16. Dugdale AHA, Curtis GC, Cripps P, et al. Effect of dietary restriction on body condition, composition and welfare of overweight and obese pony mares. Equine Vet J 2010;42:600–610. [DOI] [PubMed] [Google Scholar]
- 17. Gill JC, Pratt‐Phillips SE, Mansmann R, et al. Weight loss management in client‐owned horses. J Equine Vet Sci 2016. doi:10.1016/j.jevs.2015.12.014. [Google Scholar]
- 18. Carter RA, McCutcheon LJ, Valle E, et al. Effects of exercise training on adiposity, insulin sensitivity, and plasma hormone and lipid concentrations in overweight or obese, insulin‐resistant horses. Am J Vet Res 2010;71:314–321. [DOI] [PubMed] [Google Scholar]
- 19. McCutcheon LJ, Geor RJ, Pratt SE, et al. Effects of prior exercise on components of insulin signalling in equine skeletal muscle. Equine Vet J Suppl 2006;S36:330–334. [DOI] [PubMed] [Google Scholar]
- 20. Gordon ME, McKeever KH, Betros CL, et al. Exercise‐induced alterations in plasma concentrations of ghrelin, adiponectin, leptin, glucose, insulin, and cortisol in horses. Vet J 2007;173:532–540. [DOI] [PubMed] [Google Scholar]
- 21. Giles SL, Rands SA, Nicol CJ, et al. Obesity prevalence and associated risk factors in outdoor living domestic horses and ponies. Peer J 2014;2:e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Potter SJ, Bamford NJ, Harris PA, et al. Comparison of weight loss, with or without dietary restriction and exercise, in Standardbreds, Andalusians and mixed breed ponies. J Equine Vet Sci Elsevier 2013;33:339. [Google Scholar]
- 23. Hampson BA, de Laat MA, Monot J, et al. Adaption of horses to a novel dynamic feeding system: Movement and behavioural responses. Equine Vet J 2013;45:481–484. [DOI] [PubMed] [Google Scholar]
- 24. Henneke DR, Potter GD, Kreider JL, et al. Relationship between condition score, physical measurements and body‐fat percentage in mares. Equine Vet J 1983;15:371–372. [DOI] [PubMed] [Google Scholar]
- 25. Carter RA, Geor RJ, Staniar WB, et al. Apparent adiposity assessed by standardised scoring systems and morphometric measurements in horses and ponies. Vet J 2009;179:204–210. [DOI] [PubMed] [Google Scholar]
- 26. Bröjer J, Lindåse S, Hedenskog J, et al. Repeatability of the combined glucose‐insulin tolerance test and the effect of a stressor before testing in horses of 2 breeds. J Vet Intern Med 2013;27:1543–1550. [DOI] [PubMed] [Google Scholar]
- 27. Argo CM, Curtis GC, Grove‐White D, et al. Weight loss resistance: A further consideration for the nutritional management of obese Equidae. Vet J 2012;194:179–188. [DOI] [PubMed] [Google Scholar]
- 28. Dugdale AH, Curtis GC, Milne E, et al. Assessment of body fat in the pony: Part II. Validation of the deuterium oxide dilution technique for the measurement of body fat. Equine Vet J 2011;43:562–570. [DOI] [PubMed] [Google Scholar]
- 29. Brunzell JD, Hazzard WR, Porte D, et al. Evidence for a common, saturable, triglyceride removal mechanism for chylomicrons and very low density lipoproteins in man. J Clin Investigat 1973;52:1578–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Borghouts LB, Keizer HA. Exercise and insulin sensitivity: A review. Int J Sports Med 2000;21:1–12. [DOI] [PubMed] [Google Scholar]
- 31. Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: The American college of sports medicine and the American diabetes association: Joint position statement. Diabetes Care 2010;33:e147–e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Menzies‐Gow NJ, Wray H, Bailey SR, et al. The effect of exercise on plasma concentrations of inflammatory markers in normal and previously laminitic ponies. Equine Vet J 2014;46:317–321. [DOI] [PubMed] [Google Scholar]
- 33. Clarke J, Janssen I. Is the frequency of weekly moderate‐to‐vigorous physical activity associated with the metabolic syndrome in Canadian adults? Appl Physiol Nutr Metab 2013;38:773–778. [DOI] [PubMed] [Google Scholar]
- 34. Lacombe VA, Hinchcliff KW, Devor ST. Effects of exercise and glucose administration on content of insulin‐sensitive glucose transporter in equine skeletal muscle. Am J Vet Res 2003;64:1500–1506. [DOI] [PubMed] [Google Scholar]
- 35. Dela F, Mikines KJ, von Linstow M, et al. Effect of training on insulin‐mediated glucose uptake in human muscle. Am J Physiol 1992;263:E1134–E1143. [DOI] [PubMed] [Google Scholar]
- 36. Mikines KJ, Sonne B, Farrell PA, et al. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol 1988;254:E248–E259. [DOI] [PubMed] [Google Scholar]
- 37. Zisser H, Gong P, Kelley CM, et al. Exercise and diabetes. Int J Clin Pract Suppl 2011;170:71–75. [DOI] [PubMed] [Google Scholar]
- 38. McGowan CM, Dugdale AH, Pinchbeck GL, et al. Dietary restriction in combination with a nutraceutical supplement for the management of equine metabolic syndrome in horses. Vet J 2013;196:153–159. [DOI] [PubMed] [Google Scholar]
- 39. Bruynsteen L, Moons CP, Janssens GP, et al. Level of energy restriction alters body condition score and morphometric profile in obese Shetland ponies. Vet J 2015;206:61–66. [DOI] [PubMed] [Google Scholar]
- 40. Pedersen LR, Olsen RH, Jurs A, et al. A randomized trial comparing the effect of weight loss and exercise training on insulin sensitivity and glucose metabolism in coronary artery disease. Metabolism 2015;64:1298–1307. [DOI] [PubMed] [Google Scholar]
- 41. Salter RE, Hudson RJ. Feeding ecology of horses in Western Alberta. J Range Manag 1979;32:221–225. [Google Scholar]
- 42. Hampson BA, de Laat MA, Mills PC, et al. Distances travelled by feral horses in ‘outback’ Australia. Equine Vet J 2010;42:582–586. [DOI] [PubMed] [Google Scholar]
- 43. Hillyer MH, Taylor FG, Proudman CJ, et al. Case control study to identify risk factors for simple colonic obstruction and distension colic in horses. Equine Vet J 2002;34:455–463. [DOI] [PubMed] [Google Scholar]
- 44. Feige K, Furst A, Eser MW. Effects of housing, feeding and use on equine health with emphasis on respiratory and gastrointestinal diseases. Schweiz Arch Tierheilkd 2002;144:348–355. [DOI] [PubMed] [Google Scholar]
- 45. Bachmann I, Audige L, Stauffacher M. Risk factors associated with behavioural disorders of crib‐biting, weaving and box‐walking in Swiss horses. Equine Vet J 2003;35:158–163. [DOI] [PubMed] [Google Scholar]
- 46. Sillence M, Noble G, McGowan C. Fast food and fat fillies: The ills of western civilisation. Vet J 2006;172:396–397. [DOI] [PubMed] [Google Scholar]


