Abstract
Background:
Medial patellofemoral ligament (MPFL) reconstruction is performed to prevent recurrent instability, but errors in femoral fixation can elevate graft tension.
Hypothesis:
Errors related to femoral fixation will overconstrain the patella and increase medial patellofemoral pressures.
Study Design:
Controlled laboratory study.
Methods:
Five knees with patellar instability were represented with computational models. Kinematics during knee extension were characterized from computational reconstruction of motion performed within a dynamic computed tomography (CT) scanner. Multibody dynamic simulation of knee extension, with discrete element analysis used to quantify contact pressures, was performed for the preoperative condition and after MPFL reconstruction. A standard femoral attachment and graft resting length were set for each knee. The resting length was decreased by 2 mm, and the femoral attachment was shifted 5 mm posteriorly. The simulated errors were also combined. Root-mean-square errors were quantified for the comparison of preoperative patellar lateral shift and tilt between computationally reconstructed motion and dynamic simulation. Simulation output was compared between the preoperative and MPFL reconstruction conditions with repeated-measures Friedman tests and Dunnett comparisons against a control, which was the standard MPFL condition, with statistical significance set at P < .05.
Results:
Root-mean-square errors for simulated patellar tilt and shift were 5.8° and 3.3 mm, respectively. Patellar lateral tracking for the preoperative condition was significantly larger near full extension compared with the standard MPFL reconstruction (mean differences of 8 mm and 13° for shift and tilt, respectively, at 0°), and lateral tracking was significantly smaller for a posterior femoral attachment (mean differences of 3 mm and 4° for shift and tilt, respectively, at 0°). The maximum medial pressure was also larger for the short graft with a posterior femoral attachment than for standard MPFL reconstruction, with a significant increase in the mean value of 1.6 MPa at 30°.
Conclusion:
MPFL reconstruction reduces lateral tracking, but nonanatomic femoral fixation and overtensioning the graft overcorrect patellar tracking and increase pressure applied to medial patellar cartilage.
Clinical Relevance:
Errors in femoral fixation and graft tensioning can lead to postoperative loss of flexion and overloading of medial cartilage.
Keywords: patellar instability, computational simulation, MPFL reconstruction, patellar tracking, cartilage pressure
Medial patellofemoral ligament (MPFL) reconstruction is performed to stabilize the patella in patients with recurrent patellar instability. Intraoperatively, the graft is typically positioned to limit patellar lateral displacement to approximately 1 trochlear quadrant.8,30 A fixation point on the femur is chosen based on anatomometric criteria, with the goal of approximating the native MPFL attachment and maintaining the distance between graft attachment points as the knee flexes. Multiple techniques have been developed to identify the anatomic attachment point on the femur.34,42
Several in vitro and computational simulation studies have been performed to evaluate the influence of MPFL reconstruction on patellofemoral mechanics. While reconstruction of the MPFL has been shown to limit lateral patellar displacement,29,31,39 if there are elevated levels of graft tension (due to initial overtensioning or nonanatomic femoral fixation), the overconstrained patella experiences increased pressure in the region of the medial cartilage.3,11,31,40 A primary limitation of the prior studies is that none accounted for the amount of lateral maltracking10,44 associated with patellar instability or trochlear dysplasia that is common for patients with recurrent instability9,23,38 and correlated with lateral tracking.5,15
The current study was performed with 2 primary goals. The first was to characterize the influence of MPFL reconstruction on patellar tracking and pressure applied to cartilage in knees with patellar instability. The second was to assess the influence of errors that could occur during femoral fixation of the graft on patellofemoral mechanics. The study particularly focused on fixing the graft too far posteriorly on the femur and overtensioning the graft, which could occur if the graft attachment rotates posteriorly during fixation with an interference screw or is pushed into the femoral tunnel during fixation, respectively. Dynamic simulation of knee function was utilized to address the goals, allowing characterization of patellar tracking and pressure applied to cartilage with models based on the anatomy of knees with patellar instability. Simulated dynamic patellar tracking was compared with dynamic patellar tracking for the subjects on which the models were based10 to assess the accuracy of the computational simulations.
Methods
Subjects
Simulation of knee motion was based on 5 computational models reconstructed from subjects with recurrent patellar instability. The study was approved by the Institutional Review Board of the Johns Hopkins Medical Institutions, where the subjects were treated. Each treated subject had a history of symptomatic recurrent lateral patellar dislocation episodes. Two subjects had a previous medial imbrication and lateral release. These were included based on the assumption that the medial imbrication failed with continued instability. The patella was dislocatable during examination under anesthesia for 1 of these knees, with 4 quadrants of lateral translation recorded for the other. Two of the remaining 3 knees were dislocatable under anesthesia, with 3 quadrants of lateral translation recorded for the other knee. No subject with a previous tibial tuberosity realignment procedure was included. The subjects included 3 females. The mean age (±SD) was 21 ± 7 years. Based on computed tomography (CT) evaluation, the mean tibial tuberosity to trochlear groove distance was 17 mm (range, 14-21 mm), and patella alta (Insall-Salvati index >1.2) was identified for 1 knee. The mean sulcus angle based on the most proximal axial magnetic resonance image (MRI) slice of the trochlear groove including patellofemoral cartilage contact was 163° (range, 150°-171°). Values greater than 150° are beyond the 95% CI for normal knees, indicating that almost all of our subjects had some degree of trochlear dysplasia.9
Computational Reconstruction of Knee Extension
Dynamic CT imaging (Aquilion ONE scanner; Toshiba Medical Systems) acquired during knee extension against gravity as part of clinical evaluation was utilized to characterize knee kinematics.10 The rotating gantry acquired 21 volumes of 320 axial images (0.5-mm separation, 512 × 512–pixel in-plane resolution) over 10 seconds as the knee extended from the maximum flexion angle that could be achieved within the scanner to full extension. Computational models of the femur, tibia, and patella were reconstructed (3D Doctor; Able Software Corp) from 5 or 6 volumes that spanned the extension range. A single model of the femur, patella, and tibia was transferred to all positions of knee flexion using an iterative closest-point algorithm.4 A local coordinate system was created for the femur based on the transepicondylar axis and long axis, with similar coordinate systems created for the patella and tibia,10 allowing characterization of knee kinematics based on the floating axis convention.19
Dynamic Simulation of Knee Extension
The computational models representing each knee were used as the basis for dynamic simulation of knee extension. MRI scans of each knee collected for clinical evaluation (proton density–weighted sagittal scans, slice thickness ranging from 0.6 to 4.0 mm) allowed reconstruction of the femur, tibia, and patella and the cartilage attached to each bone. An iterative closest-point algorithm4 was used to align each bone reconstructed from MRI to the respective bone from CT with the knee in the most flexed position (approximately 50°) to align cartilage surfaces with the CT bone models. The cartilage surfaces were merged with the bones. Because of the varying quality of the clinical MRI scans, the articular surfaces of the merged models were smoothened (3-matic; Materialise) to visual satisfaction for multibody dynamics simulation (Recurdyn; FunctionBay Inc) (Figure 1). The anterior and posterior cruciate ligaments, the medial and lateral collateral ligaments, and the posterior joint capsule were represented by multiple tension-only springs with damping6,32 and governed by previously described nonlinear force-strain relationships, including prestrain with the knee extended.6,37 The patellar tendon was represented by 5 springs assigned a total stiffness of 2000 N/mm.14 The lateral retinaculum was represented by 2 springs with a total stiffness of 2 N/mm.11 Interaction at the tibiofemoral and patellofemoral articulations was governed by simplified Hertzian contact20,21 according to the equation
where F c is the contact force in the cartilage, k is the contact stiffness coefficient, δ is the penetration of surfaces, δ′ is the penetration velocity, n is the compliance exponent, and B(δ) is the damping coefficient. The contact parameters were k = 500 N/mm, B(δ) = 5 N·s/mm, and n = 1.5.7
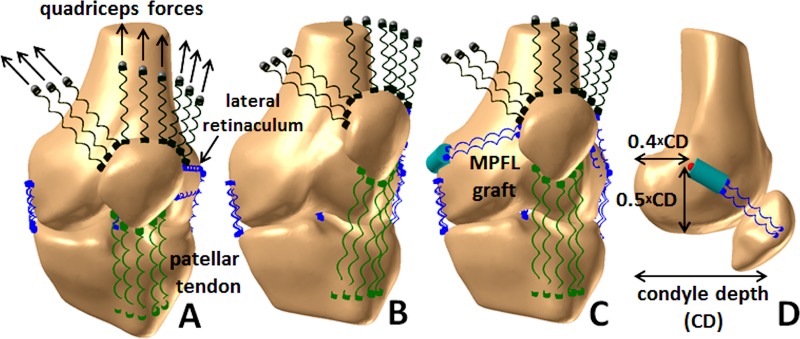
Figure 1.
Multibody dynamic simulation model of the knee. Patellar tracking is shown as a left knee extends from (A) a flexed position to (B) full extension in the preoperative condition and (C) for the knee extended with an MPFL graft wrapping around the femoral condyle. (D) The standard attachment of the MPFL graft based on the depth of the medial condyle42 is also shown. MPFL, medial patellofemoral ligament.
Quadriceps muscle forces were divided among the vastus medialis obliquus (VMO), vastus lateralis, and the combination of the vastus intermedius, rectus femoris, and vastus medialis longus. The forces originated at points attached to the femur, based on the typical muscle fiber orientation for each component of the quadriceps,17 and were applied to the patella through springs with a total stiffness of 1350 N/mm.36 Forces were divided among the quadriceps components based on previously measured ratios.24,45 The force applied by the VMO was approximately 50% lower than normal to represent weakness characteristic of patellofemoral disorders.13 Medial and lateral hamstrings forces applied one-third of the total quadriceps force to the tibia along the long axis of the femur.12,25 With the femur fixed in space and mass added to the tibia to represent the moment of the lower limb about the knee, a total quadriceps force that initiated knee extension against gravity was applied, extending the knee from 50° to 0° of flexion.
Dynamic knee extension was simulated for each knee in a preoperative condition and after MPFL reconstruction. The native MPFL was considered to be ruptured due to recurrent instability and was not represented in the preoperative condition. For the 2 knees with a previous lateral release, the lateral retinaculum was not included for comparison with knee kinematics from the subjects. The lateral retinaculum was included for comparisons between the simulated conditions for consistency across all models and to resist overcorrection from MPFL reconstruction. For MPFL reconstruction, a dual-strand semitendinosus tendon graft was represented with 2 springs. To approximate the native MPFL, the graft attachment on the patella spanned from the medial edge of the VMO attachment to the medial edge of the patella.22,42,43 For standard MPFL reconstruction, the graft was fixed to the femur at a position approximating the anatomic attachment, at a distance of 50% of the anterior-posterior depth of the medial condyle from the distal boundary of the medial condyle and 40% of the depth from the posterior boundary (Figure 1).42 Springs representing the MPFL graft were assigned a total stiffness of 100 N/mm.11 The springs extended from the patellar attachment to a wrapping surface fixed to the medial femoral condyle. The portion of the graft from the wrapping surface to the femoral attachment rotated as a rigid body. The resting length of the graft was set to allow 1 quadrant of lateral patellar translation with respect to the deepest point of the trochlear groove with the knee flexed to 25°.8,16 Patellofemoral and tibiofemoral kinematics were processed using the same coordinate axes used to characterize motion during dynamic CT imaging. The total force within the MPFL graft was also quantified.
Errors related to femoral fixation of the graft were also represented for simulation of knee extension. To simulate the graft rotating around the femoral tunnel, the femoral attachment was shifted 5 mm posteriorly (without altering the resting length). The 5-mm distance was based on a 7-mm graft tunnel, accounting for the width of the graft reducing the maximum change in the attachment point. To simulate the graft being pushed into the femoral tunnel, the graft was shorted by 2 mm. The combination of a short graft and a posterior femoral attachment was also represented.
Computational Pressure Distribution
The patellofemoral contact pressure distribution was postprocessed from the simulated motion using a previously validated discrete element analysis method.14 The pressure distribution was quantified at 5° intervals of the simulated knee extension from 50° to 15°. The patella was typically proximal to the trochlear groove at lower flexion angles. The articular surface on the patella was represented by a layer of at least 5000 elements. The cartilage thickness for each element was determined by the shortest distance to the underlying bone. A spring with an elastic modulus of 4 MPa and a Poisson ratio of 0.45 was positioned at each patellar element. The force within each spring was based on linear elastic theory for a homogeneous and isotropic material according to the equation
where E is the elastic modulus, A is the area covered by the spring, γ is the Poisson ratio, h is the combined thickness of the patellar element and nearest element on the femur, and d is the compression of the spring. Compression was determined by overlap of the patellar cartilage with the femoral cartilage. The position of the patella was iteratively adjusted to balance the compressive force, lateral force, and tilt moment applied by the quadriceps muscles, lateral retinaculum, patellar tendon, and graft with the reaction forces and moments from the cartilage. The maximum pressure applied to the lateral facet and medial facet and the position of the center of pressure with respect to a point centered between the most medial and lateral points on the patella were quantified to represent the pressure distribution.
Statistical Analysis
Assessment of the simulated kinematics focused on patellar lateral tilt and shift as the parameters most relevant to patellar instability. Each data point for patellar lateral shift and tilt from reconstruction of motion in the scanner was compared with the corresponding simulation data from the same knee at the same flexion angle. Root-mean-square errors between the kinematics from subjects and simulated data were quantified. Kinematics data from the subjects were also interpolated at 10° intervals and averaged for comparison of variations with the knee flexion angle to averaged simulation data.
The simulated kinematics and pressure distribution data were compared between the preoperative and MPFL reconstruction conditions using nonparametric repeated-measures Friedman tests. Nonparametric analyses were used because Kolmogorov-Smirnov tests indicated the data were not normally distributed. The data were compared at every 5° of knee flexion. Post hoc comparisons were performed with a nonparametric version of a Dunnett comparison against a control, using the standard MPFL condition as the control for the preoperative condition and all variations in the MPFL graft. Statistical significance was set at P < .05 for all comparisons. With 5 models, the study was designed for a statistical power of 0.85 based on an estimated effect size of 2.018 for repeated-measures changes in patellar tracking related to surgical realignment10 and pressure increases related to errors in graft tension and femoral fixation.11
Results
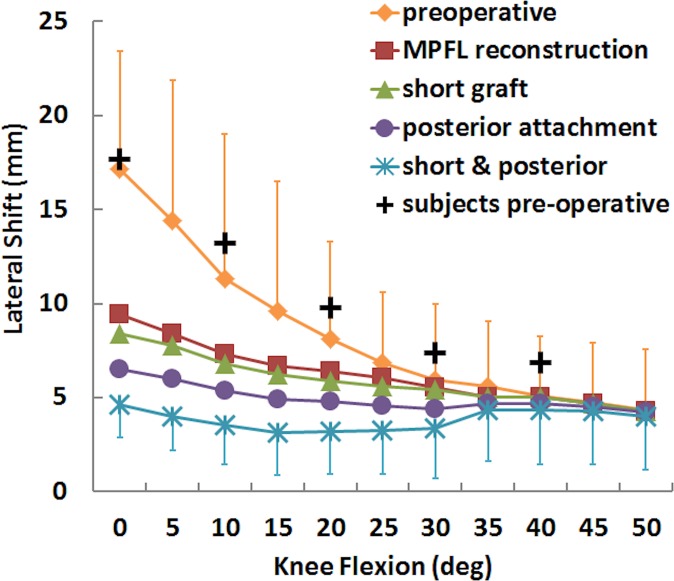
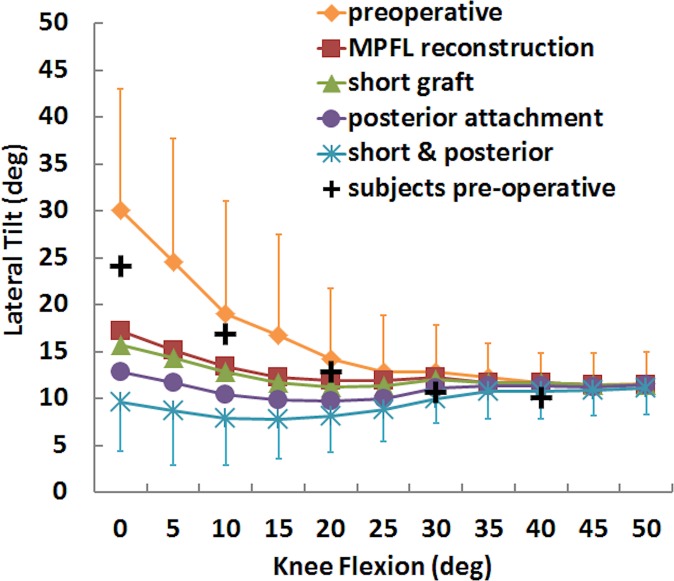
Variations in preoperative patellar lateral shift and tilt with the flexion angle were similar for motion from the subjects and simulated motions (Figures 2 and 3). The root-mean-square errors over all data points were 5.8° and 3.3 mm for patellar tilt and shift, respectively.
Figure 2.
Mean (±SD) patellar lateral shift for the preoperative condition, standard MPFL reconstruction, and MPFL reconstruction with a short graft and a posterior femoral attachment. Data points are also shown representing motion of the subjects within the dynamic scanner. MPFL, medial patellofemoral ligament.
Figure 3.
Mean (±SD) patellar lateral tilt for the preoperative condition, standard MPFL reconstruction, and MPFL reconstruction with a short graft and a posterior femoral attachment. Data points are also shown representing motion of the subjects within the dynamic scanner. MPFL, medial patellofemoral ligament.
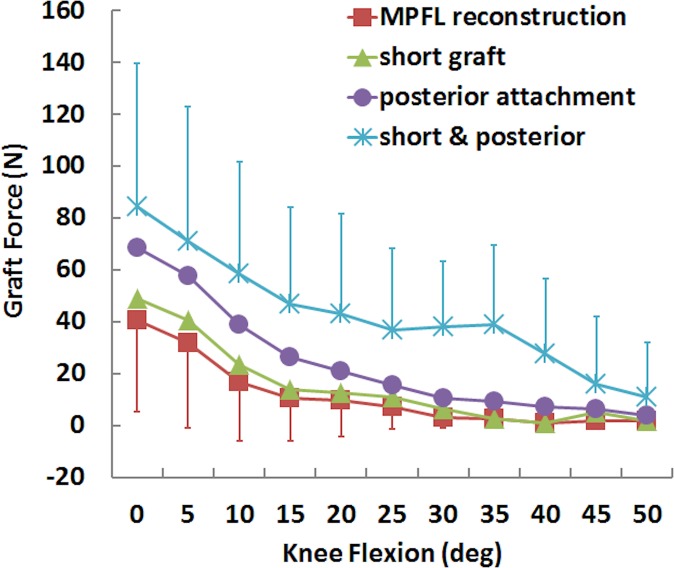
For the simulated kinematics, standard MPFL reconstruction reduced lateral patellar tracking, while a short graft and posterior fixation further reduced lateral tracking from the standard reconstruction condition. Patellar lateral shift and tilt were significantly smaller for the standard MPFL reconstruction than for the preoperative condition at nearly all flexion angles from 0° to 40° and 0° to 30°, respectively (Table 1). For mean patellar shift and tilt, the maximum differences were 8 mm and 13°, respectively, with the knee at full extension (Figures 2 and 3). Moving the femoral attachment posteriorly further decreased lateral tracking, with significant differences at several flexion angles, primarily near full extension. The combination of a short graft and posterior attachment point significantly decreased lateral shift and tilt at all flexion angles from 0° to 40°. The combined errors decreased the mean lateral shift and tilt at full extension by 5 mm and 8°, respectively, compared with standard MPFL reconstruction. The graft force was significantly larger for posterior fixation combined with a short graft than standard reconstruction from 0° to 30° and 40° of knee flexion, with a difference of 44 N for the combined errors at 0° of flexion (Figure 4). Significant differences in graft force were also noted at the same flexion angles for a posterior attachment.
TABLE 1.
Flexion Angles at Which Data Points Are Significantly Different From Standard MPFL Reconstructiona
| Flexion Angle | |||||||||||
| 0° | 5° | 10° | 15° | 20° | 25° | 30° | 35° | 40° | 45° | 50° | |
| Preoperative | |||||||||||
| Lateral shift | |||||||||||
| Lateral tilt | |||||||||||
| Max medial pressure | |||||||||||
| Center of pressure | |||||||||||
| Posterior attachment | |||||||||||
| Lateral shift | |||||||||||
| Lateral tilt | |||||||||||
| Graft force | |||||||||||
| Max medial pressure | |||||||||||
| Center of pressure | |||||||||||
| Short and posterior | |||||||||||
| Lateral shift | |||||||||||
| Lateral tilt | |||||||||||
| Graft force | |||||||||||
| Max medial pressure | |||||||||||
| Center of pressure | |||||||||||
aStatistically significantly different from standard medial patellofemoral ligament (MPFL) reconstruction: light gray cells, P < .05; dark gray cells, P < .01. No significant differences were identified for the short graft alone or for maximum lateral pressure measurements. Max, maximum.
Figure 4.
Mean (±SD) graft force for a standard medial patellofemoral ligament (MPFL) reconstruction and MPFL reconstruction with a short graft and a posterior femoral attachment.
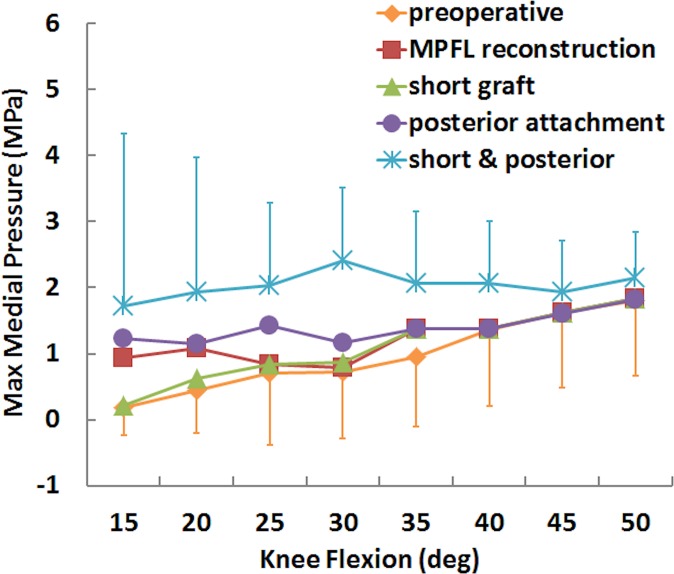
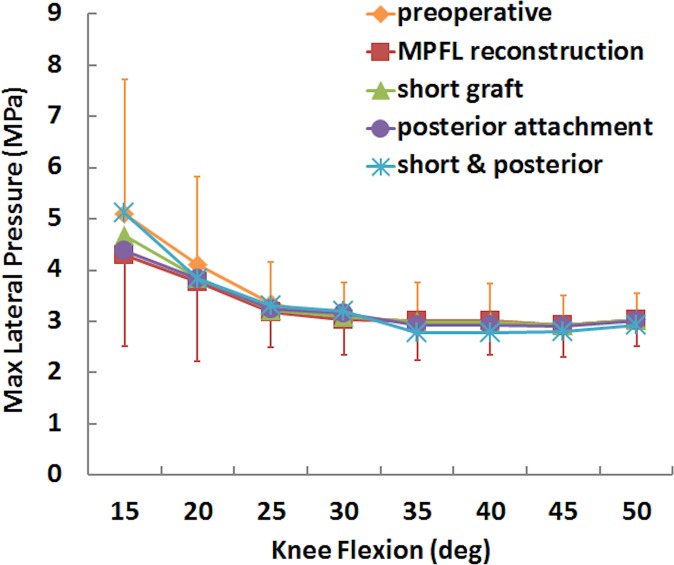
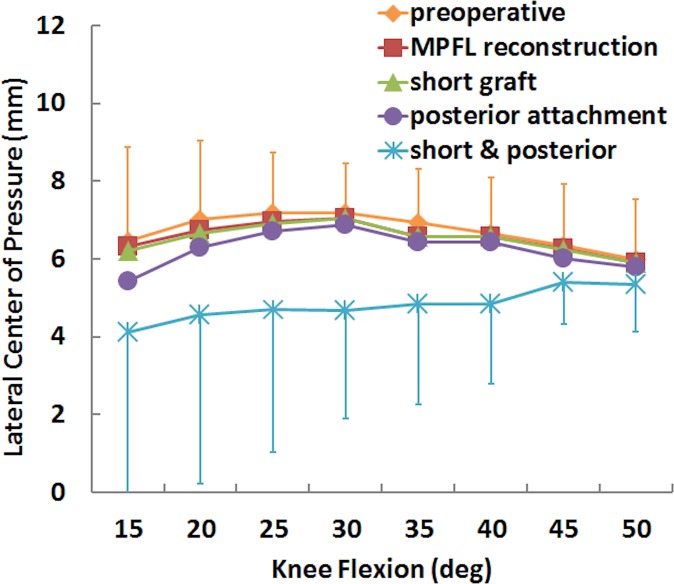
For the simulated pressure distribution, the combination of a short graft with posterior fixation increased the maximum medial pressure and shifted the center of pressure medially. The maximum medial pressure was significantly larger for the short graft with a posterior femoral attachment than for standard MPFL reconstruction from 30° to 40° of knee flexion (Table 1), with an increase in the mean value from 0.8 to 2.4 MPa at 30° (Figures 5 and 6). The position of the center of pressure was significantly less lateral for a short graft with a posterior femoral attachment than for standard MPFL reconstruction at 25° and 30° of knee flexion, with a mean change of 2 mm at 30° (Figure 7). No significant differences in maximum lateral pressure were identified (P > .11) (Figure 8).
Figure 5.
Mean (±SD) maximum medial pressure for the preoperative condition, standard medial patellofemoral ligament (MPFL) reconstruction, and MPFL reconstruction with a short graft and a posterior femoral attachment.
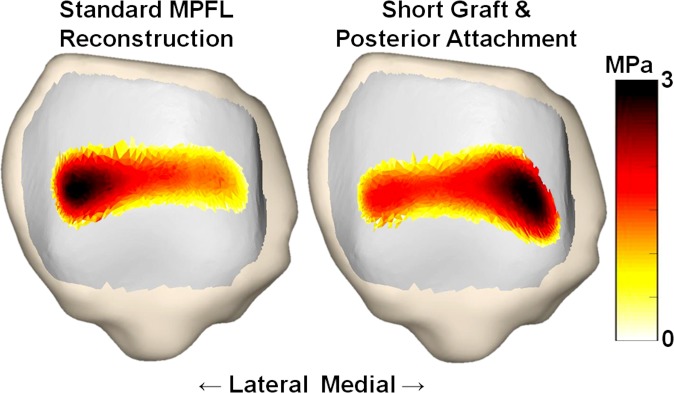
Figure 6.
Contact pressure patterns for 1 patella at 30° of flexion for a standard medial patellofemoral ligament (MPFL) reconstruction and reconstruction with a short graft and a posterior femoral attachment.
Figure 7.
Mean (±SD) maximum lateral pressure for the preoperative condition, standard medial patellofemoral ligament (MPFL) reconstruction, and MPFL reconstruction with a short graft and a posterior femoral attachment.
Figure 8.
Mean (±SD) lateral center of pressure for the preoperative condition, standard medial patellofemoral ligament (MPFL) reconstruction, and MPFL reconstruction with a short graft and a posterior femoral attachment.
Discussion
The results of the study indicate that standard MPFL reconstruction decreases patellar lateral tracking, but a short graft and posterior fixation further decrease patellar lateral shift and tilt and shift contact pressure medially on the patella. Previous in vitro experimental studies also indicated that MPFL reconstruction decreases elevated levels of patellar lateral tilt and shift.29,31,39 However, the previous studies were performed on knees with normal patterns of patellar tracking. With the MPFL transected, the greatest mean increases in patellar lateral shift and tilt from a flexed position to an extended position were approximately 8 mm and 8°, respectively.31 In contrast, for the preoperative condition of the current study, patellar lateral shift and tilt increased by 13 mm and 19°, respectively, from 50° of flexion to full extension. The current study is the first the authors are aware of to evaluate MPFL reconstruction as a means to reduce lateral tracking utilizing knees from patients with patellar instability.
A short graft and posterior fixation alter patellar tracking and contact pressures due to elevated graft forces. A previous computational study showed a similar increase in graft tension and medial shift in pressure distribution due to the combination of overtensioning the graft and nonanatomic femoral fixation.11 Previous in vitro experimental studies have also shown that nonanatomic femoral fixation and elevated levels of graft tension overcorrect patellar tracking and increase pressure applied to medial cartilage.3,31,40,41 Because the previous studies were also performed with knees that did not exhibit patellar instability, the results needed to be verified for knees with lateral patellar maltracking and trochlear dysplasia. The current results further support the previous evidence indicating that nonanatomic femoral attachment and overtensioning of an MPFL graft overcorrect patellar tracking and elevate pressure applied to medial cartilage.
The current study also indicated that the multibody dynamic simulation technique is capable of producing patellar tracking patterns typical of patients with patellar instability. The most important characteristic of the simulations is the ability to represent the large values of patellar lateral shift and tilt, particularly with the knee extended, as compared with normal knees. The large levels of patellar shift and tilt with the knee near full extension have been shown to be correlated with the trochlear dysplasia and the lateral position of the tibial tuberosity.5,15 Previous studies have shown computational simulation can produce dynamic patellar tracking patterns similar to in vitro experimental data.1,2,32 The root-mean-square error for patellar shift from a recently described explicit finite element model was less than 3 mm,1 compared with 3.3 mm for the current study. The previous study did not provide a root-mean-square error for patellar tilt at flexion angles less than 50°, but the mean simulated lateral tilt was approximately 5° larger than the in vitro tilt near full extension, similar to the difference between simulated patellar tilt and tilt measured from the subjects for the current study. The current study produced accuracy similar to the previous study even though the previous study had the advantages of validating models based on normal knees and known muscle forces and orientations. The dynamic modeling techniques developed for the current study will be valuable for future studies that require representation of the tracking patterns related to instability for evaluation of treatment options.
The current study also included computational replication of the patellofemoral contact pressure distribution. The discrete element analysis technique utilized to characterize pressure patterns has previously been validated against in vitro experimental measurements characterizing the influence of altering forces applied to the patella on the pressure distribution.14 The data from the subjects did not allow for measurement of patellofemoral pressures for further validation.
The current study focused on 5 mm of posterior displacement of the femoral attachment site for the graft and 2 mm of shortening of the resting length of the graft. The errors were selected to replicate possible errors from securing an MPFL graft to the femur with an interference screw. The interference screw is tightened after measuring out the appropriate graft length, which for the current study was based on allowing 1 quadrant of patellar lateral translation with the graft at the resting length. The graft can rotate with the screw around the perimeter of the femoral tunnel,28,33 with the most dramatic change moving the attachment point on the femur from an anterior position on the tunnel to a posterior position. Previous studies have shown that proximal and distal malpositioning of the femoral tunnel increases medial patellar pressures.11,40,41 For the previous studies, the graft length was set at the proximal or distal tunnel, while the current study focused on a change in the fixation point after the graft length was set, causing the largest changes to occur for the largest deviation in fixation point at the posterior position. The graft can also be pushed into the femoral tunnel as the screw is turned, with the resting length of the graft outside of the tunnel shortened to represent this condition.
Overcorrecting patellar kinematics and increasing medial contact pressures may adversely influence postoperative function. Increasing medial contract pressures is a particular concern due to the prevalence of medial cartilage fibrillation and erosion associated with recurrent instability due to contact between the medial facet of the patella and the lateral condyle of the femur.27 Loss of flexion and medial pain have been noted as consequences of MPFL reconstruction with an overtensioned graft or nonanatomic femoral attachment,26 although a previous study identified similar values for range of motion and subjective assessment of outcome for knees with properly positioned and malpositioned femoral attachment points.35
Limitations of the study should be noted. Many properties of the dynamic simulation models were assigned based on previously published data and anatomic landmarks. Parameters assigned to the models based on previous studies include properties of all soft tissues, ligament attachment points, and muscle forces and orientations. Only clinical MRI scans were available for reconstruction of cartilage surfaces. Because of the relatively low resolution of the MRI scans for a majority of the knees, smoothening of the cartilage surfaces was necessary to represent articular anatomy and allow the models to run. These limitations highlight the importance of determining the accuracy of the simulation models by comparing simulated kinematics to kinematics data based on motion of the subjects the models were based on. For representation of the postoperative condition, properties assigned to the graft represent the state immediately after reconstruction, while graft elongation or fibrosis may occur during healing. No true normal tracking pattern could be assigned to the knees with patellar instability, so standard MPFL reconstruction was used to represent the control condition. The simulated motions represent patellar tracking patterns related to patellar instability, but conditions that actually produce instability were not represented. The number of models required a relatively large effect size to identify significant differences, limiting the flexion range for identifying differences for some parameters. Although increased medial pressure values were identified with errors in graft fixation, the long-term effects of increased pressure on the medial facet are currently unknown. The models represented the inhomogeneity in anatomy typical of patellar instability, requiring additional studies to determine how the relationship between MPFL graft fixation and patellar tracking varies with anatomy.
Conclusion
The current study indicates that fixing an MPFL graft at a standardized position on the femur with proper graft tensioning dramatically decreases patellar lateral shift and tilt near full extension, but rotation of the graft around an interference screw to a posterior position on the femur and overtensioning the graft results in overcorrection of patellar tracking and increased pressure applied to medial patellar cartilage. The current study represents the first time the effects of MPFL reconstruction and errors in femoral fixation have been demonstrated in an environment that represents knees with patellar instability. The dynamic simulation of knee motion used to evaluate MPFL reconstruction was shown to produce patellar tracking patterns representative of knees with instability. Care should be taken to avoid migration of the point of femoral fixation and elevated graft tension when securing an MPFL graft.
Acknowledgment
Imaging data to create the models were provided by Dr Andrew Cosgarea and the Department of Radiology at Johns Hopkins University. Their assistance is greatly appreciated.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: Funding for the study was provided by MedShape. K.E.S. is employed by and owns stock in MedShape. K.G. owns stock in MedShape. J.F. receives consulting fees from and has stock options in MedShape.
References
- 1. Ali AA, Shalhoub SS, Cyr AJ, et al. Validation of predicted patellofemoral mechanics in a finite element model of the healthy and cruciate-deficient knee. J Biomech. 2016;49:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baldwin MA, Langenderfer JE, Rullkoetter PJ, Laz PJ. Development of subject-specific and statistical shape models of the knee using an efficient segmentation and mesh-morphing approach. Comput Methods Programs Biomed. 2010;97:232–240. [DOI] [PubMed] [Google Scholar]
- 3. Beck P, Brown NA, Greis PE, Burks RT. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35:1557–1563. [DOI] [PubMed] [Google Scholar]
- 4. Besl PJ, McKay HD. A method for registration of 3-D shapes. IEEE Trans Pattern Anal Mach Intel. 1992;14:239–256. [Google Scholar]
- 5. Biyani R, Elias JJ, Saranathan A, et al. Anatomical factors influencing patellar tracking in the unstable patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2014;22:2334–2341. [DOI] [PubMed] [Google Scholar]
- 6. Blankevoort L, Kuiper JH, Huiskes R, Grootenboer HJ. Articular contact in a three-dimensional model of the knee. J Biomech. 1991;24:1019–1031. [DOI] [PubMed] [Google Scholar]
- 7. Bloemker KH, Guess TM, Maletsky L, Dodd K. Computational knee ligament modeling using experimentally determined zero-load lengths. Open Biomed Eng J. 2012;6:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38:2248–2254. [DOI] [PubMed] [Google Scholar]
- 9. Charles MD, Haloman S, Chen L, Ward SR, Fithian D, Afra R. Magnetic resonance imaging-based topographical differences between control and recurrent patellofemoral instability patients. Am J Sports Med. 2013;41:374–384. [DOI] [PubMed] [Google Scholar]
- 10. Elias JJ, Carrino JA, Saranathan A, Guseila LM, Tanaka MJ, Cosgarea AJ. Variations in kinematics and function following patellar stabilization including tibial tuberosity realignment. Knee Surg Sports Traumatol Arthrosc. 2014;22:2350–2356. [DOI] [PubMed] [Google Scholar]
- 11. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34:1478–1485. [DOI] [PubMed] [Google Scholar]
- 12. Elias JJ, Faust AF, Chu YH, Chao EY, Cosgarea AJ. The soleus muscle acts as an agonist for the anterior cruciate ligament. An in vitro experimental study. Am J Sports Med. 2003;31:241–246. [DOI] [PubMed] [Google Scholar]
- 13. Elias JJ, Kilambi S, Goerke DR, Cosgarea AJ. Improving vastus medialis obliquus function reduces pressure applied to lateral patellofemoral cartilage. J Orthop Res. 2009;27:578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elias JJ, Saranathan A. Discrete element analysis for characterizing the patellofemoral pressure distribution: model evaluation. J Biomech Eng. 2013;135:81011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elias JJ, Soehnlen NT, Guseila LM, Cosgarea AJ. Dynamic tracking influenced by anatomy in patellar instability. Knee. 2016;23:450–455. [DOI] [PubMed] [Google Scholar]
- 16. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21:1180–1184. [DOI] [PubMed] [Google Scholar]
- 17. Farahmand F, Senavongse W, Amis AA. Quantitative study of the quadriceps muscles and trochlear groove geometry related to instability of the patellofemoral joint. J Orthop Res. 1998;16:136–143. [DOI] [PubMed] [Google Scholar]
- 18. Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. [DOI] [PubMed] [Google Scholar]
- 19. Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105:136–144. [DOI] [PubMed] [Google Scholar]
- 20. Guess TM, Liu H, Bhashyam S, Thiagarajan G. A multibody knee model with discrete cartilage prediction of tibio-femoral contact mechanics. Comput Methods Biomech Biomed Engin. 2013;16:256–270. [DOI] [PubMed] [Google Scholar]
- 21. Guess TM, Thiagarajan G, Kia M, Mishra M. A subject specific multibody model of the knee with menisci. Med Eng Phys. 2010;32:505–515. [DOI] [PubMed] [Google Scholar]
- 22. Kita K, Horibe S, Toritsuka Y, et al. Effects of medial patellofemoral ligament reconstruction on patellar tracking. Knee Surg Sports Traumatol Arthrosc. 2012. 20:829–837. [DOI] [PubMed] [Google Scholar]
- 23. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41:575–581. [DOI] [PubMed] [Google Scholar]
- 24. Makhsous M, Lin F, Koh JL, Nuber GW, Zhang L-Q. In vivo and noninvasive load sharing among the vasti in patellar malalignment. Med Sci Sports Exerc. 2004;36:1768–1775. [DOI] [PubMed] [Google Scholar]
- 25. Mani S, Kirkpatrick MS, Saranathan A, Smith LG, Cosgarea AJ, Elias JJ. Tibial tuberosity osteotomy for patellofemoral realignment alters tibiofemoral kinematics. Am J Sports Med. 2011;39:1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nelitz M, Williams RS, Lippacher S, Reichel H, Dornacher D. Analysis of failure and clinical outcome after unsuccessful medial patellofemoral ligament reconstruction in young patients. Int Orthop. 2014;38:2265–2272. [DOI] [PubMed] [Google Scholar]
- 27. Nomura E, Inoue M. Cartilage lesions of the patella in recurrent patellar dislocation. Am J Sports Med. 2004;32:498–502. [DOI] [PubMed] [Google Scholar]
- 28. Offerhaus C, Balke M, Braas M, Pennig D, Gick S, Höher J. Knee laxity in anterior cruciate ligament reconstruction: The influence of graft rotation using interference screw fixation [in German]. Orthopade. 2015;44:231–237. [DOI] [PubMed] [Google Scholar]
- 29. Ostermeier S, Holst M, Bohnsack M, Hurschler C, Stukenborg-Colsman C, Wirth CJ. In vitro measurement of patellar kinematics following reconstruction of the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2007;15:276–285. [DOI] [PubMed] [Google Scholar]
- 30. Parikh SN, Nathan ST, Wall EJ, Eismann EA. Complications of medial patellofemoral ligament reconstruction in young patients. Am J Sports Med. 2013;41:1030–1038. [DOI] [PubMed] [Google Scholar]
- 31. Philippot R, Boyer B, Testa R, Farizon F, Moyen B. Study of patellar kinematics after reconstruction of the medial patellofemoral ligament. Clin Biomech (Bristol, Avon). 2012;27:22–26. [DOI] [PubMed] [Google Scholar]
- 32. Purevsuren T, Elias JJ, Kim K, Kim YH. Dynamic simulation of tibial tuberosity realignment: model evaluation. Comput Methods Biomech Biomed Engin. 2015;18:1606–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saithna A, Chizari M, Morris G, Anley C, Wang B, Snow M. An analysis of the biomechanics of interference screw fixation and sheathed devices for biceps tenodesis. Clin Biomech (Bristol, Avon). 2015;30:551–557. [DOI] [PubMed] [Google Scholar]
- 34. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35:801–804. [DOI] [PubMed] [Google Scholar]
- 35. Servien E, Fritsch B, Lustig S, et al. In vivo positioning analysis of medial patellofemoral ligament reconstruction. Am J Sports Med. 2011;39:134–139. [DOI] [PubMed] [Google Scholar]
- 36. Shah KS, Saranathan A, Koya B, Elias JJ. Finite element analysis to characterize how varying patellar loading influences pressure applied to cartilage: model evaluation. Comput Methods Biomech Biomed Engin. 2015;18:1509–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shin CS, Chaudhari AM, Andriacchi TP. The influence of deceleration forces on ACL strain during single-leg landing: a simulation study. J Biomech. 2007;40:1145–1152. [DOI] [PubMed] [Google Scholar]
- 38. Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43:921–927. [DOI] [PubMed] [Google Scholar]
- 39. Stephen JM, Dodds AL, Lumpaopong P, Kader D, Williams A, Amis AA. The ability of medial patellofemoral ligament reconstruction to correct patellar kinematics and contact mechanics in the presence of a lateralized tibial tubercle. Am J Sports Med. 2015;43:2198–2207. [DOI] [PubMed] [Google Scholar]
- 40. Stephen JM, Kaider D, Lumpaopong P, Deehan DJ, Amis AA. The effect of femoral tunnel position and graft tension on patellar contact mechanics and kinematics after medial patellofemoral ligament reconstruction. Am J Sports Med. 2014;42:364–372. [DOI] [PubMed] [Google Scholar]
- 41. Stephen JM, Kittl C, Williams A, et al. Effect of medial patellofemoral ligament reconstruction method on patellofemoral contact pressures and kinematics. Am J Sports Med. 2016;44:1186–1194. [DOI] [PubMed] [Google Scholar]
- 42. Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40:1871–1879. [DOI] [PubMed] [Google Scholar]
- 43. Tanaka MJ, Bollier MJ, Andrish JT, Fulkerson JP, Cosgarea AJ. Complications of medial patellofemoral ligament reconstruction: common technical errors and factors for success: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94:e87. [DOI] [PubMed] [Google Scholar]
- 44. Williams AA, Elias JJ, Tanaka MJ, et al. The relationship between tibial tuberosity-trochlear groove distance and abnormal patellar tracking in patients with unilateral patellar instability. Arthroscopy. 2016;32:55–61. [DOI] [PubMed] [Google Scholar]
- 45. Zhang L-Q, Wang G, Nuber GW, Press JM, Koh JL. In vivo load sharing among the quadriceps components. J Orthop Res. 2003;21:565–571. [DOI] [PubMed] [Google Scholar]