Abstract
Brain tumors represent the most common solid tumors in childhood, accounting for almost 25% of all childhood cancer, second only to leukemia. Childhood CNS tumors encompass a wide variety of diagnoses, from benign to malignant. Any brain tumor can be associated with significant morbidity, even when low grade, and mortality from childhood CNS tumors is disproportionately high compared to other childhood malignancies. Management of children with CNS tumors requires knowledge of the unique aspects of care associated with this particular patient population, beyond general oncology care. Pediatric brain tumor patients have unique needs during treatment, as cancer survivors, and at end of life. A multidisciplinary team approach, including advanced practice nurses with a specialty in neuro-oncology, allows for better supportive care. Knowledge of the unique aspects of care for children with brain tumors, and the appropriate interventions required, allows for improved quality of life.
Keywords: Pediatric, Neuro-oncology, Brain Tumor, Quality of Life
Introduction
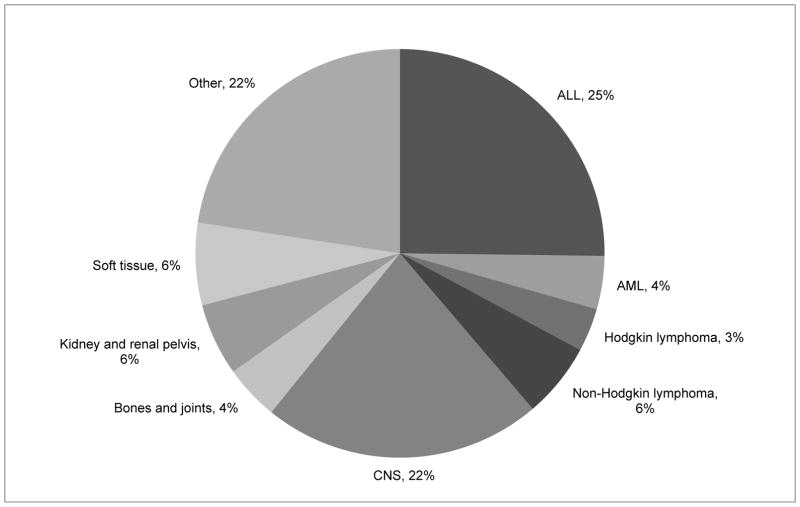
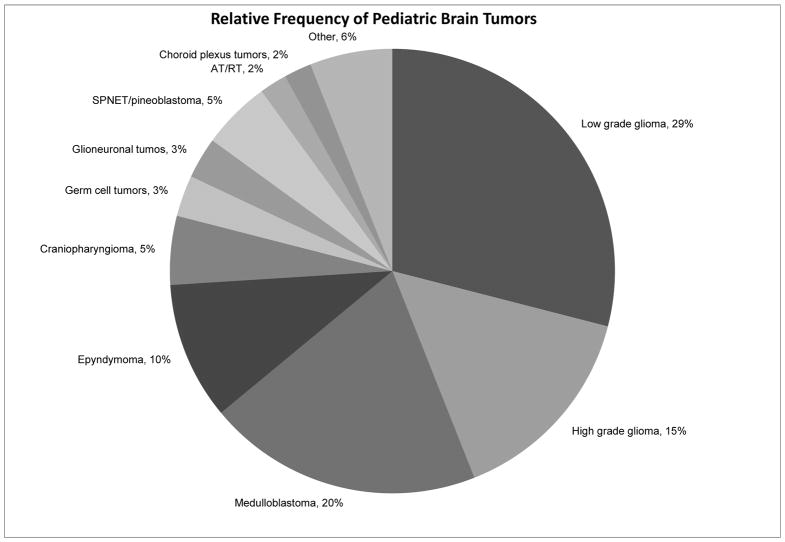
CNS tumors are the second most common pediatric malignancy, second only to ALL, and represent the most common solid tumors in childhood. Overall, CNS tumors account for 22% of all pediatric cancer diagnoses (figure 1). Within the spectrum of CNS tumors there is a myriad of diagnoses (figure 2), ranging from benign to malignant, from low to high grade. While certain diagnoses, such as diffuse intrinsic pontine glioma, are known to have dismal survival rates, even a low grade astrocytoma with an excellent prognosis can have significant morbidity with potential to dramatically affect quality of life. Depending on the histiologic diagnosis the plans for oncologic treatment, survivorship, and end of life care vary widely.
Figure 1.
U.S. Cancer Incidence in Patients <15 years of age. U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2011 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2014. Available at: www.cdc.gov/uscs.
Figure 2.
Relative Frequency of Pediatric Brain Tumors. Data from Rickert CH, Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv System 2001; 17:503–11.
Throughout the spectrum of care these patients may have a combination of neurologic deficits including speech and swallowing impairments affecting communication and nutrition, vision changes, motor and sensory deficits, and impairments in balance and coordination. Headaches, fatigue, seizures, stroke, endocrinopathies, and neurocognitive and behavioral changes can develop at diagnosis, after surgery, after treatment, or at end of life. These deficits can improve and even resolve, but for the majority of patients they are chronic or evolving issues that impair function and require referrals to specialists in rehabilitative medicine, neuro-ophthalmology, neurology, endocrinology, psychiatry, and neuropsychiatry. With appropriate interventions and management pediatric patients with CNS tumors can have improved quality of life during treatment, survivorship, and at end of life.
Treatment
ND is a 10 year old boy with a two month history of slowly increasing fatigue, headaches and diplopia. Three days prior to presenting to the local ER he had two episodes of jerking of all extremities and uncontrollable laughter. He then developed lethargy, severe headache, and vomiting, and presented acutely to his local emergency room. Brain MRI confirmed a large midline mass occupying the pineal region and 3rd ventricle, with resulting hydrocephalus. ND was transferred to the Pediatric Intensive Care Unit and started on dexamethasone and levetiracetam. Elevated serum alpha fetoprotein confirmed the diagnosis of a malignant CNS germ cell tumor. A ventriculoperitoneal shunt was placed. Spine MRI was negative and a lumbar puncture could not be performed due to extent of hydrocephalus. An endocrine work-up was negative. Residual deficits included mild somnolence, diplopia, Parinaud’s syndrome, and nystagmus. Standard chemotherapy for CNS germ cell tumor was started emergently. Post chemotherapy MRI revealed residual disease and he underwent second look surgery, which confirmed residual tumor was mature teratoma. ND proceeded to radiation therapy, consisting of whole ventricle radiation with a boost to the tumor bed. He was referred to neuro-ophthalmology for vision deficits and to physical therapy for general weakness. He received home instruction and an individualized educational plan was developed to allow continued tutoring during treatment. Following completion of treatment he successfully transitioned back to school full time and was active in sports with accommodations for residual vision impairments.
Pediatric brain tumors encompass a wide range of pathologic classification. It is imperative to understand the pathology of the tumor because it directly impacts the child’s extent of disease evaluation, treatment and prognosis. The WHO classification system for pediatric central nervous system neoplasms is used to specify the histology and grade of tumors. With our increased understanding of the molecular signatures of many CNS tumors, the WHO classification will be updated in the future to include this information, which may be helpful in predicting prognosis, and possibly suggest new, targeted therapies. Staging of tumors is based upon location and by dissemination within and beyond the central nervous system.1 This information guides the team as they begin to care for the newly diagnosed child or adolescent and their family. Helping families and, in an age appropriate approach, the child or adolescent, understand the significance of the pathology is an important first step in their treatment.
Clinical Presentation
The diagnosis of a CNS neoplasm starts with a detailed history elicited by the practitioner. Practitioners must be sensitive to the parents’ anxiety as the questions are asked, particularly if the child has been unwell for a few weeks or months. A meticulous physical and neurological examination is performed. In general, a child who presents with rapidly progressing symptoms in a short time period is likely to have a rapidly growing malignant brain tumor, often associated with surrounding edema. The size and the location of the tumor contribute to the presenting symptomatology. Symptoms occur because of acute or chronic increased intracranial pressure and/or the infiltration or compression of surrounding areas of the central nervous system. Non-localizing symptoms that occur because of intracranial pressure can include headache, vomiting, changes in personality, diplopia from cranial nerve six (abducens) palsy, and papilledema and, in young children, rapidly increasing head circumference. Headaches that are new or have changed in nature from previous headaches, wake the child from sleep, occur upon awakening and worsen with the Valsalva maneuver are considered red flags.2 Headaches accompanied by nausea or vomiting, particularly if vomiting relieves the pain, are particularly concerning. Localizing symptoms occur because of the tumor’s location although there can be some overlap of symptoms. As an example, tumors in the cerebral hemispheres can cause headache, personality changes, memory loss, seizures, focal, motor or sensory deficits, hearing and visual field changes. Midline and suprasellar tumors can present with endocrinopathies, visual impairment and increased intracranial pressure. Cerebellar lesions causing hydrocephalus can also present with signs and symptoms of raised intracranial pressure but may also result in ataxia, cranial neuropathies, hemiparesis and dysdiadochokinesis.3, 4 (Table 1)
Table 1.
Cardinal symptoms of CNS tumors in children and adolescents
| Cause | Cardinal symptom |
|---|---|
| Elevated intracranial pressure | Vomiting (despite empty stomach), headache, personality changes, cranial nerve VI palsy, papilledema and visual impairment, sunset phenomenon, macrocephaly |
| Cerebellar tumor | Unsteady gait, scanning speech, ataxia, nystagmus, intention tremor, dysdiadochokinesis |
| Brain stem tumor/infiltration | Horizontal ophthalmoplegia, cranial nerve palsies, spastic palsies, long tract signs |
| Cerebellopontine tumor/extension | Facial paralysis, hearing loss, torticollis |
| Cerebral hemispheric tumor | Cerebral seizures (e.g., complex partial seizures), pareses, paralyses, sensory impairments |
| Suprasellar tumor/chiasma/hypothalamus tumors | Vision loss, narrowing of visual field, nystagmus |
| Tumor of hypophyseal and hypothalamic region | Short stature, diabetes insipidus, disordered pubertal development, eating disorders |
| Diencephalic tumors/infiltration | Diencephalic syndrome: cachexia in infants who sometimes appear euphoric |
| Pineal/midbrain tumor | Parinaud syndrome with vertical ophthalmoplegia |
| Spinal tumors and metastases | Back pain, scoliosis, transverse symptoms, pyramidal tract signs, but also flaccid paralysis |
Reprinted from Fruehwald, M. and Rutkowski, S. Tumors of the CNS in Children and Adolescents Deutsches Arzteblatt International 2011; 108(22): 390–397.
Children with certain genetic syndromes or family history such as NF-1,NF-2, tuberous sclerosis, Li-Fraumeni syndrome, Gardner syndrome, Turcot syndrome and Gorlin syndrome have a predisposition for developing CNS tumors and must be monitored appropriately.5
Diagnostic Evaluation
If the neurological exam reveals deficits, neuro-imaging, preferably with MRI with and without gadolinium is performed. Visual and auditory aides such as music and movies are now available during MRI, and may help children tolerate a lengthy MRI. If a child is medically unstable or too young or anxious to lie quietly for an MRI, a CT of the brain, ideally with and without iodinated contrast, can be done. Neuro-radiologists may also recommend other imaging techniques tools such as diffusion-tensor imaging, dynamic contrast enhanced imaging, functional MRI, spectroscopy and a fluorodeoxyglucose (FDG) PET to further help characterize the lesion.6, 7 Our institution is using a MRI hydrocephalus protocol which includes single shot fast spin echo imaging, axial diffusion weighted imaging and axial apparent diffusion coefficient imaging and, sometimes, susceptibility weighted imaging. The images are clear, scan time is short and there is no risk of radiation exposure. This scan, however, may not be practical for the very young, anxious or medically unstable child so we continue to use non-contrast CT scans to assess for hydrocephalus with these patients. Further work-up is dictated by the pathology of the primary tumor and may include lumbar puncture, bone marrow aspirate and biopsy, and bone scan. In primary CNS germ cell tumors, serum and cerebrospinal fluid alpha fetoprotein and human chorionic gonadotropin levels can be diagnostic.8 Knowing the full extent of disease at diagnosis is vital for treatment planning and prognosis.
Surgery
Neurosurgical intervention is the initial treatment modality for the majority of pediatric brain tumors, depending upon location of the lesion, clinical status of the patient and disease dissemination. The goals for surgical intervention include obtaining a tissue diagnosis, removing or debulking the tumor,9 and restoring normal CSF flow via ventriculoperitoneal shunt or endoscopic third ventriculostomy. Intraoperative MRI may assist in achieving complete resection while decreasing morbidity by guiding the neurosurgeon away from vital structures/tracts. Endoscopic third ventriculostomy may prevent the placement of shunt hardware, decreasing the possibility of infection and the remote chance for extracranial disease spread.9 In general, complete resections improve survival rates. However, the risk of morbidity in some cases is too high to consider surgery a safe option. With a diffuse intrinsic pontine glioma, for example, diagnosis has traditionally been based on clinical presentations and MRI findings; with the advent of molecular profiling and safer neurosurgical techniques, diffuse intrinsic pontine glioma is being more frequently biopsied. Molecular profiling may suggest a targeted therapy if standard therapeutic options fail. With hypothalamic chiasmatic gliomas, primary surgical resection is not recommended because of the risk of visual impairment.10 Emergency neurosurgical interventions may be necessary with obstructive hydrocephalus, hemorrhage, or mass effect. Early diagnosis and treatment of increased intracranial pressure with the goal of optimizing cerebral perfusion and maintaining cerebral metabolic demands is critical to preventing brain injury.11
Neurosurgical procedures carry intraoperative and post-operative risks. Complications can include infection, blood loss, neurological morbidity, hematoma and edema. Cerebellar mutism or posterior fossa syndrome can occur 24–48 hours post-operatively after posterior fossa surgery. While there is a range of the severity of this syndrome, the child can develop mutism, hypotonia, emotional lability, and neurocognitive impairment.12 There is no treatment protocol and recovery varies. Zolpidem has been used successfully to increase wakefulness, accelerate the resolution of the mutism and improve the irritability.13 Rehabilitative services are critical and should be initiated as soon as the child is medically stable. Prior to discharge, patient and caretakers receive extensive personalized teaching from the practitioner so that they can recognize neurological deterioration and seek immediate medical attention. In addition if indicated, the practitioner reviews the management of seizures and anticonvulsants and of diabetes insipidus and intranasal vasopressin. Steroid tapering is documented on a home diary and the families are aware of the possibility of hypertension, hyperglycemia, oral candidiasis and dramatic mood swings.
Radiation Therapy
Radiation therapy is a vital component of the multimodal plan for the management of most malignant brain tumors in children over the age of 5 years. The pathology, location of the tumor, degree of surgical resection, disease dissemination and age of the child will determine the amount and type of radiation. Most centers use photon energy delivered either to a focal area or to the entire craniospinal axis.14 Proton therapy, the most recent advancement in radiation therapy, delivers the same total dose but it can reduce the dose that may affect healthy tissue because of less scatter.15 It has the potential to safely escalate higher doses to the high risk tumors. Proton beam RT may offer similar cure rates with fewer side effects but more head to head studies need to be published.16 In most cases, radiation therapy is given daily for approximately 6 weeks which allows the child/adolescent to tolerate a large cumulative dose. Delivering radiation therapy to a young or anxious child is more complicated because it involves daily anesthesia, although it is safely done in many centers. Immediate side effects of radiation therapy can include nausea, anorexia, and headache, which can be effectively addressed with medication. Skin changes in the field of radiation can result in reddened and irritated patches that respond to water base emollients and heal slowly after the therapy is completed. The plethora of long-term deficits that may be caused by radiation therapy alone or in combination with chemotherapy have been extensively documented. The goal of using conformal radiation therapy techniques to deliver precise doses to the tumor site(s) while sparing healthy tissue and structures will ideally lessen both acute and long term radiation induced toxicities. Radiation therapy can also be used in a palliative setting and is a helpful tool that, in one study, resulted in a 58% overall response rate for neurological symptoms with no palliative patient experiencing significant toxicity. The symptoms improved with a median response time of about a week.17
Chemotherapy
The use of chemotherapy is guided by the tumor’s pathology, extent of disease and age of the child. Challenges in adequately treating CNS tumors include penetrating the blood brain barrier and drug resistance.14 Chemotherapy can be used prior to surgery, in combination with radiation therapy, as adjuvant therapy and to treat recurrent disease. It can be given daily or weekly during radiation therapy as a radiation sensitizer in certain tumors. High dose multi agent protocols, in combination with autologous stem cell rescue, in the very young (in most cases, less than age 3) are utilized to delay the use of radiation therapy until the brain is more developed. Lower doses of chemotherapy agents can be given for longer periods of time to treat low-grade gliomas that are not resectable.18 Certain tumors such as medulloblastoma respond with improved survival rates to multimodal therapy.19 Single agent chemotherapy such as temozolomide is usually given concurrently with radiation therapy for high grade gliomas and then used as adjuvant therapy. Although adjuvant therapy has slightly improved survival for high grade gliomas, the overall prognosis remains dismal.20 Chemotherapy has proven not to be effective in the treatment of diffuse pontine gliomas and, rarely is used in the treatment of ependymomas.
Clinical Trials
Although traditional cytotoxic chemotherapy such as alkylating agents has been used for decades, research in the treatment of CNS tumors is now focused on the development of biological agent and therapies targeted to intratumoral mutations.14 Understanding the biology of various tumors including the important molecules and pathways responsible for the oncogenesis of tumors is crucial for the development of new agents.21 New biologic agents are currently being studied in phase 1 and 2 clinical trials and potentially have better CNS penetration and fewer toxicities than conventional chemotherapy.22, 23 Ongoing studies are crucial to the understanding of the toxicities and efficacy of these new agents when used alone or in combination with other agents or treatment modalities. It is critical that practitioners help families understand the importance of enrolling in a clinical trial. In this way, clinicians increase enrollment, share information about the small number of these patients, and advance the knowledge and use of these new agents. The family and the child (age appropriate) must be educated about compliance, possible side effects, and their management, and when the child needs to be seen emergently.
Multi-disciplinary Interventions
Children with CNS tumors have a high likelihood of experiencing a variety of disabilities related to their tumor and the effects of the aforementioned various treatment modalities. Rehabilitative services such as occupation and physical therapy are needed by many children for weakness ranging from a hemiplegia of the arm and leg to a subtle change in fine motor skills. Occupational and speech therapists can assist the child who has swallowing and speech difficulties to recover full function. The variety of possible ophthalmologic deficits must be carefully monitored. A child with diplopia may benefit from alternating eye patching so as to minimize the discomfort, while a child with visual loss will need special accommodations to help him orient to his environment. Neuro-ophthalmologists may recommend prism glasses for Parinaud’s syndrome. Early recognition and treatment of endocrinopathies such as diabetes insipidus or SIADH will prevent further neurological complications. Neurocognitive baseline testing will help detect and address early neuro-toxic effects such as memory deficits. Continued education is important to the child and the parent to maintain normalcy during treatment, and can be accommodated using 504 plans or individualized education plans. The social worker and psychologists help the child and family to work though the emotional and social issues that arise from having a brain tumor.24
Treating a child with a primary CNS lesion requires a multi-disciplinary approach and involves family members. Ideally treatment should be administered at an academic medical center so that all levels of care are coordinated. Teaching and support are ongoing as the team and family relationship develops. The ideal goal of treatment is to eradicate the disease while sparing healthy tissue and structures. Early interventions are vital to help each child to reach their highest potential and live a fulfilling, happy life.
Survivorship
MF is an 8-year-old girl diagnosed at age 5 with standard risk medulloblastoma. Neuroimaging studies demonstrated a midline fourth ventricular mass without evidence of metastatic disease (see Figure 3). She underwent a gross total resection of the tumor and on post-operative day 2 developed posterior fossa syndrome. She received induction therapy with external beam radiation therapy consisting of 2340 cGy craniospinal radiation with a boost to the tumor bed of 3240 cGy for a total dose of 5580 cGy to the tumor bed, along with concurrent weekly vincristine. Estimated dose to the hypothalamic pituitary axis was 2800 cGy. Maintenance chemotherapy consisted of cisplatin, lomustine and vincristine. One year after completion of treatment the patient was given an individualized survivorship care plan, including a treatment summary and comprehensive plan outlining the recommended follow up for potential late effects (see Table 2). MF has residual complications including dysarthria, high frequency hearing loss, hemiparesis, ataxia, neurocognitive dysfunction, and fine and gross motor deficits. She developed growth hormone deficiency and hypothyroidism, which are managed by an endocrinologist. Formal neuropsychological testing revealed decreased processing speed as evidenced by learning difficulty. Arrangements were made for appropriate supportive services and educational accommodations at school, including speech, occupational, and physical therapies. She made steady strides with continued rehabilitation. Ongoing issues with debilitating low self-esteem and anxiety related to diagnosis and late effects persist for which she sees a psychologist.
Figure 3.
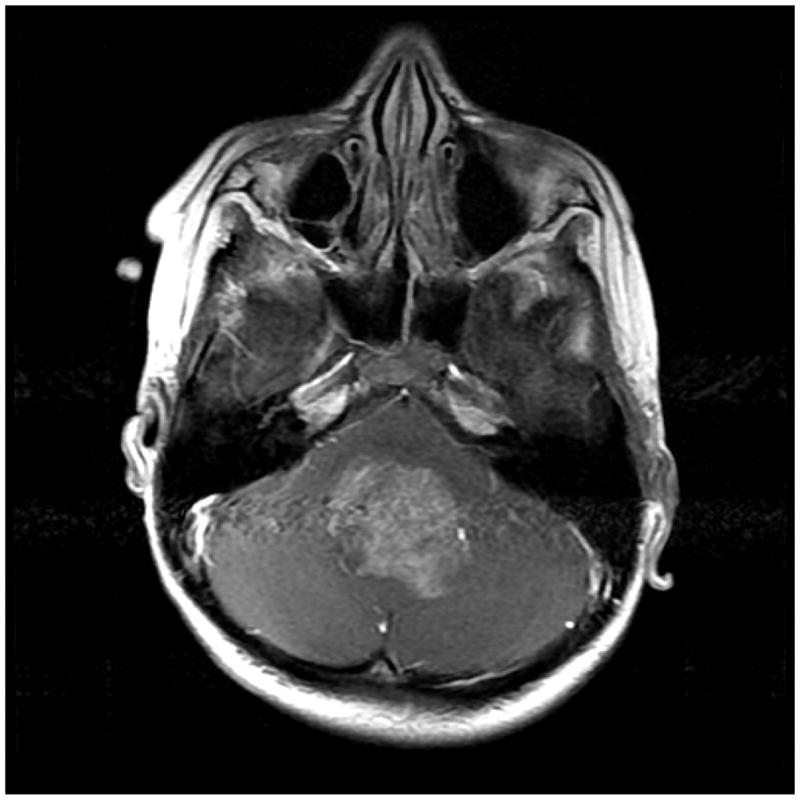
MRI image of medulloblastoma at diagnosis (Axial T1 weighted image with contrast).
Table 2.
Individualized Survivorship Care Plan: Summary of Recommended Follow Up for a child treated for medulloblastoma
| POTENTIAL LATE EFFECT | SCREENING RECCOMENDATIONS * |
|---|---|
| Any treatment history | Complete physical exam annually |
| Neurocognitive dysfunction | Neuropsychological evaluation, baseline and as indicated |
| Hypothalmic pituitary dysfunction: adrenocorticotropic hormone (ACTH), growth hormone (GH), precocious puberty; FSH /LH deficiency | Assessment of hypothalamic pituitary axis: morning cortisol, GH stimulation test as indicated, FSH/LH, monitor growth and pubertal development |
| Hearing problems | Audiogram baseline and as indicated |
| Eye problems | Ophthalmology exam annually |
| Thyroid problems | TSH/FTI, inspect and palpate thyroid |
| Pulmonary dysfunction | Pulmonary Function Test (PFT) every 3– 5 years |
| Kidney dysfunction | BUN/Creatinine, BP, UA annually |
| Gonadal dysfunction / Infertility | FSH/LH, estradiol, monitor pubertal development |
| Metabolic syndrome (type II diabetes, insulin resistance, abnormal lipids) | Monitor weight, height, BMI (body mass index), fasting glucose, insulin, lipid panel annually |
| Musculoskeletal alteration / Decreased spinal growth | Monitor sitting height |
| Altered bone health | Bone density evaluation as indicated |
| Secondary malignancies | CBC annually, inspect and palpate radiation areas, dermatology exam annually |
| Dental problems | Dental exam and cleaning every 6 months |
Screening recommendations from Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancer version 4.0. http://www.survivorshipguidelines.org/pdf/LTFUGuidelines_40.pdf. October 2013. Accessed 12/01/14
Advancements in treatment modalities have dramatically improved survival rates for children who have been diagnosed with a brain tumor. The five year relative survival rate following diagnosis of a primary malignant brain tumor for children between 0–19 years of age is 73%.25 Long-term survivorship has prompted many new treatment regimens aimed at reducing late effects while maintaining the survival rates. We have learned that “less is more”: fewer radical surgical procedures, lower doses of radiation therapy, smaller volumes of radiation therapy, and fewer and less intensive courses of chemotherapy.26
Unfortunately, many childhood brain tumor survivors are at significant risk for adverse long-term health-related complications, referred to as late effects. Most of these late effects can be attributed to direct neurologic damage to the developing brain caused by the tumor itself, surgery, the long-term toxicity of chemotherapy, or the effects of irradiation on the central nervous system. In general, children who are treated at a young age and those who receive the most intensive therapy (i.e. high doses of radiation combined with high doses of chemotherapy) are more likely to develop late effects. Late effects may develop months to years after completion of cancer treatment.27
In addition to the survivorship issues affecting the general pediatric oncology population, brain tumor survivors are at significant risk for endocrinopathies, neurocognitive dysfunction, neurological or neurosensory dysfunction, peripheral neuropathy, hearing and vision problems, seizures, stroke, headaches, secondary malignancies, and psychosocial complications. Table 3 provides a summary of the most common potential late effects, risk factors, and recommended health screening for pediatric CNS tumor survivors .28
Table 3.
Common potential late effects, associated risk factors and health screening recommendations for pediatric CNS (central nervous system) tumsor survivors
| Potential Late Effect | Risk Factor | Health Screening Recommendations ** |
|---|---|---|
Altered Bone Composition
|
Chemotherapy: antimetabolite (methotrexate Corticosteroids: (dexamethasone Radiation: Brain |
History: joint pain, swelling, immobility, limited range of motion Physical: musculoskeletal exam Imaging: bone density evaluation |
Cerebrovascular Complications
|
Radiation: Brain (≥18cGy) |
History: hemiparesis, hemiplegia, weakness, aphasia Physical: neurologic exam Measurements: blood pressure Imaging: MRI Brain, MRA Referral: Neurology, Physical and Occupational therapy |
Dental Problems
|
Any chemotherapy Radiation: Brain |
History: dry mouth Physical: oral exam Referral: Dental |
|
Endocrine: Gonadal Dysfunction Female:
|
Chemotherapy: alkylating agents (busulfan, cyclophosphamide, ifosfamide, lomustine, procarbazine, thiotepa) and heavy metals (carboplatin, cisplatin Radiation: Spine |
History (All): pubertal onset, tempo of puberty, sexual function History (Females): menstruation, pregnancy Physical (All): tanner staging Physical (Male): testicular volume Screening Labs (Females): FSH/LH, Estradiol level Screening Labs (Males): FSH/LH, Morning Testosterone, Sperm analysis Referral (All): Reproductive endocrinology Referral (Females): Gynecology, Referral (Males): Urology |
Endocrine: Hormonal Deficiency
|
Radiation: Brain | See above |
Endocrine: Metabolic Syndrome
|
Radiation: Brain Neurosurgery: Brain (suprasellar region) |
History: polyuria, polydipsia, polyphagia Measurements: Height, Weight, BMI Labs: Na, K, glucose, Cl, CO2, fasting lipid panel, insulin, HgA1C, Referral: Endocrinology |
Endocrine: Hypothalamic Pituitary Dysfunction
|
Radiation: Brain (≥ 18cGy Radiation: Brain (≥ 30cGy) |
History: previous growth velocity, fatigue, onset of puberty, nutritional status Physical (All): tanner staging Physical (Male): testicular volume Measurements: height, weight, BMI (Note the corresponding percentile on growth chart Imaging: bone age Labs (All): CBC, Electrolytes, LFTs, FSH/LH, TSH, Free T4, Labs (Male): testosterone level Referral: Endocrinology |
Endocrine: Hypothalamic Pituitary Dysfunction
|
Radiation: Brain (≥ 30cGy) |
History: anorexia, dehydration, hypoglycemia, lethargy, unexplained HTN Labs: morning cortisol |
Endocrine: Hypothalamic Pituitary Dysfunction
|
Radiation: Brain (≥ 30cGy) |
History: fatigue, wt gain cold intol, constipation, weight gain, dry skin, brittle hair, depressed mood PE: ht, wt, hair, skin, thyroid Labs: Free T4 |
Endocrine: Hypothalamic Pituitary Dysfunction
|
Radiation: Brain (≥ 40cGy) |
History: galactorrhea or decreased libido Labs: prolactin if pt has galactorrhea, or decreased libido |
Endocrine: Obesity
|
Radiation: Brain |
History: diet and activity level Measurements: height, weight, BMI, blood pressure Referral: Nutritionist |
Endocrine: Thyroid Problems
|
Radiation: Brain/Spine |
History: fatigue, cold intolerance, constipation, weight gain, dry skin, brittle hair, depressed mood Physical: hair, skin, thyroid exam Measurements: height, weight (Note the corresponding percentile on growth chart Labs: TSH, Free T4 Referral: Endocrinology |
ENT Problems
|
Radiation: Brain |
History: rhinorrhea, nasal discharge Referral: ENT |
Eye Problems
|
Corticosteroids: (dexamethasone Radiation: Brain Chemotherapy: busulfan |
History: visual changes (decreased acuity, diplopia Physical: eye exam (visual acuity, fundoscopic exam for lens opacity Referral: Ophthalmologist |
GI: Bowel Dysfunction
|
Neurosurgery: Spinal Cord |
History: Chronic constipation, Fecal soiling Physical: Rectal exam Referral: GI |
GU: Bladder Dysfunction
|
Radiation: Spine (lumbar, sacral, cauda equina Neurosurgery: Spinal Cord |
History: urinary urgency/frequency, urinary incontinence/retention, dysuria, nocturia, abnormal urinary stream Measurements: blood pressure Labs: BUN, Creatinine, Na, K, Cl, CO,Ca, Mg, PO4; Urinalysis Referral: Urology |
GU: Urinary Tract Dysfunction
|
Chemotherapy: alkylating agents (cyclophosphamide, ifosfamide Radiation: Spine (sacral, total spine) |
History: Urinary urgency/frequency, urinary incontinence/retention, dysuria, nocturia, abnormal urinary stream Measurements: blood pressure Labs: BUN, Creatinine, Na, K, Cl, CO, Ca, Mg, PO4, Urinalysis Referral: Urology |
GU: Renal
|
Chemotherapy:, carboplatin, cisplatin, cyclophosphamide |
Measurement: blood pressure Labs: same as above Referral: Nephrology |
Hearing Problems
|
Chemotherapy: heavy metals (carboplatin, cisplatin) |
History: hearing difficulty, tinnitus, vertigo Physical: otoscopic exam Screening: audiogram Referral: Audiology; speech therapy for patients with hearing loss. |
Ototoxicity
|
Radiation Brain >30 | See above |
Musculoskeletal Alteration: Growth Problems
|
Radiation: Spine (all fields) |
Measurements: limb lengths, sitting height, height, weight Referral: Orthopedic |
Musculoskeletal Alteration: Spine Deformity
|
Radiation: Spine Neurosurgery: Spinal Cord (laminectomy, laminoplasty) |
Physical: spine exam for scoliosis and kyphosis Referral: Orthopedic |
Neurocognitive Dysfunction
|
Chemotherapy: antimetabolite (methotrexate Radiation: Brain Neurosurgery: Brain |
History: educational and/or vocational progress Physical: neurologic exam Screening: formal neuropsychological evaluation Referral: Neuropsychology |
Neurological: Clinical leukoencephalopathy
|
Chemotherapy: antimetabolite (methotrexate), cytarabine Radiation: Brain ≥24cGy Neurosurgery: Brain |
History: cognitive, motor, sensory deficits, seizures, other neurologic symptoms Physical: neurologic exam Imaging: MRI Brain, MRA Referral: Neurology |
Neurological: Impaired CSF Diversion
|
Neurosurgery: Brain |
Imaging: X-ray shunt series (skull and abdominal images Referral: Neurosurgery |
Neurological: Motor and/or sensory deficits
|
Neurosurgery: Brain |
Physical: neurologic exam Referral: Neurology, Physiatrist/rehabilitation medicine specialist, speech, physical, occupational therapies |
Neurosensory Problems: Peripheral motor and/or sensory neuropathy
|
Chemotherapy with plant alkaloids (vinblastine, vincristine)and heavy metals (carboplatin, cisplatin) |
History: areflexia, weakness, foot drop, paresthesias, dysesthesias Physical: neurologic exam Referral: Neurologist and physical therapy for symptomatic neuropathy |
Pulmonary Dysfunction
|
Chemotherapy: alkylating agents (lomustine, busulfan) |
History: Cough, SOB, DOE, wheezing, poor growth Measurements: height, weight Physical: Pulmonary exam Screening: PFTs (complete pulmonary function testing) |
Psychosocial/Behavioral
|
Any cancer experience |
History: psychosocial assessment (educational and/or vocational progress, social withdrawal Referral: Psychiatry |
Second Malignancy
|
Chemotherapy: epipodophyllotoxins (etoposide Chemotherapy: high dose alkylating agents (cyclophosphamide, ifosfamide, lomustine) |
History: fatigue, bleeding, easy bruising Physical: dermatological exam (pallor, petechiae, purpura Labs: CBC, bone marrow exam as indicated Referral: Oncology |
Second Malignancy
|
Chemotherapy: high dose alkylating agents (cyclophosphamide, ifosfamide, lomustine) |
History: hematuria, urinary urgency, urinary frequency, dysuria Labs: Urinalysis Referral: Urology |
Second Malignancy
|
Radiation: Brain/Spine |
Physical: thyroid exam Referral: Endocrinology |
Second Malignancy
|
Radiation: Brain |
History: headaches, vomiting, cognitive, motor or sensory deficits, seizures and other neurologic symptoms; skin changes (i.e. moles Physical: Neurologic exam, Ophthalmology exam, Dermatological exam Imaging: MRI Brain Referral: Oncology, Neurology, Neurosurgery, Dermatology |
Sexual Dysfunction
|
Neurosurgery: Spinal Cord |
History (Males): erectile/ejaculatory dysfunction History (Females): altered, diminished or loss of sensation, dyspareunia Referral (Males): Urology Referral (Females): Gynecology Referral (All): counseling |
Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancer version 4.0. http://www.survivorshipguidelines.org/pdf/LTFUGuidelines_40.pdf. October 2013. Accessed 12/01/14
Late Effects: Endocrinopathies
More than 40% of pediatric patients treated for CNS tumors develop endocrinopathies, which can be attributed to the disruption of the hypothalamic/pituitary axis due to tumor or radiation.29 A retrospective study in an established cohort of childhood cancer survivors with 748 participants treated with cranial radiation observed for a mean of 27.3 years revealed that the estimated point prevalences was 46.5% for growth hormone deficiency, 10.8% for lutenizing hormone/follicle-stimulating hormone deficiency, 7.5% for thyroid stimulating hormone deficiency and 4% for adrenocorticotropic hormone deficiency and the cumulative incidence increased with follow up.30
Growth hormone deficiency is common among pediatric brain tumor survivors, occurring in nearly 100% of patients who receive a radiation dose of 36 Gy or more to the pituitary region, and is less common with radiation doses of 18–24 Gy, but may not become evident until 10 years after treatment.31, 32 Concern has been raised about growth hormone deficiency replacement therapy having a causal role in recurrence or second malignancies. A report from the Childhood Cancer Survivor Study that evaluated the association between growth hormone replacement therapy and the development of secondary CNS malignancy concluded that there was no statistically significant increased overall risk of the occurrence of a secondary CNS malignancy associated with growth hormone replacement therapy. Specifically, occurrence of meningiomas and gliomas were not associated with growth hormone replacement therapy.33 Neuroendocrine consequence in brain tumor survivors is complex and is best managed by an endocrinologist who works in joint collaboration with the treating oncologist.
Late Effects: Neurological
Neurocognitive dysfunction in brain tumor survivors can vary from mild to devastating. Cranial irradiation has been associated with the highest risk of long-term cognitive morbidity particularly in younger children and those receiving a higher dose.34 Neurocognitive morbidity in attention, executive functioning, processing speed, working memory and memory are most apparent and contribute to declines in intellectual and academic abilities.35 Over time, skills fail to emerge at key developmental stages; thus greater time since treatment is also associated with poorer neuropsychological outcomes.36 In a large unselected sample of long term survivors of brain tumors, the incidence of severe neurocognitive impairment was 5% and the overall mean IQ for the sample was significantly below the normative mean.34 Given the high rate of neurocognitive dysfunction, all survivors should be referred for neuropsychological evaluation. Early educational, cognitive and pharmacological interventions are needed to maximize potential for recovery.35
A childhood cancer survivor study evaluating the long-term neurologic and neurosensory sequelae in 1,607 adult survivors of a primary CNS tumor diagnosed between 1970 and 1986, found survivors were at significant risk for hearing impairment, legal blindness, cataracts, double vision, and seizure disorder compared to a sibling cohort. Neurosensory impairment was reported in 17% of patients. Radiation exposure greater then 50 cGy to the posterior fossa was associated with a higher likelihood developing any hearing impairment. Coordination problems were reported in 49%, and motor control problems in 26%. Radiation exposure of a minimum 50 cGy to the frontal brain region had a moderately elevated risk for motor problems. Seizure disorders were reported in 25% of brain tumor survivors, including 6.5% who had a first occurrence of seizure greater than 5 years off treatment. Radiation dose of 30 cGy or more was associated with a two-fold elevated risk for a late seizure.37 Treatment of these problems is challenging and complex. Referral to a neuro-ophthalmologist for prism eyeglasses, audiologist for hearing aid and epileptologist for seizure management may be needed.
Cranial irradiation increases the risk of stroke or TIA (transient ischemic attack) in pediatric brain tumor survivors. The incidence of neurovascular events in this population is 100-fold higher than in the general pediatric population. Cranial irradiation induced late vasculopathy is an important risk factor. Median time from first radiation to first event was 4.9 years.38 Providers need to focus on primary stroke prevention.
Off therapy headaches are also common in brain tumor patients. A retrospective review of off-therapy headaches in 81 brain tumor patients over two years showed that almost half of the patients with pre-diagnosis headaches had recurrent off therapy headaches. A thorough medical history and neurological exam to determine the cause of the headache is critical as a headache may be a sign of tumor recurrence or second CNS malignancy. If the exam is significant for papilledema, hypertension or any abnormal neurological finding, diagnostic imaging with a MRI of the brain is indicated. If there is no tumor recurrence, then focus can be on determining the underlying trigger and treating symptoms. Prophylactic medication may be warranted to reduce headache frequency.39
Late Effects: Secondary Tumors
Development of a secondary benign or malignant CNS tumor is an infrequent but extremely serious late effect. A report from the Childhood Cancer Survivor Study showed that exposure to radiation therapy is the most important risk factor for the development of a new CNS tumor in survivors. The majority of secondary malignancies are either gliomas which tend to occur at a mean of 9 years post initial diagnosis or meningiomas which tend to occur at a mean of 17 years post diagnosis. The higher risk of subsequent glioma in children irradiated at a very young age may reflect greater susceptibility of the developing brain to radiation.40 Other secondary malignancy includes sarcomas, non-melanoma skin cancers and thyroid cancer.41 The majority of secondary malignancies occur in or near the radiation field. In addition to neurological examinations to detect early signs of a new or tumor recurrence, patients should be referred for routine surveillance dermatological examinations.28
Late Effects: Psychosocial
Children with brain tumors and those who experience insults to their CNS as a result of cancer or cancer treatment are at considerably high risk for adverse psychosocial outcomes.42, 43 Survivors who have had brain or CNS tumors or who have had intensive CNS therapy appear to be most at risk for cognitive, social, and adjustment difficulties. Studies have noted that the social skills of brain tumor patients tend to be adversely affected by treatment and that patients with cognitive impairments tend to feel and be perceived as more isolated than peers44 and to have more behavioral problems to a degree that is much higher than that of other pediatric cancer survivors.45
A recent publication studied the long-term impact of childhood CNS tumors and their treatment on the self perception of adult survivors. The cohort included 697 Swedish survivors diagnosed with a primary CNS tumor during 1982–2001. The study concluded that the experience of having a CNS tumor combined with late effects from treatment could negatively alter self perception and self esteem, vital for mental health and quality of survival. Therefore, care and psychosocial follow-up of survivors should include measures for identifying disturbances and for assessing the need for psychosocial intervention.46
Long Term Follow Up Guidelines
The Institute of Medicine and National Research Council recommend in their report entitled From Cancer Patient to Cancer Survivor: Lost in Transition, (Institute of Medicine) that a summary of treatment and a comprehensive plan for follow up is given to the patient and primary care provider upon completion of treatment. Such a survivorship plan will identify potential late effects and provide guidance on follow-up care, prevention, and health maintenance.47
The Children’s Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers 28 were developed as a resource for healthcare professionals who provide ongoing care to survivors of pediatric malignancies. They are appropriate for asymptomatic survivors of childhood, adolescent and young adult cancers presenting for routine risk based medical follow-up. More extensive evaluations are performed, as clinically indicated, for survivors presenting with signs and symptoms suggesting illness or organ dysfunction. Healthcare professionals who do not regularly care for survivors of pediatric malignancies are encouraged to consult with a pediatric oncology long-term follow-up center if any questions arise when reviewing or using these guidelines.
Lifelong medical surveillance with a multi-disciplinary team approach is essential to maximize health and improve quality of life.26 Some pediatric cancer centers have a dedicated survivorship clinic that provides comprehensive care, education and counseling to survivors once the treatment phase is over. However, the majority of brain tumor survivors may not have access to a specialized survivorship clinic. Primary health care providers and subspecialists need to be knowledgeable of potential late effects and appropriate screening for early identification and intervention.
End of Life Care
DG is a 7 year old boy with recurrent brain and spine ependymoma, first diagnosed five years ago. Since diagnosis he has undergone multiple brain and spine tumor resections, focal proton radiation and palliative re-irradiation, and has received over five different chemotherapy regimens, including investigational agents. He maintained a good quality of life during treatment, attending school and engaging with family and friends in various activities outside the home. In the past two weeks DG has become less interactive, only occasionally opening his eyes to look at a book while his mother reads. At times he becomes agitated. He is not tolerating his oral medications, including steroids and seizure medications. He has severe dysarthria and tetraplegia. His parents agreed that quality of life has rapidly diminished, and agreed to discontinue palliative chemotherapy and transition to hospice care. His parent’s main concerns at this time are the inability to communicate with DG, his periods of irritability, and the complete reliance he has on them for all personal needs.
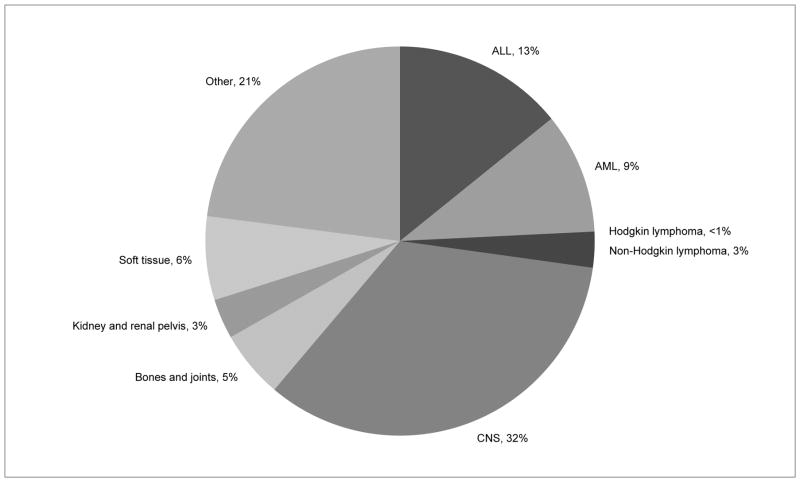
Cancer is the leading cause of death from disease in children. While cure rates for pediatric CNS tumors continue to improve overall, the overall mortality rate is greater than 25% (see figure 4),48 and certain subtypes of CNS tumors have dismal survival rates. Diffuse intrinsic pontine glioma has a median survival rate of less than 1 year after diagnosis. The 5 year survival rate for glioblastoma multiforme is 5–15%, and is 20–40% for anaplastic astrocytoma.49 These statistics highlight the importance of providing active end of life care for children with CNS tumors.
Figure 4.
U.S. Deaths in patients <15 years of age as percentage of total cancer Deaths 2007–2011. U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2011 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2014. Available at: www.cdc.gov/uscs.
Common Symptoms
In the general pediatric cancer population the most commonly reported symptoms causing distress at end of life are fatigue, pain, and dyspnea.50 In addition to these symptoms, children with CNS tumors have unique end of life care needs specific to the brain tumor population, including dysphagia and dysarthria, hearing and vision loss, paralysis, seizures, agitation, headache, and cognitive and behavioral changes.51–54 There are limited studies examining the end of life care needs specific to children with CNS tumors. More detailed adult studies exist, which highlight the unique end of life needs of adults with CNS tumors,55–58 and the data parallel the limited information available in children with CNS tumors. The details of these studies from children and adults are summarized in Table 4.
Table 4.
Frequency of Common Symptoms in CNS Tumor Patients at EOL
| Study/Symptom | Seizures | Speech problems | Dysphagia | Agitation, delirium or confusion | Neurologic deficits (CN, paralysis, gait) | Headache pain | Other |
|---|---|---|---|---|---|---|---|
| Vallero et al.,55 2014 39 children, mixed CNS tumors |
41% | __ | __ | __ | 69% had CN deficit, 41% confined to bed w/ paralysis or changes in cognition/ consciousness | __ | 83% were on steroids, 58% receiving palliative sedation for agitation, seizures, dysphagia, dyspnea |
| Goldman et al.,56 2006 185 children, 59 w/ CNS tumors |
39% | 76% | 62% | __ | __ | 64% | 69% had increased oral secretions; 62% had hearing or vision problem |
| Pace et al.,57 2009 169 adults, majority GBM |
30% | __ | 85% | 15% | __ | 36% | 85% had drowsiness |
| Faithfull et al.,58 2005, 39 adults w glioma | 56% | 64% | __10% | 51% | 77% had poor mobility, 62% hemiparesis | 62% | __ |
| Sizoo et al.,59 2010 55 adults, mixed CNS tumors |
45% | __ | 71% | 29% | 51% had focal neurologic deficits , | 33% | 33% with progressive cognitive decline, 87% had drowsiness or impaired consciousness |
| Gofton et al.,60 2012, 168 adults, mixed CNS tumors | 57% | 27% | __14% | 27% | 65% had gait impairment, 58% had motor deficit | 26% | 22% had vision loss, 62% had cognitive/personality changes |
One retrospective chart review of 39 children who died from CNS tumors at a single center found that 69% had cranial nerve deficits at end of life, 41% needed lengthy bed rest due to paraplegia, tetraplegia, or deterioration of consciousness and psycho-cognitive skills, and 41% had seizures. Steroids were administered in 83% of these patients, and all had at least one steroid side effect (e.g. cushingoid, weight gain, irritability). No patient had uncontrolled pain, though 51% received morphine, and 58% received palliative sedation, for agitation, seizures, dysphagia, and dyspnea.53 Another study of children with cancer determined that children with CNS tumors had speech problems (76%), increased oral secretions (69%), headache (64%), swallowing difficulties (62%), hearing/vision problems (62%) and seizures (39%).54
In one adult study the most frequent symptoms observed in the last four weeks of life for 169 patients (majority had glioblastoma mutliforme) were epilepsy (30%), headaches (36%), drowsiness (85%), dysphagia (85%), and agitation and delirium (15%).55 A retrospective review of 39 adult patients with primary malignant glioma reported that end of life patients had poor mobility (77%), limited communication (64%), hemiparesis (62%), headaches (62%), seizures (56%) and confusion (51%).56 Another review of 55 adults patients with CNS tumors, two thirds with glioblastoma multiforme, patients were documented to have drowsiness/progressive loss of consciousness (87%), dysphagia (71%), progressive focal neurologic deficits (motor deficits, coordination loss, aphasia) (51%), seizures (45%), headache (33%), progressive cognitive deficits (memory loss, personality changes, apathy, problems in executive functioning and understanding) (33%), and confusion/agitation (29%).57 And in a retrospective review of 168 patients at end of life, with CNS tumors of all types, patients were found to have gait impairment (65%), cognitive/personality change (62%), motor deficit (58%), seizures (57%), aphasia (27%), delirium (27%), headache (26%), and vision changes (22%). This study also reported that personality changes were a challenge for caregivers, and fatigue and depression were strongly correlated, in over 50% of the patients.58 In each of these studies the authors concluded that adults with malignant brain tumors have specific symptoms during the end of life phase, which differ from terminally ill patients dying of other cancers.
The progressive physical impairments in children with CNS tumors cause a negative impact on mood, behavior and cognition. Inappropriate and impulsive behaviors can be difficult to control, and the lack of ability to communicate is frustrating, and often a significant turning point for parent and child. The unique challenges for children with CNS tumors at end of life lead to a palliative experience more similar to those with progressive degenerative neurologic conditions.59, 60 Providers for children with CNS tumors need to learn how to manage these unique symptoms, in order to provide the best comfort and support for both the patient and families.
Dysphagia
The high frequency of dysphagia, 62–85%, in patients with CNS tumors at end of life emphasizes the need for creative methods to administer medications. This is especially important for antiepileptic drugs, antiemetics, opioids, steroids and anxiolytics, all commonly used at end of life in patients with CNS tumors. Pharmacists often may be able to compound medications into dosage forms that are not commercially available, for nasal, sublingual, or rectal use.
For sublingual administration the liquid volume should be kept to 1–2ml. Immediate release tablets can be crushed and mixed with water, to ease administration. Allow 5–10 minutes between sublingual doses or before eating and drinking, to promote absorption.61
Drugs administered into the upper part of the rectum will be absorbed by the superior rectal vein, which empties into the portal vein and subsequently the liver, allowing rapid hepatic metabolism and making less drug available to the systemic circulation.62 In adults drug volumes of 10–25ml are easily retained, and the volume should be less than 60–80ml to decrease chances of spontaneous expulsion. The rectal area is an alkaline environment, and alkaline preparations such as aqueous and alcohol solutions will be better absorbed than suppositories or suspensions. Inserting tablets rectally is an option, and may be an easier route than a liquid. If using tablets use immediate release, and if the rectum is dry insert 10ml of warm water to promote dissolution. Multiple tablets can be enclosed in a gelatin capsule, either crushed or whole, to eliminate the need for multiple insertions. Enteric-coated tablets should not be inserted rectally, as they require an acidic environment to dissolve. Alcoholic (elixirs) or parenteral formulations repeatedly administered sublingually or rectally can be irritating to the oral or rectal mucosa.61, 62
Transdermal medications have not been proven to be effective beyond providing local pain relief.61, 63 While observational studies have found some benefit to topical medications, research studies show there is inconsistent drug absorption and bioavailability; relief may be due instead to other factors, such as the soothing therapy of touch when a topical is being applied.61 Continued research is needed to engineer effective formulations of transdermal medication. In addition, providers should not forget about the benefits of non-pharmaceutical care, such as talk therapy, massage, acupressure or aromatherapy.
Communication
Impaired communication is a significant source of distress in children and families at end of life, occurring in 27–76% of patients with CNS tumors, and can be due to dysarthria, aphasia, or hearing deficits.59, 60 However there are no studies examining the communication needs of children with CNS tumors at end of life. Augmentative and alternative communication devices can be cost prohibitive, and a child at end of life may not have the time required to be fitted for and taught to use the device.64, 65 More simple means of communication include writing on a dry erase board, using an alphabet or picture boards, and communication applications on computers and touch devices.64 While these devices have limitations based on the patient’s neurologic deficits, at the very end of life these devices may be useful on a very basic level to indicate wants and desires. More studies need to be performed examining the benefit of these techniques in children with CNS tumors and communication impairment at end of life.
Agitation & Mood
Agitation, mood fluctuations, and impulsive and inappropriate behaviors also cause significant distress for patient and caregivers at end of life.51, 60 A change in behavior has been noted in the majority of children with CNS tumors at end of life, much more often than in children with hematologic or other solid tumors.51 Benzodiazepines usage has been reported in almost 60% of children with a CNS tumor at end of life, to alleviate anxiety or to induce sedation for terminal dyspnea or agitation.53 Agitation is a documented issue for adults with CNS tumors, occurring in 15–51% of these patients at end of life. Providers should be prepared to deal with agitation and mood and behavior changes in their pediatric patients at end of life. Symptom management may be achieved with non-pharmacological techniques, such as addressing causes of discomfort, soothing children with gentle touch and calming voices, use of familiar objects to provide comfort, and providing frequent reminders of orientation, such as clocks, calendar, or bulletin boards. Agitation may continue in spite of these measures. A review of medications may identify a possible source of agitation, confusion, or behavior changes. Medications changes must be balanced with managing other symptoms, such as headache or seizures.66 In cases where the aforementioned actions do not help, or cannot be utilized, benzodiazepines, neuroleptics, and opioids can be given in doses to improve mood and agitation. Palliative sedation may be an acceptable outcome to manage symptoms, and should be discussed with the family.55, 66
Headaches
Headache occurs in up to 64% of children with CNS tumors at end of life,55 and in 26–62% of adults with CNS tumors at end of life. Headache pain is often controlled by steroids, but may require additional pain relief from non-opioid or opioid pain relievers,55 particularly if steroids are contributing to agitation.
Seizures
Seizures are a frequent symptom in patients with brain tumors, with a reported incidence of 30–57% at end of life. They may develop during the end of life period, or the incidence and severity can increase. Dysphagia, altered consciousness, and agitation may prohibit treatment with oral medications. Uncontrolled seizures will negatively influence quality of life, cause distress to caregivers, and can further contribute to neurologic deficits and cognitive or behavioral problems.66, 67 Furthermore, uncontrolled seizures may necessitate hospital admission, when a patient or their family may wish for death at home. Providers can consider modifying antiepileptic drug regimens in advance, or having a plan in place in the event the patient can no longer tolerate oral antiepileptic drugs. Intramuscular, subcutaneous, sublingual, nasal, or rectal preparations should be considered. Rectal medications have the benefit of simple education and ease of use. Providers should be aware that rectal absorption of AEDs can vary between individuals, for some drugs the bioavailability is lower rectally and the dose may need to be increased. Rectal administration never leads to higher absorption rates, and doses should not be decreased when switching from oral to rectal preparation.68
Most liquid antiepileptic drugs can be given rectally, but consideration of the formulation being used is important. It is helpful to have a clinical pharmacist to help decide on the best preparation and any changes in dosing.66 Diazepam and lorazepam may also be given rectally, and lorazepam is absorbed nasally, though there is limited data available. Midazolam is available nasally and buccally. 68
In any decision regarding antiepileptic drug use the side effects should be considered and discussed with the family, as some medications can cause agitation or mood changes, sedation, or further weight gain.66–68 There is no proven benefit to prophylactic antiepileptic drugs, even in end of life care, and discontinuing prophylactic seizure medications is appropriate to prevent unnecessary side effects and to ease patient care.68 Finally, if a patient continues to have breakthrough seizures despite AEDs, or who newly develops seizures during end of life, consideration of the risks and benefits of withdrawing treatment can also be discussed. In some cases repeated focal seizures with minimal residual effects on the patient may be preferred to the complications that can arise from antiepileptic drugs.66
Autopsy
Autopsy should be considered in all children with CNS tumors at end of life. Even in the case where a gross total resection may be obtained at diagnosis, multiple tumor recurrences can occur, not all will be resected or biopsied, and at the end of life the most resistant tumors that have led to death may have never been biopsied or resected. Autopsy samples can provide information about the most aggressive component of a tumor, allow for larger specimens to be collected or for examination of multiple sites of disease, and can reveal mechanisms of tumor progression and information on the genetics of a tumor. Autopsy is especially helpful in cases where tumors are not often biopsied or resected, such as diffuse intrinsic pontine glioma.
Autopsy may provide an opportunity for a positive feeling from the death of a child, the knowledge that their death may advance research into curative therapy. Studies of have shown that the majority of families of a child with a CNS tumor would prefer to be offered an autopsy, and that none who consented to autopsy regretted the decision. The best time to initiate the discussion of autopsy is when the conversation has turned from curative to palliative treatment. The least desirable time is immediately before or after death. In most cases families prefer that the primary provider initiate the discussion.
Physicians may be uncomfortable afraid they will cause distress, and may be uncertain of autopsy protocols and procedures, or uncertain of how tumor can be used for further research. Providers for children with CNS tumors should discuss with funeral directors, social workers and therapists, hospital pathologists, and researchers how to coordinate autopsy and tumor donation, how the sample can be used for research, and the best time and manner in which to approach the family. With a plan in place prior to discussion the family will appreciate the advanced knowledge and be able to make an informed decision.69, 70
Discussion
For children with CNS tumors the complexity of care throughout treatment and survivorship, or at end of life, requires a strong multidisciplinary team knowledgeable in the care of this unique population and their families throughout the spectrum of illness.
Treatment requires a multimodal approach including surgery, radiation therapy, chemotherapy, and investigational agents. Innovative methods of diagnosis, treatment, and supportive care must continue to be developed, with the goal of reducing toxicity and long term sequelae. Beyond treatment for the tumor it is important to maximize quality of life for the patient and family’s general well being. Referrals for psychosocial care, rehabilitation, and to specialists in neuro-ophthalmology, endocrinology, and neuropsychiatry, are as essential as primary treatment for the tumor.
Following completion of treatment, survivorship care requires more than monitoring for tumor recurrence. While advancements in treatment have resulted in improved survival rates childhood brain tumor survivors are at significant risk for adverse late effects, which can occur months to years following completion of treatment, often requiring management for the patient’s lifetime. Extensive survivorship studies summarize the known late effects unique to childhood brain tumor survivors, such as neurocognitive dysfunction, psychosocial dysfunction, endocrinopathies, persistent neurologic sequelae including focal deficits, seizures, headache and stroke, and secondary malignancy. Lifelong medical surveillance for early identification of and intervention for late effects can improve overall quality of life and maximize health. An ongoing goal must be to provide adequate resources to allow these patients to reach their fullest potential.
Despite advances in treatment and improved survival rates, greater than 25% of children with CNS tumors will succumb to their disease. Children dying from CNS tumors have unique end of life symptoms such as impaired communication, dysphagia impairing medication administration, paralysis, headaches, seizures, and cognitive and behavioral changes, all of which add to the distress of the patient and the family. More research is needed to better understand and identify the unique aspects of care for child dying of a brain tumor.
The neurologic deficits experienced by pediatric CNS tumor population are chronic or evolving issues that can impair function and quality of life and require referrals to specialists and long term management. With proper intervention and further research, pediatric patients with CNS tumors can have improved quality of life during treatment, survivorship, and at end of life.
Acknowledgments
We wish to thank the staff and patients of the Department of Pediatrics at Memorial Sloan Kettering Cancer Center. We also wish to thank Joseph Olechnowicz for editorial assistance.
Funding: No funding was used in the completion of this manuscript.
Footnotes
Author Contributions
All authors wrote sections of the manuscript and provided critical review of the entire manuscript.
Declaration of conflicting interests The authors have no conflicts of interest in relation to this manuscript.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta neuropathologica. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn K, Finkel A. It IS a tumor -- current review of headache and brain tumor. Current pain and headache reports. 2014;18:421. doi: 10.1007/s11916-014-0421-8. [DOI] [PubMed] [Google Scholar]
- 3.Duffner PK. Diagnosis of brain tumors in children. Expert review of neurotherapeutics. 2007;7:875–85. doi: 10.1586/14737175.7.7.875. [DOI] [PubMed] [Google Scholar]
- 4.Fruhwald MC, Rutkowski S. Tumors of the central nervous system in children and adolescents. Deutsches Arzteblatt international. 2011;108:390–7. doi: 10.3238/arztebl.2011.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford J. Childhood brain tumors. Pediatrics in review / American Academy of Pediatrics. 2013;34:63–78. doi: 10.1542/pir.34-2-63. [DOI] [PubMed] [Google Scholar]
- 6.Braynt SO, Soonmee C, Barkovich AJ. Modern Imaging of Pediatric Brain Tumors. In: Gupta N, Banerjee A, Haas-Kogan D, editors. Pediatric CNS Tumors. 2. Berlin: Springer-Verlag; 2010. [Google Scholar]
- 7.Brandao LA, Poussaint TY. Pediatric brain tumors. Neuroimaging clinics of North America. 2013;23:499–525. doi: 10.1016/j.nic.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Kyritsis AP. Management of primary intracranial germ cell tumors. Journal of neuro-oncology. 2010;96:143–9. doi: 10.1007/s11060-009-9951-z. [DOI] [PubMed] [Google Scholar]
- 9.Heuer GG, Jackson EM, Magge SN, Storm PB. Surgical management of pediatric brain tumors. Expert review of anticancer therapy. 2007;7:S61–8. doi: 10.1586/14737140.7.12s.S61. [DOI] [PubMed] [Google Scholar]
- 10.Walker DA, Liu J, Kieran M, et al. A multi-disciplinary consensus statement concerning surgical approaches to low-grade, high-grade astrocytomas and diffuse intrinsic pontine gliomas in childhood (CPN Paris 2011) using the Delphi method. Neuro-oncology. 2013;15:462–8. doi: 10.1093/neuonc/nos330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitfield AF, Carroll AB, Kissoon N. Emergency management of increased intracranial pressure. Pediatric emergency care. 2012;28:200–4. doi: 10.1097/PEC.0b013e318243fb72. quiz 5–7. [DOI] [PubMed] [Google Scholar]
- 12.Ross SG, Northman L, Morris M, Green AL, Ullrich NJ. Cerebellar mutism after posterior fossa tumor resection: case discussion and recommendations for psychoeducational intervention. Journal of pediatric oncology nursing : official journal of the Association of Pediatric Oncology Nurses. 2014;31:78–83. doi: 10.1177/1043454213518975. [DOI] [PubMed] [Google Scholar]
- 13.Shyu C, Burke K, Souweidane MM, et al. Novel use of zolpidem in cerebellar mutism syndrome. Journal of pediatric hematology/oncology. 2011;33:148–9. doi: 10.1097/MPH.0b013e3182053a1a. [DOI] [PubMed] [Google Scholar]
- 14.Fleming AJ, Chi SN. Brain tumors in children. Current problems in pediatric and adolescent health care. 2012;42:80–103. doi: 10.1016/j.cppeds.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Sreeraman R, Indelicato DJ. Proton therapy for the treatment of children with CNS malignancies. CNS oncology. 2014;3:149–58. doi: 10.2217/cns.14.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merchant TE, Farr JB. Proton beam therapy: a fad or a new standard of care. Current opinion in pediatrics. 2014;26:3–8. doi: 10.1097/MOP.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 17.Rahn DA, 3rd, Mundt AJ, Murphy JD, Schiff D, Adams J, Murphy KT. Clinical outcomes of palliative radiation therapy for children. Practical radiation oncology. 2014 doi: 10.1016/j.prro.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Minturn JE, Fisher MJ. Gliomas in children. Current treatment options in neurology. 2013;15:316–27. doi: 10.1007/s11940-013-0225-x. [DOI] [PubMed] [Google Scholar]
- 19.Gerber NU, Mynarek M, von Hoff K, Friedrich C, Resch A, Rutkowski S. Recent developments and current concepts in medulloblastoma. Cancer treatment reviews. 2014;40:356–65. doi: 10.1016/j.ctrv.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Cage TA, Mueller S, Haas-Kogan D, Gupta N. High-grade gliomas in children. Neurosurgery clinics of North America. 2012;23:515–23. doi: 10.1016/j.nec.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Khatua S, Sadighi ZS, Pearlman ML, Bochare S, Vats TS. Brain tumors in children--current therapies and newer directions. Indian journal of pediatrics. 2012;79:922–7. doi: 10.1007/s12098-012-0689-9. [DOI] [PubMed] [Google Scholar]
- 22.Nageswara Rao AA, Scafidi J, Wells EM, Packer RJ. Biologically targeted therapeutics in pediatric brain tumors. Pediatric neurology. 2012;46:203–11. doi: 10.1016/j.pediatrneurol.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilday JP, Bartels UK, Bouffet E. Targeted therapy in pediatric low-grade glioma. Current neurology and neuroscience reports. 2014;14:441. doi: 10.1007/s11910-014-0441-0. [DOI] [PubMed] [Google Scholar]
- 24.Vargo M. Brain tumor rehabilitation. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2011;90:S50–62. doi: 10.1097/PHM.0b013e31820be31f. [DOI] [PubMed] [Google Scholar]
- 25.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro-oncology. 2014;16(Suppl 4):iv1–63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw S. Endocrine late effects in survivors of pediatric brain tumors. Journal of pediatric oncology nursing : official journal of the Association of Pediatric Oncology Nurses. 2009;26:295–302. doi: 10.1177/1043454209343180. [DOI] [PubMed] [Google Scholar]
- 27.Turner CD, Rey-Casserly C, Liptak CC, Chordas C. Late effects of therapy for pediatric brain tumor survivors. Journal of child neurology. 2009;24:1455–63. doi: 10.1177/0883073809341709. [DOI] [PubMed] [Google Scholar]
- 28.Children's Oncology Group. Long-Term Follow-up Guidelines for Survivors of Childhood Adolescent, and Young Adult Cancers. Monrovia, CA: Children's Oncology Group; 2013. version 4.0 ed. [Google Scholar]
- 29.Gurney JG, Kadan-Lottick NS, Packer RJ, et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer. 2003;97:663–73. doi: 10.1002/cncr.11095. [DOI] [PubMed] [Google Scholar]
- 30.Chemaitilly W, Li Z, Huang S, et al. Anterior hypopituitarism in adult survivors of childhood cancers treated with cranial radiotherapy: a report from the st jude lifetime cohort study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:492–500. doi: 10.1200/JCO.2014.56.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brennan BM, Rahim A, Mackie EM, Eden OB, Shalet SM. Growth hormone status in adults treated for acute lymphoblastic leukaemia in childhood. Clinical endocrinology. 1998;48:777–83. doi: 10.1046/j.1365-2265.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- 32.Littley MD, Shalet SM, Beardwell CG, Robinson EL, Sutton ML. Radiation-induced hypopituitarism is dose-dependent. Clinical endocrinology. 1989;31:363–73. doi: 10.1111/j.1365-2265.1989.tb01260.x. [DOI] [PubMed] [Google Scholar]
- 33.Patterson BC, Chen Y, Sklar CA, et al. Growth hormone exposure as a risk factor for the development of subsequent neoplasms of the central nervous system: a report from the childhood cancer survivor study. The Journal of clinical endocrinology and metabolism. 2014;99:2030–7. doi: 10.1210/jc.2013-4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reimers TS, Ehrenfels S, Mortensen EL, et al. Cognitive deficits in long-term survivors of childhood brain tumors: Identification of predictive factors. Medical and pediatric oncology. 2003;40:26–34. doi: 10.1002/mpo.10211. [DOI] [PubMed] [Google Scholar]
- 35.Askins MA, Moore BD., 3rd Preventing neurocognitive late effects in childhood cancer survivors. Journal of child neurology. 2008;23:1160–71. doi: 10.1177/0883073808321065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore BD., 3rd Neurocognitive outcomes in survivors of childhood cancer. Journal of pediatric psychology. 2005;30:51–63. doi: 10.1093/jpepsy/jsi016. [DOI] [PubMed] [Google Scholar]
- 37.Packer RJ, Gurney JG, Punyko JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:3255–61. doi: 10.1200/JCO.2003.01.202. [DOI] [PubMed] [Google Scholar]
- 38.Campen CJ, Kranick SM, Kasner SE, et al. Cranial irradiation increases risk of stroke in pediatric brain tumor survivors. Stroke; a journal of cerebral circulation. 2012;43:3035–40. doi: 10.1161/STROKEAHA.112.661561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson AH, Jordan C, Mazewski CM. Off-therapy headaches in pediatric brain tumor patients: a retrospective review. Journal of pediatric oncology nursing : official journal of the Association of Pediatric Oncology Nurses. 2009;26:354–61. doi: 10.1177/1043454209340323. [DOI] [PubMed] [Google Scholar]
- 40.Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Journal of the National Cancer Institute. 2006;98:1528–37. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 41.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. Journal of the National Cancer Institute. 2001;93:618–29. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 42.Boman K, Bodegard G. Long-term coping in childhood cancer survivors: influence of illness, treatment and demographic background factors. Acta paediatrica. 2000;89:105–11. doi: 10.1080/080352500750029167. [DOI] [PubMed] [Google Scholar]
- 43.Mulhern RK. Neuropsychological Late Effects. In: Bearison D, Mulhern RK, editors. Pediatric Psychooncology. New York: Oxford University Press; 1994. pp. 99–121. [Google Scholar]
- 44.Vannatta K, Gartstein MA, Short A, Noll RB. A controlled study of peer relationships of children surviving brain tumors: teacher, peer, and self ratings. Journal of pediatric psychology. 1998;23:279–87. doi: 10.1093/jpepsy/23.5.279. [DOI] [PubMed] [Google Scholar]
- 45.Carpentieri SC, Meyer EA, Delaney BL, et al. Psychosocial and behavioral functioning among pediatric brain tumor survivors. Journal of neuro-oncology. 2003;63:279–87. doi: 10.1023/a:1024203323830. [DOI] [PubMed] [Google Scholar]
- 46.Hornquist L, Rickardsson J, Lannering B, Gustafsson G, Boman KK. Altered self-perception in adult survivors treated for a CNS tumor in childhood or adolescence: population-based outcomes compared with the general population. Neuro-oncology. 2014 doi: 10.1093/neuonc/nou289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medicine Io. National Research Council: From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 48.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 49.Mueller S, Chang S. Pediatric brain tumors: current treatment strategies and future therapeutic approaches. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2009;6:570–86. doi: 10.1016/j.nurt.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolfe J, Grier HE, Klar N, et al. Symptoms and suffering at the end of life in children with cancer. The New England journal of medicine. 2000;342:326–33. doi: 10.1056/NEJM200002033420506. [DOI] [PubMed] [Google Scholar]
- 51.Pritchard M, Burghen E, Srivastava DK, et al. Cancer-related symptoms most concerning to parents during the last week and last day of their child's life. Pediatrics. 2008;121:e1301–9. doi: 10.1542/peds.2007-2681. [DOI] [PubMed] [Google Scholar]
- 52.Arland LC, Hendricks-Ferguson VL, Pearson J, Foreman NK, Madden JR. Development of an in-home standardized end-of-life treatment program for pediatric patients dying of brain tumors. Journal for specialists in pediatric nursing : JSPN. 2013;18:144–57. doi: 10.1111/jspn.12024. [DOI] [PubMed] [Google Scholar]
- 53.Vallero SG, Lijoi S, Bertin D, et al. End-of-life care in pediatric neuro-oncology. Pediatric blood & cancer. 2014;61:2004–11. doi: 10.1002/pbc.25160. [DOI] [PubMed] [Google Scholar]
- 54.Goldman A, Hewitt M, Collins GS, Childs M, Hain R United Kingdom Children's Cancer Study Group/Paediatric Oncology Nurses' Forum Palliative Care Working G. Symptoms in children/young people with progressive malignant disease: United Kingdom Children's Cancer Study Group/Paediatric Oncology Nurses Forum survey. Pediatrics. 2006;117:e1179–86. doi: 10.1542/peds.2005-0683. [DOI] [PubMed] [Google Scholar]
- 55.Pace A, Di Lorenzo C, Guariglia L, Jandolo B, Carapella CM, Pompili A. End of life issues in brain tumor patients. Journal of neuro-oncology. 2009;91:39–43. doi: 10.1007/s11060-008-9670-x. [DOI] [PubMed] [Google Scholar]
- 56.Faithfull S, Cook K, Lucas C. Palliative care of patients with a primary malignant brain tumour: case review of service use and support provided. Palliative medicine. 2005;19:545–50. doi: 10.1191/0269216305pm1068oa. [DOI] [PubMed] [Google Scholar]
- 57.Sizoo EM, Braam L, Postma TJ, et al. Symptoms and problems in the end-of-life phase of high-grade glioma patients. Neuro-oncology. 2010;12:1162–6. doi: 10.1093/neuonc/nop045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gofton TE, Graber J, Carver A. Identifying the palliative care needs of patients living with cerebral tumors and metastases: a retrospective analysis. Journal of neuro-oncology. 2012;108:527–34. doi: 10.1007/s11060-012-0855-y. [DOI] [PubMed] [Google Scholar]
- 59.Zelcer S, Cataudella D, Cairney AE, Bannister SL. Palliative care of children with brain tumors: a parental perspective. Archives of pediatrics & adolescent medicine. 2010;164:225–30. doi: 10.1001/archpediatrics.2009.284. [DOI] [PubMed] [Google Scholar]
- 60.Cataudella DA, Zelcer S. Psychological experiences of children with brain tumors at end of life: parental perspectives. Journal of palliative medicine. 2012;15:1191–7. doi: 10.1089/jpm.2011.0479. [DOI] [PubMed] [Google Scholar]
- 61.Weiland A. Topical compunds: reviewing the evidence. Hospiscript. 2012;8:1–2. [Google Scholar]
- 62.Warren DE. Practical use of rectal medications in palliative care. Journal of pain and symptom management. 1996;11:378–87. doi: 10.1016/0885-3924(96)00012-7. [DOI] [PubMed] [Google Scholar]
- 63.LeBon B, Zeppetella G, Higginson IJ. Effectiveness of topical administration of opioids in palliative care: a systematic review. Journal of pain and symptom management. 2009;37:913–7. doi: 10.1016/j.jpainsymman.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 64.Fager S, Bardach L, Russell S, Higginbotham J. Access to augmentative and alternative communication: new technologies and clinical decision-making. Journal of pediatric rehabilitation medicine. 2012;5:53–61. doi: 10.3233/PRM-2012-0196. [DOI] [PubMed] [Google Scholar]
- 65.Korner S, Sieniawski M, Kollewe K, et al. Speech therapy and communication device: impact on quality of life and mood in patients with amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis & frontotemporal degeneration. 2013;14:20–5. doi: 10.3109/17482968.2012.692382. [DOI] [PubMed] [Google Scholar]
- 66.Wusthoff CJ, Shellhaas RA, Licht DJ. Management of common neurologic symptoms in pediatric palliative care: seizures, agitation, and spasticity. Pediatric clinics of North America. 2007;54:709–33. xi. doi: 10.1016/j.pcl.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 67.Pace A, Villani V, Di Lorenzo C, et al. Epilepsy in the end-of-life phase in patients with high-grade gliomas. Journal of neuro-oncology. 2013;111:83–6. doi: 10.1007/s11060-012-0993-2. [DOI] [PubMed] [Google Scholar]
- 68.Krouwer HG, Pallagi JL, Graves NM. Management of seizures in brain tumor patients at the end of life. Journal of palliative medicine. 2000;3:465–75. doi: 10.1089/jpm.2000.3.4.465. [DOI] [PubMed] [Google Scholar]
- 69.Baker JN, Windham JA, Hinds PS, et al. Bereaved parents' intentions and suggestions about research autopsies in children with lethal brain tumors. The Journal of pediatrics. 2013;163:581–6. doi: 10.1016/j.jpeds.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alabran JL, Hooper JE, Hill M, et al. Overcoming autopsy barriers in pediatric cancer research. Pediatric blood & cancer. 2013;60:204–9. doi: 10.1002/pbc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]