Abstract
Background
Dietary phosphate and protein restriction decreases plasma PTH and FGF‐23 concentrations and improves survival time in azotemic cats, but has not been examined in cats that are not azotemic.
Hypothesis
Feeding a moderately protein‐ and phosphate‐restricted diet decreases PTH and FGF‐23 in healthy older cats and thereby slows progression to azotemic CKD.
Animals
A total of 54 healthy, client‐owned cats (≥ 9 years).
Methods
Prospective double‐blinded randomized placebo‐controlled trial. Cats were assigned to test diet (protein 76 g/Mcal and phosphate 1.6 g/Mcal) or control diet (protein 86 g/Mcal and phosphate 2.6 g/Mcal) and monitored for 18 months. Changes in variables over time and effect of diet were assessed by linear mixed models.
Results
A total of 26 cats ate test diet and 28 cats ate control diet. There was a significant effect of diet on urinary fractional excretion of phosphate (P = 0.045), plasma PTH (P = 0.005), and ionized calcium concentrations (P = 0.018), but not plasma phosphate, FGF‐23, or creatinine concentrations. Plasma PTH concentrations did not significantly change in cats fed the test diet (P = 0.62) but increased over time in cats fed the control diet (P = 0.001). There was no significant treatment effect of the test diet on development of azotemic CKD (3 of 26 (12%) test versus 3 of 28 (11%) control, odds ratio 1.09 (95% CI 0.13–8.94), P = 0.92).
Conclusions and Clinical Importance
Feeding a moderately protein‐ and phosphate‐restricted diet has effects on calcium‐phosphate homeostasis in healthy older cats and is well tolerated. This might have an impact on renal function and could be useful in early chronic kidney disease.
Keywords: CKD, Feline, FGF‐23, Nutrition, PTH, Renal/Urinary tract
Abbreviations
- BCS
body condition score
- BSH
British Shorthair
- CI
confidence interval
- CKD
chronic kidney disease
- DHA
docosahexaenoic acid
- DLH
domestic longhair
- DSH
domestic shorthair
- EPA
eicosapentaenoic acid
- FE
fractional excretion
- FGF‐23
fibroblast growth factor 23
- FOS
fructo‐oligo‐saccharides
- GFR
glomerular filtration Rate
- GLM
New Zealand green‐lipped mussel extract
- iCa
ionized calcium
- LoD
limit of detection
- MCS
muscle condition score
- NFE
nitrogen‐free extract
- PTH
parathyroid hormone
- SBP
systolic blood pressure
- SRHP
secondary renal hyperparathyroidism
- TT4
total thyroxine
- UPC
urine protein‐to‐creatinine ratio
- USG
urine specific gravity
The prevalence of chronic kidney disease (CKD) in cats is high1 and increases with age,2 making it likely that a large number of older cats could have nonazotemic CKD. Thirty‐one percent of nonazotemic cats ≥9 years of age develop azotemic CKD by 12 months.3 Previous studies have looked at biomarkers for early CKD,4, 5, 6, 7, 8 risk factors for CKD9, 10, and predictors of azotemia development,3, 10 but at present, identification of cats with nonazotemic CKD remains difficult.
In humans, the collection of clinical, biochemical, and imaging abnormalities ultimately leading to renal osteodystrophy is now termed “chronic kidney disease‐mineral and bone disorder” (CKD‐MBD).11 Cats have disruption of calcium‐phosphate homeostasis in CKD, with hyperphosphatemia, hyperparathyroidism, decreased calcitriol and ionized calcium,12 and increased FGF‐23 concentrations.13 Additionally, cats with late‐stage CKD have decreased bone quality, with an increased number of bone resorption cavities in their femurs and decreased bone mineral density compared to healthy cats.14 Taken together, these data support the use of the term CKD‐MBD in cats with CKD. In humans, CKD‐MBD biochemical changes occur early in CKD to maintain a normal serum phosphate concentration in the face of declining glomerular filtration rate (GFR). Similarly, both parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF‐23) are increased in healthy older cats that develop CKD within 12 months compared to cats remaining nonazotemic,15, 16 suggesting that stimulation of phosphaturic hormones occurs to help maintain phosphate balance in the preazotemic stages of CKD.
Dietary phosphate restriction is considered the mainstay of treatment for naturally occurring azotemic CKD in cats. Commercially available diets containing low protein (≤60.2 g/MCal) and phosphate (≤1.0 g/MCal) reduce plasma PTH concentration and improve survival time in azotemic cats compared to cats eating maintenance adult diets with approximately 120 g/MCal protein and 4.8 g/MCal phosphate.17 Additionally, a diet marketed for cats with CKD containing restricted protein (≤67.4 g/MCal) and phosphate (≤1.2 g/MCal) reduces the incidence of uremic crises and renal‐related deaths when compared to a maintenance diet containing protein >92.0 g/MCal and phosphate ≥1.8 g/MCal.18 Plasma FGF‐23 concentrations significantly decrease after 28–56 days of feeding a diet containing low protein ≤66.5 g/MCal and phosphate ≤1.1 g/MCal in cats with stable azotemia.19 However, feeding a protein (69.7 g/MCal)‐ and phosphate (1.0 g/MCal)‐restricted diet instead of a high‐protein (129.4 g/MCal) and high‐phosphate (3.4 g/MCal) diet to young adult cats (2–3 years of age) over a short time period (4 weeks) resulted in higher plasma creatinine and phosphate concentrations, which was postulated to be because of a decrease in glomerular filtration rate (GFR).20
The benefit of phosphate and protein restriction in healthy older cats is unknown. The overall aim (primary outcome) of this study was to examine whether feeding a moderately protein (76 g/MCal)‐ and phosphate (1.6 g/MCal)‐restricted diet to cats ≥9 years of age could prevent progression to azotemic CKD, compared to feeding a maintenance diet with protein 86g/MCal and phosphate 2.6 g/MCal. More specifically, to examine whether moderate phosphate restriction would result in decreased fractional excretion of phosphate and reduced plasma PTH and FGF‐23 concentrations in healthy cats.
Materials and Methods
Animal Selection
Cats were recruited prospectively into this randomized double‐blinded controlled trial. The study protocol was approved by the Ethics and Welfare Committees of the Royal Veterinary College (URN 2011 1113) and Royal Canin. Clients of the Beaumont Sainsbury Animal Hospital were invited to bring apparently healthy cats ≥9 years of age to the clinic for free screening, with the incentive of receiving free food for 18 months for eligible cats. All cats seen between October 2011 and January 2013 were screened. Owners were requested to withhold food from their cat for at least 8 hours before appointments. The protocol followed at each visit is outlined in Figure 1.
Figure 1.
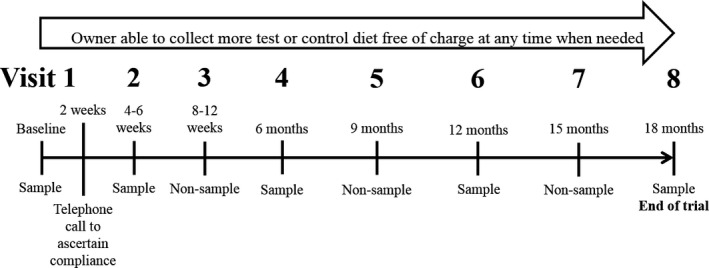
A timeline detailing when owners were requested to bring their cats into the clinic during the trial. All visits included history taking (including the completion of questionnaires detailing dietary history), measurement of systolic blood pressure, physical examination including assessment of bodyweight and body condition score, and dispensing of more diet as required. Sampling visits additionally included jugular venepuncture and cystocentesis for acquisition of blood and urine samples, respectively.
Cats were considered eligible for the trial if they were healthy on physical examination and had no outward signs of illness reported by their owners, with the exception of signs of osteoarthritis. Auscultation of a heart murmur, documentation of dental calculus ± gingivitis, and palpation of small kidneys were not exclusion criteria. Cats with plasma creatinine concentration >2 mg/dL were excluded unless they had a concurrent USG of ≥1.035. If no cystocentesis sample could be collected, the cat was reevaluated 1–2 weeks later and cats with persistent azotemia were excluded, unless a USG ≥1.035 was documented. Cats were excluded if they had long‐term medical problems within the last 6 months; a plasma total thyroxine (TT4) >40 nmol/L; recent recurrent lower urinary tract problems; or were currently being fed on a diet formulated for urinary tract conditions. Cats diagnosed with systemic hypertension, defined as systolic blood pressure (SBP) >160 mmHg with concurrent evidence of hypertensive retinopathy or mean SBP >170 mmHg on two occasions without evidence of hypertensive retinopathy, were treated with amlodipine besylate1 and reevaluated before enrollment.
Randomization and Blinding
Owners of cats that met the inclusion criteria were invited to take part in the 18‐month clinical trial. Consent was obtained from all owners. Cats were entered onto a randomization list by the same veterinary nurse for all cases, in strict chronologic order. The only exception was that eligible cats from the same household were assigned to the same diet (in total 4 households, each with 2 cats).
The food manufacturer assigned code names to the diets and labeled plain white bags with the code, a feeding guide, and an expiry date. The code was unblinded only after all analyses had been completed.
Test and Control Diets Used
The test diet used was a commercially available dry diet formulated for cats, marketed as a “senior” diet.2 To manufacture the control diet, any ingredients not normally present in an adult maintenance diet for cats were removed from the test diet, and protein and phosphate concentrations were adjusted to be similar to commercial adult maintenance diets. Both diets were formulated with the same ingredients with the exception of a few additional ingredients in the test diet; the nutritional composition and ingredients for each diet are shown in Table 1. The cholecalciferol (1000 IU/kg) and iodine (5 ppm) contents of both diets were the same, and both were formulated to induce a urine pH of 6–6.5 to reduce the incidence of struvite urolithiasis. Both diets provided all nutrients in excess of the National Research Council‐recommended nutrient requirements for cats.21 The test diet was lower in protein, higher in omega (ω)‐3 polyunsaturated fatty acids (PUFA), and lower in calcium and phosphate when compared to the control diet. Owners were given guidance on weight of the dry trial diet to feed per day based on bodyweight. The amount was tailored to help achieve weight loss in cats with a body condition score >6 of 9. Owners were encouraged not to feed any other food. Owners insistent on feeding a wet food were requested to feed a maximum of 50 g per day, to avoid all fish‐flavored foods and to avoid other “senior” diets. To maximize compliance, owners were asked to only rarely provide other foods (e.g., chicken, dairy) and treats. Owners were requested to keep a daily diary of all foods given to their cat during the trial period. Owners were encouraged to ensure that at least 66% of their cat's daily food intake (by volume) was the assigned diet. Because dry food has approximately 4 times more calories per gram than wet food, this was to ensure that the majority of the calorific intake of the cat (≥approximately 80%) would come from the study diets. In a previous study, feeding a protein (60.3 g/Mcal)‐ and phosphate (1.0 g/Mcal)‐restricted diet as at least two‐third of the daily energy requirements was sufficient to see a significant effect on plasma phosphate concentration in cats with azotemic CKD.17, 22
Table 1.
Ingredients for the test and control diets and mean dietary composition from three batches of each diet, as provided by the manufacturer. Nutrients expressed per Mcal of metabolizable energy with energy calculated by the National Research Council 2006 equation. Underlined ingredients were only present in the test diet and not in the control diet. Nutrients highlighted in bold illustrate the main differences between the two diets
| Nutrient | Test Diet Mean ± SD | Control Diet Mean ± SD |
|---|---|---|
| Energy (Kcal/Kg) | 3822 ± 29 | 3736 ± 15 |
| Water (g/Mcal) | 13.9 ± 1.59 | 15.3 ± 2.34 |
| Protein (g/Mcal) | 76.0 ± 1.13 | 85.5 ± 2.16 |
| Fat (g/Mcal) | 37.2 ± 1.13 | 36.7 ± 1.25 |
| Fatty Acids ω3 (g/Mcal) | 2.34 ± 0.61 | 0.95 ± 0.15 |
| Fatty Acids ω6 (g/Mcal) | 9.49 ± 0.85 | 10.1 ± 1.29 |
| Fatty Acids ω6/ω3 | 4.18 ± 0.74 | 10.7 ± 1.18 |
| C18:2 Linoleic Acid ω6 (g/Mcal) | 9.09 ± 0.81 | 9.84 ± 1.24 |
| C18:3 Gamma‐linoleic Acid ω6 (g/Mcal) | 0.09 ± 0.02 | 0.04 ± 0.00 |
| C20:4 Arachidonic Acid ω6 (g/Mcal) | 0.14 ± 0.02 | 0.15 ± 0.04 |
| C20:5 EPA (g/Mcal) | 0.88 ± 0.33 | 0.03 ± 0.01 |
| C22:6 DHA (g/Mcal) | 0.46 ± 0.07 | 0.03 ± 0.01 |
| NFE (g/Mcal) | 106 ± 2.44 | 97.9 ± 2.70 |
| Starch (g/Mcal) | 90.5 ± 2.59 | 80.7 ± 1.03 |
| Cellulose (g/Mcal) | 13.2 ± 1.32 | 14.4 ± 0.69 |
| Total dietary fibre (g/Mcal) | 28.9 ± 1.22 | 31.6 ± 1.74 |
| Minerals (g/Mcal) | 15.3 ± 0.87 | 17.9 ± 0.37 |
| Calcium (g/Mcal) | 1.93 ± 0.18 | 2.87 ± 0.19 |
| Phosphate (g/Mcal) | 1.59 ± 0.05 | 2.61 ± 0.15 |
| Sodium (g/Mcal) | 1.27 ± 0.08 | 1.20 ± 0.09 |
| Potassium (g/Mcal) | 2.09 ± 0.13 | 2.10 ± 0.12 |
| Chloride (g/Mcal) | 2.31 ± 0.08 | 1.73 ± 0.18 |
| Ingredients | Maize, wheat gluten, maize flour, dehydrated poultry protein, wheat, maize gluten, animal fats, rice, vegetable fibres, hydrolyzed animal proteins, chicory pulp, fish oil, soya oil, minerals, tomato (source of lycopene), psyllium husks and seeds, FOS, GLM 0.3%, hydrolyzed yeast (source of mannan‐oligo‐saccharides), hydrolyzed crustaceans (source of glucosamine), borage oil, marigold extract (source of lutein), hydrolyzed cartilage (source of chondroitin) | |
NFE, nitrogen‐free extract; FOS, fructo‐oligo‐saccharides; GLM, New Zealand green‐lipped mussel extract; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Study Follow‐Up
The schedule of clinic visits for this 18‐month trial is shown in Figure 1. At each visit, owners were questioned about dietary compliance and perceived palatability. The cats had their systolic blood pressure measured by Doppler, as previously described, and had a physical examination including measurement of bodyweight. Body condition score (BCS) was determined from 1 to 9 and muscle condition score (MCS) scored as normal (4), mild (3), moderate (2), or severe (1) muscle loss by the MCS system proposed by WSAVA.3
The following data were obtained at visits 1, 2, 4, 6, and 8. Blood samples were obtained by jugular venepuncture and urine samples collected by cystocentesis. Ionized calcium (iCa) was measured on whole blood immediately after sampling with a portable analyzer.4 Heparinized plasma was submitted to an external laboratory for biochemical analysis (and TT4 analysis on visits 1 and 6).5 Urinalysis and sediment examination were performed in house. Residual heparinized plasma, EDTA plasma, and urine were stored at −80°C. Batch analysis was performed at the external laboratory for urine protein‐to‐creatinine ratio (UPC) and urine phosphate. Batch analysis was performed in house to measure plasma FGF‐236 and PTH7 concentrations, as previously described.13 These data were also obtained at other time points if a cat became unwell during the study.
Development of azotemic CKD, death/euthanasia, and reaching visit 8 on the allocated intervention were considered trial end points. Renal azotemia was defined as a plasma creatinine concentration >2 mg/dL with concurrent urine specific gravity (USG) <1.035, or persistent azotemia on two consecutive occasions at least 2 weeks apart without evidence of a prerenal cause. Owners were instructed to follow the trial protocol until confirmation of azotemia in cases where no urine sample was obtained. If USG was documented to be >1.035, the study protocol was continued regardless of the plasma creatinine concentration. The trial was terminated if a cat refused to eat the trial diet; an owner repeatedly failed to attend appointments; a cat developed a significant medical condition requiring additional treatment; or adverse effects were observed that required cessation of the diet. All reasons for trial termination were recorded. Cases where no data were obtained while on the assigned intervention were excluded from analyses. Data for all other cases were included until the study end point or termination of the trial.
Statistical Analysis
Assuming 33% of cats on the control diet would develop azotemic CKD during the study period, and that <5% cats would develop azotemia on the test diet, with a 5% type I error rate and power of 80%, a sample size calculation suggested 30 cats would be required in each group. Statistical analyses were performed by SPSS.8 Statistical significance was determined as P < .05. Normality of variables was assessed by visual inspection of histograms. Results are reported as mean ± SD for normally distributed variables or as median [25th, 75th percentiles] for data not normally distributed. Variables at baseline were compared between groups by independent t‐tests or Mann‐Whitney U‐tests, as appropriate. Proportions were compared between groups by a Fisher's exact test. Treatment effect reported as an odds ratio with 95% confidence intervals (CI).
Fractional excretion of phosphate was calculated by the following formula:
Linear mixed‐effects models were constructed to compare the change in continuous variables across all visits and study the effect of diet on the changes identified. Variables with highly skewed distributions were logarithmically transformed. Data from all visits, including additional recheck visits, if performed, were included. Case number was treated as a random factor; all other variables were fixed factors. Time was treated as a continuous covariate with the unit expressed as month (28 days). Quadratic terms for time (time2) were included in initial models to account for potential nonlinear changes of variables over time and subsequently removed if not significant. Contrast analysis was performed posthoc to examine differences between the two diet groups at specific time intervals in variables with a significant diet*time interaction.
Generalized estimating equations by ordinal logistic link function were constructed to compare the change in categorical variables across all visits and the effect of diet. An exchangeable correlation structure was used to account for correlation among repeated measures from the same cat. Fixed factors and covariates were included as described above.
Results
One hundred and forty‐five cats were assessed for eligibility for the trial with a median age of 12.8 [11.2, 13.8] (range 9.0–21.0) years. Case enrollment, diet allocation, and follow‐up are summarized in a flow diagram as per the CONSORT 2010 statement23 (Fig 2). Additional information not included in Figure 2 is outlined below.
Figure 2.
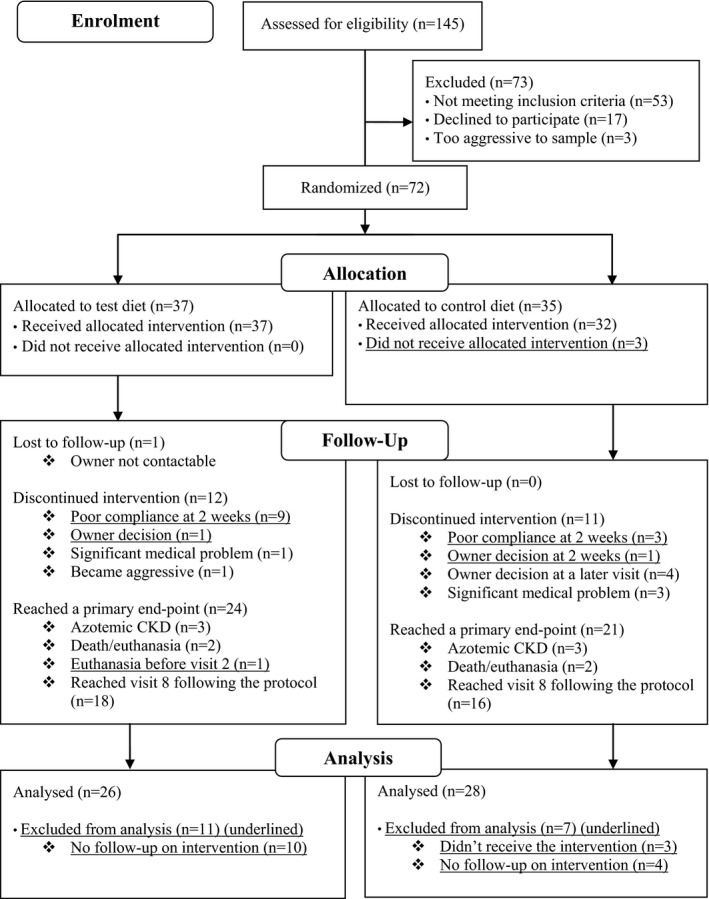
CONSORT 2010 Flow Diagram detailing study population recruitment and handling.
Reasons for not meeting the inclusion criteria included a diagnosis of azotemic CKD (n = 21), borderline CKD diagnosis with conflicting results on follow‐up (n = 6), TT4 > 40 nmol/L (n = 16), recurrent lower urinary tract problems/eating a urinary diet (n = 4), eating a prescription diet9 (n = 1), taking long‐term medication for suspected cardiovascular disease (n = 2), a diagnosis of diabetes mellitus (n = 2), and chronic eye problems (n = 1).
Medical problems diagnosed during the trial, which necessitated trial termination, were congestive heart failure (n = 1) for a cat on the test diet and severe weight loss (n = 1), hepatitis (n = 1), and diabetes mellitus (n = 1) for cats on the control diet.
Medications administered during the trial to cats assigned to the test diet included short‐term antibiotics for a cat bite abscess (n = 1), amlodipine besylate (n = 1), long‐term NSAIDs for osteoarthritis (n = 5), and short‐term NSAIDs (n = 3). Additionally, one cat received medications for pancarpal arthrodesis surgery for a short period of time, which occurred between visits 4 and 6. Medications administered during the trial to cats assigned to the control diet included amlodipine besylate (n = 1), clomipramine hydrochloride to manage urine spraying (n = 1), antibiotics for a urinary tract infection (n = 1), and long‐term NSAIDs for osteoarthritis (n = 1). Additionally, methimazole10 was administered to one cat that was diagnosed with hyperthyroidism (TT4 64.1 nmol/L) at visit 6 and was promptly and successfully treated medically, becoming euthyroid (TT4 30.1 nmol/L) within 2 months and remaining euthyroid at visits 7 and 8. There was no significant difference between the proportion of cats requiring long‐term NSAIDs between groups (test group 19% and control group 4%; P = 0.067).
Twenty‐six cats that received test diet and 28 cats that received control diet were included in analyses (Fig 2). Baseline variables were similar between groups, although UPC measurements were significantly higher in cats assigned to receive control diet (see Table 2).
Table 2.
Comparison of variables at visit 1 (baseline) for cats assigned to each diet. The only significant P value (P < .05) is highlighted in bold
| Test diet (n = 26) | Control diet (n = 28) | P value | |||
|---|---|---|---|---|---|
| Mean ± SD or Median [25th, 75th percentiles] | n | Mean ± SD or Median [25th, 75th percentiles] | n | ||
| Age (years) | 12.2 [11.2, 13.5] | 26 | 12.1 [11.1, 13.9] | 28 | 0.92 |
| Creatinine (mg/dl) | 1.5 ± 0.2 | 26 | 1.6 ± 0.2 | 28 | 0.73 |
| Urea (mg/dl) | 28.3 ± 5.6 | 26 | 27.2 ± 4.5 | 28 | 0.41 |
| Ionized calcium (mg/dl) | 1.27 ± 0.08 | 20 | 1.3 ± 0.06 | 24 | 0.24 |
| Total calcium (mg/dl) | 9.76 ± 0.60 | 26 | 9.88 ± 0.60 | 28 | 0.48 |
| Phosphate (mg/dl) | 3.88 ± 1.21 | 26 | 3.50 ± 0.50 | 28 | 0.14 |
| PTH (pg/ml) | 7.7 [2.6, 10.1] | 25 | 6 [2.6, 11.4] | 28 | 0.36 |
| FGF‐23 (pg/ml) | 153.4 [105.1, 208.6] | 25 | 124.3 [99.2, 209.9] | 28 | 0.31 |
| SBP (mmHg) | 132 ± 25 | 26 | 133 ± 22 | 28 | 0.88 |
| Weight (Kg) | 4.96 ± 1.29 | 26 | 4.54 ± 0.93 | 28 | 0.17 |
| BCS (1 to 9) | 6 ± 1.4 | 26 | 6 ± 1.41 | 28 | 0.34 |
| UPC | 0.13 [0.11, 0.17] | 13 | 0.20 [0.15, 0.24] | 15 | 0.045 |
| USG | 1.048 ± 0.01 | 13 | 1.042 ± 0.014 | 15 | 0.23 |
| TT4 (nmol/l) | 22.8 ± 7.9 | 26 | 22.6 ± 8.9 | 28 | 0.91 |
| Sex | 18 MN, 8 FN | 12 MN, 16 FN | |||
| Breeds | 23 DSH, 3 DLH | 21 DSH, 5 DLH, 1 BSH, 1 Chinchilla X | |||
n, number of cats in group; SBP, systolic blood pressure; BCS, body condition score; UPC, urine protein‐to‐creatinine ratio; USG, urine specific gravity; TT4, plasma total thyroxine concentration; MN, male neutered; FN, female neutered; DSH, domestic shorthair; DLH, domestic longhair; BSH, British Shorthair.
Effect of Diet Over Time
The median [range] timings for visits 2–8 were visit 2: 35 days [28–56], visit 3: 105 days [70–140], visit 4: 189 days [140–233], visit 5: 287 days [245–322], visit 6: 368 days [315–413], visit 7: 462 days [420–497], and visit 8: 553 days [474–595]. On six occasions, additional visits occurred during the study period for the following reasons: to confirm the presence of azotemic CKD (4 visits) or to assess control of hyperthyroidism (2 visits). On 24 occasions, cats were not presented to the clinic, of which 23 of 24 were nonsample visits; whenever possible, compliance with the assigned diet was checked by telephone in this circumstance.
The proportion of cats lost from the trial because of poor compliance was not significantly different between groups (9 of 37 test vs. 3 of 32 control, P = 0.10). For the 54 cats included in the analyses, the amount of diet eaten was recorded as <66% on 11 of 284 occasions, but was always ≥50%. There was no difference in percentage of diet eaten between the groups (P = 0.74) or change in the percentage of diet eaten over time (P = 0.69). There was no difference between the proportion of cats in each group that died or were euthanized (2 of 26 test vs. 2 of 28 control, P = 0.34) during the study (see Fig 2). Assessment of the primary outcome found no difference in the number or proportion of cats that developed azotemic CKD in each group (3 of 26 (12%) test vs. 3 of 28 (11%) control, odds ratio 1.09 (95% CI 0.13–8.94), P = 0.92).
The majority of cats in this study ate some wet food in addition to the study diet provided: 17 of 26 cats assigned to the test diet and 18 of 28 cats assigned to the control diet. In all cases, the wet foods offered were not marketed as a “senior” diet. A variety of brands were offered, with Whiskas® being the most commonly fed brand. The majority of cats were also offered some “human” foods, but in all cases, the foods offered were small amounts, offered occasionally, and no cat received these foods as a substantial part of their diet. The most common foods offered were chicken (28 cats) and dairy (milk, cream, yoghurt, or cheese) (17 cats). Additional dry foods were offered to a small number of cats: 6 of 26 cats on the test diet and 2 of 28 on the control diet. However, only 2 cats (one on each diet) received more than a few kibbles of alternative dry food per day.
A summary of variables examined over time and the effects of diet are shown in Table 3a and b. Variables that did not change over time and no effect of diet was seen: plasma phosphate, logFGF‐23 (Fig 3) and potassium concentrations, SBP, or logUPC. Although UPC concentrations were higher in the control diet group at baseline, there was no difference between the groups at any other time points (P > 0.57). Total calcium (P = 0.032) and TT4 concentrations (P = 0.007) increased, while BCS (P < 0.001), bodyweight (P < 0.001), MCS (P < 0.001), USG (P = 0.047), and plasma creatinine (P = 0.030) decreased over time for all cats, independent of diet fed (Fig 3).
Table 3.
(a and b): Linear mixed model analysis of changes in variables during the study period showing (a) summary of P values for terms included in the models and (b) summary of intercepts and slopes (n = 54)
| Variable | Diet | Time | Diet*Time | Time2 |
|---|---|---|---|---|
| (a) | ||||
| Weight (Kg) | 0.20 | 0.98 | 0.067 | 0.011 |
| Ionised calcium (mmol/L) | 0.48 | 0.59 | 0.018 | 0.022 |
| FE phosphate (%) | 0.61 | 0.021 | 0.045 | 0.040 |
| BCS (1 to 9) | 0.80 | <0.001 | NS | NS |
| MCS | 0.52 | <0.001 | NS | NS |
| Total T4 (nmol/L) | 0.75 | 0.007 | NS | NS |
| Creatinine (mg/dL) | 0.66 | 0.030 | NS | NS |
| Total calcium (mg/dL) | 0.58 | 0.032 | NS | NS |
| USG | 0.84 | 0.047 | NS | NS |
| logUPC | 0.39 | 0.090 | NS | NS |
| SBP (mmHg) | 0.39 | 0.29 | NS | NS |
| logFGF23 (pg/ml) | 0.11 | 0.32 | NS | NS |
| Potassium (mEq/L) | 0.95 | 0.76 | NS | NS |
| Urea (mg/dL) | 0.92 | 0.84 | NS | NS |
| % study diet eaten | 0.82 | 0.84 | NS | NS |
| Phosphate (mg/dL) | 0.35 | 0.89 | NS | NS |
| Variable | Test Diet | Control Diet | ||||
|---|---|---|---|---|---|---|
| Intercept | Slope of Time | Slope of Time2 | Intercept | Slope of Time | Slope of Time2 | |
| (b) | ||||||
| Weight (Kg) | 4.94 ± 0.21 | (−0.003) ± 0.006 | (−0.008) ± 0.003 | 4.56 ± 0.20 | 0.004 ± 0.006 | (−0.008) ± 0.003 |
| Ionised calcium (mmol/L) | 1.28 ± 0.013 | 0.0002 ± 0.002 | 0.0002 ± 8.02E‐05 | 1.30 ± 0.013 | (−0.002) ± 0.002 | 0.0002 ± 8.02E−05 |
| FE phosphate (%) | 40.5 ± 2.8 | (−1.83) ± 0.64 | 0.066 ± 0.032 | 38.6 ± 2.7 | (−1.15) ± 0.65 | 0.066 ± 0.032 |
| BCS (1 to 9) | 6.05 ± 0.22 | (−0.021) ± 0.006 | NA | 5.97 ± 0.21 | (−0.021) ± 0.006 | NA |
| MCS (1 to 4) | 3.69 ± 0.075 | (−0.016) ± 0.004 | NA | 3.63 ± 0.073 | (−0.016) ± 0.004 | NA |
| Total T4 (nmol/L) | 22.9 ± 1.8 | 0.30 ± 0.11 | NA | 22.1 ± 1.8 | 0.30 ± 0.11 | NA |
| Creatinine (mg/dL) | 1.51 ± 0.05 | (−0.004) ± 0.002 | NA | 1.54 ± 0.05 | (−0.004) ± 0.002 | NA |
| Total calcium (mg/dL) | 9.89 ± 0.11 | 0.009 ± 0.004 | NA | 9.81 ± 0.11 | 0.009 ± 0.004 | NA |
| USG | 1.046 ± 0.003 | (−0.0002) ± 0.0001 | NA | 13045 ± 0.003 | (−0.0002) ± 0.0001 | NA |
| logUPC | (−0.82) ± 0.04 | 0.002 ± 0.001 | NA | (−0.78) ± 0.03 | 0.002 ± 0.001 | NA |
| SBP (mmHg) | 130.5 ± 2.8 | (−0.12) ± 0.11 | NA | 133.8 ± 2.8 | (−0.12) ± 0.11 | NA |
| logFGF23 (pg/mL) | 2.20 ± 0.05 | (−0.001) ± 0.001 | NA | 2.09 ± 0.05 | (−0.001) ± 0.001 | NA |
| Potassium (mEq/L) | 3.99 ± 0.05 | 0.0007 ± 0.0023 | NA | 3.99 ± 0.049 | 0.0007 ± 0.0023 | NA |
| Urea (mg/dL) | 27.5 ± 1.02 | (−0.007) ± 0.04 | NA | 27.4 ± 0.99 | (−0.007) ± 0.04 | NA |
| % study diet eaten | 84.9 ± 2.6 | (−0.014) ± 0.069 | NA | 84.1 ± 2.5 | (−0.014) ± 0.069 | NA |
| Phosphate (mg/dL) | 3.73 ± 0.09 | 0.0007 ± 0.005 | NA | 3.61 ± 0.09 | 0.0007 ± 0.005 | NA |
Outcome variables showing significant change over time and between groups (P < .05) are highlighted in bold. The unit used for time was month (28 days). Diet represents cats assigned to test or control diet.
The term diet*time2 was not significant in any models and is therefore not included in the table. A significant effect of time2 indicates a significant nonlinear change in both groups during the study period. If time2 was not significant (NS), this term was removed from the model.
A significant interaction in diet*time indicates the rate of change of the outcome variable differed between diets and therefore indicates a difference between feeding test or control diet. If diet*time was not significant, it suggests that any change in the outcome variable occurred in all cats independent of which diet they were assigned to and the term was therefore removed from the model.
For models where diet*time was NS, a significant change in time indicates the outcome variable significantly increased or decreased during the study period and that the change seen was linear.
For linear changes over time, the slope of time2 is not applicable (NA).
Figure 3.
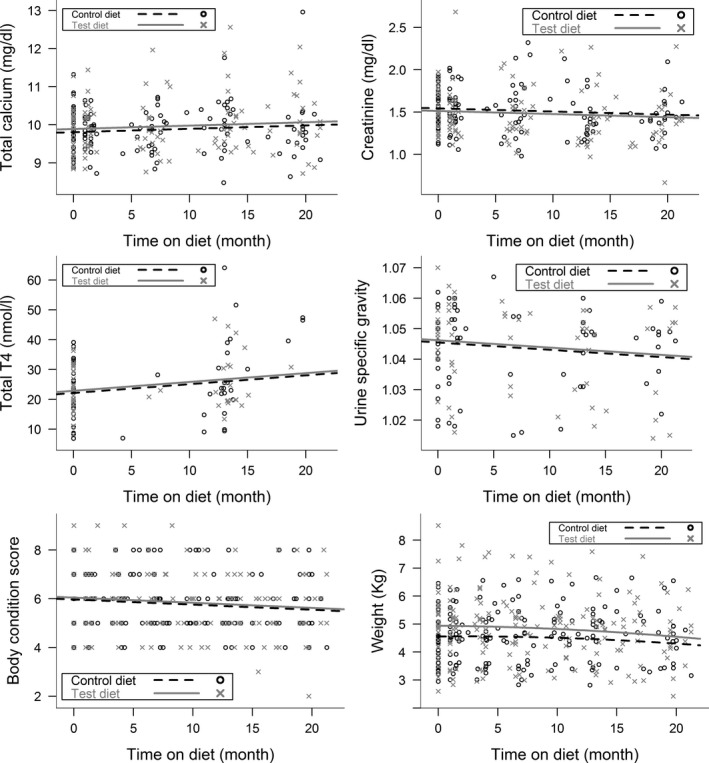
Scatter plots of plasma total calcium, creatinine and TT4 concentrations, USG, bodyweight and BCS for cats eating the test or control diets during the study period. All changes over time were linear (except for bodyweight) and were independent of which diet the cats were eating. There was a significant increase in plasma total calcium (P = 0.032) and TT4 (P = 0.007) concentrations and a significant decrease in plasma creatinine concentrations (P = 0.030), body condition score (P < 0.001), and urine specific gravity (P = 0.047) during the study period. Bodyweight decreased significantly over time (P = 0.011), and this change was independent of which diet the cats were eating. The change over time was nonlinear.
FE phosphate changed significantly over time for both groups (P = 0.040); in both cases, the change was nonlinear and the two diets had different effects (see Fig 4). Posthoc comparisons indicated that FE phosphate was significantly higher in cats eating control diet by 15 months (P = 0.045) and for the remainder of the study period (all P < 0.05).
Figure 4.
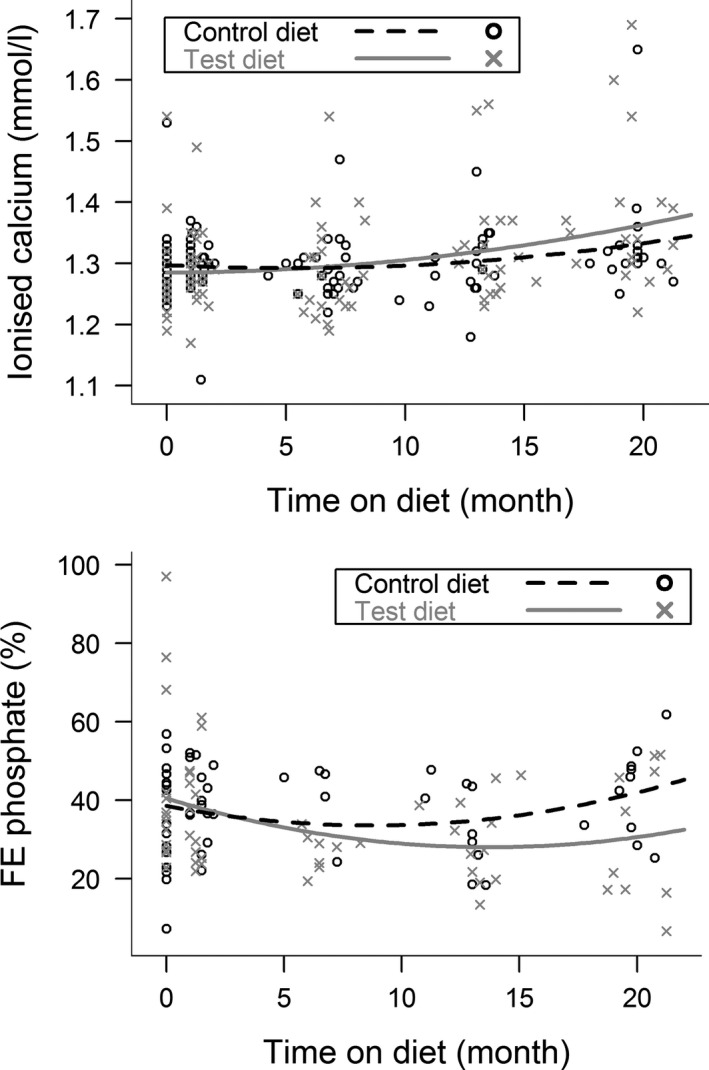
Scatter plots of whole blood ionized calcium concentration and FE phosphate for cats eating the test and control diets during the study period. All changes over time were nonlinear. Ionized calcium increased significantly over time (P = 0.022), and there was a difference between cats eating test diet and cats eating control diet over time (P = 0.018). However, post hoc comparisons revealed no significant difference between the cats on the different diets at any individual time points during the study period. FE phosphate initially decreased and subsequently increased during the study period (P = 0.040), and there was a difference between cats eating test diet and cats eating control diet over time (P = 0.045). Post hoc comparisons found that FE phosphate was significantly lower in cats eating test diet at the 15 month time point (P = 0.045) and for the remainder of the study period.
Ionized calcium concentrations increased over time for both groups (P = 0.022). Although the magnitude of the increase was greater for the test diet group, the size of the effect was small and posthoc comparisons found no significant difference between the groups at any individual time points (Fig 4). Ionized hypercalcemia (>1.4 mmol/L) was present in two cases at visit 1; one assigned to each diet and was persistent for the cat on test diet but normalized for the remainder of the trial for the cat on control diet. A further 6 cats developed ionized hypercalcemia during the study period. One cat assigned to control diet developed ionized hypercalcemia from visit 4 onwards, with total hypercalcemia from visit 6 onwards. Five cats eating test diet developed ionized hypercalcemia, including 3 cases at visit 8 (total calcium normal), 1 cat from visit 4 onwards (total calcium always normal), and the cat that had a pancarpal arthrodesis was transiently hypercalcemic for both ionized and total calcium following surgery. Plasma PTH concentrations were below the limit of detection (LoD) of the assay for all cats at visits where ionized hypercalcaemia was demonstrated, with the exception of one cat diagnosed with azotemic CKD at visit 8 (plasma PTH concentration 14.0 pg/mL). Plasma FGF‐23 concentrations were increased throughout the study period for the cat that was persistently hypercalcemic from baseline. Plasma FGF‐23 concentrations increased to above reference interval in two cats (one on each diet) that demonstrated persistent ionized hypercalcemia from visit 4 onwards. The proportion of cats that developed ionized hypercalcemia while eating test diet was higher than for cats eating control diet, but this failed to reach statistical significance (5 of 26 test vs. 1 of 28 control, P = 0.067).
Of the 240 PTH measurements obtained, 100 (41.6%) were below the LoD of the assay. PTH was therefore analyzed as a categorical variable based on a previously derived reference interval (RI)19 by four categories: (1) below the assay LoD (<5.2 pg/mL), (2) RI lower half (5.2–8.8 pg/mL), (3) RI upper half (8.9–17.6 pg/mL), and (4) above the RI (>17.6 pg/mL), to allow inclusion of all measurements. There was a significant difference in plasma PTH concentrations between the two diets during the study period (P = 0.005); feeding test diet was associated with no change in PTH over time [odds ratio (OR) 0.99, 95% confidence interval (CI) 0.96–1.03, (P = 0.62)], and feeding control diet was associated with a 7% increase in the odds of progressing to a higher PTH category per month of the study period [OR 1.07, 95% CI 1.03–1.12, (P = 0.001)] (Fig 5).
Figure 5.
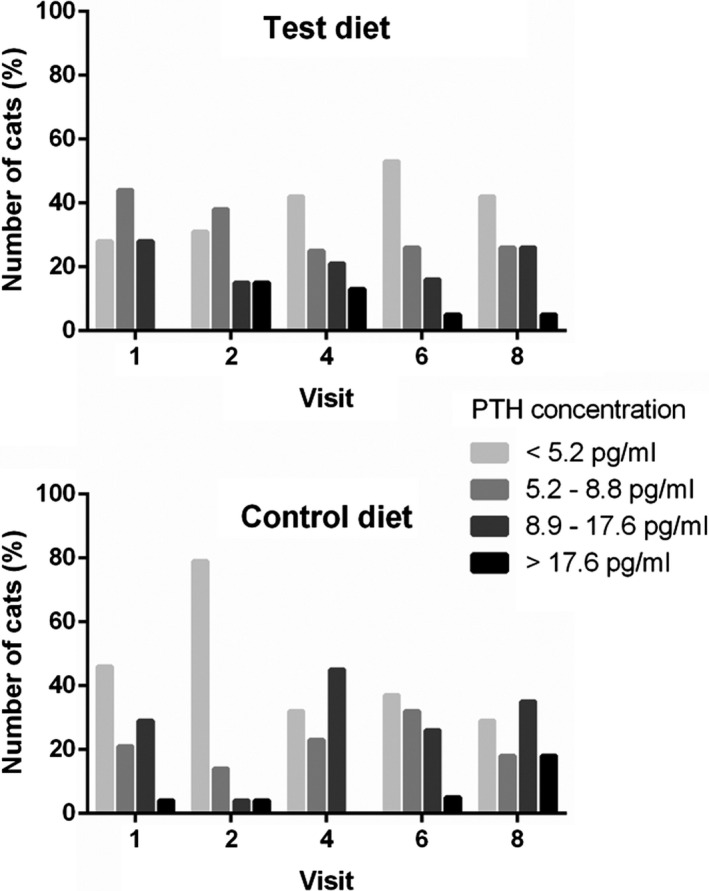
Bar charts of the percentage of cats in each PTH category at each sampling visit for cats eating the test and control diets during the study period. There was a significant difference between cats assigned to the test vs. control diet over the study period (P = 0.005). Cats fed the test diet demonstrated no significant change in PTH category during the study period (P = 0.62). Cats fed the control diet demonstrated a significant increase of 7% in the odds of moving up a PTH category for every month of the study period (P = 0.001).
Discussion
In the present study, feeding the moderately protein‐ and phosphate‐restricted test diet to healthy older cats was associated with lower FE phosphate and more stable PTH concentrations but a slightly greater increase in ionized calcium when compared to the control diet. There was no difference in the proportion of cats developing azotemic CKD between groups. Some cats from both groups were lost before visit 2 because of poor compliance; however, compliance for all remaining cats was excellent for the entire 18 month study.
The groups were well matched at baseline; however, UPC was higher in the cats assigned to the control diet group. We consider this likely to be a type I error, as there was no difference in UPC between groups at any other time point and no effect of diet on UPC over time.
FE phosphate was significantly lower in cats eating the test diet compared to those eating the control diet. This was expected, because maintaining stable total body phosphate should require a decrease in phosphate excretion if phosphate intake is reduced (and GFR does not change). Exact phosphate intake during the study could not be determined in these cats because reported dietary intake by owners could not be verified. This is a potential limitation; however, the results more accurately reflect the situation in the clinic than results from studies that use laboratory cats. Interestingly, FE phosphate was not significantly different between the groups until the 15‐month time point and at the beginning of the study period it decreased in all cats. This suggests that dietary phosphate load was higher or more bioavailable in the varied baseline diets than both the test and control diets. This may be because of the feeding of more high‐protein “treats” such as meat, fish, and dairy products before the study. On enrollment, owners were requested not to feed fish and to minimize treats, particularly in overweight cats. Ideally all cats would have had a wash‐in period before the start of the study in which they all received the same diet. This was not performed as it would have required additional sampling in these healthy cats and might have reduced compliance with the study protocol in these client‐owned animals. However, given the long timecourse of this study, it need not be considered a substantial study limitation.
Plasma phosphate and FGF‐23 concentrations remained stable for all cats during the study. It is possible that no difference was seen between the groups because there was not enough difference in phosphate content between the two diets. Alternatively, it could be expected that plasma phosphate concentrations would not change in these healthy cats, because plasma phosphate concentration does not change when feeding a more markedly phosphate‐restricted diet (<1.1 g/Mcal) to CKD cats that are normophosphatemic for their IRIS stage.19 However, plasma FGF‐23 concentrations do decrease significantly in all CKD cats fed a diet with <1.1 g/Mcal phosphate.19 Additionally, short‐term dietary phosphate restriction (maximum study duration 4 weeks) in healthy people decreases FGF‐23 concentrations.24, 25, 26, 27 Therefore, plasma FGF‐23 concentrations were expected to decrease in cats fed the test diet in the present study. As plasma phosphate concentrations were stable for all cats, the lack of change in FGF‐23 might be an appropriate physiologic response, but FE of phosphate decreased on both diets. Additionally, there was no decrease in PTH concentrations in either group. This raises the question of what was responsible for the change in phosphate excretion if not FGF‐23 (or PTH), unless the timing of sampling, or the assay used for FGF‐23 measurement prevented subtle changes in this hormone being detected.
Circulating FGF‐23 concentrations do not change acutely postprandially in healthy humans28 or those with early CKD29 and do not demonstrate a circadian rhythm in healthy humans or hemodialysis patients.30, 31 These observations have not been examined in the cat, but to minimize the effect of eating and of circadian variation on all parameters, owners were telephoned the day before every appointment to remind them to starve their cats for at least 8 hours and appointments were conducted in the mornings. Studies of murine models,32, 33 of healthy humans25, 26 and CKD patients,34, 35 have revealed variable responses of urinary phosphate excretion, serum phosphate, and serum FGF‐23 concentrations with changes in dietary phosphate load. One study of 16 normophosphatemic stage 3 or 4 CKD patients randomized in a 2 × 2 factorial design to diets with different phosphate loads (P) ± a phosphate binder (PB) (750 mg P + PB, 1500 mg P + placebo, 750 mg P + placebo, 1500 mg P + PB daily) for 2 weeks found no significant changes in serum phosphate or FGF‐23 concentrations in any group, but significant reductions in 24‐hour phosphate excretion were seen in all groups except the group receiving the highest dietary phosphate load (1500 mg + placebo).36 It seems, therefore, that dietary phosphate load can alter urinary phosphate excretion via a mechanism independent of FGF‐23. Infusion of phosphate into the duodenum and intravenous infusion of duodenal extracts into rats induce a rapid increase in FE phosphate.37 This is independent of FGF‐23, PTH, and the phosphatonin sFRP‐4 and is unchanged by denervation of the kidney. Therefore, it has been proposed that the duodenal mucosa secretes a phosphatonin‐like substance which induces a rapid increase in the fractional excretion of phosphorus.37 However, to date, the substance(s) involved in this gut‐renal axis remain unknown. Furthermore, although the presence of a phosphate‐sensing mechanism in various organs has been postulated,38 this also remains to be elucidated.
Cats fed the test diet had no change in plasma PTH concentrations throughout the trial, whereas PTH concentration increased in cats fed the control diet. The majority of these increases occurred within the reference interval. It is difficult to assess the definitive cause of this difference between groups. The control diet had a slightly lower calcium:phosphate (Ca:P) ratio (1.10) than the test diet (1.21), which may have been a stimulus of PTH secretion; however, ionized calcium increased over time in both groups. Alternatively, the change in PTH for cats eating the control diet may reflect an age‐related increase. Age has previously been shown to be a predictor of increasing PTH in both humans39, 40 and cats.15 PTH is increased at baseline for cats that develop azotemia over 12 months, compared to cats remaining nonazotemic.15 It is not possible to assess the effect that these differences in PTH concentration had, if any, on the progression of CKD in the present study, as the proportion of cats that developed azotemia was low in both diet groups. Additionally, measurement of PTH in this study was hampered by poor sensitivity of the assay, resulting in 41.6% of samples being below the LoD. Unfortunately, currently, there are no validated PTH assays for use with feline samples that do not have poor sensitivity.41 The assay used in this study measures intact PTH and has been validated previously.42, 43 An assay measuring biologically active “whole” PTH molecule has also been validated, but has not been found to offer advantage over the intact PTH assay.42
Total calcium concentrations increased in all cats during the study (independent of diet). Both diets could have had greater calcium bioavailability than the mixture of diets fed at baseline or could have resulted in a similar increase in calcium absorption, bone resorption, or reduced calcium excretion. Alternatively, total calcium may increase with age. In contrast, apparently healthy cats >10 years of age in Belgium have significantly lower total calcium concentrations than cats 6–10 years old.44 Another study found a negative effect of age on plasma total calcium concentrations in 525 healthy purebred cats, but deemed it to be clinically irrelevant when establishing a reference range.45 Ionized calcium concentrations also increased during the study period, but there was a significant difference in this change between the groups. The increase in calcium does not appear to be PTH‐mediated, as the test diet was associated with stable PTH but a greater increase in ionized calcium. Additionally, it does not appear to be vitamin D‐mediated as both diets had the same vitamin D concentration, and both diets had were mildy acidifying (urine pH 6–6.5) and therefore will not have induced difference in urinary calcium excretion. Development of ionized hypercalcemia occurred in five cats eating test diet and only one cat eating control diet; however, this difference was not significant. It is possible that these cats developed idiopathic hypercalcemia, independent of dietary calcium/phosphate load. However, feeding a substantially phosphate‐restricted renal diet has previously been associated with development of ionized hypercalcemia in azotemic cats.22 It is plausible that moderate dietary phosphate restriction could induce ionized hypercalcemia via the same (undetermined) mechanism in healthy cats and although the consequence of this is unclear, it should be considered when advising on diets for healthy older cats, as it might increase the risk of developing calcium‐containing uroliths.
TT4 concentrations gradually increased for all cats during the study, but only one cat was diagnosed with hyperthyroidism. A previous prospective study reported an annual incidence of hyperthyroidism diagnosis of 7.4% in cats with a median age of 12 years;46 however, cats in the present study were excluded if on screening, their TT4 > 40 nmol/L. The consequence of the gradual increase in TT4 seen during the present study is unknown, but were this increase to continue, more of these cats would go on to become hyperthyroid over time, which would not be unexpected because increasing age is an independent risk factor for development of feline hyperthyroidism.47
Bodyweight and BCS decreased in both groups during the study period. At enrollment, 61% (33 of 54) of the cats had a BCS of 6 of 9 or greater and owners were instructed how much to feed their cats and encouraged to weigh the food. Thus, part of the observed reduction in bodyweight may indicate successful reduction in body fat. However, MCS also decreased over time in both groups. Although data on sarcopenia in cats are currently lacking, it is well recognized in humans and to a lesser extent in dogs that lean body mass declines during aging,48 which could be one explanation for the decrease in MCS seen during this study period. Alternatively, lean body mass of cats can be affected by protein intake;49 however, additional studies are required to investigate this relationship in older cats.
Plasma creatinine concentration decreased during the study in cats on both diets. The mean decrease in creatinine was small (0.12 mg/dL) and is likely a result of the reduction in lean body mass or is possibly secondary to an increase in GFR mediated by the increase TT4. USG significantly decreased gradually over time for cats on both diets, but in isolation is an unreliable indicator of renal function as this change could have been secondary to other changes, for example, reduced protein intake. A cross‐sectional study of apparently healthy older cats found no significant difference between serum creatinine concentrations or USG in cats >10 years and cats 6–10 years of age; however, in agreement with the results of the present study, mean creatinine and USG were slightly lower in the older cats and the paired design of the present study gives it greater power to detect differences.44
The test diet was higher in ω‐3 PUFA than the control diet, which at high doses have renoprotective effects in experimental canine models of kidney disease,50 although it is not possible from this study to assess whether the increased concentration of ω‐3 PUFA had effects on the cats’ renal function. It is also possible that this difference could have had an impact on calcium‐phosphate homeostasis as there is emerging evidence that ω‐3 PUFA supplementation might reduce bone turnover in postmenopausal women.51, 52 Markers of bone turnover were not examined in this study; however, ionized calcium concentrations increased more greatly in the test diet group, which could be a result of decreased calcium deposition in bone. Further studies into the effects of ω‐3 PUFA in cats are warranted.
This trial recruited client‐owned cats to improve its generalizability to veterinary clinical practice. A limitation of this study is that the randomization of cats to either test or control diet was skewed for cats in multicat households; however, this was considered necessary to ensure compliance with the assigned diet. The study was not subjected to an intention‐to‐treat analysis because of the duration of the trial. Failure of cats to eat the assigned diet would have had a substantial impact on the comparison between test and control diets and bias the estimate of the effects of feeding the test diet. Therefore, to address the aims of this study, it was considered judicious to analyze data only from cats that had at least one follow‐up visit on the intervention. Data on compliance are presented to show what level of acceptance of dietary change can be achieved in cats of this age bearing in mind the incentive to owners of receiving diet free of charge. The majority of cats ate other foods during the study. Although fish was not consumed (because of the high ω3 PUFA content), and quantities of fresh meat and dairy were low, 17–18 cats in each group regularly ate a wet maintenance diet cat food. This is a limitation of the study as it is difficult to quantify the additional phosphate consumed by these cats, which may have impacted on the ability to see an effect of the test diets on calcium‐phosphate homeostasis. Future studies aiming to examine the effect of feline diets in client‐owned cats should be aware that pet owners may not be willing to feed one diet type exclusively.
Various medications were administered to cats during the study period in both groups, as needed based on clinical judgement. The most frequently prescribed medications were NSAIDs for osteoarthritis. It is difficult to assess the impact these medications may have had, if any, on the study outcomes. However, the proportion of cats requiring long‐term NSAIDs was not significantly different between groups and the need for pain relief to manage osteoarthritis commonly occurs in older cats. Therefore, the use of these medications in this study cohort reflects the wider management of older cats in veterinary clinical practice.
Only 11% (6 of 54) of cats were diagnosed with azotemic CKD during the present study. A previous prospective study, where no attempt was made to modify the cats’ diets during the study period, found 30.5% of cats developed azotemic CKD within 12 months.3 The disparity between these studies may be attributable to having a slightly younger population of cats in the present study, 12.2 [11.2, 13.8] years, compared to 13.0 [11.0, 15.0] years in the previous study. Given the very low rate of azotemia development in this study population, thousands of cats would have been needed to detect a significant difference in development of azotemic CKD between groups with these two diets. Recruiting very large numbers of cases for clinical veterinary studies is difficult. Further studies should therefore consider using diets with greater difference in protein and phosphate content, following cats for longer periods, or assessing renal function with GFR measurements to assess whether protein and phosphate restriction can have beneficial effects in preazotemic CKD cats.
In conclusion, feeding the test diet to healthy older cats was associated with a decrease in FE phosphate, and a greater increase in ionized calcium concentration when compared to cats fed the control diet. Cats eating the control diet had a significant increase in PTH concentration during the study period, which was not observed for cats fed the test diet. However, it is not possible to conclude whether feeding the test diet benefited renal function; further investigations in nonazotemic cats should consider using an older population, or following cats for longer than 18 months, to evaluate more thoroughly the effect of protein and phosphate restriction on renal function.
Acknowledgments
This study was performed at the Royal Veterinary College, London, UK, and funded by Royal Canin SAS, Aimargues, France.
The authors acknowledge Mrs. Nicola Lötter for her help with this study.
Conflict of Interest Declaration
V. Biourge is employed by Royal Canin. The Renal Research Clinic at the Royal Veterinary College acknowledges support from Royal Canin for its research on feline hyperphosphatemia and chronic kidney disease. R. Geddes is in receipt of an Everts Luff Trust Research Training Fellowship.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Footnotes
Istin, Pfizer, Sandwich, Kent, UK
Senior Consult Stage 1 Balance, Royal Canin SAS, Aimargues, France
ISTAT, Woodley Equipment, UK
Idexx laboratories, Wetherby, UK
FGF‐23 ELISA Kit, Kainos Laboratories, Tokyo, Japan
Total intact PTH immunoradiometric assay – coated bead version, 3KG600, Scantibodies, Santee, CA
IBM SPSS Statistics 21, IBM Corporation, Armonk, NY
Prescription diet w/d® Feline, Hills Pet Nutrition Inc., Topeka, KS
Felimazole, Dechra Veterinary Products Limited, Northwich, UK. A dose of 2.5 mg q12h was administered for 28 days, followed by a dose adjustment to 2.5 mg alternating with 5 mg q12h, which was maintained for the remainder of the study period
References
- 1. Lulich JP. Feline renal failure: Questions, answers, questions. Compend Contin Educ Vet 1992;14:127–152. [Google Scholar]
- 2. Marino CL, Lascelles BD, Vaden SL, et al. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg 2014;16:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jepson RE, Brodbelt D, Vallance C, et al. Evaluation of predictors of the development of azotemia in cats. J Vet Intern Med 2009;23:806–813. [DOI] [PubMed] [Google Scholar]
- 4. Jepson RE, Vallance C, Syme HM, Elliott J. Assessment of urinary N‐acetyl‐beta‐D‐glucosaminidase activity in geriatric cats with variable plasma creatinine concentrations with and without azotemia. Am J Vet Res 2010;71:241–247. [DOI] [PubMed] [Google Scholar]
- 5. Jepson RE, Syme HM, Markwell P, et al. Measurement of urinary cauxin in geriatric cats with variable plasma creatinine concentrations and proteinuria and evaluation of urine cauxin‐to‐creatinine concentration ratio as a predictor of developing azotemia. Am J Vet Res 2010;71:982–987. [DOI] [PubMed] [Google Scholar]
- 6. Jepson RE, Coulton GR, Cowan ML, et al. Evaluation of mass spectrometry of urinary proteins and peptides as biomarkers for cats at risk of developing azotemia. Am J Vet Res 2013;74:333–342. [DOI] [PubMed] [Google Scholar]
- 7. van Hoek I, Daminet S, Notebaert S, et al. Immunoassay of urinary retinol binding protein as a putative renal marker in cats. J Immunol Methods 2008;329:208–213. [DOI] [PubMed] [Google Scholar]
- 8. Hall JA, Yerramilli M, Obare E, Jewell DE. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J Vet Intern Med 2014;28:1676–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greene JP, Lefebvre SL, Wang M, et al. Risk factors associated with the development of chronic kidney disease in cats evaluated at primary care veterinary hospitals. J Am Vet Med Assoc 2014;244:320–327. [DOI] [PubMed] [Google Scholar]
- 10. Finch NC. Predicting the Development of Azotaemia in Geriatric Cats [thesis]. London (UK): University of London; 2011. [Google Scholar]
- 11. Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006;69:1945–1953. [DOI] [PubMed] [Google Scholar]
- 12. Barber PJ, Elliott J. Feline chronic renal failure: Calcium homeostasis in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract 1998;39:108–116. [DOI] [PubMed] [Google Scholar]
- 13. Geddes RF, Finch NC, Elliott J, Syme HM. Fibroblast growth factor 23 in feline chronic kidney disease. J Vet Intern Med 2013;27:234–241. [DOI] [PubMed] [Google Scholar]
- 14. Shipov A, Segev G, Meltzer H, et al. The effect of naturally occurring chronic kidney disease on the micro‐structural and mechanical properties of bone. PLoS ONE 2014;9:e110057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finch NC, Syme HM, Elliott J. Parathyroid hormone concentration in geriatric cats with various degrees of renal function. J Am Vet Med Assoc 2012;241:1326–1335. [DOI] [PubMed] [Google Scholar]
- 16. Finch NC, Geddes RF, Syme HM, Elliott J. Fibroblast growth factor 23 (FGF‐23) concentrations in cats with early nonazotemic chronic kidney disease (CKD) and in healthy geriatric cats. J Vet Intern Med 2013;27:227–233. [DOI] [PubMed] [Google Scholar]
- 17. Elliott J, Rawlings JM, Markwell PJ, Barber PJ. Survival of cats with naturally occurring chronic renal failure: Effect of dietary management. J Small Anim Pract 2000;41:235–242. [DOI] [PubMed] [Google Scholar]
- 18. Ross SJ, Osborne CA, Kirk CA, et al. Clinical evaluation of dietary modification for treatment of spontaneous chronic kidney disease in cats. J Am Vet Med Assoc 2006;229:949–957. [DOI] [PubMed] [Google Scholar]
- 19. Geddes RF, Elliott J, Syme HM. The effect of feeding a renal diet on plasma fibroblast growth factor 23 concentrations in cats with stable azotemic chronic kidney disease. J Vet Intern Med 2013;27:1354–1361. [DOI] [PubMed] [Google Scholar]
- 20. Backlund B, Zoran DL, Nabity MB, et al. Effects of dietary protein content on renal parameters in normal cats. J Feline Med Surg 2011;13:698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. NRC . Nutrient Requirements of Cats and Dogs. Washington, D.C.: National Academies; [Oxford: Oxford Publicity Partnership, distributor]; 2006. [Google Scholar]
- 22. Barber PJ, Rawlings JM, Markwell PJ, Elliott J. Effect of dietary phosphate restriction on renal secondary hyperparathyroidism in the cat. J Small Anim Pract 1999;40:62–70. [DOI] [PubMed] [Google Scholar]
- 23. Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 2010;63:834–840. [DOI] [PubMed] [Google Scholar]
- 24. Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor‐23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab 2005;90:1519–1524. [DOI] [PubMed] [Google Scholar]
- 25. Burnett SM, Gunawardene SC, Bringhurst FR, et al. Regulation of C‐terminal and intact FGF‐23 by dietary phosphate in men and women. J Bone Miner Res 2006;21:1187–1196. [DOI] [PubMed] [Google Scholar]
- 26. Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor‐23 concentrations in healthy men. J Clin Endocrinol Metab 2006;91:3144–3149. [DOI] [PubMed] [Google Scholar]
- 27. Sigrist M, Tang M, Beaulieu M, et al. Responsiveness of FGF‐23 and mineral metabolism to altered dietary phosphate intake in chronic kidney disease (CKD): Results of a randomized trial. Nephrol Dial Transplant 2013;28:161–169. [DOI] [PubMed] [Google Scholar]
- 28. Nishida Y, Taketani Y, Yamanaka‐Okumura H, et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int 2006;70:2141–2147. [DOI] [PubMed] [Google Scholar]
- 29. Isakova T, Gutierrez O, Shah A, et al. Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J Am Soc Nephrol 2008;19:615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Larsson T, Nisbeth U, Ljunggren O, et al. Circulating concentration of FGF‐23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 2003;64:2272–2279. [DOI] [PubMed] [Google Scholar]
- 31. Trivedi H, Szabo A, Zhao S, et al. Circadian variation of mineral and bone parameters in end‐stage renal disease. J Nephrol 2015;28:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang S, Gillihan R, He N, et al. Dietary phosphate restriction suppresses phosphaturia but does not prevent FGF23 elevation in a mouse model of chronic kidney disease. Kidney Int 2013;84:713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saito H, Maeda A, Ohtomo S, et al. Circulating FGF‐23 is regulated by 1 alpha,25‐dihydroxyvitamin D‐3 and phosphorus in vivo. J Biol Chem 2005;280:2543–2549. [DOI] [PubMed] [Google Scholar]
- 34. Isakova T, Barchi‐Chung A, Enfield G, et al. Effects of dietary phosphate restriction and phosphate binders on FGF23 levels in CKD. Clin J Am Soc Nephrol 2013;8:1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spatz C, Roe K, Lehman E, Verma N. Effect of a non‐calcium‐based phosphate binder on fibroblast growth factor 23 in chronic kidney disease. Nephron Clin Pract 2013;123:61–66. [DOI] [PubMed] [Google Scholar]
- 36. Isakova T, Gutiérrez OM, Smith K, et al. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant 2011;26:584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Berndt T, Thomas LF, Craig TA, et al. Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc Natl Acad Sci USA 2007;104:11085–11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kumar R. Phosphate sensing. Curr Opin Nephrol Hypertens 2009;18:281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kamycheva E, Sundsfjord J, Jorde R. Serum parathyroid hormone level is associated with body mass index. The 5th Tromsø study. Eur J Endocrinol 2004;151:167–172. [DOI] [PubMed] [Google Scholar]
- 40. Muntner P, Jones TM, Hyre AD, et al. Association of serum intact parathyroid hormone with lower estimated glomerular filtration rate. Clin J Am Soc Nephrol 2009;4:186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parker VJ, Gilor C, Chew DJ. Feline hyperparathyroidism: Pathophysiology, diagnosis and treatment of primary and secondary disease. J Feline Med Surg 2015;17:427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pineda C, Aguilera‐Tejero E, Raya AI, et al. Feline parathyroid hormone: Validation of hormonal assays and dynamics of secretion. Domest Anim Endocrinol 2012;42:256–264. [DOI] [PubMed] [Google Scholar]
- 43. Williams TL, Elliott J, Syme HM. Calcium and phosphate homeostasis in hyperthyroid cats: Associations with development of azotaemia and survival time. J Small Anim Pract 2012;53:561–571. [DOI] [PubMed] [Google Scholar]
- 44. Paepe D, Verjans G, Duchateau L, et al. Routine health screening: Findings in apparently healthy middle‐aged and old cats. J Feline Med Surg 2013;15:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reynolds BS. Breed dependency of reference intervals for plasma biochemical values in cats. J Vet Intern Med 2010;24:809. [DOI] [PubMed] [Google Scholar]
- 46. Wakeling J, Elliott J, Syme H. Evaluation of predictors for the diagnosis of hyperthyroidism in cats. J Vet Intern Med 2011;25:1057–1065. [DOI] [PubMed] [Google Scholar]
- 47. Wakeling J, Everard A, Brodbelt D, et al. Risk factors for feline hyperthyroidism in the UK. J Small Anim Pract 2009;50:406–414. [DOI] [PubMed] [Google Scholar]
- 48. Freeman LM. Cachexia and sarcopenia: Emerging syndromes of importance in dogs and cats. J Vet Intern Med 2012;26:3–17. [DOI] [PubMed] [Google Scholar]
- 49. Laflamme DP, Hannah SS. Discrepancy between use of lean body mass or nitrogen balance to determine protein requirements for adult cats. J Feline Med Surg 2013;15:691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brown SA, Brown CA, Crowell WA, et al. Effects of dietary polyunsaturated fatty acid supplementation in early renal insufficiency in dogs. J Lab Clin Med 2000;135:275–286. [DOI] [PubMed] [Google Scholar]
- 51. Dong H, Hutchins‐Wiese H, Kleppinger A, et al. Effects of Omega‐3 polyunsaturated fatty acid supplementation on bone turnover in older women. Int J Vitam Nutr Res 2014;84:124–132. [DOI] [PubMed] [Google Scholar]
- 52. Hutchins‐Wiese HL, Picho K, Watkins BA, et al. High‐dose eicosapentaenoic acid and docosahexaenoic acid supplementation reduces bone resorption in postmenopausal breast cancer survivors on aromatase inhibitors: A pilot study. Nutr Cancer 2014;66:68–76. [DOI] [PubMed] [Google Scholar]


