Abstract
Background:
Continuous subcutaneous insulin infusion (CSII) treatment with a tubeless patch pump has not been previously evaluated in a large cohort of patients.
Methods:
This multisite, retrospective study evaluated glycemic control in patients with type 1 diabetes (n = 873) after 3 months treatment the Omnipod® insulin management system (Insulet Corporation, Billerica, MA) compared to prior treatment with multiple daily injections (MDI) (78.1%) or CSII (21.9%). The primary outcome was change in HbA1c from baseline at 3 months post–Omnipod treatment initiation. Secondary outcomes included shifts in HbA1c to target levels, change in total daily dose (TDD) of insulin and in the frequency and severity of hypoglycemic episodes.
Results:
HbA1c was significantly improved at 3 months post–Omnipod treatment for the total population (mean ± SD): −0.6% ± 1.3 (P < .001). HbA1c was also significantly lower compared to MDI: −0.3% ± 1.3, −0.4% ± 1.4, −0.8% ± 1.3 and −0.6% ± 1.3 (P = .002 to P < .001) and CSII: −0.3% ± 0.8, −1.1% ± 1.6 (P < .01), −0.4% ± 1.1 (P < .001), and −0.5% ± 1.1 (P < .001) for pediatric, adolescent, adult, and total populations, respectively. There was a 37.9% change increase in the proportion of patients ≥18 years and a 39.3% change increase in those <18 years achieving ADA treatment targets (P = .004 to P < .001). There was a 16.4% change decrease in TDD of insulin at 3 months for the total population (P < .001). The frequency of self-reported hypoglycemia decreased significantly (P < .001) by 1.0 ± 2.4 episodes per week.
Conclusions:
Treatment with the Omnipod insulin management system was associated with clinically meaningful and statistically significant improvement in glycemic control, reduction in daily insulin requirement and reduction in the frequency and severity of hypoglycemic episodes.
Keywords: CSII, MDI, patch pump, insulin, type 1 diabetes, HbA1c
Treatment with continuous subcutaneous insulin infusion (CSII) in patients with type 1 diabetes has been demonstrated to improve glycemic control,1-3 with less severe hypoglycemia,2 reduced glycemic variability,4 and improved quality of life5 compared with treatment with multiple daily insulin injections (MDI). However, the prevalence of CSII use is only estimated at up to 40% in the United States6 with a recent survey from the T1D Exchange Registry reporting approximately 60% CSII use in patients at leading treatment centers.7
Despite the clinical benefits of CSII, many patients with type 1 diabetes are reluctant to transition to or continue CSII therapy. Some data suggest that “not wanting to have the constant presence of a catheter” and “tubing interferes with activities” are significant barriers for using CSII.8,9 The Omnipod® insulin management system (Insulet Corporation, Billerica, MA) is a tubeless patch pump that offers the potential to address barriers to traditional CSII therapy.
Initial studies have demonstrated improved glycemic control and high user satisfaction among small cohorts of patients with type 1 diabetes treated with the Omnipod system.10,11 The present study evaluates glycemic outcomes in a large cohort of patients with type 1 diabetes previously treated with MDI or CSII therapy after initiating Omnipod therapy.
Methods
Design
This multicenter, retrospective study was conducted at 461 medical practices throughout the United States between October 2014 and May 2015. The study evaluated glycemic control and treatment preferences in patients with type 1 diabetes initiating Omnipod insulin management system therapy compared to prior treatment with MDI or CSII with tubed insulin pumps.
Participants
Patients with a type 1 diabetes diagnosis who were currently receiving treatment with either MDI or CSII with a tubed insulin pump and switched to the Omnipod system were eligible for inclusion in the study. Patients were categorized by age group as pediatric (0 to <13 years), adolescent (13 to <18 years) and adult (≥18 years). The protocol was granted human subjects research exemption by the Quorum Review Institutional Review Board (Seattle, WA) (QR#: 31034).
Outcome Measures
The primary end point was the change in mean HbA1c level from baseline on MDI or CSII with a tubed insulin pump (pre-Omnipod) at 3 months post–Omnipod treatment initiation. Secondary outcomes include shifts in HbA1c to target treatment levels, change from baseline in mean total daily dose (TDD) of insulin, frequency and severity of hypoglycemic episodes per week, defined as blood glucose <70 mg/dL, and patient preferences for treatment modality, also assessed at 3 months post–Omnipod treatment initiation.
Procedures
The Omnipod insulin management system consists of the Pod, a small, adhesive, waterproof (IPX8) insulin patch pump with automated cannula insertion and the personal diabetes manager (PDM) controller.12 The PDM is a wireless, hand-held device that programs the Pod with insulin-delivery instructions, controls insulin delivery and contains an integrated blood glucose meter.
Patients were fully trained in all aspects of the insulin delivery system by Insulet clinical service managers immediately prior to Omnipod treatment initiation. Demographic information, clinical characteristics and the most recent HbA1c levels prior to initiation of Omnipod therapy were recorded. Over the course of 3 months patients were seen by health care providers (HCPs) as needed according to the HCPs’ clinical judgment. At 3 months post–Omnipod initiation, the clinical service managers met with or called patients, caregivers and health care providers and/or reviewed medical charts to collect outcome measures.
Statistical Analysis
The changes in mean HbA1c level, TDD of insulin and frequency of hypoglycemic events from baseline at 3 months were evaluated by dependent T-test for repeated measures by prior treatment modality and for treatment modalities combined. Baseline HbA1c levels were stratified according to American Diabetes Association (ADA) treatment targets of <7%, and 7% to <8%, 8% to <9%, ≥9% for adults and for pediatric and adolescent patients <7.5%, 7.5% to <8.5%, >8.5%. Glycemic control was also assessed according to a Healthcare Effectiveness Data and Information Set (HEDIS) performance measurement criteria of HbA1c <8.0% (adequate control) and >9.0 (poor control) for all patients.13 The proportion of patients shifting between HbA1c categories at 3 months was evaluated by Wilcoxon signed-ranks test. Binary regression models were used to determine factors contributing to change in HbA1c level. Self-reported severity of hypoglycemic events using a 6-point Likert scale (0 = not serious to 5 = very severe) was assessed using the Wilcoxon signed-ranks test. Patient preferences for treatment modalities were summarized. All p values were 2-sided with statistical significance defined as P < .05. Data analysis was performed using SPSS Statistics V22.0 (IBM, Armonk, NY).
Results
A total of 873 patients with type 1 diabetes were included in the analysis: 682 (78.1%) were previously treated with MDI therapy and 191 (21.9%) were previously treated with CSII. The patient population by age category was: 204 (23.4%) pediatric, 110 (12.6%) adolescent and 559 (64.0%) adult. Baseline demographic and clinical characteristics are presented in Table 1.
Table 1.
Baseline Demographic and Clinical Characteristics.
| Pediatric (0 to <13 years), n = 204 | Adolescent (13 to <18 years), n = 110 | Adult (≥18 years), n = 559 | Total, n = 873 | |
|---|---|---|---|---|
| Age (years) | 8.9 ± 2.9 | 15.3 ± 1.4 | 39.0 ± 13.5 | 29.0 ± 17.4 |
| Female (%) | 52.5 | 41.8 | 55.8 | 53.2 |
| Duration of diabetes (years)a | 1.9 | 4.2 | 16.1 | 11.1 |
| (1.6-2.3) | (3.3-5.0) | (14.9-17.4) | (10.2-12.0) | |
| Prior treatment (%) | ||||
| MDI | 90.2 | 83.6 | 72.6 | 78.1 |
| CSII | 9.8 | 16.4 | 27.4 | 21.9 |
| HbA1c (%) | 8.1 ± 1.3 | 8.5 ± 2.0 | 8.4 ± 1.7 | 8.4 ± 1.6 |
| TDD of insulin, unitsb | 25.5 ± 18.0 | 52.8 ± 23.8 | 59.2 ± 33.4 | 50.6 ± 32.5 |
Values are mean ± SD unless otherwise indicated. CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injections; TDD, total daily dose.
Pediatric n = 176, adolescent n = 93, adult n = 443. bAdult n = 558.
Hemoglobin A1c
Clinically meaningful and statistically significant reductions in HbA1c from baseline were demonstrated at 3 months post–Omnipod treatment initiation for the total population: −0.6% ± 1.3 (P < .001). These improvements were seen across all age groups independent of prior treatment with MDI or CSII (P < .01 to P < .001) (Figure 1). The decrease in HbA1c at 3 months post–Omnipod treatment initiation for the total population of patients previously treated with MDI (mean ± SD) was −0.3% ± 1.3, −0.4% ± 1.4, −0.8% ± 1.3 and −0.6% ± 1.3 for pediatric, adolescent, adult and total, respectively (P = .002 to P < .001). The change in HbA1c was −0.3% ± 0.8, −1.1% ± 1.6 (P < .01), −0.4% ± 1.1 (P < .001), and −0.5% ± 1.1 (P < .001) for pediatric, adolescent, adult and total, respectively, for patients previously treated with CSII.
Figure 1.
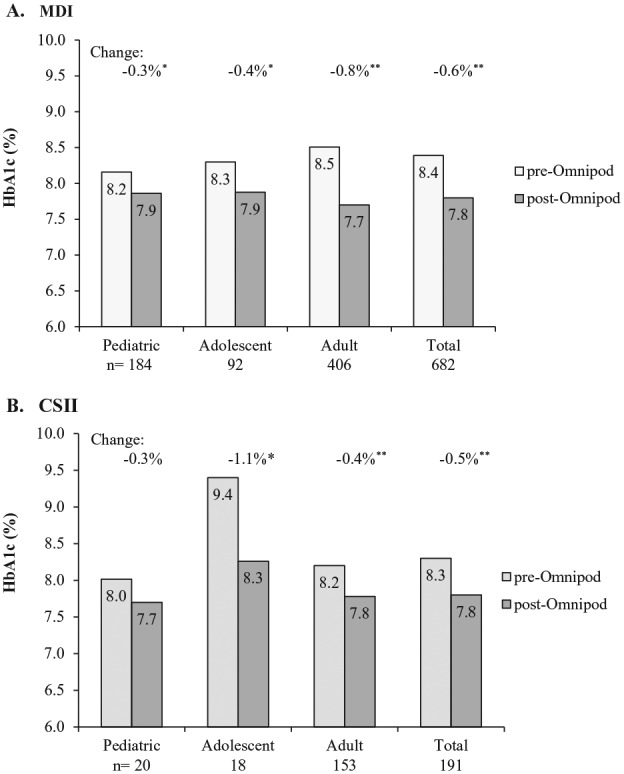
Change in HbA1c at 3 months post–Omnipod treatment initiation. (A) Change from previous treatment with multiple daily injections (MDI). HbA1c change (mean ± SD) was −0.3% ± 1.3, −0.4% ± 1.4, −0.8% ± 1.3 and −0.6% ± 1.3 for pediatric, adolescent, adult, and total, respectively. *P = .002. **P < .001. (B) Change from previous treatment with continuous subcutaneous insulin infusion (CSII). HbA1c change (mean ± SD) was −0.3% ± 0.8, −1.1% ± 1.6, −0.4% ± 1.1, and −0.5% ± 1.1 for pediatric, adolescent, adult, and total, respectively. *P < .01. **P < .001.
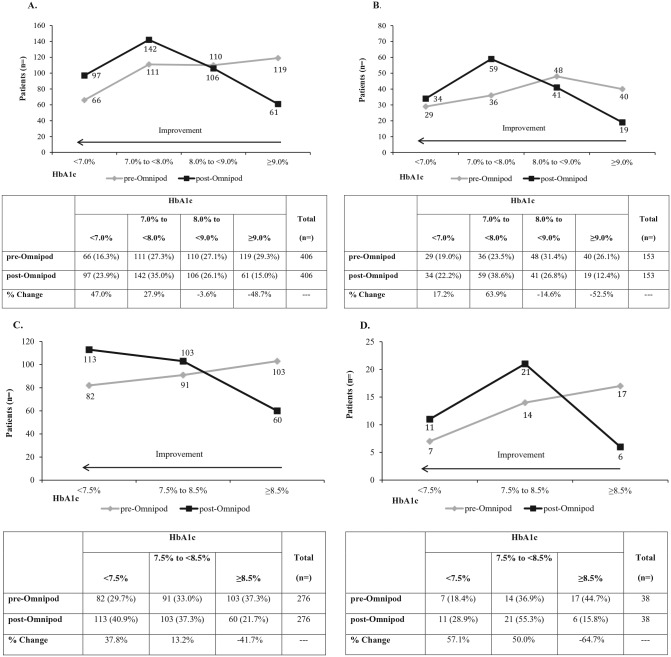
Overall, there were also clinically meaningful and statistically significant (P = .004 to P < .001) shifts in HbA1c levels of poor control to better control (Figure 2). There was a 37.9% change increase in the proportion of patients ≥18 years and a 39.3% change increase in those <18 years achieving ADA treatment targets. Significant (P < .001) shifts in HbA1c levels for all patients were also demonstrated at 3 months compared to prior treatment with MDI and CSII according to a HEDIS performance measurement criteria (Figure 3).
Figure 2.
Shifts in HbA1c levels from baseline at 3 months post–Omnipod treatment initiation—ADA treatment targets. (A) American Diabetes Association (ADA) treatment target of HbA1c <7.0% for prior treatment with multiple daily injections (MDI) for adult patients ≥18 years. (B) ADA treatment target of HbA1c <7.0% for prior treatment with continuous subcutaneous insulin infusion (CSII) for adult patients ≥18 years. (C) ADA treatment target of HbA1c <7.5% for prior treatment with MDI for patients <18 years. (D) ADA treatment target of HbA1c <7.5% for prior treatment with CSII for patients <18 years. For all panels significant improvement was demonstrated for shifts in HbA1c level across categories: P < .001 for (A), (B), (C) and P < .004 for (D). The HbA1c percentage change values at 3 months post–Omnipod treatment is presented in the tables. Percentages do not total 100 as some patients did not shift HbA1c categories.
Figure 3.
Shifts in Hba1c levels from baseline at 3 months post–Omnipod treatment initiation—HEDIS performance measurement criteria. (A) Healthcare Effectiveness Data and Information Set (HEDIS) performance measurement criteria of HbA1c <8.0% (adequate control) and >9.0 (poor control) for all patients previously treated with multiple daily injections (MDI); P < .001. (B) HEDIS performance measurement criteria for all patients previously treated with continuous subcutaneous insulin infusion (CSII); P < .001.
The HbA1c level pre-Omnipod treatment was a significant predictor of the reduction in HbA1c post–Omnipod treatment. For each HbA1c percentage point higher at baseline the odds of having a post–Omnipod reduction in HbA1c increased 2.7 times.
Total Daily Dose of Insulin
A significant reduction in TDD of insulin was demonstrated at 3 months post–Omnipod treatment initiation compared to prior treatment with MDI and CSII for the adult and total population (range −4.8 to −12.3 Units, P < .02 to P < .001) (Figure 4). A 16.4% change reduction in TDD of insulin was demonstrated at 3 months post–Omnipod treatment initiation for the population overall (P < .001).
Figure 4.
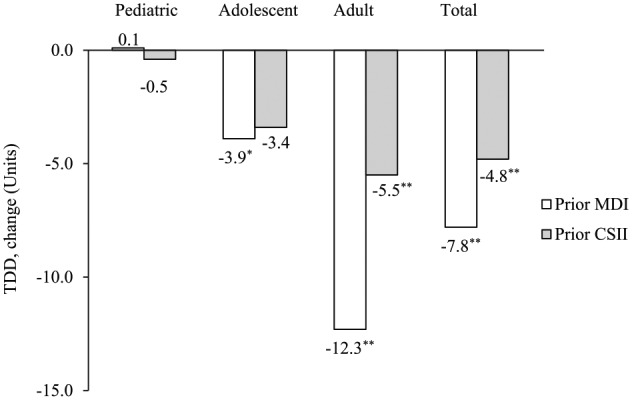
Change in TDD of insulin from prior treatment with MDI or CSII at 3 months post–Omnipod treatment initiation. CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injections; TDD, total daily dose. *P < .02. ** P < .001.
Hypoglycemic Episodes
The frequency of self-reported hypoglycemia decreased significantly (P < .001) by 1.0 ± 2.4 episodes per week, from 2.6 ± 2.8 to 1.6 ± 1.6, post–Omnipod treatment initiation for the total population. Self-reported severity of hypoglycemic episodes decreased from 2.1 ± 1.4 to 1.6 ± 1.2 (P < .001). Improvements in hypoglycemia frequency and severity were significant across all age groups independent of prior treatment modality (all P < .001).
Treatment Preference
The main reason for initiating Omnipod therapy was “did not want to be tethered to tubing”, which was reported by 73.8% and 86.4% of prior MDI and CSII treatment groups, respectively. The treatment preference with the second greatest frequency was “better glycemic control” reported by 62.8% and 24.1% of prior MDI and CSII treatment groups, respectively.
Discussion
This multiple-site, retrospective study demonstrated clinically meaningful and statistically significant improvement in HbA1c, a reduction in the TDD of insulin and a reduction in the frequency and severity of self-reported hypoglycemic events in patients treated with the Omnipod insulin management system after prior treatment with MDI or CSII. Patients reported that the tubeless feature of the pump was a significant consideration of treatment choice. This is the first report of glycemic control among a large cohort of patients treated with the Omnipod insulin management system.
The clinical benefits demonstrated in the present study are comparable to those reported in recent randomized, controlled trials and retrospective studies, which demonstrated improved glycemic control1-3,14 with less severe hypoglycemia2 in patients with type 1 diabetes treated with CSII compared with MDI treatment.
The significant shift in the proportion of patients meeting the ADA treatment targets of an HbA1c <7.0% for adults and <7.5% for pediatric and adolescent patients, independent of previous treatment modality, is an important finding of the present study. The proportion of adult patients who achieved an HbA1c <7.0% (MDI, 23.9%; CSII, 22.2%) is similar to that of patients enrolled in the T1D Exchange Registry (23.1%).7 However, the proportion of pediatric patients in the present study who achieved an ADA treatment target, approximately 40% overall, is higher than the approximately 17% reported for the T1D Exchange Registry. In addition, the significant increase in the number of patients who achieved a HEDIS performance measurement criteria of good HbA1c control13 is a relevant finding from a quality of care perspective.
The results of the present study also indicate that patients with poor HbA1c control, independent of prior treatment modality, exhibited the greatest improvement in HbA1c level after initiating Omnipod treatment. This suggests that poor glycemic control may be an important consideration for patients to initiate CSII versus attempting to achieve better control on MDI. Patient treatment preferences are also an important consideration.
In addition, an interesting finding was the difference reported by prior treatment groups regarding their main reasons for selecting Omnipod treatment. While better glycemic control was indicated by more than 60% of prior MDI patients compared to approximately 25% of prior CSII patients, the patch pump feature was the primary selection criterion in both groups.
A key strength of the present study is assessment of glycemic control in a large cohort of nearly 900 patients treated with the Omnipod system in the real-world, clinical setting. However, several limitations are noteworthy. First, due to the retrospective study design, there is the potential for selection bias as it was not feasible to assess all patients who had initiated Omnipod therapy during the study period. Self-reported data for some outcome measures is also a limitation that can be addressed in prospective studies. In addition, it was not possible to collect glucose data to quantify hypoglycemia, or detailed information regarding insulin regimens, including changes in basal and bolus insulin dosages or frequency of bolusing, due to logistical limitations. Finally, a longer study period should be a focus of future research to confirm the durability of the observed HbA1c improvements. This will likely provide greater insights regarding the specific impact of Omnipod use independent of other factors that may have influenced patient behaviors and outcomes, for example, remedial education that occurs when patients switch treatment modalities.
Conclusions
This large, multisite, retrospective study demonstrated that treatment with the Omnipod insulin management system in patients with type 1 diabetes was associated with clinically meaningful and statistically significant improvements in glycemic control, a reduction in daily insulin requirement and a reduction in the frequency and severity of hypoglycemic episodes at 3 months compared to prior treatment with MDI or CSII.
Acknowledgments
The authors thank Dr. Jack Gallagher and the analytical team of Clarity Pharma Research LLC for performing the statistical analysis.
Footnotes
Abbreviations: ADA, American Diabetes Association; CSII, continuous subcutaneous insulin infusion; HbA1c, glycosylated hemoglobin A1c; HCP, health care provider; HEDIS, Healthcare Effectiveness Data and Information Set; MDI, multiple daily insulin injections; PDM, personal diabetes manager; TDD, total daily dose.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JEL and HZ are employees of Insulet Corporation. CGP has received consulting fees from Dexcom, Inc, Insulet Corporation, Roche Diabetes Care, and Sanofi.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this study was provided by Insulet Corporation.
References
- 1. Diabetes Control and Complications Trial Study Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [DOI] [PubMed] [Google Scholar]
- 2. Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med. 2008;25:765-774. [DOI] [PubMed] [Google Scholar]
- 3. Jeitler K, Horvath K, Berghold A, et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia. 2008;51:941-951. [DOI] [PubMed] [Google Scholar]
- 4. Pickup JC, Kidd J, Burmiston S, Yemane N. Determinants of glycaemic control in type 1 diabetes during intensified therapy with multiple daily insulin injections or continuous subcutaneous insulin infusion: importance of blood glucose variability. Diabetes Metab Res Rev. 2006;22:232-237. [DOI] [PubMed] [Google Scholar]
- 5. Ruiz-de-Adana MS, Dominguez-Lopez ME, Gonzalez-Molero I, Soriguer F, Anarte MT, Rojo-Martinez G. Comparison between a multiple daily insulin injection regimen (basal once-daily glargine plus mealtime lispro) and continuous subcutaneous insulin infusion (lispro) using continuous glucose monitoring in metabolically optimized type 1 diabetes patients: a randomized open-labelled parallel study. Med Clin (Barc). 2015;146:239-246. [DOI] [PubMed] [Google Scholar]
- 6. Pickup J. Insulin pumps. Int J Clin Pract Suppl. 2011:16-19. [DOI] [PubMed] [Google Scholar]
- 7. Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38:971-978. [DOI] [PubMed] [Google Scholar]
- 8. Seereiner S, Neeser K, Weber C, et al. Attitudes towards insulin pump therapy among adolescents and young people. Diabetes Technol Ther. 2010;12:89-94. [DOI] [PubMed] [Google Scholar]
- 9. Kraus A, Runion A, Perez-Nieves M, Jian D, Schuster D, Curtis BH. Insulin pumps: the patient perspective. Poster presented at: American Diabetes Association; June 5-9, 2015 Boston, MA. [Google Scholar]
- 10. Zisser H, Jovanovic L. OmniPod Insulin Management System: patient perceptions, preference, and glycemic control. Diabetes Care. 2006;29:2175. [DOI] [PubMed] [Google Scholar]
- 11. Lebenthal Y, Lazar L, Benzaquen H, Shalitin S, Phillip M. Patient perceptions of using the OmniPod system compared with conventional insulin pumps in young adults with type 1 diabetes. Diabetes Technol Ther. 2012;14:411-417. [DOI] [PubMed] [Google Scholar]
- 12. User guide: OmniPod Insulin Management System. Insulet Corporation. 2015. [Google Scholar]
- 13. Centers for Medicare and Medicaid Services. Health insurance marketplace. Quality rating system measure technical specifications. September 2014. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityInitiativesGenInfo/Downloads/2015-QRS-Measure-Technical-Specifications.pdf. Accessed December 18, 2015.
- 14. Misso ML, Egberts KJ, Page M, O’Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010:CD005103. [DOI] [PMC free article] [PubMed] [Google Scholar]