Abstract
Objective
Historically the rates of postpartum glucose tolerance testing for women with gestational diabetes (GDM) average a suboptimal 33%. Barriers include the need for new mothers to miss work and/or arrange for childcare in order to engage in a two-hour test at a commercial lab. This pilot study was initiated to test the theory that a home testing regimen would be accepted by patients and increase the rate of postpartum glucose assessments relative to published rates, without requiring additional health-care staff or resources to achieve this goal.
Study design
Six weeks postpartum, women with GDM from an academic private practice were asked to check fingerstick blood glucose (FAST Protocol) four times a day for two days, and then obtain an oral glucose tolerance test (OGTT). The physician consultants saw the women each month during pregnancy and arranged the postpartum testing.
Results
Two of 69 refused to be consented. Twelve of the remaining 67(18%) women completed both the FAST regimen and the OGTT, three completed only the OGTT and five completed only the FAST regimen for a final follow-up rate of 20/67 (30%). The demands of caring for a newborn, or the annoyance of fingersticks, were barriers to compliance.
Conclusions
In spite of intense physician involvement, this home testing regimen was not associated with an increase in the rates of women participating in postpartum glucose assessments.
Keywords: diabetes, general medicine, high-risk pregnancy
Introduction
After a pregnancy complicated by gestational diabetes (GDM), 20–30% of women have impaired glucose tolerance or persistent diabetes mellitus (DM), but the number of women returning for recommended testing is suboptimal, averaging 33% (range 9–95%).1–26
The incidence of GDM has doubled meaning more women are in need of testing.6,27–30 Direct feedback from our patients and a focus group established that busy new mothers, many of whom return to the workforce, find the standard two-hour-75-gram oral glucose tolerance test (OGTT) inconvenient.31 The American Diabetes Association, in the spirit of capturing those at risk, promoted the fasting plasma glucose (FPG), and the American College of Obstetrics and Gynecology (ACOG) also recommends an OGTT or a FPG.1,32 However, while FPG requires less of a time commitment, the sensitivity may be inadequate.13,26,33,34 The Agency for Healthcare Research and Quality (AHRQ) has defined diagnosis of DM after a history of GDM as a future research need for the management of GDM.35,36 In that context, and because women with GDM possess a glucose monitor that they used for at least the last 12 weeks of their pregnancy, we hypothesized that among women educated and familiar with home glucose monitoring, a postpartum home testing regimen consisting of eight home fingerstick blood glucose values (fasting, and 2 hours after each meal), taken over two days, eight-weeks postpartum (the Fingerstick Assessment of Sugars Two-months postpartum or FAST Protocol) would be accepted as convenient. The primary objective of this pilot study was to determine if the FAST protocol could increase the rate of postpartum glucose testing in this cohort, compared with historical controls.
Materials and methods
Population and standard treatment
This was a cohort study of consecutive English speaking women >18 years of age with GDM who were recruited at antepartum visits during 2007, or during an inpatient consult immediately after delivery from a private Obstetric Medicine Practice at St Peter's University Hospital in New Brunswick, NJ, USA. All women in the cohort had private insurance. The low overall testing rate at 12 months was interpreted as an indication that devoting additional time to patient recruitment and tracking outside of routine clinical care was not likely to yield meaningful results. That and the loss of a physician to a different practice were the reasons why recruitment was ended after one year.
Once diagnosed with GDM, patients received standard counselling about diet and monitoring of fingerstick sugars at home. ACOG guidelines call for glucose monitoring either one hour (goal <130–140 mg/dL) (Box 1) or two hours (goal <120 mg/dL) after meals. Based on the evidence that, relative to preprandial monitoring, postprandial monitoring was associated with lower rates of neonatal hypoglycemia, large for gestational age infants and caesarean delivery because of cephalopelvic disproportion, in our centres we monitor sugars four times a day: fasting, and two hours after each meal.37,38 If, despite adherence to the appropriate diet, fasting bloods sugar were consistently and significantly >95 mg/dL or postprandial blood sugar >120 mg/dL, then pharmacological therapy was recommended with either glyburide or insulin. Blood sugars were faxed to our office each week, reviewed by a physician, feedback regarding the values was provided and patients were scheduled to be seen in the office by a physician at least once every four weeks until delivery. Demographic information was collected, including self-reported race category.
Box 1.
Convert mg/dL to mmol/L.
| 120 mg/dL = 6.7 mmol/L |
| 130–140 mg/dL = 7.2–7.8 mmol/L |
| mg/dL × 0.0555 = mmol/L |
| mmol/L × 18.0182 = mg/dL |
Postpartum protocol
When contacted by the obstetrician, the treating physicians saw the women in the hospital after delivery, reviewed the protocol, provided a prescription for an OGTT and provided an office appointment to review the results. They were instructed that six weeks postpartum they should check fingerstick blood glucose (FSBG) four times a day (fasting and 2 hours after meals) for two days (the FAST protocol), go for the OGTT a week later and follow up in the office at eight weeks to review the results. To improve compliance, they were provided with a sheet that listed the dates for the tests and office visit. The physicians reviewed the patient list and attempted to contact women whose due date had passed, or who had not followed up for the postpartum office visit to encourage them to complete the FAST protocol and obtain the postpartum OGTT. If contacted, patients were asked to provide a reason for non-adherence with the testing regimen. The FSBG values were reviewed at a follow-up visit as part of their routine care and no incentive was offered.
Endpoints
The primary endpoint was to increase the percentage of women in this cohort who had a postpartum glucose tolerance assessment above the historical average of 33%. The secondary endpoint was: if the percentage tested was higher than historical controls and those women complied with both tests (FAST and OGTT), the eight FAST glucose values would be analysed to determine cut-off point that could maximize the sensitivity of the new protocol to predict which women would have an abnormal OGTT.
Rationale for the FAST protocol
This was initiated within a private practice setting without additional staff or resources. To facilitate convenience and generalizability, women were not given a specific glucose load, so we hypothesized that (a) eight values could balance sensitivity versus specificity, and (b) two days would seem reasonable to patients who had checked sugars four times a day for 12 weeks, and therefore would promote compliance.
The effect of fragmented care was avoided as the treating physicians were responsible for arranging postpartum testing and seeing the patients to review the results. IRB approval was obtained. No funding was received. A specific rate of postpartum follow-up was not targeted as this was a pilot study. All assessments and follow up were conducted by the study physicians. Stata 9.0 (Statcorp, College Station, TX, USA) was used to calculate mean values with standard deviations; mean values were compared using t-test or Wilcoxon-Rank Sum for non-parametric data.
Results
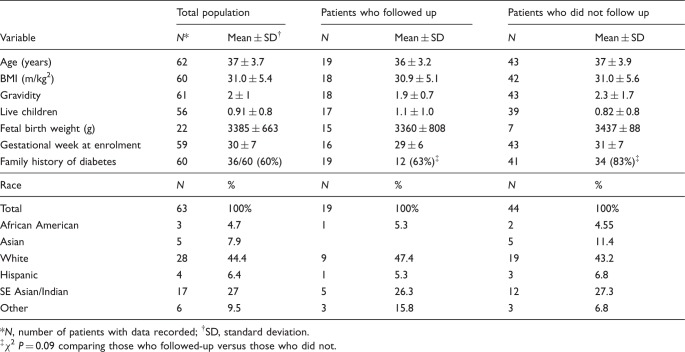
All women had private medical insurance: 69 were approached, two refused enrolment. Self-described race categories were available on 63/67 women, and they were diverse (Table 1). The mean gravidity was 2 (range 0–9) and the mean number of live children was 1 (range 0–3). Twenty (34%) of the women with a prior pregnancy had GDM, and 14/20 required insulin in the past. There were no significant differences in mean age, BMI or gestational age at enrolment.
Table 1.
Patient demographics.
| Total population |
Patients who followed up |
Patients who did not follow up |
||||
|---|---|---|---|---|---|---|
| Variable | N* | Mean ± SD† | N | Mean ± SD | N | Mean ± SD |
| Age (years) | 62 | 37 ± 3.7 | 19 | 36 ± 3.2 | 43 | 37 ± 3.9 |
| BMI (m/kg2) | 60 | 31.0 ± 5.4 | 18 | 30.9 ± 5.1 | 42 | 31.0 ± 5.6 |
| Gravidity | 61 | 2 ± 1 | 18 | 1.9 ± 0.7 | 43 | 2.3 ± 1.7 |
| Live children | 56 | 0.91 ± 0.8 | 17 | 1.1 ± 1.0 | 39 | 0.82 ± 0.8 |
| Fetal birth weight (g) | 22 | 3385 ± 663 | 15 | 3360 ± 808 | 7 | 3437 ± 88 |
| Gestational week at enrolment | 59 | 30 ± 7 | 16 | 29 ± 6 | 43 | 31 ± 7 |
| Family history of diabetes |
60 | 36/60 (60%) | 19 | 12 (63%)‡ | 41 | 34 (83%)‡ |
| Race |
N | % | N | % | N | % |
| Total | 63 | 100% | 19 | 100% | 44 | 100% |
| African American | 3 | 4.7 | 1 | 5.3 | 2 | 4.55 |
| Asian | 5 | 7.9 | 5 | 11.4 | ||
| White | 28 | 44.4 | 9 | 47.4 | 19 | 43.2 |
| Hispanic | 4 | 6.4 | 1 | 5.3 | 3 | 6.8 |
| SE Asian/Indian | 17 | 27 | 5 | 26.3 | 12 | 27.3 |
| Other | 6 | 9.5 | 3 | 15.8 | 3 | 6.8 |
*N, number of patients with data recorded; †SD, standard deviation.
‡ χ 2 P = 0.09 comparing those who followed-up versus those who did not.
In spite of monthly antepartum appointments and weekly reminder phone calls by the treating physicians, of the 67 participants only 20 (30%) had any kind of postpartum testing (FAST or OGTT). Twelve (18%) completed both an OGTT and the FAST protocol, five (7%) only the FAST protocol and three only the OGTT (Figure 1). The other women declined further testing or ignored attempts to arrange follow-up. The demands of caring for a newborn or the annoyances of fingersticks were cited by the women as deterrents to completing any kind of postpartum testing. None of the women contacted stated loss of health insurance, but not all non-compliant women were able to be contacted and others did not return messages.
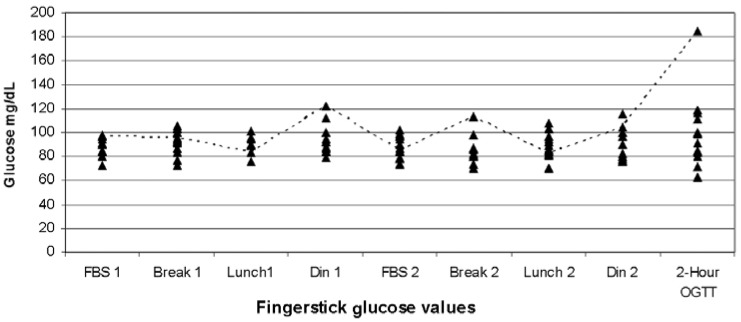
Figure 1.
Plot of 13 Patients who completed the FAST protocol and the oral glucose tolerance test. Patient 2 (dashed line) is highlighted as she was the only woman to have impaired glucose tolerance on the two-hour-75 gm OGTT (185 mg/dL), but her two fasting values and all postprandial values were normal. Fasting values for all patients (FBS1, FBS2) were ≤99 mg/dL. *FBS, fasting blood sugar. Break, breakfast. Din, dinner. OGTT, oral glucose tolerance test result. Breakfast, lunch and dinner values were taken two hours after meals.
The rate of testing for women treated with diet alone was 36% while it was 28% for those treated with a medication (insulin or glyburide). Parity did not appear to influence the follow-up rate as the mean number of live children at home was not different between those that followed up and those that did not (Table 1). More women who did not return for testing had a family history of diabetes than women who did return, but the difference was not significant, χ 2 P = 0.09 (Table 1).
From the FAST results, all fasting sugars were ≤99 mg/dL and among the 12 women who completed both the FAST protocol and the OGTT, there were six with a FAST value ≥104 mg/dL (Figure 1). Five of those (patients 1, 3, 5, 8 and 12) had only a single value ≥104 mg/dL and all had a normal OGTT; Patient 2 had three values ≥104 mg/dL with impaired glucose tolerance. The postprandial blood sugars ranged from 70 to 185 mg/dL. If the definition of an ‘abnormal’ FAST protocol was set as three values ≥104 mg/dL, compared with the OGTT, the specificity is 100% (95% confidence interval [C.I.] 72–100%). However, the sensitivity is 100%, with an impractically large 95% CI of 2.5–100% indicating that the sample size is too small to allow conclusions about the clinical utility of this protocol.
Conclusions
In spite of avoiding fragmentation of care during the ante- and postpartum periods the FAST home-testing protocol, compared with historical rates, did not facilitate an increase in the rate of postpartum glucose assessments within this GDM cohort. Five women who did not go for an OGTT followed the FAST protocol (7.4% absolute increase in postpartum glucose assessments, relative increase of 33%). However, this increase is tempered by the fact that the total rate was similar to historical controls. Additionally, the analysis of those who went for both tests yielded a wide confidence interval regarding the sensitivity. The FBG was <99 mg/dL in the one woman with impaired glucose tolerance.
Prior studies have found that use of insulin was associated with higher rates of postpartum glucose intolerance and compliance with screening rates, but the use of medication was not associated with a different rate of testing in this study.39 Although not statically significant, compared with those who returned for testing, more of those who did not return had a family history of diabetes. This is consistent with findings from a focus group that identified feelings of emotional stress due to adjusting to a new baby, and the fear of receiving a diabetes diagnosis at the visit as barriers to attending follow-up appointments.31 A number of articles and reviews have listed conflicting findings regarding specific patient demographics and associations with postpartum testing rates, such as Hispanic women being tested 2% more or 10% less than White women. We think broader system-based changes are needed to improve testing, subsequently we did not analyse every patient demographic for an association with testing.2,39
Post hoc, it is possible that the request to obtain eight home glucose values, as opposed to a lower number, was a reason for non-compliance. However, the objective was to evaluate if two more days would be perceived as a significant burden after 12 weeks of monitoring. More data points would provide additional power for a potential sensitivity/specificity analysis. Criticizing eight finger-stick values as being an insensitive diagnostic tool only leaves one with a single value FBG, a test reported to have a wide range of sensitivity or the OGTT, a test with a low-compliance rate.
The request to obtain two postpartum tests could have been an impediment, but because only five women completed the FAST protocol and not the OGTT, it appears that the need to go to a lab was not the primary barrier for the 47 women who did not complete any kind of postpartum testing. This is contrary to our working hypothesis. Additionally, direct postpartum phone calls did not improve the rate, and treatment with medication was not associated with a differential response further suggesting that those 47 were not motivated regardless of where it was done. Theoretically, extending the time for postpartum testing beyond 6–12 weeks could improve testing rates, but interestingly our literature review determined this is not the case. Retrospective studies reporting historical testing rates at 6-months (36%) and 1-year (33%) were the same as those reporting short-term follow-up (35%).14,40,41 Prospective studies allocating staff to patient contact systems, a resource we did not have, increased the testing rates to an average of 60% in all time frames.2,42,43 However, based on a prospective randomized trial, these rates could be due to the timeframe in which patient contact efforts are focused. This Canadian project demonstrated that sending reminder letters to the patient and/or their primary care physicians up to one year after delivery increased the testing rate from 14% (no letter sent) up to 60% (letter sent to patient and physician).25
Any new protocol effective at improving testing rates would have to be considered convenient, but would not require perfect sensitivity compared with the OGTT. If a new postpartum regimen was only 90% sensitive, but women assigned to it had a follow-up rate 50% higher than the OGTT group, the higher compliance would facilitate detection of a greater number of women with impaired glucose tolerance or DM.
In conclusion, this home testing regimen, while in line with the AHRQ future research need for GDM, was not associated with an overall postpartum testing rate higher than historical controls. A few more women completed the home testing portion suggesting that future efforts utilizing home testing could bypass some barriers, but not all. The prevalence of obesity, DM and GDM are increasing, therefore continued innovation is required to address the many barriers to postpartum testing and develop new protocols that will promote higher testing rates and identification of women with pre- or overt diabetes.
Declaration of conflicting interests
None.
Funding
None.
Ethical approval
The Institutional Review Board of Saint Peters University Hospital, New Brunswick, NJ, USA. All patients provided written informed consent.
Guarantor
MPC.
Contributorship
MPC was responsible for study design, ethical approval, recruitment, interpretation of data and authorship of the first draft. BGL participated in study design, recruitment and authorship. ERP and ME were responsible for recruitment and authorship. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
References
- 1. ACOG Committee Opinion No. 435: postpartum screening for abnormal glucose tolerance in women who had gestational diabetes mellitus. Obstet Gynecol 2009; 113: 1419–21. [DOI] [PubMed] [Google Scholar]
- 2. Ferrara A, Peng T, Kim C. Trends in postpartum diabetes screening and subsequent diabetes and impaired fasting glucose among women with histories of gestational diabetes mellitus: a report from the Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care 2009; 32: 269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stasenko M, Liddell J, Cheng YW, Sparks TN, Killion M, Caughey AB. Patient counseling increases postpartum follow-up in women with gestational diabetes mellitus. Am J Obstet Gynecol 2011; 204: 522 e521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hunt KJ, Conway DL. Who returns for postpartum glucose screening following gestational diabetes mellitus? Am J Obstet Gynecol 2008; 198: 404 e401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dietz PM, Vesco KK, Callaghan WM, et al. Postpartum screening for diabetes after a gestational diabetes mellitus-affected pregnancy. Obstet Gynecol 2008; 112: 868–74. [DOI] [PubMed] [Google Scholar]
- 6. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational diabetes. Obstet Gynecol 2001; 98: 525–38. [PubMed] [Google Scholar]
- 7. Russell MA, Phipps MG, Olson CL, Welch HG, Carpenter MW. Rates of postpartum glucose testing after gestational diabetes mellitus. Obstet Gynecol 2006; 108: 1456–62. [DOI] [PubMed] [Google Scholar]
- 8. Smirnakis KV, Chasan-Taber L, Wolf M, Markenson G, Ecker JL, Thadhani R. Postpartum diabetes screening in women with a history of gestational diabetes. Obstet Gynecol 2005; 106: 1297–303. [DOI] [PubMed] [Google Scholar]
- 9. Kwong S, Mitchell RS, Senior PA, Chik CL. Postpartum diabetes screening: adherence rate and the performance of fasting plasma glucose versus oral glucose tolerance test. Diabetes Care 2009; 32: 2242–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanir HM, Sener T, Gurer H, Kaya M. A ten-year gestational diabetes mellitus cohort at a university clinic of the mid-Anatolian region of Turkey. Clin Exp Obstet Gynecol 2005; 32: 241–4. [PubMed] [Google Scholar]
- 11. Blatt AJ, Nakamoto JM, Kaufman HW. Gaps in diabetes screening during pregnancy and postpartum. Obstet Gynecol 2011; 117: 61–8. [DOI] [PubMed] [Google Scholar]
- 12. Lawrence JM, Black MH, Hsu JW, Chen W, Sacks DA. Prevalence and timing of postpartum glucose testing and sustained glucose dysregulation after gestational diabetes mellitus. Diabetes Care 2010; 33: 569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reinblatt SL, Morin L, Meltzer SJ. The importance of a postpartum 75 g oral glucose tolerance test in women with gestational diabetes. J Obstet Gynaecol Can 2006; 28: 690–4. [DOI] [PubMed] [Google Scholar]
- 14. Stasenko M, Cheng YW, McLean T, Jelin AC, Rand L, Caughey AB. Postpartum follow-up for women with gestational diabetes mellitus. Am J Perinatol 2010; 27: 737–42. [DOI] [PubMed] [Google Scholar]
- 15. Clark HD, van Walraven C, Code C, Karovitch A, Keely E. Did publication of a clinical practice guideline recommendation to screen for type 2 diabetes in women with gestational diabetes change practice? Diabetes Care 2003; 26: 265–8. [DOI] [PubMed] [Google Scholar]
- 16. Kim C, Herman WH, Vijan S. Efficacy and cost of postpartum screening strategies for diabetes among women with histories of gestational diabetes mellitus. Diabetes Care 2007; 30: 1102–6. [DOI] [PubMed] [Google Scholar]
- 17. Beischer NA, Wein P, Sheedy MT. A follow-up program for women with previous gestational diabetes mellitus. Med J Aust 1997; 166: 353–7. [DOI] [PubMed] [Google Scholar]
- 18. Albareda M, Caballero A, Badell G, et al. Diabetes and abnormal glucose tolerance in women with previous gestational diabetes. Diabetes Care 2003; 26: 1199–205. [DOI] [PubMed] [Google Scholar]
- 19. Dacus JV, Meyer NL, Muram D, Stilson R, Phipps P, Sibai BM. Gestational diabetes: postpartum glucose tolerance testing. Am J Obstet Gynecol 1994; 171: 927–31. [DOI] [PubMed] [Google Scholar]
- 20. Jang HC, Yim CH, Han KO, et al. Gestational diabetes mellitus in Korea: prevalence and prediction of glucose intolerance at early postpartum. Diabetes Res Clin Pract 2003; 61: 117–24. [DOI] [PubMed] [Google Scholar]
- 21. Kjos SL, Buchanan TA, Greenspoon JS, Montoro M, Bernstein GS, Mestman JH. Gestational diabetes mellitus: the prevalence of glucose intolerance and diabetes mellitus in the first two months post partum. Am J Obstet Gynecol 1990; 163(Part 1): 93–8. [DOI] [PubMed] [Google Scholar]
- 22. Ogonowski J, Miazgowski T. The prevalence of 6 weeks postpartum abnormal glucose tolerance in Caucasian women with gestational diabetes. Diabetes Res Clin Pract 2009; 84: 239–44. [DOI] [PubMed] [Google Scholar]
- 23. Pallardo F, Herranz L, Garcia-Ingelmo T, et al. Early postpartum metabolic assessment in women with prior gestational diabetes. Diabetes Care 1999; 22: 1053–8. [DOI] [PubMed] [Google Scholar]
- 24. Schaefer-Graf UM, Buchanan TA, Xiang AH, Peters RK, Kjos SL. Clinical predictors for a high risk for the development of diabetes mellitus in the early puerperium in women with recent gestational diabetes mellitus. Am J Obstet Gynecol 2002; 186: 751–6. [DOI] [PubMed] [Google Scholar]
- 25. Clark HD, Graham ID, Karovitch A, Keely EJ. Do postal reminders increase postpartum screening of diabetes mellitus in women with gestational diabetes mellitus? A randomized controlled trial. Am J Obstet Gynecol 2009; 200: 634 e631–7. [DOI] [PubMed] [Google Scholar]
- 26. Cypryk K, Czupryniak L, Wilczynski J, Lewinski A. Diabetes screening after gestational diabetes mellitus: poor performance of fasting plasma glucose. Acta Diabetol 2004; 41: 5–8. [DOI] [PubMed] [Google Scholar]
- 27. Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care 2005; 28: 579–84. [DOI] [PubMed] [Google Scholar]
- 28. Murphy NJ, Bulkow LR, Schraer CD, Lanier AP. Prevalence of diabetes mellitus in pregnancy among Yup'ik Eskimos, 1987–1988. Diabetes Care 1993; 16: 315–7. [DOI] [PubMed] [Google Scholar]
- 29. Amankwah KS, Prentice RL, Fleury FJ. The incidence of gestational diabetes. Obstet Gynecol 1977; 49: 497–8. [PubMed] [Google Scholar]
- 30. Standards of medical care in diabetes – 2008. (POSITION STATEMENT). Diabetes Care 2008; 31: S12–54. [DOI] [PubMed] [Google Scholar]
- 31. Bennett WL, Ennen CS, Carrese JA, et al. Barriers to and facilitators of postpartum follow-up care in women with recent gestational diabetes mellitus: a qualitative study. J Womens Health (Larchmt) 2011; 20: 239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997; 20: 1183–97. [DOI] [PubMed] [Google Scholar]
- 33. Costa A, Carmona F, Martinez-Roman S, Quinto L, Levy I, Conget I. Post-partum reclassification of glucose tolerance in women previously diagnosed with gestational diabetes mellitus. Diabet Med 2000; 17: 595–8. [DOI] [PubMed] [Google Scholar]
- 34. Avignon A, Radauceanu A, Monnier L. Nonfasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes. Diabetes Care 1997; 20: 1822–6. [DOI] [PubMed] [Google Scholar]
- 35. Tan HH, Tan HK, Lim HS, Tan AS, Lim SC. Gestational diabetes mellitus: a call for systematic tracing. Ann Acad Med Singapore 2002; 31: 281–4. [PubMed] [Google Scholar]
- 36. Bennett WL, Nicholson WK, Saldanha IJ, Wilson LM, McKoy NA, Robinson KA. Future Research Needs for the Management of Gestational Diabetes SourceRockville (MD): Agency for Healthcare Research and Quality (US); Report No.: 11-EHC005-EF. AHRQ Comparative Effectiveness Reviews. Seehttp://www.ncbi.nlm.nih.gov/books/NBK51282(last checked 27 February 2013). [PubMed] [Google Scholar]
- 37. de Veciana M, Major CA, Morgan MA, et al. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med 1995; 333: 1237–41. [DOI] [PubMed] [Google Scholar]
- 38. Gestational diabetes mellitus. Diabetes Care 2004; 27(Suppl. 1): S88–90. [DOI] [PubMed] [Google Scholar]
- 39. Tovar A, Chasan-Taber L, Eggleston E, Oken E. Postpartum screening for diabetes among women with a history of gestational diabetes mellitus. Prev Chronic Dis 2011; 8: A124–A124. [PMC free article] [PubMed] [Google Scholar]
- 40. Agarwal MM, Punnose J, Dhatt GS. Gestational diabetes: implications of variation in post-partum follow-up criteria. Eur J Obstet Gynecol Reprod Biol 2004; 113: 149–53. [DOI] [PubMed] [Google Scholar]
- 41. Picon MJ, Murri M, Munoz A, Fernandez-Garcia JC, Gomez-Huelgas R, Tinahones FJ. Hemoglobin A1c versus oral glucose tolerance test in postpartum diabetes screening. Diabetes Care 2012; 35(8): 1648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu SH, Park S, Kim HS, et al. The prevalence of GAD antibodies in Korean women with gestational diabetes mellitus and their clinical characteristics during and after pregnancy. Diabetes Metab Res Rev 2009; 25: 329–34. [DOI] [PubMed] [Google Scholar]
- 43. Shea AK, Shah BR, Clark HD, et al. The effectiveness of implementing a reminder system into routine clinical practice: does it increase postpartum screening in women with gestational diabetes? Chronic Dis Can 2011; 31: 58–64. [PubMed] [Google Scholar]