Abstract
Australasia is a region with a high incidence of type 1 diabetes (T1D). There are approximately 140 000 individuals with T1D, and of these 10 000 are children. Although the region covers a huge geographical area, most children with T1D are managed by tertiary academic centers in the major capital cities. Local longitudinal data collection has been in place for several decades in most of these centers, however ongoing national data collection had not been attempted. In 2012, with funding from the Juvenile Diabetes Research Foundation (JDRF) Australian Type 1 Clinical Research Network, a national collaboration was formed to provide ongoing longitudinal collection of T1D patient characteristics and outcomes. The initial phase of this collaboration, known as the Australasian Diabetes Data Network or ADDN, was led by the Australasian Paediatric Endocrine Group (APEG) and thus included only children and adolescents. The next phase, commenced in 2016, will see adult sites added through collaboration with the Australian Diabetes Society (ADS). As most of the initial centers had longitudinal data collection in place the model employed was to establish the transfer and collation of data already collected into a central database. This required the definition of a common data dictionary, ethics and governance procedures and the employment of technology to enable efficient and accurate information transfer and accessibility. The ADDN project received widespread support from the diabetes research community with study investigators representing 20 pediatric centers across the region. The first phase focused on the 5 largest centers and at the end of 2015 these centers were uploading patient data to the ADDN database on a quarterly basis resulting in 5271 patients with 83 506 diabetes visits.
Keywords: database, information, longitudinal, pediatric
Australia is one of the high incidence countries for type 1 diabetes (T1D): there are approximately 10 000 children (aged <18 years) with T1D nationally and 1000 new onset cases per annum.1 The majority of these cases receive clinical care in tertiary academic centers or from clinical teams linked to tertiary centers.2 Incidence registries in Australia have documented an increased rate of T1D over 4 decades and provided insights into the epidemiology of the disease.3-7 In addition to investigating disease epidemiology, the value of prospectively monitoring patient characteristics and outcomes was recognized. In parallel, the availability of databases in the clinical setting led to the establishment of local datasets in the late 1980s and early 1990s. These have facilitated and informed clinical care, service planning and research.8-13
In 2012 the recognition of the benefits of national collaboration along with advances in information technology led to the formation of the Australasian Diabetes Data Network (ADDN; www.addn.org.au). This initiative, funded through the Juvenile Diabetes Research Foundation (JDRF) Australian Type 1 Clinical Research Network (T1DCRN, www.t1dcrn.org.au), sought to establish a national pediatric diabetes registry and was led in its first phase by the Australasian Paediatric Endocrine Group (APEG). Critical technological direction, database development and maintenance were provided by the University of Melbourne eResearch group. More recently the Australian Diabetes Society (ADS) has joined the collaboration, a development that will see adult cases included in the registry and will provide valuable data on outcomes across the life span.
This article describes the initial phase of ADDN that focused on major pediatric centers within Australia. A staged expansion to include regional sites, centers managing adults with T1D and New Zealand is planned for the next phase, which commenced in 2016. Although Australasia covers a huge geographical area, the majority of children with T1D are managed by tertiary academic centers in the major capital cities. As most of these centers had longitudinal data collection in place the model employed was to establish the collection and collation of these data into a central database on an on-going basis. This required the definition of a common data dictionary, ethics and governance procedures and the employment of technology to enable efficient and accurate information transfer and accessibility.
Objective and Specific Aims
The overall objective of ADDN is to provide national data on people with diabetes—to enhance service planning and clinical care through audit and benchmarking, and to facilitate research and collaboration.
Specific aims include:
Long-term monitoring of diabetes outcomes in the Australasian population with T1D across the life span.
Linkage of biobank samples (DNA, plasma, serum) with a detailed registry of clinical phenotype in a population based sample of T1D.
Population based recruitment of young people for trials in new onset and established T1D.
Enrolment of patient cohorts for specific cross-sectional and longitudinal research.
Methods
Overview
The initial phase included the design and development of a central database with a web-based user interface and the ingestion of data from 5 tertiary pediatric centers across Australia—Princess Margaret Hospital for Children (PMH) in Western Australia, Children’s Hospital at Westmead (CHW) in New South Wales, Royal Children’s Hospital (RCH) in Victoria, Lady Cilento Children’s Hospital (LCCH) in Queensland, and Women’s and Children’s Hospital (WCH) in South Australia.
The project was overseen by a steering committee including representatives from each of the 5 phase 1 centers, APEG and JDRF. The group investigated potential database systems available at the time, prior to deciding to custom build a system to leverage a number of existing center-based clinical and research databases already in place.
University of Melbourne’s eResearch group was appointed following a competitive selection process to build the ADDN database and user interface. User requirements were gathered through a series of workshops and consultation.
A data dictionary was developed to define the 130+ data points to be captured in the ADDN database. Data points related to either a patient or to a patient visit and included demographic and clinical characteristics, therapeutic interventions, comorbidities and complications, documentation of biobanked samples and consent to be contacted for future research. Where possible, International Society for Pediatric and Adolescent Diabetes (ISPAD)/APEG/ADS definitions and standard classifications were used. The data dictionary was defined in close cooperation with the diabetes research community.
Implementation of ADDN
The specification for the ADDN registry included several key capabilities: an intuitive and user-friendly interface; data ingestion from existing center-based databases; a range of queries and reports; the overall performance of the system, the hosting and back-up of the data; user documentation and training; and, importantly, support for enhanced security including data integrity, data privacy, and data auditability.
It was critical to support extraction of data from existing center-based clinical databases to avoid double data entry. Electronic medical records and personally controlled electronic health records (PCEHR) are being progressively rolled out across Australasia but currently there is a wide range of IT systems being used for pediatric diabetes patient management. These systems are predominantly custom-built applications developed using various versions of SQL Server and Microsoft Access.
Data extracts are produced locally and uploaded at quarterly intervals with center-specific data sets mapped to the core data model as defined by the data dictionary. The core data model has been essential to underpin the ADDN work, with the software systems and processes focused on ensuring that heterogeneous data sets from the different centers are systematically aligned with this model. To support this, standardized data extraction routines were developed and aligned with local IT systems. The canonical data schema was represented in XML, with versions in other formats provided by centers where technical expertise was limited.
Guarantees of confidentiality and protection of access to data are fundamental to ADDN. Identifying data are not loaded to ADDN and only the contributing diabetes data centers are aware of the identity of individuals on the registry. To allow patients to be tracked across multiple centers as the project progresses, individual participants must be uniquely identifiable. This will be achieved by using a defined set of personally identifying information to create a unique data linkage key that is stored in the local data set and loaded to ADDN together with the deidentified patient data.
Architecture of ADDN
The ADDN architecture has been developed to be scalable. It has been realized by an n-tier service-oriented architecture adopting the Model-View-Controller (MVC) paradigm14 which provides a clean separation of the data layer (database/data) from the application logic and security and the front-end user interface. The underlying database technology is MySQL, which provides a robust, open source relational database. It provides direct support for a service–oriented architecture with Representational State Transfer–based services utilizing the Spring IO Platform for security and data persistence.15 The high-level structure of the ADDN architecture is shown in Figure 1. The system supports a range of data processing pipelines that enable Extraction, Transformation and Loading (ETL) capabilities. This includes support for data extraction from the heterogeneous diabetes data resources, which are transformed into the standardized/agreed ADDN data model for subsequent querying and analysis through the ADDN user interface.
Figure 1.
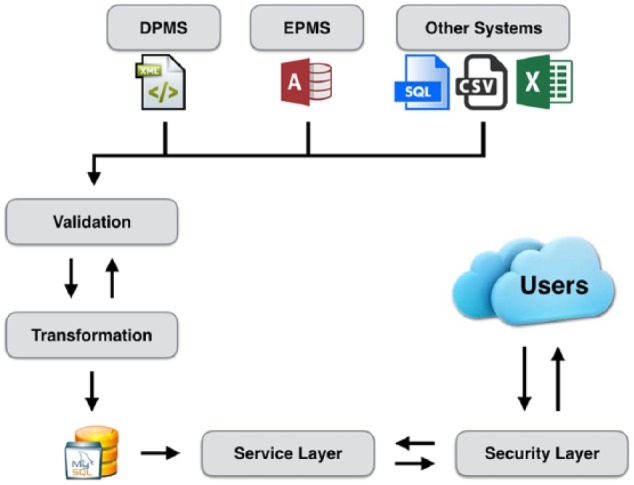
High level ADDN architecture. Local database DPMS (Diabetes Patient Management System) is used at PMH (WA), RCH (Vic), and WCH (SA); EPMS (Endocrine Patient Management System) is used at CHW (NSW).
User Interface, Reports, and Data Interrogation
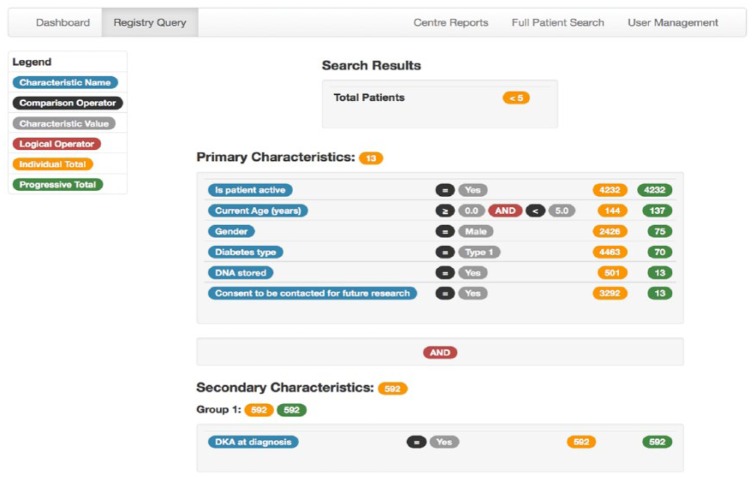
The user interface to the ADDN system provides a range of capabilities reflecting the needs of the ADDN community. The front page includes a report of the patients loaded to the registry summarized by center and diabetes type and for each center, the date of the most recent upload. ADDN includes options for the interrogation and analysis of the data. The number of patients matching a range of primary and secondary characteristics can be determined using a Registry Query tool to assist with study planning (Figure 2). The tool shows the number of individuals resulting from the application of each filter, providing a richer understanding of the underlying data.
Figure 2.
Query builder interface.
The ADDN system also supports a range of reports targeted to specific centers and the functionality to search across all individual patients using any of the data points that are defined in the data model. Specific values and upper and lower bounds can be specified for data points where applicable. To support the validation of data the ADDN model includes a quality and completeness report, detailed reporting of a random selection of individual patients, and a set of predefined reports of summary information on a range of measures. The same reports can also be run for data aggregated from all centers, allowing benchmarking of individual centers against the total group, highlighting missing data and other data quality issues.
To support data export from the registry for approved research purposes, the front-end web information has been formatted so that the HTML on the page can be copied directly. Data can be saved in various formats such as Excel or Comma Separated Variable for analysis in a range of statistical packages including Stata and SPSS.
Security
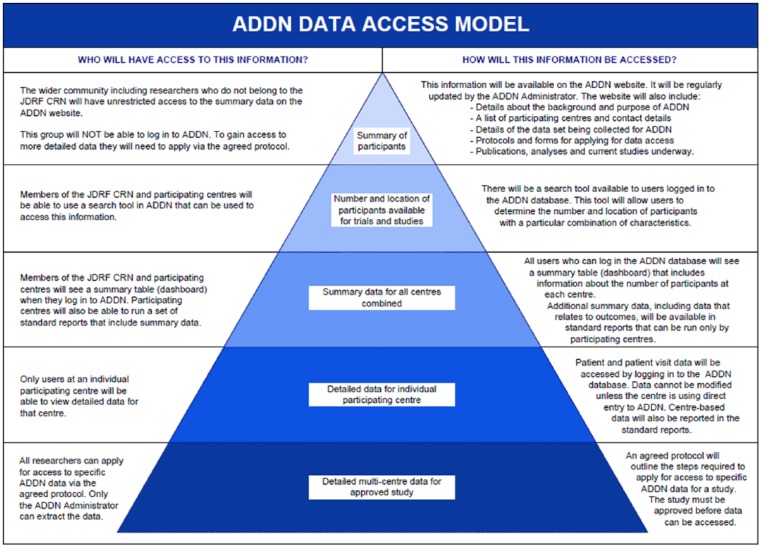
ADDN users are authenticated using individual usernames and passwords. All users are assigned a security level and the ADDN records to which they have access are determined according to the ADDN Data Access Model (Figure 3):
Figure 3.
Model for data access.
Administrator security allows access to all of the deidentified data within the ADDN database. Individuals with this role can run standard reports for individual centers and for aggregated data, perform data queries and extraction and have access to administration functions such as adding new users. This role is restricted to system administrators and lead investigators on ADDN.
JDRF T1DCRN members are able to view the front page report of patients loaded to the registry summarized by center and diabetes type. They are also able to use the Registry Query tool to find the number of patients (across all centers) that match selected criteria. This group of users do not have access to individual patient level data.
In addition to the access granted to JDRF T1DCRN members, center-based users will have detailed access to all of the data within their center. Patient records can be searched and viewed individually and standard reports can be run for the center.
The wider community, including researchers who do not belong to the JDRF T1DCRN, will be able to view a summary of aggregated data on an ADDN website to be developed in the next phase of the ADDN project.
Researchers apply to the ADDN Committee to use the data and if approved, they are provided with a data extract of the relevant fields. Protocols cover the application process, security, use and disposal of ADDN data.
Ethics
Institutional regulatory board (ethics) approval was obtained by each center according to center-specific requirements. Newly diagnosed patients were consented prospectively and existing patients were consented retrospectively as they attended routine clinic appointments.
Governance and Data Access
Governance, publication and data access protocols were developed based on input from US-based T1D Exchange Clinic Registry, BioGrid Australia and a range of Australian cohort studies unrelated to diabetes. The governance protocol includes sections on the phases of ADDN, scope, roles and responsibilities, risk management, performance assessment and ADDN committee details such as composition, term of office and member obligations. The publication and data access protocol include sections on the use of ADDN data, publication of findings and authorship.
Results
The ADDN project received widespread support from the diabetes research community with study investigators representing 20 pediatric centers across Australia (Appendix A). The first phase of ADDN was completed at the end of June 2015, reaching all agreed milestones within the set timeframe and within budget. Major achievements included the consent of >6000 patients, the development and refinement of a common data dictionary (Appendix B), and the establishment of a secure, centralized database populated with clinical and demographic data from 5 major pediatric centers across Australia. At the completion of the project these centers were uploading patient data to the ADDN database on a quarterly basis resulting in 5271 patients with 83 506 diabetes visits.
Within 6 months of the project start the ADDN Committee and project managers had been appointed, ethics applications were lodged with participating centers, a database prototype was demonstrated, a shared data dictionary was agreed and a requirements specification document was completed. At 18 months local ethics approvals were obtained by each of the 5 major diabetes centers and an additional 16 diabetes centers throughout NSW and Queensland, and 1 New Zealand diabetes center.
For the majority of diabetes centers, ethics approval was granted on the basis that written patient consent be obtained. It was usual practice to obtain written consent from patients, or in the case of minors their caregivers, at the time of diagnosis for the national incidence registry. An amendment to include the option to consent to ADDN on the same consent form as that used for the national incidence registry was submitted and approved by ethics. Diabetes centers that demonstrated significant strain on resources due to the burden of obtaining consent from prevalent cases received funding to enable the allocation of dedicated staff to consent patients in clinic. In 1 center the ethics board approved the transfer of deidentified data to ADDN without the need for written consent.
The number of patients consented to ADDN increased from 1500 at 12 months to 4800 at 2 years and 6000 by the end of the first phase. There have been no participants who have withdrawn from the study. The first data were uploaded to ADDN from 2 centers within 2 years of the project start with a third center uploading data 6 months later.
One of the major challenges for meeting the ADDN project milestones was communication with the participating centers and the wider diabetes community. A substantial proportion of participants were to be drawn from prevalent cases, largely recruited through busy diabetes clinics. The technical challenges of transferring data from numerous local centers to the central ADDN database also warranted clear communication between the local IT staff and the database developers. The project required actively engaged stakeholders to proceed in a timely manner. To achieve effective communication weekly project management meetings and monthly ADDN investigator meetings were held. Face-to-face investigator meetings were held every 6 to 12 months. ADDN Newsletters were utilized to update APEG members and the research community on the project’s progress. The ADDN project has resulted in poster and oral presentations at national scientific meetings and at international scientific meetings.16-18 In a recent T1D Exchange meeting, “Global Data Collection and Outcomes” (Boston 2015), ADDN investigators were invited panelists, an important step to developing partnerships with other groups collecting diabetes data.
Conclusion
The development of prospective national datasets is increasing globally for T1D particularly in pediatrics: a phenomenon that has been driven by the recognition of the importance of monitoring outcomes and, at the same time, facilitated by the rapid evolution of information technology. As therapies change and the incidence of the disease rises it is increasingly important to obtain representative data to inform clinicians, patients and their carers, the institutions that fund treatments, policymakers and the broader community. Such data are a rich source of information that can initiate and inform research activity and facilitate study recruitment, and inform clinical practice. Although Australia has a long track record of data collection, this had predominantly been at an individual center level and the expansion to a national level was overdue. The existence of local databases meant that the most efficient approach was to integrate these systems into a central collection rather than to develop a new local and central platform. Although in concept this was straightforward, there were a number of challenges. These included the definition of a common acceptable data dictionary, the technical issues around having differing source databases, security of transfer of information and agreement on the governance of the resource. To date these have been addressed and over 50% of Australian children with T1D are now included in the ADDN prospective database that will provide important data over time.
A major challenge is integration with local databases and electronic medical records at the source centers. There are multiple systems in use and the systems used change. Addressing this challenge is complex and requires close collaboration with the IT teams at the local site with technical and clinical input from the ADDN team as well as coordination from the local clinicians.
The next phase of ADDN is to include sites managing adult patients and to expand to other pediatric centers across Australia and New Zealand. Potentially over 80% of Australasian children with T1D and a significant number of adult patients can be included in this phase. Differences in health care provision in adult patients may present a challenge and the first patients will be those who receive their clinical care in the public as opposed to private health care system. The development of nationally representative data will enable benchmarking at an international level and facilitate collaboration. The ongoing challenge is to maintain the integrity of the data, ensure its efficient use and sustainability. Whilst the resource needs to retain independence, ongoing support will be obtained not only from the research community but also from a range of agencies, including industry and health care funders, who may utilize the information to the benefit of patient care.
Australasian Diabetes Data Network (ADDN) Study Group
| Name | Organization |
|---|---|
| Professor Maria Craig | The Children’s Hospital at Westmead, Sydney |
| Clinical Professor Tim Jones | Princess Margaret Hospital for Children, Perth |
| Clinical Professor Geoff Ambler | The Children’s Hospital at Westmead, Sydney |
| Professor Jenny Batch | Lady Cilento Children’s Hospital, Brisbane |
| Dr Phil Bergman | Monash Children’s Hospital, Melbourne |
| Professor Fergus Cameron | Royal Children’s Hospital, Melbourne |
| Professor Peter Colman | Royal Melbourne Hospital, Melbourne |
| A/Prof Louise Conwell | Lady Cilento Children’s Hospital, Brisbane |
| A/Prof Andrew Cotterill | Lady Cilento Children’s Hospital, Brisbane |
| Professor Jennifer Couper | Women’s and Children’s Hospital, Adelaide |
| A/Prof Elizabeth Davis | Princess Margaret Hospital for Children, Perth |
| Professor Kim Donaghue | The Children’s Hospital at Westmead, Sydney |
| Dr Jan Fairchild | Women’s and Children’s Hospital, Adelaide |
| Dr Leonie Gray | Mater Medical Centre, Rockhampton |
| Professor Paul Hofman | University of Auckland, New Zealand |
| Dr Neville Howard | The Children’s Hospital at Westmead, Sydney |
| Dr Michelle Jack | Royal North Shore Hospital, Sydney |
| Dr Craig Jefferies | Starship Children’s Hospital, New Zealand |
| A/Prof Bruce R King | John Hunter Children’s Hospital, Newcastle |
| Dr Antony Lafferty | The Canberra Hospital, Canberra |
| Dr Robert McCrossin | Gladstone Hospital, Gladstone |
| Dr Mark Pascoe | Royal Hobart Hospital, Hobart |
| Dr Alexia Peña | The University of Adelaide |
| A/Prof Darrell Price | Pacific Private Clinic, Gold Coast |
| A/Prof Christine Rodda | Monash Children’s Hospital, Melbourne |
| Professor Richard Sinnott | eResearch, University of Melbourne |
| Dr Alan Sive | Townsville Hospital, Townsville |
| Dr Carmel Smart | John Hunter Children’s Hospital, Newcastle |
| Dr Monique Stone | Royal Darwin Hospital, Darwin |
| Dr Elaine Tham | Women’s and Children’s Hospital, Adelaide |
| Dr Charles Verge | Sydney Children’s Hospital, Sydney |
| Professor Jerry Wales | Lady Cilento Children’s Hospital, Brisbane |
| Dr Tim Warnock | Cairns Diabetes Centre, Cairns |
| Dr Judy Williams | Bundaberg Diabetes Centre, Bundaberg |
| Dr Michael Williams | Mackay Base Hospital, Mackay |
| A/Prof Esko Wiltshire | Wellington Children’s Hospital, New Zealand |
| Dr Nick Woolfield | Caboolture Hospital, Caboolture |
| Professor Sophia Zoungas | Monash Medical Centre, Melbourne |
Acknowledgments
We thank the staff of the Juvenile Diabetes Research Foundation (JDRF) Australian Type 1 Clinical Research Network in particular Dr Dorota Pawlak and Dr Maryanne Ng for ongoing support and guidance. We also gratefully acknowledge the contribution and support of the Australasian Paediatric Endocrine Group (APEG) and Lyndell Wills, APEG Secretariat.
Appendix
Appendix A.
ADDN Phase 1 Investigators and Centers (Pediatric).
| Principal Investigators | Organization |
|---|---|
| Professor Maria Craig | The Children’s Hospital at Westmead, Sydney |
| Clinical Professor Tim Jones | Princess Margaret Hospital for Children, Perth |
| Co-investigators | |
| Clinical Professor Geoff Ambler | The Children’s Hospital at Westmead, Sydney |
| Professor Jenny Batch | Lady Cilento Children’s Hospital, Brisbane |
| Dr Phil Bergman | Monash Children’s Hospital, Melbourne |
| Professor Fergus Cameron | Royal Children’s Hospital, Melbourne |
| A/Prof Louise Conwell | Lady Cilento Children’s Hospital, Brisbane |
| A/Prof Andrew Cotterill | Lady Cilento Children’s Hospital, Brisbane |
| Professor Jennifer Couper | Women’s and Children’s Hospital, Adelaide |
| A/Prof Elizabeth Davis | Princess Margaret Hospital for Children, Perth |
| Professor Kim Donaghue | The Children’s Hospital at Westmead, Sydney |
| Dr Jan Fairchild | Women’s and Children’s Hospital, Adelaide |
| Dr Leonie Gray | Mater Medical Centre, Rockhampton |
| Dr Neville Howard | The Children’s Hospital at Westmead, Sydney |
| Dr Michelle Jack | Royal North Shore Hospital, Sydney |
| Dr Bruce King | John Hunter Children’s Hospital, Newcastle |
| Dr Antony Lafferty | The Canberra Hospital, Canberra |
| Dr Robert McCrossin | Gladstone Hospital, Gladstone |
| Dr Mark Pascoe | Royal Hobart Hospital, Hobart |
| Dr Alexia Peña | Women’s and Children’s Hospital, Adelaide |
| A/Prof Darrell Price | Pacific Private Clinic, Gold Coast |
| A/Prof Christine Rodda | Monash Children’s Hospital, Melbourne |
| Dr Alan Sive | Townsville Hospital, Townsville |
| Dr Carmel Smart | John Hunter Children’s Hospital, Newcastle |
| Dr Monique Stone | Royal Darwin Hospital, Darwin |
| Dr Elaine Tham | Women’s and Children’s Hospital, Adelaide |
| Dr Charles Verge | Sydney Children’s Hospital, Sydney |
| Dr Tim Warnock | Cairns Diabetes Centre, Cairns |
| Dr Judy Williams | Bundaberg Diabetes Centre, Bundaberg |
| Dr Michael Williams | Mackay Base Hospital, Mackay |
| Dr Nick Woolfield | Caboolture Hospital, Caboolture |
| Phase 1 Clinical Centers | |
| State/territory | Name and address |
| Western Australia | Department Endocrinology and Diabetes, Princess Margaret Hospital for Children Roberts Road, Subiaco, Perth, WA 6008 |
| New South Wales | Institute of Endocrinology & Diabetes, The Children’s Hospital at Westmead Locked Bag 4001, Westmead, NSW 2145 |
| Department of Endocrinology, Sydney Children’s Hospital High St, Randwick, NSW 2031 | |
| Department Paediatric Endocrinology, Royal North Shore Hospital Reserve Road, St Leonard’s, NSW 2065 | |
| Department of Endocrinology, John Hunter Children’s Hospital Lookout Road, Newcastle, NSW 2310 | |
| Victoria | Department Endocrinology and Diabetes, Royal Children’s Hospital Flemington Road, Parkville, VIC 3052 |
| Paediatric Endocrinology and Diabetes, Monash Children’s Hospital 246 Clayton Road, Clayton, VIC 3168 | |
| South Australia | Endocrinology and Diabetes Centre, Women’s and Children’s Hospital 72 King William Road, North Adelaide, SA 5006 |
| Queensland | Department Endocrinology and Diabetes, Lady Cilento Children’s Hospital 501 Stanley Street, South Brisbane, QLD 4101 |
| Child and Adolescent Health Service, Mackay Base Hospital PO 5580, Mackay Mail Centre, QLD 4741 | |
| Cairns Diabetes Centre, North Cairns Community Health Centre 381 Sheridan St, Cairns, QLD 4870 | |
| Diabetes Centre, Bundaberg Base Hospital PO Box 34, Bundaberg, QLD 4670 | |
| Diabetes Centre, Townsville Hospital P O Box 670, Townsville, QLD 4810 | |
| Gladstone Hospital Park Street, Gladstone, QLD 4680 | |
| Pacific Private Clinic, Gold Coast 8/123 Nerang Street, Southport, QLD 4215 | |
| Rockhampton Diabetes Clinic Suite 9, Mater Medical Centre, Jessie Street, Rockhampton, QLD 4700 | |
| Diabetes Clinic, Caboolture Hospital Locked Mail Bag 3, Caboolture, QLD 4510 | |
| Tasmania | Royal Hobart Hospital Diabetes Centre 70 Collins Street, Hobart, TAS 7000 |
| Australian Capital Territory | Paediatric and Adolescent Diabetes Unit, Canberra Hospital PO Box 11, Woden ACT 2606 |
| Northern Territory | Royal Darwin Hospital 105 Rocklands Drive, Tiwi, NT 0810 |
Appendix B.
ADDN Data Dictionary (Phase 1—Pediatric Data).
| Table: Patient | ||
|---|---|---|
| Field | Description | Expected values |
| ADDN ID | Unique identifier for patient | |
| Center code | State-city-center | Valid ADDN center code |
| Local ID | Local identifier for patient not linked to identifying information (source system) | |
| Active flag | Active flag (patient is no longer active if they have transferred to another center, died, or if it is >2 years since last visit) | Yes, no |
| Date of birth | Patient DOB | Valid date |
| Gender | Gender of patient | M, F, undetermined |
| Date of consent for ADDN | Date patient consented to ADDN | ≥date of diagnosis |
| Consent to be contacted for future research | Indicates whether patient consents to be contacted re future research | Yes, no |
| Country of birth | Patient country of birth | SACC (Standard Australian Classification of Countries, ABS) lowest level (4 digits)—2nd revision, 2011 |
| Ethnicity (primary) | Patient ethnicity—primary | ASCCEG (Australian Standard Classification of Cultural and Ethnic Groups, ABS) 2nd level (2 digits) |
| Ethnicity (secondary) | Patient ethnicity—secondary | As for primary |
| Indigenous status | Indigenous status for patients | For Australian centers—Aboriginal, Torres Strait Islander (TSI), both Aboriginal and TSI, neither Aboriginal nor TSI For NZ centers—Maori, non-Maori |
| Language spoken at home | Language spoken at home | ASCL (Australian Standard Classification of Languages, ABS) 2nd level (2 digits) |
| Birth weight | Birth weight (kg) | 0.4-6.0 |
| Birth weight SDS for gestational age | Calculated from tables | −5.0-5.0 |
| Birth weight for gestational age percentile | Calculated from tables | 0.0-100.0 |
| Gestation at birth | Gestation at birth (weeks) | 20.0-43.0 |
| Mode of birth | Mode of birth | Caesarian, NVD, forceps/assisted |
| Date of menarche | Date of menarche (females only) | After date of birth + 8 years and no later than today’s date |
| Transfer date | Date patient transferred to another center | After date of diagnosis |
| Transfer to | Center patient transferred to | Tertiary, primary, private, unknown |
| ADDN withdrawal date | Date patient withdrew from ADDN | After date of diagnosis and no later than today’s date |
| Deceased date | Date of death | After date of diagnosis and no later than today’s date |
| Cause of death | Cause of death | DKA, hypoglycemia, dead in bed, sepsis, other diabetes related, not diabetes related |
| Cause of death other | Description of “other diabetes-related” cause of death | Free text |
| DNA stored | DNA stored flag | yes, no |
| HLA A-1 | HLA A-1 genotype | 15 digits |
| HLA A-2 | HLA A-2 genotype | |
| HLA B-1 | HLA B-1 genotype | |
| HLA B-2 | HLA B-2 genotype | |
| HLA C-1 | HLA C-1 genotype | |
| HLA C-2 | HLA C-2 genotype | |
| HLA DRB1-1 | HLA DRB1-1 genotype | |
| HLA DRB1-2 | HLA DRB1-2 genotype | |
| HLA DQA1-1 | HLA DQA1-1 genotype | |
| HLA DQA1-2 | HLA DQA1-2 genotype | |
| HLA DQB1-1 | HLA DQB1-1 genotype | |
| HLA DQB1-2 | HLA DQB1-2 genotype | |
| HLA DPA1-1 | HLA DPA1-1 genotype | |
| HLA DPA1-2 | HLA DPA1-2 genotype | |
| HLA DPB1-1 | HLA DPB1-1 genotype | |
| HLA DPB1-2 | HLA DPB1-2 genotype | |
| Diabetes type | Diabetes type | Type 1, type 2, gestational, monogenic, CFRD, neonatal, other |
| Diabetes type other | Description of “other” diabetes type | Free text |
| Date of diagnosis | Date of diagnosis | No later than today’s date |
| No days hospitalized at diagnosis | Number of days hospitalized at diagnosis (days) | ≥0 and <15 |
| Country at diagnosis | Country at diagnosis | SACC (Standard Australian Classification of Countries, ABS) lowest level (4 digits)-2nd revision, 2011 |
| Postcode at diagnosis | Postcode at diagnosis | Valid postcode |
| DKA at diagnosis | DKA at diagnosis (ISPAD guidelines: Hyperglycemia (blood glucose >11 mmol/L), venous pH <7.3 or bicarbonate <15 mmol/L, ketosis) | Yes, no |
| Current postcode | Current postcode | Valid postcode |
| Table: Comorbidity | ||
| Field | Description | Expected values |
| Comorbidity | Comorbidity | Valid comorbiditya |
| Comorbidity other | Comorbidity other than those defined | Free text |
| Date of diagnosis | Date of diagnosis | No later than today’s date |
| Table: Family history | ||
| Field | Description | Expected values |
| Relationship to proband | Relationship of family member to the patient | Father, mother, sibling, half-sibling, child |
| Diabetes type | Diabetes type affecting family member | Type 1, type 2, gestational, monogenic, CFRD, neonatal, other, unspecified |
| Diabetes type other | Description of “other” diabetes type | Free text |
| Autoimmune disease | Autoimmune disease affecting family member | Thyroid, celiac, other |
| Autoimmune disease other | Description of “other” autoimmune disease | Free text |
| Table: Visit | ||
| Field | Description | Expected values |
| Date of visit | Date of patient visit to clinic | Valid date |
| Diagnosis visit | Diagnosis visit flag | Yes, no |
| Center code at visit | Center code at visit | Valid ADDN center code |
| HbA1c (NGSP) | HbA1c (NGSP) (%) | 3.0-20.0 |
| HbA1c (IFFC) | HbA1c (IFFC) (mmol/mol) | 9-195 |
| Height | Height (cm) | <200.0 |
| Height SDS | Calculated from tables | −5.0-5.0 |
| Height percentile | Calculated from tables | 0.0-100.0 |
| Weight | Weight (kg) | <200.0 |
| Weight SDS | Calculated from tables | −5.0-5.0 |
| Weight percentile | Calculated from tables | 0.0-100.0 |
| BMI | Calculated from height and weight (kg/m^2) | |
| BMI SDS | Calculated from tables | −5.0-5.0 |
| BMI percentile | Calculated from tables | 0.0-100.0 |
| Waist circumference | Waist circumference (cm) | <120.0 |
| Waist to height ratio | Calculated field | |
| Systolic blood pressure | Systolic blood pressure (mmHg) | 50-250 |
| Systolic BP SDS | Calculated from tables | −5.0-5.0 |
| Systolic BP percentile | Calculated from tables | 0.0-100.0 |
| Diastolic blood pressure | Diastolic blood pressure (mmHg) | 40-120 |
| Diastolic BP SDS | Calculated from tables | −5.0-5.0 |
| Diastolic BP percentile | Calculated from tables | 0.0-100.0 |
| IAA pos | IAA antibodies positive flag | Yes, no |
| IA-2 pos | IA-2 antibodies positive flag | Yes, no |
| GAD pos | GAD antibodies positive flag | Yes, no |
| ZnT8 pos | ZnT8 antibodies positive flag | Yes, no |
| ICA pos | ICA antibodies positive flag | Yes, no |
| Insulin regimen | Insulin regimen | CSII, BD/twice daily, MDI, Other |
| Pump make/model | Pump make/model for CSII patients | Valid pump make and model |
| Pump make/model other | Pump make/model other than those defined | Free text |
| ICR | Insulin to carb ratio used for MDI patients | Yes, no |
| ISF | Insulin sensitivity factor used for MDI patients | Yes, no |
| No injections per day | Number of injections per day for MDI patients | ≤10 |
| Insulin (1) | Insulin 1 name | Valid insulin name |
| Insulin daily dose (1) | Insulin 1 daily dose (units) | 0-200 |
| Insulin (2) | Insulin 2 name | Valid insulin name |
| Insulin daily dose (2) | Insulin 2 daily dose (units) | 0-200 |
| Insulin (3) | Insulin 3 name | Valid insulin name |
| Insulin daily dose (3) | Insulin 3 daily dose (units) | 0-200 |
| Insulin (4) | Insulin 4 name | Valid insulin name |
| Insulin daily dose (4) | Insulin 4 daily dose (units) | 0-200 |
| Total daily insulin dose | Total daily insulin dose (units) | 0-300 |
| Daily basal insulin dose | Total basal insulin dose (units) | 0-200 |
| Basal insulin % | Calculated field | 0-100 |
| SMBG frequency per day | Average frequency of self-monitoring of blood glucose per day since last visit. If available use 7 day average from glucometer | 0-30 |
| CGM | Continuous glucose monitoring since last visit or if last visit>6 m ago then in the last 6 m | Real-time, retrospective, no |
| CGM % | % of time CGM worn during real-time monitoring since last visit | None, <25%, 25-50%, 50-75%, >75%, 100% |
| Severe hypoglycemia episodes | # episodes of severe hypoglycemia (coma or convulsion) since last visit | Integer |
| Moderate hypoglycemia episodes | # episodes of moderate hypoglycemia (requiring assistance but not coma or convulsion) since last visit | Integer |
| DKA episodes | # episodes of DKA since last visit | Integer |
| Biological samples | Biological samples stored flag | Yes, no |
| Tanner stage (breast) | Tanner stage (breast) (females only) | 1-5 |
| Tanner stage (genitalia) | Tanner stage (genitalia) (males only) | 1-5 |
| Tanner stage (pubic hair) | Tanner stage (pubic hair) | 1-5 |
| Right testicular volume | Right testicular volume (ml) (males only) | ≤30 |
| Left testicular volume | Left testicular volume (ml) (males only) | ≤30 |
| Fasting Lipids | Flag to indicate whether lipid results fasting | yes, no |
| Total cholesterol | Total cholesterol (mmol/L) | 1-20 |
| TG | Triglycerides (mmol/L) | 1-20 |
| HDL | HDL (mmol/L) | 1-20 |
| LDL | LDL (mmol/L) | 1-20 |
| ACR | Albumin creatinine ratio | 0.1-50.0 |
| AER | Albumin excretion rate (mg/min) | 0.1-100.0 |
| Smoking | Smoking status | Current, past, never, unknown |
| Table: Medication | ||
| Field | Description | Expected values |
| Name | Name of medication other than insulin being taken by patient | Free text |
| Start date | Start date for other medication | Valid date |
| End date | End date for other medication | After start date |
Valid comorbidities are the following: hematological—thalassemia; endocrine/nutritional/metabolic—hypothyroidism, hyperthyroidism, thyroid autoimmunity, Wolfram syndrome, Addison disease, PCOS, obesity, dyslipidemia, hypercholesterolemia, cystic fibrosis; mental/behavioral—depression, needle phobia, anxiety, anorexia, bulimia, mental retardation, autism, ADHD, ADD; nervous system—Friedreich’s ataxia, epilepsy, TIA, mononeuritis, peripheral neuropathy, myotonic dystrophy, autonomic neuropathy; eye—cataracts, nonproliferative retinopathy, proliferative retinopathy; circulatory—hypertension, angina, AMI, angioplasty, bypass graft, heart failure, stroke, carotid artery disease, claudication, amputation; respiratory—asthma; digestive—gastroparesis, coeliac disease; skin and subcutaneous—ulceration, infection; musculoskeletal—Charcot joint, osteomyelitis; genitourinary—ESRF, albuminuria, microalbuminuria, erectile dysfunction; congenital/chromosomal—Prader-Willi syndrome, Laurence-Moon-Biedl syndrome, Down syndrome, Turner syndrome, Klinefelter syndrome.
Footnotes
Abbreviations: ADDN, Australasian Diabetes Data Network ADS, Australian Diabetes Society; APEG, Australasian Paediatric Endocrine Group; T1D, type 1 diabetes.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Juvenile Diabetes Research Foundation (JDRF) Australian Type 1 Clinical Research Network.
References
- 1. Catanzariti L, Faulks K, Moon L, et al. Australia’s national trends in the incidence of type 1 diabetes in 0-14 year olds, 2000-2006. Diabet Med. 2009;26:596-601. [DOI] [PubMed] [Google Scholar]
- 2. Cameron F, Cotterill A, Couper J, et al. Short report: care of children and adolescents with diabetes in Australia and New Zealand: have we achieved the defined goals? J Paediatr Child Health. 2013;49(4):E258-E262. [DOI] [PubMed] [Google Scholar]
- 3. Taplin CE, Craig ME, Lloyd M, et al. The rising incidence of childhood type 1 diabetes in New South Wales, 1990-2002. Med J Aust. 2005;183:243-246. [PubMed] [Google Scholar]
- 4. Haynes A, Bower C, Bulsara MK, Jones TW, Davis EA. Continued increase in the incidence of childhood type 1 diabetes in a population-based Australian sample (1985-2002). Diabetologia. 2004;47:866-870. [DOI] [PubMed] [Google Scholar]
- 5. Chong JW, Craig ME, Cameron FJ, et al. Marked increase in type 1 diabetes mellitus incidence in children aged 0-14 yr in Victoria, Australia, from 1999 to 2002. Pediatr Diabetes. 2007;8:67-73. [DOI] [PubMed] [Google Scholar]
- 6. Haynes A, Bower C, Bulsara MK, et al. Perinatal risk factors for childhood type 1 diabetes in Western Australia—a population-based study (1980-2002). Diabet Med. 2007;24:564-570. [DOI] [PubMed] [Google Scholar]
- 7. Tran F, Stone M, Huang CY, et al. Population-based incidence of diabetes in Australian youth aged 10-18yr. increase in type 1 diabetes but not type 2 diabetes. Pediatr Diabetes. 2014;15(8):585-590. [DOI] [PubMed] [Google Scholar]
- 8. Lin A, Northam EA, Werther GA, Cameron FJ. Risk factors for decline in IQ in youth with type 1 diabetes over the 12 years from diagnosis/illness onset. Diabetes Care. 2015;38(2):236-242. [DOI] [PubMed] [Google Scholar]
- 9. Downie E, Craig ME, Hing S, Cusumano J, Chan AK, Donaghue KC. Continued reduction in the prevalence of retinopathy in adolescents with type 1 diabetes: role of insulin therapy and glycemic control. Diabetes Care. 2011;34(11):2368-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peña AS, Maftei O, Harrington J, et al. Lack of evidence for progression of atherosclerosis during puberty in type 1 diabetes. Pediatr Diabetes. 2016;17:199-205. [DOI] [PubMed] [Google Scholar]
- 11. Irvine KM, Gallego P, An X, et al. Peripheral blood monocyte gene expression profile clinically stratifies patients with recent-onset type 1 diabetes. Diabetes. 2012;61(5):1281-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis EA, Keating B, Byrne GC, Russell M, Jones TW. Hypoglycemia: incidence and clinical predictors in a large population-based sample of children and adolescents with IDDM. Diabetes Care. 1997;20(1):22-25. [DOI] [PubMed] [Google Scholar]
- 13. Johnson SR, Cooper MN, Jones TW, Davis EA. Long-term outcome of insulin pump therapy in children with type 1 diabetes assessed in a large population-based case-control study. Diabetologia. 2013;56(11):2392-2400. [DOI] [PubMed] [Google Scholar]
- 14. Buschmann F, Henney K, Schimdt D. Pattern-Oriented Software Architecture: On Patterns and Pattern Language. Vol. 5 New York, NY: John Wiley; 2007. [Google Scholar]
- 15. Fielding RT, Taylor RN. Principled design of the modern web architecture. ACM Trans Internet Technol. 2002;2:115-150. [Google Scholar]
- 16. Phelan H, Donaghue K, Cameron F, et al. The Australasian Diabetes Data Network (ADDN): a potential tool to facilitate transition. Paper presented at: Annual Scientific Meeting of the Australian Diabetes Society; 2014; Melbourne. [Google Scholar]
- 17. Phelan H, Donaghue K, Cameron F, et al. The Australasian Diabetes Data Network (ADDN): first steps towards a national diabetes resource. Paper presented at: Asia Pacific Endocrine Society and the Australasian Paediatric Endocrine Society Joint Meeting; 2014; Darwin. [Google Scholar]
- 18. Phelan H, Donaghue K, Cameron F, et al. The Australasian Diabetes Data Network (ADDN): first national audit data. Paper presented at: Annual Scientific Meeting of the International Society for Paediatric and Adolescent Diabetes; 2015; Brisbane. [Google Scholar]