Abstract
Aim
To compare the efficacy, tolerability and safety of celecoxib, naproxen and placebo in Asian patients with osteoarthritis (OA) of the knee.
Method
Patients of Asian descent with knee OA, aged ≥ 45 years, in a flare state with a functional capacity classification of I–III, received celecoxib 200 mg once daily, naproxen 500 mg twice daily or placebo, for 6 weeks. The change in Patient's Assessment of Arthritis Pain (week 6 vs. baseline) was the primary endpoint. Secondary endpoints, including Patient's and Physician's Global Assessments of Arthritis, Western Ontario and McMaster Universities OA Index (WOMAC), use of complementary and alternative medicines, incidence of treatment‐emergent adverse events (TEAEs) and measurements of upper gastrointestinal tolerability, were also assessed.
Results
Three hundred and sixty‐seven patients were randomized: 145 to celecoxib, 144 to naproxen and 78 to placebo. Celecoxib was as effective as naproxen in reducing OA pain (least squares mean change from baseline in visual analogue scale score [standard error] −37.1 [2.0] for celecoxib and −37.5 [2.0] for naproxen). Patient's and Physician's Global Assessment of Arthritis, WOMAC scores, Pain Satisfaction Scale and Patient Health Questionnaire‐9 showed statistically significant improvement in active treatment groups versus placebo, with the exception of naproxen WOMAC scores. Treatment‐related TEAEs occurred in 19 (13%), 34 (24%) and six (8%) patients in the celecoxib, naproxen and placebo groups, respectively.
Conclusion
Celecoxib and naproxen were comparable in their effects to reduce the signs and symptoms of knee OA in Asian patients. Celecoxib was shown to be safe and well tolerated in this patient population.
Keywords: cyclooxygenase‐2, ethnicity, nonsteroidal anti‐inflammatory drugs, race
Introduction
Osteoarthritis (OA) is a substantial public health issue in the US and the incidence of OA in ethnic minority groups in the US is underestimated.1 As the US population ages, the prevalence of OA is expected to increase in all ethnicity groups. In Asian countries, more than 16% of people will be aged over 65 years by 20402 and Asian Americans are estimated to represent 9% of the US population by 2050.3 In light of these projections, an important topic for healthcare providers will be the impact of OA in aging Asian populations.
There is evidence to suggest that symptomatic OA of the knee is the most prevalent form of OA in Asia, and may be linked to older age and female sex.2 OA of the knee is prevalent in both rural Asian communities and in affluent urban areas, and is associated with obesity.2, 4, 5, 6 Some management options, such as surgery, are inaccessible to patients in some Asian communities, particularly among those who are living in rural communities of developing countries.2 In the US Asian population who reported arthritis, 38.2% reported activity limitation, 28.2% reported work limitation and 18.5% reported severe joint pain.7 Therefore, providing effective, safe and cost‐effective long‐term solutions is paramount.
Several studies (reviewed in Edwards et al.8) have evaluated the differences in experimentally induced pain perception and threshold between subjects from different ethnicities, with inconsistencies found among populations. Race‐related differences may also exist in patients' responses to pain medications.9, 10 It has previously been demonstrated that a variant of the cytochrome P450 allele, which is predominantly found in Asian populations, can lead to significant alterations in the metabolism of certain nonsteroidal anti‐inflammatory drugs (NSAIDs).11 It is therefore conceivable that differences exist in Asian patients' responses to other treatments for OA, including celecoxib. Analyzing interracial differences between patients' responses to pain and its treatments provides important information that can help clinicians to individualize treatment regimens and clinical assessments.
We have previously reported the results from studies that investigated the efficacy and safety of two NSAIDs in patients of African American12 and Hispanic13 descent. The aim of this report is to summarize the results from a study that evaluated the efficacy and safety of celecoxib (a cyclooxygenase [COX]‐2 selective NSAID) and naproxen (a widely used NSAID) in patients of Asian descent. Given the burden associated with OA in Asia, determining the efficacy and safety of accessible treatments in an ethnically Asian American population will be useful to physicians and healthcare professionals alike.
Materials and methods
This was a 6‐week, randomized, double‐blind, placebo‐controlled study that determined the efficacy and safety of once‐daily celecoxib versus twice‐daily naproxen (active control) in Asian patients aged ≥ 45 years with defined criteria for knee OA.14 The trial was carried out in 31 centers in the US in compliance with the principles of Good Clinical Practice and the Declaration of Helsinki. Each study site received protocol approval from an institutional review board, and all patients gave written informed consent.
All patients were in an OA flare state, and within a functional capacity classification of I–III (as classified by a physician, where I = complete functional capacity with ability to carry on all usual duties without handicaps and IV = largely or wholly incapacitated with the patient bedridden or confined to a wheelchair, permitting little or no self‐care). Eligible patients were randomized in a 2 : 2 : 1 ratio to celecoxib 200 mg once daily, naproxen 500 mg twice daily, or placebo, and attended four clinic visits (screening, baseline, week 2 and week 6). Screening occurred within 1–14 days prior to the first dose of study medication, and during this period, patients discontinued use of any prior NSAID/analgesic drug. Acetaminophen (up to 2 g/day) was permitted as rescue analgesia for the treatment of arthritis symptoms during the pretreatment screening period. Patients were to discontinue use of acetaminophen at least 24 h prior to the baseline arthritis assessments.
This article presents the results of one of three clinical trials that had identical methodologies but were carried out in different ethnic groups in the US (African Americans, Hispanics and Asians). The results from the study that was carried out in an African American population12 and Hispanic population13 have been published previously.
Treatment efficacy was assessed using the change from baseline to week 6 in the Patient's Assessment of Arthritis Pain (primary outcome), which was measured using a standard visual analogue scale (VAS) ranging from 0 mm (no pain) to 100 mm (worst pain). This was performed in the evaluable population (treated patients with 70–120% treatment compliance, no major protocol violations and primary assessments at baseline and week 6) using a generalized linear model adjusted for treatment and center effects and with baseline score as a covariate. Celecoxib was regarded as effective as naproxen if the treatment difference (naproxen – celecoxib) at the lower range of the two‐sided 95% confidence interval (CI) was above −10 mm.15 The difference between active treatments and placebo was used as a control.
Change from baseline in a number of secondary measurements was also used to compare treatments. These included the Patient's and Physician's Global Assessments of Arthritis, Western Ontario and McMaster Universities OA Index (WOMAC), Pain Satisfaction Scale, and the Patient Health Questionnaire (PHQ‐9) (to week 6/early termination), and the American Pain Society pain score (to day 7). The WOMAC total domain score (range 0–96) was the sum of the pain, stiffness and physical function domain scores. These were performed in the modified intent‐to‐treat (mITT) population (randomized patients with at least one dose of study medication and post‐baseline follow‐up efficacy measure). WOMAC and questions 2–5 of the American Pain Society pain score were analyzed using a generalized linear model adjusted for treatment and center effects and with baseline score as a covariate. Patient's and Physician's Global Assessments of Arthritis, Pain Satisfaction scale, PHQ‐9 and question 1 of the American Pain Society pain score were analyzed using the Cochran‐Mantel‐Haenszel test (row‐mean‐score‐test) stratified by center. The study also recorded the use of complementary and alternative medicine at screening.
The tolerability of celecoxib versus placebo was also evaluated, by comparing treatment‐emergent treatment‐related adverse events (TEAEs) and measurements of upper gastrointestinal (UGI) tolerability. This evaluation was carried out in the safety population (randomized patients receiving at least one dose of study medication).
Results
Patient characteristics
The baseline characteristics of the 367 randomized patients self‐reported as Asian are shown in Table 1. Patients' ages ranged 42 to 90 years; most (67–68%) were female, with a medium‐high knee OA disease burden across all groups (mean pain VAS score ranged 64.4 to 65.8 mm). A total of 362 patients received treatment and 281 completed the study (Fig. 1). On average, medication compliance exceeded 80% in all treatment groups: celecoxib (87.2%), naproxen (82.1%) and placebo (86.6%). A total of 62 patients (29, 26 and seven subjects in the celecoxib, naproxen and placebo treatment groups, respectively) used rescue medication (i.e., acetaminophen) during the course of the study.
Table 1.
Baseline demographic and clinical characteristics
| Celecoxib 200 mg qd (n = 145) | Naproxen 500 mg bid (n = 144) | Placebo (n = 78) | P‐value | |
|---|---|---|---|---|
|
Age, years, mean (SD) (Range) |
65.9 (11.1) (42–90) |
64.1 (11.4) (45–88) |
63.9 (11.1) (45–88) |
0.491 |
| Female, n (%) | 97 (67) | 98 (68) | 52 (67) | 0.989 |
| Duration of OA, years, mean (SD) | 4.5 (4.0) | 4.8 (5.5) | 4.6 (4.3) | 0.619 |
| [n = 127†] | [n = 128†] | [n = 68†] | ||
|---|---|---|---|---|
| Patients' Global Assessment, n (%) | 0.1346 | |||
| Very good | 0 | 0 | 0 | |
| Good | 0 | 1 (< 1) | 0 | |
| Fair | 24 (17) | 30 (21) | 19 (24) | |
| Poor | 109 (75) | 103 (72) | 56 (72) | |
| Very poor | 12 (8) | 10 (7) | 3 (4) | |
| Physician's Global Assessment, n (%) | 0.0553 | |||
| Very good | 0 | 0 | 1 (1) | |
| Good | 0 | 1 (< 1) | 1 (1) | |
| Fair | 25 (17) | 30 (21) | 19 (24) | |
| Poor | 116 (80) | 106 (74) | 56 (72) | |
| Very poor | 4 (3) | 7 (5) | 1 (1) | |
| Functional capacity classification, n (%) | 0.5096 | |||
| I | 6 (4) | 5 (4) | 4 (5) | |
| II | 119 (83) | 118 (82) | 66 (85) | |
| III | 19 (13) | 21 (15) | 8 (10) | |
| IV | 0 | 0 | 0 | |
| VAS score, mm, mean (SD) | 64.6 (12.2) | 65.8 (11.7) | 64.4 (13.0) | 0.5984 |
| WOMAC total score, mean (SD)‡ | 50.1 (15.5) | 51.2 (14.0) | 50.5 (16.0) | 0.8121 |
†Non‐missing included only. ‡WOMAC total domain score is the sum of pain, stiffness and physical function domain scores. Continuous measures were analyzed by a general linear model with factors for treatment and center. Categorical data were analyzed using the Cochran‐Mantel‐Haenszel test, stratified by center. bid, twice daily; OA, osteoarthritis; qd, once daily; SD, standard deviation; VAS, visual analogue scale; WOMAC, Western Ontario and McMaster Universities OA Index.
Figure 1.
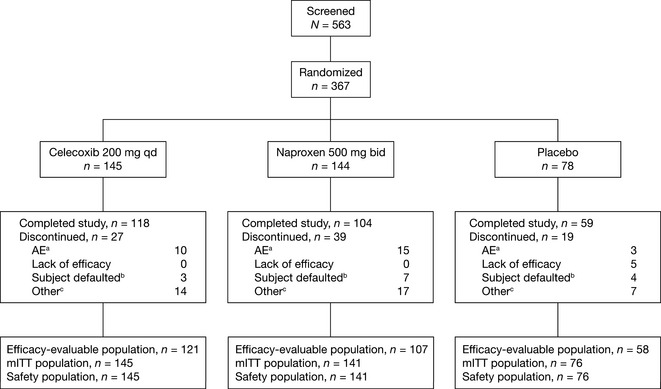
Patient disposition. AE, adverse event; bid, twice daily; mITT, modified intent‐to‐treat; qd, once daily. aIncludes both treatment‐related and non‐treatment‐related AEs. bIncludes ‘lost to follow‐up’ and ‘subject no longer willing to participate in study’. cIncludes ‘protocol violation’.
Use of complementary and alternative medicines at baseline
Of the 563 patients screened, the complementary/alternative therapies most frequently used by patients within 1 month prior to screening were dietary modifications to increase the amount of fish in diet and to avoid saturated fats or fried foods (Fig. 2).
Figure 2.
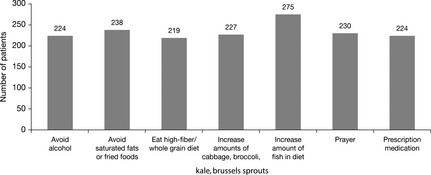
Complementary and alternative medicine used by > 200 patients 1 month prior to screening (screened patients).
Patient's Assessment of Arthritis Pain (VAS)
Both celecoxib and naproxen reduced the Patient's Assessment of Arthritis Pain (VAS) score; these improvements were clinically meaningful and suggested that celecoxib was as effective as naproxen (Table 2).
Table 2.
Patient's Assessment of Arthritis Pain (VAS in mm) at week 6 (efficacy‐evaluable population)
| Celecoxib 200 mg qd (n = 121) | Naproxen 500 mg bid (n = 107) | Placebo (n = 58) | |
|---|---|---|---|
| Baseline, mean (SE) | 65.1 (1.1) | 65.4 (1.1) | 63.7 (1.5) |
| Week 6, mean (SE) | 21.7 (1.9) | 21.9 (2.0) | 25.6 (3.1) |
| Change from baseline, LSM (SE) | −37.1 (2.0) | −37.5 (2.0) | −33.6 (2.6) |
| Naproxen – celecoxib† | Naproxen – placebo | Celecoxib – placebo | |
|---|---|---|---|
| Difference in LSM (SE) | −0.4 (2.5) | −3.9 (3.0) | −3.5 (3.0) |
| 95% CI | −5.2 to 4.5 | −9.8 to 2.1 | −9.3 to 2.3 |
| P‐value | 0.8791 | 0.2027 | 0.2403 |
†Celecoxib treatment was observed to be as effective as naproxen, based on the protocol requirements, since the lower‐bound of the two‐sided 95% CI of the treatment difference (naproxen–celecoxib) was above −10 mm (−5.2 mm). Change in VAS score from baseline to week 6 was analyzed using a generalized linear model with treatment and center effect in the model and baseline score as a covariate. bid, twice daily; qd, once daily; VAS, visual analogue scale; CI, confidence interval; LSM, least squares means; SE, standard error.
Physician's and Patients' Global Assessments of Arthritis
For the Physician's Global Assessment of Arthritis, there was a statistically significant difference between both active treatments and placebo (P < 0.05) by week 2 (Table 3). At the final visit (week 6/early termination), the majority of patients were rated as ‘good’/'very good' on this scale for celecoxib (73%) and naproxen (67%) compared with placebo (58%), but only celecoxib achieved statistical significance versus placebo at week 6 (P < 0.05). Physicians also described the arthritis condition as ‘improved’ by most celecoxib (64%; P < 0.01 vs. placebo) and naproxen (57%; P < 0.05 vs. placebo) users by the final visit.
Table 3.
Summary of Physician's Global Assessment of Arthritis (mITT population)
| Celecoxib 200 mg qd | Naproxen 500 mg bid | Placebo | |
|---|---|---|---|
| (n = 145) | (n = 141) | (n = 76) | |
| Week 2, n (%) | n = 137 | n = 130 | n = 74 |
| Very good | 4 (3) | 6 (5) | 2 (3) |
| Good | 54 (39) | 50 (39) | 21 (28) |
| Fair | 64 (47) | 63 (49) | 38 (51) |
| Poor | 15 (11) | 11 (9) | 11 (15) |
| Very poor | 0 | 0 | 2 (3) |
| Week 6, n (%) | n = 143 | n = 136 | n = 76 |
| Very good | 23 (16) | 24 (18) | 10 (13) |
| Good | 82 (57) | 66 (49) | 34 (45) |
| Fair | 27 (19) | 35 (26) | 24 (32) |
| Poor | 10 (7) | 10 (7) | 6 (8) |
| Very poor | 1 (1) | 1 (1) | 2 (3) |
| Celecoxib vs. naproxen | Celecoxib vs. placebo | Naproxen vs. placebo | |
|---|---|---|---|
| Week 2, P‐value | 0.3997 | 0.0382 | 0.0143 |
| Week 6, P‐value | 0.5151 | 0.0354 | 0.1963 |
Physician's Global Assessment of Arthritis scores were analyzed using Cochran‐Mantel‐Haenszel (row‐mean‐score‐test), stratified by center. bid, twice daily; mITT, modified intent‐to‐treat; qd, once daily.
Similar improvements were observed for the Patient's Global Assessment of Arthritis. Patients described the arthritis condition as ‘improved’ in the celecoxib (68%; P < 0.01 vs. placebo) and naproxen (59%; P < 0.01 vs. placebo) groups by the final visit.
WOMAC, Pain Satisfaction Scale, and PHQ‐9
Using the least squares mean (LSM) [SE] of change from baseline in the WOMAC scale, the celecoxib group was significantly different (P < 0.05) from the placebo group for the total (−24.9 [1.6] vs. −19.7 [2.1]), pain (−5.6 [0.4] vs. −4.3 [0.5]), and physical function (−17.3 [1.2] vs. −13.9 [1.5]) domains, but not for the stiffness domain (−2.0 [0.2] vs. −1.6 [0.2]). The mean change from baseline in the total and individual domain scores indicated improvement for naproxen versus placebo, but the differences did not achieve statistical significance.
Overall, a greater proportion of patients using active treatment compared with those using placebo responded favorably for the other secondary scores. Of note, the speed of pain relief (Pain Satisfaction Scale) was statistically significantly in favor of celecoxib compared with naproxen (P < 0.05). The PHQ‐9 (question 1: Over the past 2 weeks how often have you been bothered by loss of pleasure in activity, depression, problems with sleep, lack of energy, changes in appetite, feeling like a failure, trouble concentrating, moving slowly or becoming restless, or thoughts of being better off dead or hurting yourself?) improved in both active treatment groups (LSM change was −0.6 [celecoxib] and −0.5 [naproxen]) but worsened in the placebo group (+0.2).
Safety
Treatment‐related TEAEs were reported by 19 (13%) patients in the celecoxib group, 34 (24%) in the naproxen group, and six (8%) in the placebo group. The treatment‐related TEAEs that occurred in ≥ 2% of patients are listed in Table 4. Most TEAEs were mild to moderate in severity, with only 20 (6%) patients discontinuing due to a treatment‐related TEAE (7/145 [5%] in the celecoxib group, 12/141 [9%] in the naproxen group and 1/76 [1%] in the placebo group). There were no reports of serious AE or death. Few patients reported issues with UGI tolerability, with 5/145 (3%) in the celecoxib group, 9/141 (6%) in the naproxen group and 2/76 (3%) in the placebo experiencing a UGI event (moderate to severe nausea, abdominal pain and/or dyspepsia).
Table 4.
Treatment‐related AEs occurring in ≥ 2% of patients (in decreasing order of occurrence)
| Celecoxib 200 mg qd (n = 145) | Naproxen 500 mg bid (n = 141) | Placebo (n = 76) | |
|---|---|---|---|
| AE by preferred term, n (%) | |||
| GI | |||
| Abdominal pain | 9 (6) | 15 (11) | 3 (4) |
| Dyspepsia | 2 (1) | 7 (5) | 0 |
| Constipation | 0 | 3 (2) | 0 |
| Diarrhea | 0 | 3 (2) | 0 |
| CNS | |||
| Depression | 4 (3) | 2 (1) | 2 (3) |
| Dizziness | 0 | 3 (2) | 0 |
AE, adverse event; bid, twice daily; CNS, central nervous system; GI, gastrointestinal; qd, once daily.
Discussion
To date, most randomized studies examining the efficacy of celecoxib have focused on Caucasian and non‐Asian populations.12, 16, 17 This study confirmed that once‐daily celecoxib was as effective as twice‐daily naproxen in relieving signs and symptoms associated with OA of the knee in Asian patients. In this population celecoxib was well‐tolerated, with no differences in UGI tolerability when compared with placebo.
Aside from ethnicity, baseline characteristics of patients in this study (sex, age, duration of OA, pain assessments, functional capacity, VAS and WOMAC scores) were similar to those observed in the African American12 and Hispanic13 cohorts. In addition, similar improvements from baseline to week 6 in the Patient's Assessment of Arthritis Pain VAS were noted for the active treatment groups in Asian patients, when compared to the studies carried out in patients of different ethnicities.12, 13
Variability is known to exist in the therapeutic and adverse effects of NSAIDs in patients of different genetic backgrounds.18 A potential mediator of patient responses is cytochrome P450 (CYP), which metabolizes many drugs that are in clinical use. The highly polymorphic CYP2C9 isoform is responsible for metabolizing NSAIDs, and the CYP2C9*13 variant is known to be highly prevalent in patients of Chinese, Japanese and Korean descent but absent in African American, white and Hispanic patients. In those who have the CYP2C9*1/*13 genotype, the metabolism of lornoxicam, a COX‐1 and COX‐2 inhibitor, is markedly reduced.11, 18 Although the clinical significance is unclear, these data show that consideration of population‐specific biomarkers for predicting NSAID response should be investigated.
The low magnitude of risk for adverse events with celecoxib and naproxen in this study was consistent with what has been demonstrated in a previous report.19 A meta‐analysis of 89 randomized, controlled studies in which celecoxib was compared with placebo or nonselective NSAIDs for pain and inflammation treatment showed that there was a greater risk of GI hemorrhage (risk difference: −0.53%), GI ulceration (−0.46%), edema (−0.62%) and hypertension (−0.57%) with nonselective NSAIDs than celecoxib.19
Although there is a limited number of studies that have evaluated the safety of NSAIDs in Asian patients, differences in the frequencies of UGI events have been observed between treatments. An analysis of 12 studies that compared celecoxib with loxoprofen in Japanese patients with OA or rheumatoid arthritis (RA) showed that there were significantly fewer serious GI events, including symptomatic ulcers, with celecoxib (P < 0.05).20 An analysis of three 12‐week studies that compared twice‐daily celecoxib with diclofenac in Chinese patients with OA or RA showed that there was no statistical difference in occurrence of gastroduodenal ulcers (primary outcome) (2.8% vs. 5.1%, P = 0.083), but there were significantly fewer gastric ulcers with celecoxib (0.5% vs. 3.6%; P = 0.002).21 Consistent with these studies, the current analysis found a low rate of UGI event occurrences in the active treatment groups, and the event rate with celecoxib was the same as that observed with placebo (3%) and half the rate of the naproxen group (6%) with relative risk (95% CI) of 58% (26–129%). This 42% risk reduction did not reach statistical significance, which was likely due to the low event rate and/or the limited sample size.
This study has a number of limitations. The population was a US Asian cohort; however, patients were not stratified by their ethnicity descent (e.g., Chinese, Japanese). It is difficult to determine the effect of any country‐specific lifestyle, habitual or cultural factors on treatment outcomes as data on relating to these factors were not evaluated. Furthermore, the primary analysis did not distinguish between responders and nonresponders, which could have affected the overall change in pain relief reported. Nor did the study include a definition of a ‘minimal clinically important difference,’ and we can only assume that the large LSM reductions in the VAS scale would be clinically meaningful. However, it must be noted that the LSM change in arthritis pain (VAS) score was also high in the placebo group (−33.6 mm) compared with celecoxib (−37.1 mm) and naproxen (−37.5 mm) treatment. A similar finding was noted in the study of these treatments when carried out in Africa American patients12 and, to a lesser degree, Hispanic patients.13 A previous meta‐analysis has reported that the placebo effect in OA studies is high, and can be influenced by a number of factors, including sample size and baseline disease severity.22 Both of these factors could have had an effect in this study. A further limitation is the length of the trial; although it was consistent with other studies, many patients require longer treatment than the 6‐week treatment duration that was evaluated here. Also, we cannot rule out the effect of complementary/alternative medication use, and the rationale for and impact of rescue medication which was used by seven patients in the placebo group compared with 29 in the celecoxib group and 26 in the naproxen group.
To minimize the placebo effect in OA studies, there has been recent interest in analyzing composite pain and activity. The rationale for the pain‐activity outcome measure is based on the observation that the analgesic effect in some patients may not result in reduced pain, but might lead to increased physical activity. This measure could therefore represent a more reliable measure of the analgesic effect. A recent randomized, placebo‐controlled, crossover study of celecoxib in 63 patients (47 completers) showed that a responder (defined as a patient who had a 20% improvement in pain [numerical rating scale] or 10% improvement in activity [WOMAC function scale or actigraphy]) yielded larger differences between celecoxib and placebo.23 Although actigraphy was found to be more responsive than WOMAC, it would have been interesting to examine this composite approach in the current study.
The complementary and alternative medicines questionnaire revealed a variety of nonpharmaceutical strategies that were adopted by Asian American patients with arthritis to relieve their symptoms, such as increasing consumption of fish and avoiding saturated fat. Recognizing these patient approaches, some of which might be culture‐specific, could help devise realistic comparator studies and identify accessible treatment strategies, particularly with regard to the use of alternative medications. A comparison of Ayurvedic formulations (extracts of Tinospora cordifolia, Zingiber officinale, Emblica officinalis and Boswellia serrata), glucosamine sulfate 2 g daily and celecoxib 200 mg daily in 440 Indian patients with knee OA over a 24‐week period showed that pain relief was within the equivalence range. However, seven patients using Ayurvedic intervention were withdrawn due to increased serum glutamic pyruvic transaminase, which normalized when the intervention was stopped.24 These findings indicate that safety assessments of alternative medications are warranted, together with a definition of bioequivalence of efficacy in clinical studies. A limitation of the Chopra et al. study was in the wide definition of equivalence between treatments, which was set to ± 1.5 cm on body weight‐bearing pain (VAS).
From a cost‐effectiveness viewpoint, analyses of local models (including those from studies in Asia) indicate that celecoxib is favorable compared with nonselective NSAIDs.25 It would be interesting to further determine cost outcomes using data from this study compared with commonly used agents in the Asia‐Pacific region.
In summary, these findings show that celecoxib once daily was as effective as naproxen twice daily in treating the pain, symptoms and physical function impact of knee OA in a US Asian cohort. It was also associated with a low risk of UGI events.
Disclosure of conflicts of interest
Margaret N. Essex, Michael A. O'Connell, Regina Behar and Weihang Bao are all employees of Pfizer Inc.
Disclosure of funding
This study was sponsored by Pfizer Inc.
Authors' contributions
ME, MO'C and RB interpreted the data and drafted the paper. WB analyzed and interpreted the data and drafted the manuscript. All authors gave final approval of the version to be published.
Acknowledgement
Editorial support was provided by Kate Bradford, PhD, and Christina Campbell, PhD, of PAREXEL, and was funded by Pfizer Inc.
References
- 1. Thomas PA (2007) Racial and ethnic differences in osteoporosis. J Am Acad Orthop Surg 15 (Suppl. 1), S26–30. [DOI] [PubMed] [Google Scholar]
- 2. Fransen M, Bridgett L, March L, Hoy D, Penserga E, Brooks P (2011) The epidemiology of osteoarthritis in Asia. Int J Rheum Dis 14 (2), 113–21. [DOI] [PubMed] [Google Scholar]
- 3. Pew Research Center . US population projections: 2005–2050. Pew Research Hispanic Trends Project website. Available from URL http://www.pewhispanic.org/2008/02/11/us-population-projections-2005-2050/. Accessed 18 August 2014.
- 4. Gibson T, Hameed K, Kadir M, Sultana S, Fatima Z, Syed A (1996) Knee pain amongst the poor and affluent in Pakistan. Br J Rheumatol 35, 146–9. [DOI] [PubMed] [Google Scholar]
- 5. Haq SA, Darmawan J, Islam MN et al (2005) Prevalence of rheumatic diseases and associated outcomes in rural and urban communities in Bangladesh: a COPCORD study. J Rheumatol 32, 348–53. [PubMed] [Google Scholar]
- 6. Zeng QY, Zang CH, Li XF, Dong HY, Zhang AL, Lin L (2006) Associated risk factors of knee osteoarthritis: a population survey in Taiyuan, China. Chin Med J (Engl) 119, 1522–7. [PubMed] [Google Scholar]
- 7. Bolen J, Schieb L, Hootman JM et al (2010) Differences in the prevalence and severity of arthritis among racial/ethnic groups in the United States, National Health Interview Survey, 2002, 2003, and 2006. Prev Chronic Dis 7 (3), A64. [PMC free article] [PubMed] [Google Scholar]
- 8. Edwards CL, Fillingim RB, Keefe F (2001) Race, ethnicity and pain. Pain 94, 133–7. [DOI] [PubMed] [Google Scholar]
- 9. Ortolani O, Conti A, Ngumi ZW et al (2004) Ethnic differences in propofol and fentanyl response: a comparison among Caucasians, Kenyan Africans and Brazilians. Eur J Anaesthesiol 21, 314–9. [DOI] [PubMed] [Google Scholar]
- 10. Sibille KT, Kindler LL, Glover TL et al (2011) Individual differences in morphine and butorphanol analgesia: a laboratory pain study. Pain Med 12, 1076–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi CI, Kim MJ, Jang CG, Park YS, Bae JW, Lee SY (2011) Effects of the CYP2C9*1/*13 genotype on the pharmacokinetics of lornoxicam. Basic Clin Pharmacol Toxicol 109, 476–80. [DOI] [PubMed] [Google Scholar]
- 12. Essex MN, O'Connell M, Bhadra BP (2012) Response to nonsteroidal anti‐inflammatory drugs in African Americans with osteoarthritis of the knee. J Int Med Res 40, 2251–66. [DOI] [PubMed] [Google Scholar]
- 13. Essex MN, Behar R, O'Connell MA, Bhadra BP (2014) Efficacy and tolerability of celecoxib and naproxen versus placebo in Hispanic patients with knee osteoarthritis. Int J Gen Med 7, 227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Altman R, Asch E, Bloch D et al (1986) Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 29, 1039–49. [DOI] [PubMed] [Google Scholar]
- 15. Ehrich EW, Davies GM, Watson DJ, Bolognese JA, Seidenberg BC, Bellamy N (2000) Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol 27, 2635–41. [PubMed] [Google Scholar]
- 16. Singh G, Fort JG, Goldstein JL et al (2006) Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS‐I Study. Am J Med 119, 255–66. [DOI] [PubMed] [Google Scholar]
- 17. Williams GW, Ettlinger RE, Ruderman EM et al (2000) Treatment of osteoarthritis with a once‐daily dosing regimen of celecoxib: a randomized, controlled trial. J Clin Rheumatol 6 (2), 65–74. [DOI] [PubMed] [Google Scholar]
- 18. Bruno A, Tacconelli S, Patrignani P (2014) Variability in the response to non‐steroidal anti‐inflammatory drugs: mechanisms and perspectives. Basic Clin Pharmacol Toxicol 114 (1), 56–63. [DOI] [PubMed] [Google Scholar]
- 19. Essex MN, Zhang RY, Berger MF, Upadhyay S, Park PW (2013) Safety of celecoxib compared with placebo and non‐selective NSAIDs: cumulative meta‐analysis of 89 randomized controlled trials. Expert Opin Drug Saf 12, 465–77. [DOI] [PubMed] [Google Scholar]
- 20. Sakamoto C, Soen S (2011) Efficacy and safety of the selective cyclooxygenase‐2 inhibitor celecoxib in the treatment of rheumatoid arthritis and osteoarthritis in Japan. Digestion 83 (1–2), 108–23. [DOI] [PubMed] [Google Scholar]
- 21. Cheung R, Cheng TT, Dong Y et al (2010) Incidence of gastroduodenal ulcers during treatment with celecoxib or diclofenac: pooled results from three 12‐week trials in Chinese patients with osteoarthritis or rheumatoid arthritis. Int J Rheum Dis 13 (2), 151–7. [DOI] [PubMed] [Google Scholar]
- 22. Zhang W, Robertson J, Jones AC, Dieppe PA, Doherty M (2008) The placebo effect and its determinants in osteoarthritis: meta‐analysis of randomised controlled trials. Ann Rheum Dis 67, 1716–23. [DOI] [PubMed] [Google Scholar]
- 23. Trudeau J, Van IR, Eaton T et al (2015) Assessment of pain and activity using an electronic pain diary and actigraphy device in a randomized, placebo‐controlled crossover trial of celecoxib in osteoarthritis of the knee. Pain Pract 15, 247–55. [DOI] [PubMed] [Google Scholar]
- 24. Chopra A, Saluja M, Tillu G et al (2013) Ayurvedic medicine offers a good alternative to glucosamine and celecoxib in the treatment of symptomatic knee osteoarthritis: a randomized, double‐blind, controlled equivalence drug trial. Rheumatology (Oxford) 52, 1408–17. [DOI] [PubMed] [Google Scholar]
- 25. Huelin R, Pokora T, Foster TS, Mould JF (2012) Economic outcomes for celecoxib: a systematic review of pharmacoeconomic studies. Expert Rev Pharmacoecon Outcomes Res 12, 505–23. [DOI] [PubMed] [Google Scholar]


