Abstract
Following endocytosis, internalized molecules are found within intracellular vesicles and tubules that move along the cytoskeleton and undergo fission, as demonstrated here using primary cultured rat hepatocytes. Although the use of depolymerizing drugs has shown that the cytoskeleton is not required to segregate endocytic protein, many studies suggest that the cytoskeleton is involved in the segregation of protein in normal cells. To investigate whether cytoskeletal-based movement results in the segregation of protein, we tracked the contents of vesicles during in vitro microscopy assays. These studies showed that the addition of ATP causes fission of endocytic contents along microtubules, resulting in the segregation of proteins that are targeted for different cellular compartments. The plasma membrane proteins, sodium (Na+) taurocholate cotransporting polypeptide (ntcp) and transferrin receptor, segregated from asialoorosomucoid (ASOR), an endocytic ligand that is targeted for degradation. Epidermal growth factor receptor, which is degraded, and the asialoglycoprotein receptor, which remains partially bound to ASOR, segregated less efficiently from ASOR. Vesicles containing ntcp and transferrin receptor had reduced fission in the absence of ASOR, suggesting that fission is regulated to allow proteins to segregate. A single round of fission resulted in 6.5-fold purification of ntcp from ASOR, and 25% of the resulting vesicles were completely depleted of the endocytic ligand.
Keywords: asialoglycoprotein receptor, endosome, fission, microtubules, sorting, transferrin receptor
Proteins at the plasma membrane are continuously endocytosed and transported to intracellular early (sorting) endosomes. From early endosomes, proteins are typically sorted either to late endosomes, for subsequent degradation, or to recycling endosomes, from which they traffic back to the plasma membrane (1,2). Coat proteins and adaptor proteins appear to function in protein sorting and budding at the endosome (3,4). However, examination of endosomal protein within mammalian cells by light microscopy reveals dramatic cytoskeletal-based trafficking, tubule formation and fission (5–7), while examination by electron microscopy reveals tubular extensions corresponding to sites of protein sorting (8,9). Microtubule motor proteins are involved in the movement of endocytic proteins from the early/sorting endosomes to the perinuclear recycling vesicles and late endosomes (10,11), and it has been shown that microtubule depolymerization can delay protein degradation and block apical recycling and transcytosis (12–15). However, it has also been shown in cultured cells that the depolymerization of microtubules does not block protein recycling (16,17). Therefore, the precise role of microtubules in protein segregation is unclear. The studies presented here address this issue by examining how microtubule-based movement of endocytic material that is seen by time-lapse microscopy of living cells contributes to protein sorting and how this sorting manifests itself at the level of an individual fission event. Few reports have quantified whether cytoskeletal-based fission can result in specific segregation of different proteins. In contrast, the mechanisms of endocytic vesicle fusion and the importance of cellular SNARE protein machinery have been well established, in part because of reconstitution of fusion in vitro (18,19). It has been shown that fission can be quantitatively analyzed in vitro using time-lapse microscopy (20). This report broadens those studies with an in-depth analysis of fission and the behavior of multiple proteins in order to determine whether studies of single vesicles can provide an important tool for understanding the underlying mechanisms of protein sorting.
Four integral membrane proteins were used in these studies: transferrin receptor, asialoglycoprotein receptor (ASGPR), epidermal growth factor receptor (EGFR) and sodium (Na+) taurocholate cotransporting polypeptide (ntcp) as well as the lumenal markers, asialoorosomucoid (ASOR) and fluorescent dextran. The transferrin receptor and ASGPR are well-studied integral membrane proteins that undergo recycling back to the cell surface once internalized (21,22). In contrast, a significant portion of EGFR is transported to multivesicular bodies and degraded once it is internalized (23,24). Ntcp is a hepatocyte-specific bile acid transporter that localizes to the plasma membrane as well as a subplasma membrane intracellular pool (25,26). ASOR is a ligand for ASGPR and once internalized undergoes dissociation followed by sorting to the lysosomes where it is degraded (12). Dextran is a soluble pinocytic marker that enters cells in the fluid phase (27). These markers were selected in order to study the behavior of molecules that are targeted for either degradation or recycling during individual fission events that occur along microtubules.
Results
Vesicle motility and fission in primary hepatocytes
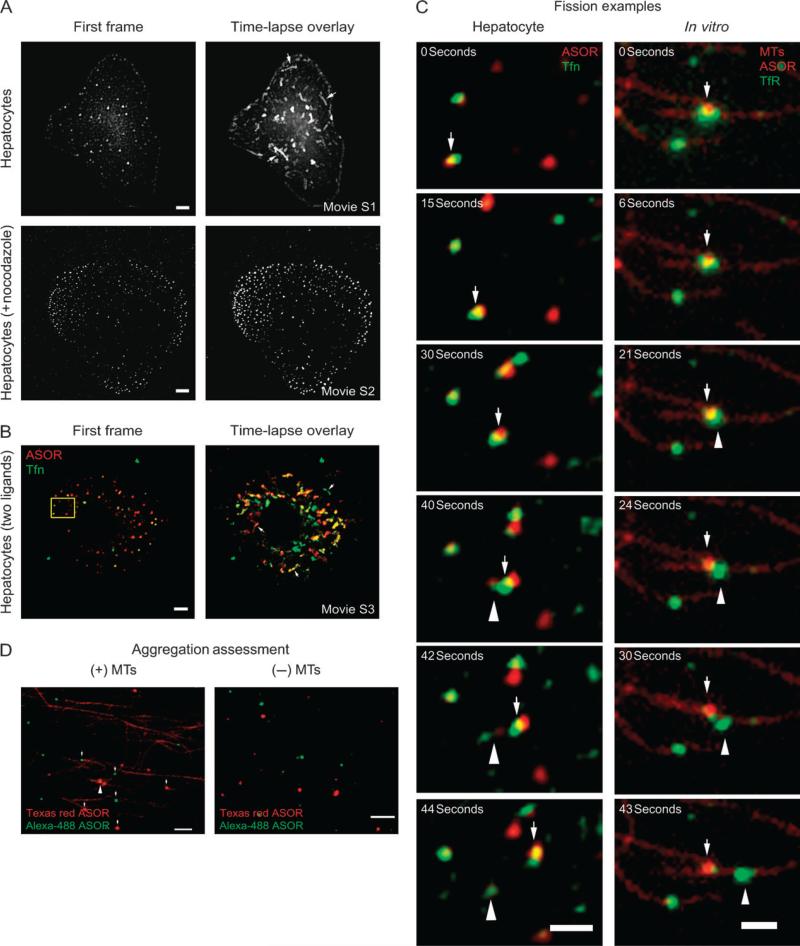
Previous studies have documented that early endosomes, late endosomes and lysosomes undergo microtubule-based movement during the processing of endocytic contents (7,28,29). However, it has been difficult to demonstrate how this movement contributes to metabolic functions such as the segregation of protein. To confirm that hepatocytes display microtubule-based movement of their early endosomes, hepatocytes were incubated with fluorescent endocytic ligand, ASOR, in the presence or absence of the microtubule-depolymerizing drug, nocodazole. Time-lapse fluorescent microscopy revealed that ASOR is present within intracellular vesicles after 5 min of uptake and that these undergo a complicated display of fusion, fission and back-and-forth movement (Movie S1). This is seen as bright streaks in the time-lapse overlay in the top panel of Figure 1A. Treatment of cells with nocodazole suspended nearly all long-distance motility events (Movie S2), as seen by the lack of bright streaks in the bottom panel of Figure 1A. Some smaller movements and vibrating activities were still observed, indicating that endosomes were not rigidly fixed and that cells were still capable of movement that did not depend on microtubules.
Figure 1. Movement and fission of endocytic vesicles in hepatocytes and in vitro.
A) Control or nocodazole-treated hepatocytes were allowed to endocytose fluorescent ASOR for 5 min. Cells were washed and imaged by time-lapse microscopy for 1- to 1.5-min, 8 min following the wash. The left panels show the first frame of the movie, and the right panels shows an overlay (maximum intensity projection) of all movie frames. Arrows point to fluorescence streaks that indicate the movement of the internalized ASOR. These streaks were absent in nocodazole-treated cells, but short-distance movement was observed as broadening of the fluorescence spots. B) Control hepatocytes were allowed to endocytose Texas red ASOR (red) and Alexa-488 transferrin (Tfn) (green) and washed, imaged and displayed as described above. Both ligands showed prominent long-distance movement as indicated by the streaks (arrows). The yellow box indicates the region of the cell that is magnified for the subsequent figure. C) On the left, fission is observed in a living hepatocyte (from Movie S5, an additional example is seen in Movie S6), while on the right, fission is observed in vitro within a microscope chamber. Arrows mark the location of ‘mother’ vesicles, while arrowheads mark the location of emerging ‘daughter’ vesicles. In the hepatocyte, a vesicle containing both ASOR (red) and Tfn (green) moves to the right and at 30 seconds begins to undergo fission, resulting in a separate daughter vesicle by 42 seconds. In the in vitro example, vesicles were bound to microtubules (MTs) (red filaments) and labeled for endocytic ASOR (red) as well as the transferrin receptor (TfR) (green), and ATP was added to initiate motility and fission at 0 seconds. Fission occurred at 21 seconds, resulting in a separate daughter vesicle by 30 seconds. D) Vesicles were assessed for the degree of aggregation in the presence or absence of MTs. A rat liver was allowed to endocytose Alexa-488 ASOR (green), and a second liver was allowed to endocytose Texas red ASOR (red), and the green and red livers were homogenized together and endocytic vesicles were purified according to protocol. Most vesicles contained either red or green fluorescence (arrows), indicating that vesicles did not aggregate, although occasional overlap was seen (arrowhead). The lack of aggregation is better seen when vesicles are imaged in the absence of microtubule (right). Scale bars, 5 μm, except in C, 2 μm.
We next asked whether vesicles containing proteins that are targeted to different cellular locations could be seen undergoing fission while moving on microtubules. We also examined whether these fission events resulted in the segregation of one protein from the other. Hepatocytes were incubated with Alexa-488-labeled transferrin and Texas red-labeled ASOR and subsequently imaged in two fluorescence channels (Figure 1B). Transferrin remains bound to its receptor during cycles of endocytosis and recycling where it moves from the cell surface into endosomes and then back to the cell surface (21). However, ASOR is taken up by the ASGPR and released within endocytic compartments where it subsequently traffics to the lysosomes and is degraded (30). As seen in Movie S3 and zoomed in Movies S5 and S6 (Movie S4 contains the corresponding bright-field images), vesicles containing both markers were highly motile and could be seen to undergo fission. Still images from Movie S5 demonstrating fission are shown in Figure 1C.
These data indicate that a single vesicle can contain two different proteins and undergo microtubule-based movement and fission. However, these findings do not imply that these events can lead to the overall segregation of these proteins from each other. Whereas many studies indicate that microtubule-based trafficking contributes to endocytic protein sorting and the recycling of protein (10,31), other studies have demonstrated that depolymerization of the microtubule cytoskeleton does not block protein recycling (16,17). In an attempt to address these issues, we initiated studies to quantify the overall redistribution of proteins during single vesicle fission events within living cells. However, varying background fluorescence, photobleaching and the complex, three-dimensional motion of the vesicles prohibited accurate quantification of protein using live cell imaging techniques. Given these difficulties, we instead took advantage of an in vitro assay that has been developed to analyze the microtubule motor proteins that are involved in endocytic vesicle traffic (32,33).
Reconstitution of vesicle fission in vitro
A standardized in vitro motility assay was used to generate the data below. Similar assays have been used to identify and purify motor proteins, to characterize mutations of motor proteins and to show that organelles use specific sets of motor proteins and regulatory factors for cellular transport (33–36). In brief, endocytic vesicles are isolated by homogenization of rat liver followed by sucrose step-gradient centrifugation. Vesicles are then stored as single-use, frozen aliquots. Small volume (3 μL) glass microscope chambers are constructed from glass slides, and micro-tubules are polymerized from fluorescent tubulin and attached to the bottom of the chambers. Endocytic vesicles are thawed and added to the chambers where they attach to the microtubules, and the unattached contents are washed away. Fifty micromolar ATP is added to the chambers to initiate vesicle motility, and this is captured by high-resolution, wide-field, multi-fluorescence channel, time-lapse microscopy. Prior to ATP addition, chambers containing endocytic vesicles that are attached to microtubules are stored in buffer on ice where they remain active in motility assays for more than 3 h.
Two approaches are used to identify specific proteins within the endocytic vesicles: (i) the hepatic portal vein is injected with fluorescent ligand, and hepatocytes are allowed to take up the ligand prior to homogenization of the liver and (ii) the endosomes are labeled by immunofluorescence within the microscope chambers after they have bound to microtubules. Unless specifically noted, all the biochemical preparations of vesicles in the following experiments contain fluorescent-labeled ASOR that has been injected into the hepatic portal vein 5 min prior to liver homogenization. The red fluorescent vesicles within the preparation represent ‘early’ endocytic ASOR as they contain both ligand and receptor (20). This preparation also contains a multitude of other vesicles that copurify with these ASOR-containing vesicles. As described previously, these vesicles bind to microtubules and move in the presence of ATP without the addition of exogenous protein or cytosol (20,32,37). In addition, the moving vesicles undergo fission. As in living cells, fission is observed as the separation of a single region, or spot, of fluorescence. Unlike in living cells, vesicles in vitro remain within the focal plane during movement, and the history of all movements can be tracked. This facilitates the differentiation of fission from other motile events, such as the movement of two vesicles in opposite directions (e.g. vesicle crossing). Also unlike in living cells, vesicle motility by these techniques occurs in a single burst that lasts approximately 90 seconds. Subsequently, vesicles detach from microtubules or stop moving, presumably as a result of ATP hydrolysis and depletion (32) or possibly because of changes in protein phosphorylation or other factors (26). This relatively short burst of movement simplifies data collection and analysis.
Figure 1C (right panels) presents an example of fission occurring in vitro. A vesicle containing ASOR (red) and stained with antibody to the transferrin receptor (green) is attached to a microtubule (white arrow). ATP was added to the chamber, and after 21 seconds, a daughter vesicle containing only transferrin receptor emerges, moving to the right. A portion of the transferrin receptor remains with the mother vesicle. Note that the size of the vesicle and duration of fission are similar for the examples in the living cell and in vitro (Figure 1C), that is, the apparent diameter for both vesicles is approximately 1 μm and the separation into two vesicles takes less than 10 seconds of real time. Movies S7 and S8 provide additional examples of fission events between ASOR and ntcp, and ASOR, ntcp and transferrin receptor, respectively.
Fission is not the result of the movement of overlapped vesicles
It is possible that fission observed during in vitro microtubule motility assays results from the separation of overlapping or aggregated vesicles. Previous studies have shown that fission is not reduced by diluting the vesicles prior to attachment to microtubules (20), indicating that fission is not the result of overlapped, unconnected vesicles. To determine whether the vesicle isolation procedure produces aggregated vesicles, livers from rats that had endocytosed either Texas red ASOR (red) or Alexa-labeled ASOR (green) were homogenized together and vesicles were isolated using the standard protocol. Any vesicle that aggregates during this process would be revealed by overlap of red and green fluorescence. The degree of overlap was assessed in the presence and absence of microtubules, and the level of ATP-induced motility and fission was also measured. Images from these experiments are shownin Figure 1D. Of the 961 microtubule-bound vesicles scored, 671 were green and 290 were red, and of these, 32 (3.3%) showed some overlap of red and green fluorescence. Of the overlapped vesicles, only three underwent separation of green and red during motility experiments. Thus, the clustering of red and green vesicles was rare, and those that did cluster did not show a high level of separation upon addition of ATP.
Vesicle motility and fission varies as a function of protein constituents
In order to measure the segregation of protein during microtubule-based fission, we first sought to characterize the amount of motility and fission of vesicles that contained different endocytic proteins. Several groups have demonstrated that different populations of endocytic vesicles have different amounts of motility (6,38). The endocytic vesicle preparation used in these studies contains the endocytic marker, ASOR, as well as numerous other vesicles (11,32). Because motor proteins, coat proteins or other regulatory proteins have the potential to exchange between vesicles during their purification, it was possible that all vesicles would display an identical level of motility and fission in vitro. Alternatively, it was possible that the amount of fission for some vesicles would be too low to allow quantification of protein segregation.
We performed in vitro vesicle motility assays and subsequently measured the level of motility and fission for vesicles that contained the endocytic ligand, ASOR. Motility is defined as the per cent of microtubule-bound vesicles that undergo movement, while fission is defined as the per cent of motile vesicles that undergo fission. Twenty-one per cent of microtubule-bound vesicles that contained ASOR were motile (n = 67), and 14% of these underwent fission (Figure 2). These results are very similar to those obtained in previous studies that used the same procedure to purify endocytic vesicles. In those studies, 25% of the ASOR-containing vesicles were motile and 13% of the motile vesicles underwent fission [(20); n = 832]. A second endocytic marker, fluorescent dextran, was injected into the rat hepatic portal vein instead of fluorescent ASOR, and vesicles were isolated by the same procedure. Dextran is a pinocytotic fluid-phase marker frequently used to track intracellular vesicles (39,40). in vitro motility assays were performed, and the level of motility and fission of dextran-containing vesicles was measured. It was found that dextran-containing vesicles had higher motility than ASOR-containing vesicles (58.2%, n = 531, p ≤ 0.001 versus 21% for ASOR) but significantly less fission of the dextran fluorescence (8.4%, compared with 14% for ASOR, p ≤ 0.001; Figure 2). This indicates that the level of motility and fission is different for different vesicle populations, despite the fact that the vesicles were purified by the same procedure. Interestingly, the dextran-containing vesicles had higher motility than the ASOR-containing vesicles, yet fission was significantly lower. This demonstrates that fission and motility are regulated independently. Currently, it is not clear why dextran-containing vesicles would have such high levels of motility, and we do not know how these activities affect endocytic processing.
Figure 2. The level of motility and fission is different for different populations of endocytic vesicles; fission is low for vesicles that contain ntcp or transferrin receptor (TfR) but lack ASOR.
Motility and fission were measured in the in vitro motility assay for vesicles that contained ASOR [n (number of vesicles) = 67, e (number of experiments) = 5], dextran (n = 531, e = 10), ntcp but no ASOR (n = 861, e = 7), transferrin receptor (TfR) but no ASOR (n = 322, e = 4), ntcp and ASOR (n = 316, e = 22), TfR and ASOR (n = 303, e = 13), and ntcp and ASOR (n = 212, e = 15) after pretreatment with 50 μm LY294002, as indicated. Motility was scored as the per cent of microtubule-bound vesicles that underwent continuous directed movement (y-axis). Fission was scored as the per cent of moving vesicles that underwent fission (y-axis). Assays were performed using different aliquots of the same preparations of endocytic vesicles, which were purified following endocytic uptake of fluorescent ASOR into rat liver. In some cases, fluorescent dextran was substituted for ASOR (as indicated). Different populations of vesicles within the preparation are indicated on the x-axis. These were identified using fluorescent antibodies and fluorescent endocytic ASOR, which labeled subsets of the vesicles within the preparation.
As mentioned above, the endocytic vesicle preparation contains many different vesicles (11), and, when used as an endocytic ligand, a subset of the vesicles will contain ASOR. We had previously determined that many of the vesicles within the preparation contain the transmembrane protein, transferrin receptor, as well as the bile acid transporter, ntcp (26). To determine if vesicles containing either of these proteins had similar levels of motility and fission as that of the ASOR-containing vesicles, motility assays were performed for vesicles that were labeled by immunofluorescence for either transferrin receptor or ntcp. Movies resulting from these experiments were analyzed for vesicles that contained either ntcp or transferrin receptor but not ASOR. Forty-one per cent of vesicles that contained ntcp were motile, yet no fissions were detected (n = 861). Thirty-two per cent of the vesicles that contained transferrin receptor were motile (n = 322), and only 3.8% of these underwent fission (Figure 2). These data indicate that some vesicles within the preparation have a low amount of fission, despite having high motility.
Approximately 20% of the vesicles that contain either ntcp or transferrin receptor also contain ASOR (data not shown). When these vesicles were analyzed, it was found that 29.1% of the vesicles that contained ASOR and ntcp were motile (n = 316) and 26.1% underwent fission of the ntcp protein (Figure 2). Twenty point eight per cent of the vesicles that contained transferrin receptor and ASOR were motile, and 22.2% underwent fission of the transferrin receptor (n = 303; Figure 2). In both cases, the presence of the endocytic ligand corresponded with increased fission and slightly reduced motility. When vesicles containing endocytic dextran instead of endocytic ASOR were used for similar studies, it was found that ntcp, transferrin receptor and several other proteins failed to show significant colocalization with the fluorescent dextran in vitro, precluding the generation of additional data (not shown). Overall, however, these experiments demonstrated that different vesicles within the same preparation have different levels of motility and fission and that vesicles containing ASOR as well as other proteins have sufficient fission to allow an analysis of the segregation of ASOR.
Inhibition of PI3-kinase decreases fission but not motility of vesicles that contain ASOR and ntcp
To confirm that fission and motility can be regulated independently, we measured these activities following preincubation with the phosphatidylinositol 3-kinase (PI3-kinase) inhibitor, LY294002. Many reports have shown that PI3-kinase inhibition reduces protein recycling and sorting (41–43), and we therefore examined whether the fission of endocytic vesicles, which may participate in protein sorting and recycling, may also be affected. PI3-kinase activity has also been shown to be important for the formation of phospholipids that allow the attachment of motor proteins to vesicles (10,44). However, in these studies, LY294002 was added after vesicles had attached to microtubules, and the PI3-kinase activity might not be limiting for the motility of pre-attached vesicles.
The motility and fission of microtubule-bound vesicles containing ASOR and ntcp were quantified following pre-incubation with 50 μm LY294002 in the motility chamber. As can be seen in Figure 2 (rightmost bars), 34.4% of the inhibitor-treated vesicles moved along microtubules (n = 212) compared with 29.1% of untreated vesicles (n = 316), suggesting that PI3-kinase inhibition has minimal effect on the motility of vesicles that contain ASOR and ntcp (p = 0.2). However, fission was reduced from 26.1 to 9.6% (p = 0.01), indicating that PI3-kinase regulates the level of vesicle fission without affecting motility.
Segregation of ASOR from ntcp
Previous studies have shown that the bile acid transporter, ntcp, resides on the plasma membrane as well as intracellularly (25,45), and recently, we have shown that a fraction of this protein colocalizes with early endocytic ASOR but not late endocytic ASOR (26). Fission of ntcp away from ASOR in early endocytic vesicles represents a potential means by which ntcp is depleted from the late endosomes. This was examined in this study by measuring the degree to which ASOR and ntcp segregate during fission along microtubules. To obtain this data, early endocytic ASOR-containing vesicles were bound to micro-tubules in vitro and the presence of ntcp was detected on the vesicles by immunofluorescence. ATP was added to the vesicles during multi-channel, time-lapse fluorescence microscopy experiments. Subsequently, the time-lapse movies were analyzed for the presence of vesicles that contain both ntcp and ASOR, and this population was analyzed for fission. The amount of ASOR and ntcp segregating to each daughter vesicle was determined by measuring the amount of fluorescence for each fission. These data are tabulated (Supplementary Tables 1, 2 and 6) and displayed as a correlation of the two proteins (see below).
Figure 3 is a cartoon that indicates how a correlation plot can be used to view protein segregation. On the left, the figure depicts a vesicle undergoing fission into two daughter vesicles, and on the right, the per cent of protein recovered in each daughter vesicles is plotted as a correlation. Four hypothetical examples demonstrate different distributions of red and green proteins into daughter vesicles, while yellow indicates overlap of red and green in the same vesicle. In the first example, red and green proteins divide equally between the two daughter vesicles and the proteins do not segregate. The correlation plot shows that each daughter vesicle contains approximately 50% of the red and green proteins. In the second example, the proteins divide unequally into the daughter vesicles but again there is no segregation. One vesicle contains a large percentage of both red and green proteins, while the other vesicle contains a small percentage of both proteins. In this case, the daughter vesicles reside in the upper right and lower left quadrants of the correlation plot. In the third example, the proteins divide unequally into the daughter vesicles and partial segregation is achieved. One daughter vesicle contains a large percentage of red protein along with a small percentage of green protein, while the other daughter contains a small percentage of red protein along with a large percentage of green protein. These vesicles reside in the upper left and lower right quadrants of the plot. In the final example, complete segregation is achieved, and one vesicle contains 100% of the red protein and 0% of the green protein, and the other vesicle contains 0% of the red protein and 100% of the green protein. These vesicles reside in the extreme upper left and lower right of the plot.
Figure 3. Graphing the distribution of protein from vesicle fission as a correlation.
A cartoon indicates several examples of how two proteins may distribute to different vesicles during fission and how this can be plotted as a correlation. A mother vesicle containing red and green proteins is shown on the left bound to a microtubule (red filament); yellow indicates overlap of the red and green proteins. The mother vesicle undergoes fission (arrow) into two daughter vesicles, and the fraction of red and green proteins distributing to each daughter is plotted as a percentage as two dots. In the first example, protein distributes approximately equally to the daughter vesicles, and the daughter vesicles appear centrally localized on the correlation plot. In the second example, protein distributes unequally to the daughters, but red and green proteins do not segregate. These daughters localize to the lower left and upper right quadrants of the plot. In the third example, protein distributes unequally to the daughters, and red and green proteins partially segregate. Vesicles from such fissions localize to the upper left and lower right quadrants of the plot. In the final example, the proteins segregate completely, and daughter vesicles align at the extreme upper left (0 and 100%) and lower right (100 and 0%) portions of the plot. Because both daughter vesicles are included, the graph is rotationally symmetric (each vesicle is related to the other daughter of the pair by the formula: 100% – co-ordinate value).
The correlation plot therefore indicates whether proteins segregate from each other during fission, and all fissions are displayed so that the scatter in the data is revealed. The percentage of vesicles that are found in each quadrant can be included as an additional measure of the segregation of the two proteins as is frequently performed for flow cytometry analysis (46). Proteins that segregate will be negatively correlated, localizing to the upper left and lower right quadrants, while proteins that do not segregate will be positively correlated, localizing to the upper right and lower left quadrants. Because both daughter vesicles are included, the graph is rotationally symmetric (each vesicle is related to the other daughter of the pair by the formula: 100% – co-ordinate value). Also note that the correlation, plotted as a percentage (as in Figure 3), does not provide information on the total amount of fluorescence.
Figure 4A presents the correlation plot for vesicle fissions that contain ntcp and ASOR. Overall, the vesicles cluster to the upper left and lower right quadrants of the plot, indicating that the proteins are segregating. The degree of segregation can be represented by the average values of the lower left and lower right quadrants. Vesicles in these lower quadrants contain the smaller portion of ASOR. They also contain some proportion of ntcp. If protein divided equally likely into any portion and if the proteins distributed independently from one another, then the average value of the lower quadrants would be 25% for ASOR and 50% for ntcp. Twenty-five per cent is the middle value for the lower half of a population, and 50% is the middle value for a total population. However, in the actual correlation plot, these quadrants contained 11.3 ± 1.6% (mean ± SEM) of the ASOR and 72.9 ± 3.0% of the ntcp, as indicated by the triangle and dotted lines of Figure 4A. The scatter of the data indicates that the per cent of protein distributing to daughter vesicles is variable. In addition, there are many vesicles that completely lack ASOR and some that completely lack ntcp, and therefore, some fission events achieve complete segregation of the proteins. It should also be noted that if the observed fission was actually the result of independent movement of overlapped ‘pure’ vesicles (i.e. vesicles that contained only ASOR or only ntcp), then ‘mixed’ daughter vesicles (containing both ntcp and ASOR) should rarely be observed. Instead, about 60% of all daughter vesicles were mixed. In summary, these results suggest that intracellular fission produces daughter vesicles containing a broad distribution of protein, with many vesicles (32 of 130 or 25%) completely devoid of ASOR, a marker that is targeted for degradation within lysosomes.
Figure 4. Segregation of ASOR from ntcp and transferrin receptor during microtubule-based motility in vitro.
Vesicles were purified from rat liver that contained fluorescent endocytic ASOR as well as subpopulations of unlabeled vesicles as described in Figure 2. These were bound to microtubules and labeled for ntcp and transferrin receptor by immunofluorescence. Motility assays were performed and subsequently analyzed for vesicles that have undergone fission and contain ASOR and ntcp or transferrin receptor. The amount of protein present on each endocytic vesicle was measured by fluorescence intensity. The distribution of ASOR was plotted as a correlation versus ntcp or transferrin receptor in the manner outlined in the cartoon of Figure 3. Each point represents a single daughter vesicle after it has undergone fission. In (A) and (B), the per cent of protein segregating to daughter vesicles is presented, and the graph is rotationally symmetric. In (C) and (D), the total amount of fluorescence is presented. Fissions showing identical percentages were offset by 0.5% along the x-axis for visualization. A) ASOR and ntcp predominantly segregated to opposite vesicles during fission. The lower quadrants contained an average of 11.3 ± 1.6% (SEM) of the ASOR and 72.9 ± 3.0% of the ntcp, as indicated by the triangle and dotted line. B) ASOR and transferrin receptor segregated to opposite vesicles during fission, although the trend was not as strong as for ASOR and ntcp. The lower quadrants contained an average of 10.9 ± 1.7% of the ASOR and 61.0 ± 3.2% of transferrin receptor as indicated by the triangle and dotted line. C) A plot of total fluorescence (rather than per cent) of ASOR versus ntcp for the same vesicles that were used in (A). This does not reveal a negative correlation, which indicates that the negative correlation of (A) is a result of fission rather than the amount of protein on the vesicles per se. D) A plot of the total fluorescence of ASOR versus transferrin receptor for the same vesicles that were used in (B). Fluorescence is in arbitrary units for all probes. UL, UR, LL and LR are abbreviations for upper left, upper right, lower left and lower right, and the adjacent number indicates the percentage of vesicles that are found in the quadrant.
Segregation of ASOR from transferrin receptor
Several other protein markers were selected for study in order to determine whether segregation during fission is dependent on the identity of the protein and whether proteins that are targeted for different cellular destinations undergo greater segregation than those that are targeted for the same destination. Figure 4B presents a correlation plot for vesicles containing transferrin receptor and ASOR. These experiments were performed identically to the experiments containing ntcp except that antibodies to transferrin receptor were included to identify this protein. Figure 4B indicates that the transferrin receptor also segregates away from ASOR during fission. The lower right and lower left quadrants contained 10.9 ± 1.7% of the ASOR and 61 ± 3.2% of transferrin receptor, as indicated by the triangle and dotted lines. There is also a similar degree of scatter and a similar amount of vesicles that completely lack ASOR (30%).
Lack of correlation between total fluorescence in daughter vesicles
It could be argued that the degree of protein segregation that we have attributed to fission is in reality a reflection of the degree to which the proteins are present in vesicles overall. That is, perhaps those vesicles that contain a low amount of ASOR also contain a high amount of ntcp, independent of whether or not these vesicles have undergone fission. We tested this hypothesis by plotting a correlation for the total amount of fluorescence, rather than the per cent, that is present on the vesicles of the previous two data sets. Figure 4C,D shows that there is no strong overall trend for either ntcp or transferrin receptor with respect to ASOR, that is, vesicles containing either a high or a low amount of ASOR can contain a high or low amount of ntcp or transferrin receptor. It is only when the vesicles are paired because of fission (Figure 4A,B) that we see a low amount of ASOR distributing with a high amount of ntcp. Mean values were used to divide the total fluorescence data into quadrants in the manner of Figure 4A,B, and these did not reveal any unusual distributions of protein. Eighty-three per cent and 68% of the vesicles were found in the negatively correlated (upper left and lower right) quadrants in Figure 4A,B, while 47 and 55% of the vesicles were found in the negatively correlated quadrants of Figure 4C,B.
Segregation of transferrin receptor from ntcp, ASOR from ASGPR and ASOR from EGFR
Figure 5A presents a correlation plot for vesicles that contain transferrin receptor and ntcp. Unlike ASOR, transferrin receptor did not segregate from ntcp during fission. Instead, 86% of the daughter vesicles were contained in the upper right and lower left (positively correlated) quadrants of the plot. The lower left and lower right quadrants (vesicles containing the lower portion of transferrin receptor) contained on average 30 ± 4.6% of the transferrin receptor and 27 ± 4.8% of ntcp, indicating that vesicles with a low amount of transferrin receptor also contained a low amount of ntcp on average. Few vesicles (4 of 42 or 10%) completely lacked transferrin receptor. Cumulatively, these data indicate that two basolateral, plasma membrane proteins, ntcp and transferrin receptor, are able to efficiently segregate from the endocytic ligand, ASOR, during microtubule-based fission while they are unable to segregate from each other.
Figure 5. Segregation of Transferrin receptor from ntcp, ASOR from ASGPR and ASOR from EGFR during microtubule-based motility in vitro.
Vesicles were purified from rat liver that contained fluorescent endocytic ASOR as well as subpopulations of unlabeled vesicles as described in Figure 2. These were used for in vitro motility assays, and the per cent of protein segregating to individual vesicles was plotted as correlations as in Figure 4. A) Transferrin receptor and ntcp remain together (i.e. do not segregate) during fission. The lower left and lower right quadrants (vesicles containing the lower portion of transferrin receptor) contained on average 30 ± 4.6% of the transferrin receptor and 27 ± 4.8% of ntcp. B) ASOR and ASGPR partially segregate during fission. The lower quadrants contained on average 11 ± 3.8% of the ASOR and 46 ± 6.8% of the ASGPR. C) ASOR and EGFR partially segregate during fission. The lower quadrants contained on average 18 ± 3.7% of the ASOR and 40 ± 6.3% of the EGFR. UL, UR, LL and LR are abbreviations for upper left, upper right, lower left and lower right, and the adjacent number indicates the percentage of vesicles that are found in the quadrant. The triangles and dotted lines indicate average values for the lower quadrants.
ASGPR, the receptor for ASOR, was chosen as a fourth marker for the analysis of protein segregation. ASGPR–ASOR defines a classical pathway whereby receptor binds to ligand at the cell surface, releases ligand intracellularly and the ligand is targeted to lysosomes where it is degraded, while the receptor is recycled back to the cell surface (47,48). Further studies showed that approximately half of the ligand dissociates from the receptor rapidly (t½ ~ 2.5 min) following endocytic uptake, while the other half dissociates slowly (t½ ~ 50 min). Therefore, for reasons that are still not understood, a significant portion of ASOR remains bound to ASGPR during endocytic processing and vesicular traffic (49,50).
We used the in vitro assay to address whether ASOR that is located within endocytic vesicles after 5 min of uptake undergoes efficient segregation from ASGPR during microtubule-based fission. Figure 5B shows that segregation between ASOR and ASGPR was not as efficient as the segregation of ASOR from either ntcp or transferrin receptor. On average, 11 ± 3.8% of ASOR and 46 ± 6.8% of ASGPR were present in the lower quadrants of the correlation plot. A similar level of segregation of ASGPR from ASOR was also observed in a previous study (20). This level of segregation was intermediate between the segregation of ASOR from ntcp (proteins that strongly segregated) and transferrin receptor from ntcp (proteins that did not segregate). The percentage of vesicles in the negatively correlated upper left and lower right quadrants totaled 83, 35 and 14% for the segregation of ASOR from ntcp, ASOR from ASGPR and transferrin receptor from ntcp, respectively. Interestingly despite moderate overall segregation, 29% of vesicles completely lacked ASOR as a result of fission of vesicles containing ASGPR and ASOR. These studies suggest that ASOR is not completely free to segregate from ASGPR within the endocytic vesicles and that the slowly dissociating [‘state 1’ (48)] receptors are present within endocytic vesicles that can bind to and move along microtubules.
The EGFR was chosen as a fifth marker for the study of endocytic segregation as it is an integral membrane receptor that follows a different intracellular trafficking pathway than either transferrin receptor or ASGPR. Whereas the transferrin receptor and ASGPR are recycled to the plasma membrane following endocytosis, the EGFR is taken up by clathrin-mediated endocytosis and transported to the inner membranes of multivesicular bodies where a significant portion of the receptor moves to lysosomes and is degraded (23,24,51). Figure 5C shows that the EGFR does not sort away from the degraded ligand, ASOR, during microtubule-based fission. On average, 18 ± 3.7% of the ASOR and 40 ± 6.3% of the EGFR were present in the lower quadrants of the correlation plot, and only 40% of the vesicles were present in the negatively correlated upper left and lower right quadrants of Figure 5C. These studies suggest that the different endocytic sorting pathways of EGFR and transferrin receptor are reflected in their different sorting behavior with respect to ASOR.
Summary of the sorting behavior of the proteins
Table 1 summarizes the sorting of the different combinations of proteins that were studied in this report. Each fission is the division of a single vesicle into two vesicles, and therefore, the amount of protein sorting to each vesicle was plotted as per cent of total (Figures 4A,B and 5A–C). Strong segregation would be indicated by a low percentage of one protein sorting into the same vesicles that contain a high percentage of the other protein. This is shown in Table 1 as the ‘average per cent of protein recovered in one daughter vesicle’ and corresponds to the average per cent of protein in the lower left and lower right quadrants of the correlation plots. About 11.3% of the ASOR and 72.9% of the ntcp were found in the same vesicles, indicating that a single round of fission produced a 6.5-fold purification of ntcp (72.9/11.3%) on average.
Table 1.
Summary of the degree of protein segregation during fission
| Protein pair analyzed | Average per cent protein recovered in one daughter vesiclea | Fold purificationb | n c | Per cent of fissions with protein segregationd |
|---|---|---|---|---|
| ASOR | 11.3 ± 1.6 | 6.5 | 65 | 83 |
| Ntcp | 72.9 ± 3.0 | |||
| ASOR | 10.9 ± 1.7 | 5.6 | 72 | 68 |
| TfR | 61.0 ± 3.2 | |||
| ASOR | 18.4 ± 3.7 | 2.2 | 15 | 40 |
| EGFR | 39.7 ± 6.3 | |||
| ASOR | 11.2 ± 3.8 | 4.1 | 17 | 35 |
| ASGPR | 45.8 ± 6.8 | |||
| TfR | 29.9 ± 4.6 | 0.9 | 21 | 14 |
| Ntcp | 27.4 ± 4.8 |
This is the average per cent protein ± SEM in the lower left and lower right quadrants of Figures 4A,B and 5A–C. These are the daughter vesicles from each fission that contain the smaller portion of the first listed protein.
This is the purification of the second listed protein from the first in these vesicles, compared with the pre-fission vesicle, obtained by dividing the percentages in the previous column.
The number of fissions.
This is the per cent of vesicles located within the negatively correlated quadrants of Figures 4A,B and 5A–C. In these fissions, the majority of one protein was found in the vesicle containing the minority of the other protein.
Table 1 ranks the proteins according to their tendency to segregate during fission. Ntcp segregated strongly from ASOR, transferrin receptor segregated slightly less strongly, and EGFR and ASGPR had moderate segregation from ASOR. Transferrin receptor, however, did not segregate from ntcp. This can be seen in the column that lists the ‘per cent fissions with protein segregation’ and corresponds to the per cent of vesicles that are contained in the negatively correlated, upper left and lower right quadrants. The degree of segregation was significantly different for all protein markers with p-values of 0.01, <0.005 and <0.005 when comparing the per cent of ntcp segregating from ASOR to the per cent of transferrin receptor, EGFR and ASGPR segregating from ASOR. These data indicate that proteins have different degrees of segregation during fission, and it suggests that microtubule-based fission events are participating in the segregation of protein.
The degree of segregation does not correlate with vesicle fluorescence intensity or size
A population of ‘highly sorted’ daughter vesicles was analyzed for their fluorescence intensity and motility. Daughter vesicles arising from fission were considered highly sorted if they contained at least 80% of a single marker protein and less than 20% of the ‘opposing’ marker proteins. For ASOR, both ntcp and transferrin receptor were considered opposing marker proteins, while for ntcp and transferrin receptor, ASOR was considered the opposing marker protein (Table 2). The highly sorted vesicles were brighter on average than the remaining vesicles, indicating that protein segregation did not occur into dim vesicles. The highly sorted vesicles also had equal or greater standard deviation of fluorescence, indicating that protein did not segregate into vesicles of uniform intensity. Overall, this analysis did not reveal any correlation between fluorescence intensity and the degree of protein sorting. Furthermore, the total fluorescence correlation plots of Figure 4C,D showed that fluorescence intensities were dispersed. These results are consistent with the concept that protein segregates to vesicles that have a broad distribution in size and intensity (52).
Table 2.
Properties of the highly sorted vesiclesa
| Protein | Highly sorted vesicles | Remaining vesicles |
|---|---|---|
| ASOR | ||
| Mean intensity | 337.7 | 244.5 |
| SD | 305.8 | 355.3 |
| Per cent moving | 41 | 52 |
| n | 27 | 172 |
| Ntcp | ||
| Mean intensity | 399.5 | 301.3 |
| SD | 335.3 | 347.3 |
| Per cent moving | 70 | 54 |
| n | 20 | 90 |
| Transferrin receptor | ||
| Mean intensity | 717.9 | 477.8 |
| SD | 585.9 | 478.0 |
| Per cent moving | 86 | 50 |
| n | 14 | 112 |
These are a subset of fissions where proteins strongly sorted from each other. They contained at least 80% of the listed protein and less than 20% of the ‘opposite’ protein. For ASOR, the opposite protein was considered as both ntcp and TfR, and for ntcp and TfR, the opposite protein was considered as ASOR.
The transmembrane proteins, ntcp and transferrin receptor, were highly motile during fission
Fissions were observed as the division of a single vesicle into two daughter vesicles. Typically, one of the daughter vesicles was motile, while the other remained stationary, although in some fissions (6%) both daughters were motile (Supplementary Tables). A review of the data shows that some proteins were less abundant in the moving vesicles than others. For instance, on average, 66% of the daughter vesicles in the lower left and lower right quadrants of Figures 4A,B and 5A–C were motile. These are the daughter vesicles that contained the smaller amount of ASOR from the fission. The trend is more apparent when looking at fissions that strongly segregated protein. Seventy-two per cent of daughter vesicles that contained either ntcp or transferrin receptor but were completely depleted of ASOR after fission were motile (n = 60, culled from Tables S1–S6). However, only 38% of the vesicles that contained ASOR but were completely depleted of ntcp were motile (n = 16, p = 0.01), and 20% of vesicles that contained ASOR but were completely depleted of transferrin receptor were motile (n = 11, p < 0.001). This is also seen in the analysis of ‘highly sorted’ vesicles of Table 2. Seventy per cent and 86% of the highly sorted ntcp- and transferrin receptor-containing vesicles, respectively, were motile compared with 41% of the highly sorted ASOR-containing vesicles (n = 27, p = 0.03 and p < 0.001, respectively, compared with highly sorted ASOR-containing vesicles). These data show that fissions were frequently the result of the transmembrane proteins, ntcp and transferrin receptor, moving away from the ligand, ASOR, that is located on the lumenal side of the vesicle. This is consistent with the concept of ‘geometric’ protein sorting in which transmembrane proteins are believed to be concentrated into tubules that bud or pull away from the endocytic lumen, resulting in protein segregation (8,53).
Discussion
This study demonstrates that microtubule-based motility results in segregation of endocytic protein and that the degree of segregation is specific to the particular protein. In living cells, internalized ligands were seen to undergo multiple microtubule-based vesicle fissions, resulting in apparent segregation of protein (Figure 1). However, varying background fluorescence, photobleaching and the complex, three-dimensional motion of the vesicles prevented accurate quantification of protein fluorescence. Instead, we used endocytic vesicles prepared from rat liver to recapitulate microtubule-based traffic within in vitro microscopy assays. This allowed direct quantification of protein with fluorescent antibodies and fluorescent-tagged ligands. Previous reports have shown that movement of the vesicles is dependent on specific sets of motor proteins and that the inhibition of motor proteins blocks fission (11,20,26,33). Fission resulted from splitting and movement of at least one daughter vesicle along the microtubule in every case (Supplementary Tables).
It is possible that fission under these conditions is dependent only on the activity of motor proteins. However, we found that fission is regulated differently than vesicle motility. For instance, vesicles containing transferrin receptor and ASOR had decreased motility but increased fission compared with vesicles containing transferrin receptor but no ASOR. Also, PI3-kinase inhibition reduced fission but not motility (Figure 2). This suggests that fission on micro-tubules results from the co-ordinated activity of motor proteins with other cellular machineries. These may include proteins that have been shown to be involved in vesicle budding and protein sorting such as coatomers, adaptors and ADP ribosylation factor 1 (54). It should be emphasized that our studies were performed without addition of exogenous proteins and that soluble material is removed during the vesicle purification and microscopy assays. The vesicles themselves had all the machinery required to produce motility, fission and protein segregation.
Analyses of protein levels on vesicles undergoing fission suggest that proteins segregate during fission only when they are destined for different cellular compartments. Ntcp strongly segregated away from ASOR (Table 1), and this was achieved because ASOR sorted asymmetrically, leaving many ntcp vesicles completely devoid of ASOR (Figure 4A). The transferrin receptor showed a similar but somewhat weaker segregation from ASOR (Figure 4B; Table 1), whereas ntcp and transferrin receptor, proteins that are both recycled to the plasma membrane, remained together during fission (Figure 5A). ASOR showed moderate segregation from its receptor, ASGPR. Although the receptor is recycled and the ligand is degraded, a significant portion of ASOR remains bound to receptor following endocytosis and only dissociates slowly (49). Similar slowly dissociating pools of ligand are seen for many if not most receptor–ligand pairs, and a full understanding of this phenomenon remains to be elucidated. EGFR, an integral membrane protein that is targeted for degradation (23), also failed to segregate efficiently from ASOR. It is interesting to consider that neither ASGPR nor EGFR segregates efficiently from ASOR in vitro. However, within cells, ASGPR is recycled to the plasma membrane, while EGFR is degraded. Analysis of the data indicates that, although their overall segregation from ASOR is similar, more ‘pure’ ASGPR vesicles are generated than pure EGFR vesicles (Figure 5B,C). This suggests that the generation of pure vesicles is an important step for protein segregation, and we speculate that these vesicles may be directly targeted to the recycling compartment and plasma membrane.
The geometric model of protein segregation proposes that vesicles enriched in transmembrane receptors are physically pulled away from lumenal protein (53). Agreeing with this, we found that fissions frequently produced a motile daughter vesicle that was enriched in transmembrane protein (Table 2). A recent study that tracked the movement of endocytic vesicles within cells showed that ligands can pre-sort to dynamic and static endosome populations and that the dynamic population is more rapidly processed for degradation (6). We also observed populations of vesicles with different rates of motility. However, in our studies, vesicles containing proteins that are destined for recycling (ntcp and transferrin receptor) had similar or higher motility than vesicles containing proteins or molecules that are destined for degradation (ASOR and dextran; Figure 2). Many factors can regulate motility (5,26,55), and additional studies will be needed to understand this regulation. The recycling of membrane transporters such as GLUT4 or Na+/H+ exchanger (42,43) has been found to be dependent on PI3-kinase activity, and in this study, it is shown that PI3-kinase inhibition reduces fissions by more than half without affecting vesicle motility. Studies from our laboratory have found that inhibition of the microtubule-based minus-ended motor KIFC1 decreased fission (33) and that rab4 may have a role (55) in regulation of the fission machinery. Activation of the molecular motors by protein kinases (56,57) and concomitant involvement of the Rab proteins represent potential mediators of membrane fission and vesicle processing.
Materials and Methods
Chemicals and reagents
Commercial antibodies used were transferrin receptor (number 13-6800; Zymed laboratories) and EGFR (number sc-03; Santa Cruz Biotechnology). In-house-generated antibodies used were ASGPR, to the C-terminal cytoplasmic tail (20), ntcp, to amino acids 337–350 at the cytoplasmic C-terminus (26). Primary antibodies were used at 0.33–20 μg/mL. Fluorescent secondary antibodies were from Jackson ImmunoResearch. ASOR was prepared from human orosomucoid (Sigma-Aldrich) by acid hydrolysis (58) and labeled with Texas red sulfonyl chloride or Alexa-488 (Invitrogen Corporation) as described (32). Some ASOR was labeled with 125I by a chloramine-T method to a specific activity of 10 000–15 000 c.p.m./ng (59). Amino dextran (Cat. no. D1861; Invitrogen Corporation) was labeled with Texas red sulfonyl chloride. Labeled and unlabeled tubulin was from Cytoskeleton. All other reagents were from Sigma-Aldrich.
Fluorescent ASOR-containing endocytic vesicles were prepared from rat liver as previously described (11,20,26,32,60,61). For some experiments, the portal veins of two livers were separately injected with either Texas red or Alexa-488 ASOR, and these were homogenized together and vesicles were isolated according to protocol. Taxol-stabilized fluorescent micro-tubules were polymerized from rhodamine–tubulin as previously described (11). Assay of vesicle motility on microtubules was as described (20,32,60). Antibodies were screened for their appropriateness in immunofluorescence assays by Western blot and whole cell immunofluorescence (37). For a given day's experiment, 10–15 chambers with microtubule-bound, immunofluorescence-stained endosomes were assembled and stored on ice, and the chambers were sequentially placed on a microscope stage maintained at 37°C. Time-lapse multi-fluorescence channel microscopy was then initiated, and 50 μm ATP was added to induce movement and fission of the endosomes.
Immunofluorescence analysis in vitro
Detection of protein on unfixed endocytic vesicles by monoclonal or affinity-purified polyclonal antibody followed by fluorescence secondary antibody was performed as described (60). Wide-field imaging was performed at 37°C using a 60×, 1.4 numerical aperture Olympus objective on an Olympus 1X71 inverted microscope equipped for multi-channel fluorescence with a Lambda DG-4 excitation source and Lambda 10-2 emission filter wheel (Sutter Instruments). Data were collected through a CoolSnap HQ cooled charge-coupled device camera (Photometrics, Roper Scientific) using metamorph software (Molecular Devices). Time-lapse movies were captured in 1–3 channels (Cy2, Cy3 and Cy5). Some images were captured on a BioRad Radiance confocal microscope in the Analytical Imaging Facility (Albert Einstein College of Medicine). Movies were analyzed using imagej software (National Institutes of Health public domain; http://rsb.info.nih.gov/ij/) and adobe photoshop v 6.0 (Adobe Systems Inc.). Protein signal was measured as the signal intensity minus background multiplied by the area. Background intensity was measured and averaged from three neighboring regions. To adjust for different imaging conditions (e.g. exposure time and excitation intensity), fluorescence was normalized so that each set of experiments gave equal median vesicle fluorescence for a given protein. For total fluorescence correlation plots, 10 units (in arbitrary units) were added to each vesicle to allow plotting on a logarithmic scale. The p-values for correlation coefficients were from Student's t-test. The Supplementary Tables present all the data from different sets of experiments that were performed either with different combinations of antibodies or after pre-incubation with 50 mm LY294002. In the correlation plots, fissions were culled from all experimental sets where the listed proteins were used, regardless of the presence of other proteins.
ASOR uptake in cultured rat hepatocytes
Rat hepatocytes were isolated by collagenase perfusion as described (55), attached to coverslip-bottomed dishes (MatTek Corporation) that were coated with 0.5 mg/mL Matrigel (BD Biosciences) and cultured overnight in Hepatozyme media (Invitrogen Corporation). Dishes were treated with 10 μg/mL Texas red-labeled ASOR and, in some experiments, 20 μg/mL Alexa-488 transferrin (Invitrogen Corporation) for 5 min, washed and imaged at 37°C with 200-millisecond exposure times. Images were processed with a SpotEnhancing filter (Daniel Sage, Biomedical Imaging Group). For some experiments, cells were cooled to 4°C in the presence or absence of 30 μm nocodazole for 30 min, followed by warming to 37°C and exposure to ASOR as described above in continuous exposure to nocodazole.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health grants DK41918 and DK23026.
Footnotes
Supplemental materials are available as part of the online article at http://www.blackwell-synergy.com
References
- 1.Gruenberg J. The endocytic pathway: a mosaic of domains. Nat Rev Mol Cell Biol. 2001;2:721–730. doi: 10.1038/35096054. [DOI] [PubMed] [Google Scholar]
- 2.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 3.Wessels E, Simpson JC. Impact of live cell imaging on coated vesicle research. Semin Cell Dev Biol. 2007;18:412–423. doi: 10.1016/j.semcdb.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 5.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 6.Lakadamyali M, Rust MJ, Zhuang X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell. 2006;124:997–1009. doi: 10.1016/j.cell.2005.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matteoni R, Kreis TE. Translocation and clustering of endosomes and lysosomes depends on microtubules. J Cell Biol. 1987;105:1253–1265. doi: 10.1083/jcb.105.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geuze HJ, Slot JW, Strous GJ, Lodish HF, Schwartz AL. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983;32:277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- 9.Harding C, Levy MA, Stahl P. Morphological analysis of ligand uptake and processing: the role of multivesicular endosomes and CURL in receptor-ligand processing. Eur J Cell Biol. 1985;36:230–238. [PubMed] [Google Scholar]
- 10.Hoepfner S, Severin F, Cabezas A, Habermann B, Runge A, Gillooly D, Stenmark H, Zerial M. Modulation of receptor recycling and degradation by the endosomal kinesin KIF16B. Cell. 2005;121:437–450. doi: 10.1016/j.cell.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Bananis E, Nath S, Gordon K, Satir P, Stockert RJ, Murray JW, Wolkoff AW. Microtubule-dependent movement of late endocytic vesicles in vitro: requirements for dynein and kinesin. Mol Biol Cell. 2004;15:3688–3697. doi: 10.1091/mbc.E04-04-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolkoff AW, Klausner RD, Ashwell G, Harford J. Intracellular segregation of asialoglycoproteins and their receptor: a prelysosomal event subsequent to dissociation of the ligand-receptor complex. J Cell Biol. 1984;98:375–381. doi: 10.1083/jcb.98.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breitfeld PP, McKinnon WC, Mostov KE. Effect of nocodazole on vesicular traffic to the apical and basolateral surfaces of polarized MDCK cells. J Cell Biol. 1990;111:2365–2373. doi: 10.1083/jcb.111.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunziker W, Male P, Mellman I. Differential microtubule requirements for transcytosis in MDCK cells. EMBO J. 1990;9:3515–3525. doi: 10.1002/j.1460-2075.1990.tb07560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maples CJ, Ruiz WG, Apodaca G. Both microtubules and actin filaments are required for efficient postendocytotic traffic of the polymeric immunoglobulin receptor in polarized Madin-Darby canine kidney cells. J Biol Chem. 1997;272:6741–6751. doi: 10.1074/jbc.272.10.6741. [DOI] [PubMed] [Google Scholar]
- 16.Koval M, Pagano RE. Lipid recycling between the plasma membrane and intracellular compartments: transport and metabolism of fluorescent sphingomyelin analogues in cultured fibroblasts. J Cell Biol. 1989;108:2169–2181. doi: 10.1083/jcb.108.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGraw TE, Dunn KW, Maxfield FR. Isolation of a temperature-sensitive variant Chinese hamster ovary cell line with a morphologically altered endocytic recycling compartment. J Cell Physiol. 1993;155:579–594. doi: 10.1002/jcp.1041550316. [DOI] [PubMed] [Google Scholar]
- 18.Block MR, Glick BS, Wilcox CA, Wieland FT, Rothman JE. Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc Natl Acad Sci U S A. 1988;85:7852–7856. doi: 10.1073/pnas.85.21.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spang A, Schekman R. Reconstitution of retrograde transport from the Golgi to the ER in vitro. J Cell Biol. 1998;143:589–599. doi: 10.1083/jcb.143.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bananis E, Murray JW, Stockert RJ, Satir P, Wolkoff AW. Microtubule and motor-dependent endocytic vesicle sorting in vitro. J Cell Biol. 2000;151:179–186. doi: 10.1083/jcb.151.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunn KW, McGraw TE, Maxfield FR. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endo-some. J Cell Biol. 1989;109:3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang T, Wolkoff AW, Stockert RJ. Adaptor heat shock protein complex formation regulates trafficking of the asialoglycoprotein receptor. Am J Physiol Gastrointest Liver Physiol. 2006;290:G369–G376. doi: 10.1152/ajpgi.00204.2005. [DOI] [PubMed] [Google Scholar]
- 23.Dikic I. Mechanisms controlling EGF receptor endocytosis and degradation. Biochem Soc Trans. 2003;31:1178–1181. doi: 10.1042/bst0311178. [DOI] [PubMed] [Google Scholar]
- 24.Renfrew CA, Hubbard AL. Degradation of epidermal growth factor receptor in rat liver. Membrane topology through the lysosomal pathway. J Biol Chem. 1991;266:21265–21273. [PubMed] [Google Scholar]
- 25.Mukhopadhayay S, Ananthanarayanan M, Stieger B, Meier PJ, Suchy FJ, Anwer MS. cAMP increases liver Na+-taurocholate cotransport by translocating transporter to plasma membranes. Am J Physiol. 1997;273:G842–G848. doi: 10.1152/ajpgi.1997.273.4.G842. [DOI] [PubMed] [Google Scholar]
- 26.Sarkar S, Bananis E, Nath S, Anwer MS, Wolkoff AW, Murray JW. PKCzeta is required for microtubule-based motility of vesicles containing the ntcp transporter. Traffic. 2006;7:1078–1091. doi: 10.1111/j.1600-0854.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 27.Marbet P, Rahner C, Stieger B, Landmann L. Quantitative microscopy reveals 3D organization and kinetics of endocytosis in rat hepatocytes. Microsc Res Tech. 2006;69:693–707. doi: 10.1002/jemt.20337. [DOI] [PubMed] [Google Scholar]
- 28.Herman B, Albertini DF. A time-lapse video image intensification analysis of cytoplasmic organelle movements during endosome trans-location. J Cell Biol. 1984;98:565–576. doi: 10.1083/jcb.98.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goltz JS, Wolkoff AW, Novikoff PM, Stockert RJ, Satir P. A role for microtubules in sorting endocytic vesicles in rat hepatocytes. Proc Natl Acad Sci U S A. 1992;89:7026–7030. doi: 10.1073/pnas.89.15.7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stockert RJ. The asialoglycoprotein receptor: relationships between structure, function, and expression. Physiol Rev. 1995;75:591–609. doi: 10.1152/physrev.1995.75.3.591. [DOI] [PubMed] [Google Scholar]
- 31.Driskell OJ, Mironov A, Allan VJ, Woodman PG. Dynein is required for receptor sorting and the morphogenesis of early endosomes. Nat Cell Biol. 2007;9:113–120. doi: 10.1038/ncb1525. [DOI] [PubMed] [Google Scholar]
- 32.Murray JW, Bananis E, Wolkoff AW. Reconstitution of ATP-dependent movement of endocytic vesicles along microtubules in vitro: an oscillatory bidirectional process. Mol Biol Cell. 2000;11:419–433. doi: 10.1091/mbc.11.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nath S, Bananis E, Sarkar S, Stockert RJ, Sperry AO, Murray JW, Wolkoff AW. Kif5B and Kifc1 interact and are required for motility and fission of early endocytic vesicles in mouse liver. Mol Biol Cell. 2007;18:1839–1849. doi: 10.1091/mbc.E06-06-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vale RD, Reese TS, Sheetz MP. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985;42:39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Endow SA, Higuchi H. A mutant of the motor protein kinesin that moves in both directions on microtubules. Nature. 2000;406:913–916. doi: 10.1038/35022617. [DOI] [PubMed] [Google Scholar]
- 36.Gill SR, Schroer TA, Szilak I, Steuer ER, Sheetz MP, Cleveland DW. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J Cell Biol. 1991;115:1639–1650. doi: 10.1083/jcb.115.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray JW, Wolkoff AW. Assay of Rab4-dependent trafficking on microtubules. Methods Enzymol. 2005;403:92–107. doi: 10.1016/S0076-6879(05)03009-0. [DOI] [PubMed] [Google Scholar]
- 38.Ichikawa T, Yamada M, Homma D, Cherry RJ, Morrison IE, Kawato S. Digital fluorescence imaging of trafficking of endosomes containing low-density lipoprotein in brain astroglial cells. Biochem Biophys Res Commun. 2000;269:25–30. doi: 10.1006/bbrc.2000.2261. [DOI] [PubMed] [Google Scholar]
- 39.Petiot A, Faure J, Stenmark H, Gruenberg J. PI3P signaling regulates receptor sorting but not transport in the endosomal pathway. J Cell Biol. 2003;162:971–979. doi: 10.1083/jcb.200303018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy RF. Analysis and isolation of endocytic vesicles by flow cytometry and sorting: demonstration of three kinetically distinct compartments involved in fluid-phase endocytosis. Proc Natl Acad Sci U S A. 1985;82:8523–8526. doi: 10.1073/pnas.82.24.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Dam EM, Ten Broeke T, Jansen K, Spijkers P, Stoorvogel W. Endocytosed transferrin receptors recycle via distinct dynamin and phosphatidylinositol 3-kinase-dependent pathways. J Biol Chem. 2002;277:48876–48883. doi: 10.1074/jbc.M206271200. [DOI] [PubMed] [Google Scholar]
- 42.Malide D, Cushman SW. Morphological effects of wortmannin on the endosomal system and GLUT4-containing compartments in rat adipose cells. J Cell Sci. 1997;110:2795–2806. doi: 10.1242/jcs.110.22.2795. [DOI] [PubMed] [Google Scholar]
- 43.Kurashima K, Szabo EZ, Lukacs G, Orlowski J, Grinstein S. Endosomal recycling of the Na+/H+ exchanger NHE3 isoform is regulated by the phosphatidylinositol 3-kinase pathway. J Biol Chem. 1998;273:20828–20836. doi: 10.1074/jbc.273.33.20828. [DOI] [PubMed] [Google Scholar]
- 44.Klopfenstein DR, Tomishige M, Stuurman N, Vale RD. Role of phosphatidylinositol(4,5)bisphosphate organization in membrane transport by the Unc104 kinesin motor. Cell. 2002;109:347–358. doi: 10.1016/s0092-8674(02)00708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dranoff JA, McClure M, Burgstahler AD, Denson LA, Crawford AR, Crawford JM, Karpen SJ, Nathanson MH. Short-term regulation of bile acid uptake by microfilament-dependent translocation of rat ntcp to the plasma membrane. Hepatology. 1999;30:223–229. doi: 10.1002/hep.510300136. [DOI] [PubMed] [Google Scholar]
- 46.Krutzik PO, Clutter MR, Nolan GP. Coordinate analysis of murine immune cell surface markers and intracellular phosphoproteins by flow cytometry. J Immunol. 2005;175:2357–2365. doi: 10.4049/jimmunol.175.4.2357. [DOI] [PubMed] [Google Scholar]
- 47.Morell AG, Irvine RA, Sternlieb I, Scheinberg IH, Ashwell G. Physical and chemical studies on ceruloplasmin. V. Metabolic studies on sialic acid-free ceruloplasmin in vivo. J Biol Chem. 1968;243:155–159. [PubMed] [Google Scholar]
- 48.Weigel PH, Yik JH. Glycans as endocytosis signals: the cases of the asialoglycoprotein and hyaluronan/chondroitin sulfate receptors. Biochim Biophys Acta. 2002;1572:341–363. doi: 10.1016/s0304-4165(02)00318-5. [DOI] [PubMed] [Google Scholar]
- 49.Oka JA, Weigel PH. Recycling of the asialoglycoprotein receptor in isolated rat hepatocytes. Dissociation of internalized ligand from receptor occurs in two kinetically and thermally distinguishable compartments. J Biol Chem. 1983;258:10253–10262. [PubMed] [Google Scholar]
- 50.Stockert RJ, Potvin B, Tao L, Stanley P, Wolkoff AW. Human hepatoma cell mutant defective in cell surface protein trafficking. J Biol Chem. 1995;270:16107–16113. doi: 10.1074/jbc.270.27.16107. [DOI] [PubMed] [Google Scholar]
- 51.Waterman H, Yarden Y. Molecular mechanisms underlying endocytosis and sorting of ErbB receptor tyrosine kinases. FEBS Lett. 2001;490:142–152. doi: 10.1016/s0014-5793(01)02117-2. [DOI] [PubMed] [Google Scholar]
- 52.Luini A, Ragnini-Wilson A, Polishchuck RS, De Matteis MA. Large pleiomorphic traffic intermediates in the secretory pathway. Curr Opin Cell Biol. 2005;17:353–361. doi: 10.1016/j.ceb.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 53.Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 54.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–682. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 55.Bananis E, Murray JW, Stockert RJ, Satir P, Wolkoff AW. Regulation of early endocytic vesicle motility and fission in a reconstituted system. J Cell Sci. 2003;116:2749–2761. doi: 10.1242/jcs.00478. [DOI] [PubMed] [Google Scholar]
- 56.Dillman JF III, Pfister KK. Differential phosphorylation in vivo of cytoplasmic dynein associated with anterogradely moving organelles. J Cell Biol. 1994;127:1671–1681. doi: 10.1083/jcb.127.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okada Y, Sato-Yoshitake R, Hirokawa N. The activation of protein kinase A pathway selectively inhibits anterograde axonal transport of vesicles but not mitochondria transport or retrograde transport in vivo. J Neurosci. 1995;15:3053–3064. doi: 10.1523/JNEUROSCI.15-04-03053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stockert RJ, Haimes HB, Morell AG, Novikoff PM, Novikoff AB, Quintana N, Sternlieb I. Endocytosis of asialoglycoprotein-enzyme conjugates by hepatocytes. Lab Invest. 1980;43:556–563. [PubMed] [Google Scholar]
- 59.Hudgin RL, Pricer WE, Jr, Ashwell G, Stockert RJ, Morell AG. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J Biol Chem. 1974;249:5536–5543. [PubMed] [Google Scholar]
- 60.Murray JW, Bananis E, Wolkoff AW. Immunofluorescence micro-chamber technique for characterizing isolated organelles. Anal Biochem. 2002;305:55–67. doi: 10.1006/abio.2002.5655. [DOI] [PubMed] [Google Scholar]
- 61.Murray JW, Wolkoff AW. In vitro motility system to study the role of motor proteins in receptor-ligand sorting. Methods Mol Biol. 2007;392:143–158. doi: 10.1007/978-1-59745-490-2_10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.