Summary
Reasons for performing study
Lungeing is commonly used as part of standard lameness examinations in horses. Knowledge of how lungeing influences motion symmetry in sound horses is needed.
Objectives
The aim of this study was to objectively evaluate the symmetry of vertical head and pelvic motion during lungeing in a large number of horses with symmetric motion during straight line evaluation.
Study design
Cross‐sectional prospective study.
Methods
A pool of 201 riding horses, all functioning well and considered sound by their owners, were evaluated in trot on a straight line and during lungeing to the left and right. From this pool, horses with symmetric vertical head and pelvic movement during the straight line trot (n = 94) were retained for analysis. Vertical head and pelvic movements were measured with body mounted uniaxial accelerometers. Differences between vertical maximum and minimum head (HDmax, HDmin) and pelvic (PDmax, PDmin) heights between left and right forelimb and hindlimb stances were compared between straight line trot and lungeing in either direction.
Results
Vertical head and pelvic movements during lungeing were more asymmetric than during trot on a straight line. Common asymmetric patterns seen in the head were more upward movement during push‐off of the outside forelimb and less downward movement during impact of the inside limb. Common asymmetric patterns seen in the pelvis were less upward movement during push‐off of the outside hindlimb and less downward movement of the pelvis during impact of the inside hindlimb. Asymmetric patterns in one lunge direction were frequently not the same as in the opposite direction.
Conclusions
Lungeing induces systematic asymmetries in vertical head and pelvic motion patterns in horses that may not be the same in both directions. These asymmetries may mask or mimic fore‐ or hindlimb lameness.
Keywords: horse, circle, gait analysis, lunge, symmetry
Introduction
Lameness is very common in sport horses and the main reason for interruption of their athletic careers 1, 2, 3. Lungeing is a common aid in lameness evaluations and at prepurchase examinations and there may be large differences in the severity of apparent lameness between straight trot, left and right rein 4. The effect of circular trotting on the kinematics of the horse has been studied in small numbers of horses under particular surface conditions 5, 6, 7, 8, 9, 10. Recognising that vertical head and trunk movement symmetry is decreased during lungeing on a hard surface, compared with straight trot, is important to avoid incorrectly declaring a horse as lame 8. Clayton and Sha 5 detected changes in torso orientation and limb loading during lungeing compared with straight path movement. Hobbs et al. 7 found an increase in duty factor (stance duration/stride duration) for the inside forelimb and increased limb and body tilt during lungeing. Kinetic asymmetries were also seen in horses during lungeing. Chateau et al. 11 and Pfau et al. 9, 10 detected that speed, ground surface and circle radius affect movement symmetry. Circle‐dependent asymmetries also affected the degree of lameness seen at lungeing in horses with induced fore‐ and hindlimb lameness 12.
Accordingly, knowledge of any systematic biomechanical changes as a function of circular motion is important to distinguish between normal asymmetric movements caused by the circular path and asymmetric movements due to pain in lame horses. The aim of this study was to evaluate the effect of lungeing on the vertical movement symmetry of the head and pelvis in a large sample of horses in regular work using a body mounted inertial sensor‐based system. The specific objective was to quantify vertical movement symmetry of the head and pelvis during lungeing in both directions, controlling for surface, in comparison with straight line trot.
Materials and methods
Horses
Initially, 201 horses (Sweden, n = 100; United States, n = 101) were screened. Each horse was measured at the trot, in a straight line and during lungeing to the left and the right. Horses were evaluated on grass, sand‐ or gravel‐based surfaces available at the evaluation site and lunged in circles with diameters between 10 and 20 m suitable for the environment and comfortable for the horse. All horses were ridden regularly and functioning well in their work.
Instrumentation
Each horse was instrumented with a commercially available system (Lameness locator)1 consisting of 3 sensors (dimensions 3.8 × 2.5 × 1.3 cm, mass 30 g) 13. Single axis accelerometers were attached, one each, to the poll by either taping to the crown piece of the halter or by attaching to a head bumper and to the most dorsal position of the pelvic midline between the tubera sacrale with a hook and loop patch and tape. A single axis gyroscope was strapped to the dorsal surface of the right forelimb pastern with elastic cloth tape. The accelerometers were positioned with the vertical axis oriented upwards. Data were digitally sampled at 200 Hz in real time and analysed by the software of the motion analysis system (Lameness locator)1.
Data analysis
Raw uniaxial acceleration signals from head and pelvis sensors, aligned with the global vertical axis in standing position, were first transformed into displacement signals using a custom designed, error‐correcting, double integration technique and the signal from the right forelimb gyroscope used for stride splitting 13. In the displacement signal, local maxima and minima were identified (2 per stride). Difference in minimum head (HDmin) and pelvis (PDmin) height during left and right stance phases and difference in maximum head (HDmax) and pelvis (PDmax) height after left and right stance phases were computed per stride (Fig 1). The mean amplitude and sign of HDmin, HDmax, PDmin and PDmax for all strides in a trial were calculated from the signal resulting in either a positive or negative value for each variable. Positive values indicate right and negative values left fore‐ or hindlimb asymmetry HDminright/left, HDmaxright/left, PDminright/left and PDmaxright/left 14 (Fig 1).
Figure 1.
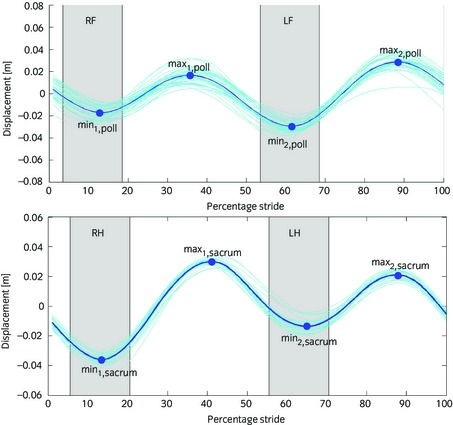
Illustration of vertical head and pelvis movement over a stride cycle and definitions of symmetry measures for head (HDmin, HDmax) and pelvis (PDmin, PDmax). Typical examples of a right forelimb (RF) lame horse (top, poll) and of a left hindlimb (LH) lame horse (bottom, sacrum) are shown. The movement patterns are used based on published evidence on fore‐ 14 or hindlimb 26 lameness. For ease of interpretation, positive values of HDmin and PDmin (representing patterns commonly seen in right forelimb or hindlimb lame horses) are referred to as HDminright and PDminright, negative values as HDminleft and PDminleft. Positive values for HDmax and PDmax represent typical patterns of right forelimb and right hindlimb lameness, negative values of left forelimb and left hindlimb lameness. The grey bars labelled LF, RF, LH, RH indicate the limb in contact with the ground at the time of the measured displacement minima (min1,poll, min2,poll, min1,sacrum, min2,sacrum) for ease of interpretation of sidedness of observed asymmetries between the 2 halves of the stride. The stride cycle here starts at approximately foot contact of the RH limb.
All horses with no evidence of asymmetry outside thresholds defined by Keegan et al. 13 during a straight line trot evaluation were selected for further study. According to these criteria, horses with absolute differences larger than 6 mm in the forelimbs and 3 mm in the hindlimbs and with standard deviations (s.d.) less than their respective means were excluded from further analysis. For each horse all trials collected were used to determine mean HDmin, HDmax, PDmin and PDmax for left lunge, right lunge and straight. The numbers of horses with right or left asymmetries for each activity were determined and compared between lungeing directions.
Descriptive statistics were calculated. Normalities of HDmin, HDmax, PDmin and PDmax in the whole dataset were assessed using Shapiro–Wilk's test. If significant, the best Box‐Cox transformation was selected for further modelling. Mixed models 15 were constructed for the outcomes HDmin, HDmax, PDmin and PDmax with horse as random effect and activity as fixed effect using the mean of all trials in each activity. The covariance structure was set to compound symmetry. Pairwise comparison P values ≤0.05 were considered significant.
To investigate whether the type of surface influenced the results, a second mixed model analysis was performed using all trials with activity and surface (soft/hard/grass) included as fixed effects. The random effect was horse and the covariance structure variance component.
Results
The population
Of the 201 horses, 94 horses had consistent symmetric vertical torso motion while trotting in a straight line and thus were retained for quantitative analysis during lungeing. In Sweden, the 45 horses belonged to 30 different owners (range 1–6 horses/owner, median one horse/owner). In the United States, the 49 horses belonged to 9 different owners (range 2–19 horses/owner, median 4 horses/owner). There were 53 Warmblood‐type horses, one Coldblood horse, 19 Quarter Horse or Quarter Horse‐type horses, 14 ponies and 7 crossbreeds or of unknown breed.
For each activity, 1–8 trials (median; straight = 2, left = 3.5 and right = 3.5), depending on the compliance and training of the horse, were collected and a total of 676 trials for the 94 horses. For straight line trotting there were 30 (±10) strides evaluated per trial. For lungeing to the left and right there were 36 (±9) and 35 (±7) strides evaluated per trial, respectively. For the straight line measurements 39 horses were examined on a hard surface, 24 on grass and 48 on a soft surface (17 horses on both hard and soft). Ten horses were lunged on hard gravel (one horse on both hard and soft), 24 on grass and 61 on a soft gravel‐based surface.
Effect of lungeing direction
Mean HDmaxleft, HDmaxright, HDminleft, HDminright, PDmaxleft, PDmaxright and PDminleft, PDminright values for all 94 horses, by activity (n = 282), are shown in Table 1. Figure 2 shows least square means from the mixed models. HDmin, HDmax and PDmin were modelled untransformed while PDmax was raised to a power of 1.5 to achieve a normal distribution. All but one (PDmax the left‐straight comparison P value = 0.28) pairwise, between activity comparisons were significant. Lungeing to the left introduced HDminright and HDmaxleft asymmetries and lungeing to the right HDminleft and HDmaxright asymmetries. These patterns were reversed for vertical pelvic movement where lungeing to the left caused PDminleft and PDmaxright asymmetries. When lungeing to the right, PDminright and PDmaxleft asymmetries were seen.
Table 1.
Fore‐ and hindlimb asymmetries for straight, left and right lungeing direction in 94 riding horses perceived by their owners to be sound
| Variable | n | Mean | s.d. | Percentiles | |||
|---|---|---|---|---|---|---|---|
| 5th | 25th | 75th | 95th | ||||
| Straight | |||||||
| HDminright | 51 | 4.7 | 4.3 | 0.7 | 1.6 | 7.5 | 12.4 |
| HDminleft | 43 | −4.7 | 3.2 | −9.9 | −7.0 | −2.2 | −0.8 |
| HDmaxright | 45 | 3.9 | 3.0 | 0.6 | 1.3 | 5.7 | 8.4 |
| HDmaxleft | 49 | −3.8 | 3.2 | −9.1 | −4.8 | −1.6 | −0.6 |
| PDminright | 44 | 1.4 | 1.1 | 0.0 | 0.7 | 2.1 | 3.8 |
| PDminleft | 50 | −1.8 | 1.2 | −4.1 | −2.7 | −1.0 | −0.2 |
| PDmaxright | 55 | 2.0 | 1.3 | 0.2 | 1.0 | 2.8 | 4.2 |
| PDmaxleft | 39 | −1.8 | 1.5 | −4.9 | −2.8 | −0.5 | 0.0 |
| Left circle | |||||||
| HDminright | 57 | 9.0 | 7.7 | 0.9 | 3.5 | 13.0 | 28.0 |
| HDminleft | 37 | −9.3 | 7.3 | −27.0 | −13.8 | −2.9 | −1.0 |
| HDmaxright | 27 | 6.5 | 5.2 | 1.2 | 3.2 | 6.9 | 18.5 |
| HDmaxleft | 67 | −7.3 | 5.3 | −17.1 | −10.2 | −3.0 | −0.4 |
| PDminright | 20 | 2.9 | 3.5 | 0.2 | 0.8 | 3.8 | 11.6 |
| PDminleft | 74 | −6.1 | 4.1 | −14.1 | −8.3 | −3.2 | −0.7 |
| PDmaxright | 57 | 3.3 | 2.8 | 0.2 | 1.3 | 4.5 | 8.7 |
| PDmaxleft | 37 | −3.2 | 2.2 | −6.8 | −4.4 | −1.3 | −0.5 |
| Right circle | |||||||
| HDminright | 40 | 5.7 | 4.3 | 0.6 | 2.6 | 7.6 | 15.1 |
| HDminleft | 54 | −8.3 | 6.8 | −21.9 | −11.5 | −2.3 | −0.9 |
| HDmaxright | 66 | 6.4 | 4.0 | 0.5 | 2.8 | 9.2 | 13.6 |
| HDmaxleft | 28 | −7.8 | 4.9 | −18.6 | −10.0 | −4.2 | −2.1 |
| PDminright | 74 | 5.7 | 4.0 | 0.5 | 2.4 | 8.0 | 14.4 |
| PDminleft | 20 | −2.9 | 2.5 | −8.7 | −4.1 | −1.3 | −0.3 |
| PDmaxright | 37 | 2.6 | 2.0 | 0.1 | 0.9 | 4.2 | 6.3 |
| PDmaxleft | 57 | −3.9 | 3.5 | −12.5 | −5.3 | −1.7 | −0.1 |
HD = head; PD = pelvis.
Figure 2.
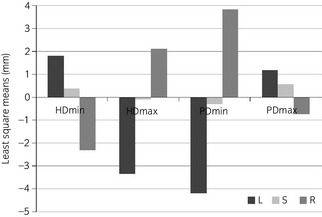
Least square means (mm) from mixed model analysis of HDmin, HDmax, PDmin and PDmax (n = 94 horses). Significant differences were found with all pairwise comparisons between left and right (P<0.0001), for HDmin, left vs. straight (P = 0.04) and straight vs. right (P = 0.04), for HDmax and PDmin left vs. straight and straight vs. right (P<0.0001) and for PDmax straight vs. right (P<0.0001) but not PDmax left vs. straight (P = 0.28). HD = head; PD = pelvis; L = left; S = straight; R = right.
The numbers of horses with left/right HDmin/HDmax and PDmin/PDmax, respectively for right and left rein are presented in Tables 2 and 3. In 41% (n = 39) of the horses, head movement asymmetries of the same type (inner or outer) when lungeing on the opposite rein (diagonal of Table 2) were seen. Likewise, 46% (n = 43) of the horses had opposite pelvic movement asymmetries when lungeing on the opposite rein (diagonal of Table 3).
Table 2.
The numbers of horses with forelimb asymmetries on both circles observed in 94 riding horses perceived by their owners to be sound
| Left circle | |||||
|---|---|---|---|---|---|
| HDminleft HDmaxleft | HDminleft HDmaxright | HDminright HDmaxleft | HDminright HDmaxright | Total | |
| Right circle | |||||
|
HDminright
HDmaxright |
19 | 2 | 4 | 9 | 34 |
|
HDminright
HDmaxleft |
1 | 1 | 2 | 2 | 6 |
|
HDminleft
HDmaxright |
6 | 1 | 5 | 10 | 22 |
|
HDminleft
HDmaxleft |
6 | 1 | 11 | 14 | 32 |
| Total | 32 | 5 | 22 | 35 | 94 |
The diagonal (bold) shows the number of horses with bidirectional asymmetries. HD = head.
Table 3.
The number of horses with hindlimb asymmetries on both circles observed in 94 riding horses perceived by their owners to be sound
| Left circle | |||||
|---|---|---|---|---|---|
| PDminleft PDmaxleft | PDminleft PDmaxright | PDminright PDmaxleft | PDminright PDmaxright | Total | |
| Right circle | |||||
|
PDminright
PDmaxright |
13 | 16 | 0 | 1 | 30 |
|
PDminright
PDmaxleft |
11 | 24 | 4 | 5 | 44 |
|
PDminleft
PDmaxright |
1 | 1 | 2 | 3 | 7 |
|
PDminleft
PDmaxleft |
5 | 3 | 1 | 4 | 13 |
| Total | 30 | 44 | 7 | 13 | 94 |
The diagonal (bold) shows the number of horses with bidirectional asymmetries. PD = pelvis.
The most common bidirectional asymmetry (i.e. same asymmetry pattern seen in both directions) for head movement (n = 19) was inner limb HDmax and HDmin asymmetries (i.e. HDmaxleft and HDminleft lungeing to the left and HDmaxright and HDminright lungeing to the right). Two bidirectional asymmetries were uncommonly seen HDminleft and HDmaxright lungeing to the left (n = 5) and HDminright and HDmaxleft lungeing to the right (n = 6). The most common bidirectional asymmetry for pelvic movement (n = 24) PDmaxright and PDminleft lungeing to the left and PDmaxleft and PDminright lungeing to the right. Very few horses had bidirectional asymmetries opposite of this pattern, with PDmaxleft and PDminright lungeing to the left (n = 7) and PDmaxright and PDminleft lungeing to the right (n = 7).
Comparison with straight line thresholds
Seven horses (7%) had both HDmin and HDmax below the straight line threshold during lungeing in both directions. Thirty‐four of the horses (36%) had HDmin and/or HDmax values above the threshold but only during lungeing in one direction and 53 of the horses (56%) while lungeing in both directions. The largest percentage of horses (n = 46, 49%) had either HDmax and HDmin of the same limb, inner for both left lunge and right lunge or HDmaxleft and HDminright for left lunge and/or HDmaxright and HDminleft for right lunge.
Four horses (4%) had PDmax and PDmin values below the straight line trot threshold on both reins. Thirty horses (32%) had PDmax and/or PDmin above this threshold when lungeing in one direction and 60 (64%) horses had PDmax and/or PDmin above this threshold on both reins. The most common asymmetric pelvic pattern was seen with PDminleft lungeing to the left or PDminright lungeing to the right (n = 69, 73% of horses). The second most common asymmetric pattern seen was PDmaxright lungeing to the left or PDmaxleft lungeing to the left (n = 45, 48% of horses).
Effect of surface
Surface was insignificant in all models using the 676 trials and the mixed model estimates, with surface included as a confounder, were close to those from the mean value analysis (n = 282, Supplementary Item 1).
Discussion
Sample population
This study shows that the circular path during lungeing creates asymmetric movement of both head and pelvis compared with straight line trot in horses with symmetrical straight line motion patterns and perceived to be sound by their owners/trainers. We used the latter inclusion criteria for our initial screening phase in order to include horses functioning well in regular training and exclude horses with known lameness problems. However, in the absence of a gold standard to define soundness on the lunge, objective straight line measurements were used as our final inclusion criteria, which should make our results more repeatable. We believe that our quantitative assessment with very stringent thresholds of movement asymmetry on the straight is an alternative approach to visual lameness scoring, avoiding the inherent variability and interobserver disagreement shown in previous studies both on the straight 16, 17 and on the lunge 18.
A recent study suggests that horses observed to be outside these stringent thresholds during straight line assessment show more asymmetry when assessed on the lunge compared with horses within these limits 10. This gives further support to our quantitative approach of selecting horses based on their movement symmetry on the straight to reduce the likelihood of including lame horses. However, bilateral lameness with equal amplitudes of head and pelvic movement, between right and left halves of the stride, can be difficult to detect from straight line trot. Objective motion analysis has shown good success in detecting the ‘lamer’ limb in bilaterally lame horses when compared against kinetic analysis 19. Nevertheless, it is possible that some of the 94 horses selected for further study had bilateral forelimb or hindlimb lameness.
In the current study, a large proportion of horses were excluded on the basis of the stringent threshold for straight line measurements and this proportion is in accordance with the percentage of lameness identified visually in the horses studied by Greve and Dyson 20. These observations beg the questions: Are these horses really lame or simply asymmetric? And if they are lame, does this constitute a potential welfare issue for competition horses? Further studies are needed to investigate the significance of asymmetric head and pelvic movement screening examinations based on trot in a straight line and to determine whether these asymmetries are a reflection of biological variation or related to pain that may require intervention.
Commonly observed symmetry patterns on the lunge
The results of this study indicate that lungeing increases both vertical head and pelvic movement asymmetry compared with straight line trot and this may be interpreted as lameness. The patterns and amplitudes of asymmetric head movement are more variable and less predictable than those of vertical pelvic movement asymmetry. Also, for both head and pelvic movement asymmetry, the patterns seen while lungeing in one direction were not the same as lungeing in the other direction that also may be interpreted as lameness.
The most common head movement patterns mimicked an apparent outside forelimb lameness (57 horses HDminright for left rein and in 54 horses HDminleft for right rein) followed by an apparent inside forelimb lameness (37 horses HDminleft on left rein, 40 horses HDminright on right rein) (Table 2). Small amounts of head movement asymmetry mimicking outside forelimb lameness should not be unexpected even in sound horses. Starke et al. 8 found more horses mimicking inside forelimb lameness with a smaller sample size on a hard surface. Differences between surfaces may play a significant role here, although the pattern mimicking inside forelimb lameness was also found on a compliant arena surface 9. While the analysis in the current study was controlled for surface, we decided not to analyse the interaction between trial and surface because the material was imbalanced with respect to surfaces and the surface designation was not optimal thus the functional properties may have both differences and similarities within the groups.
Interpretation of our HDmax data is less straightforward. Our assessment of the side of asymmetry is based on a previously described pattern for horses with induced weightbearing lameness 14 showing both a reduction in downward and upward movement of the head during the lame limb stance phase. It has also been suggested that horses with forelimb lameness will raise the head more if the pain occurs primarily during forelimb propulsion at the end of stance 4 to unload the forelimb by shifting weight caudally. This would result in the opposite sign of HDmax (negative for RF lameness, positive for LF lameness) than the assumptions used in the current study.
In general, asymmetries for both HDmin and HDmax are small and hence may be below the threshold of human perception 21. Technological differences, quantifying movement in the dorsoventral direction using uniaxial accelerometers as used here or in the vertical based on a full inertial measuring unit 9, 10, could also contribute to small differences when the horses are leaning into the circle.
The most consistent hindlimb finding during lungeing was less downward movement of the pelvis (PDmin) during the stance phase on the inner hindlimb, mimicking inner hindlimb lameness, often in both directions. These horses most often also had less upward movement of the pelvis after outer hindlimb stance. When combining PDmin and PDmax asymmetries for both reins (ignoring asymmetry magnitudes) almost half of the population were symmetrically asymmetric (Table 3).
Fore‐ and hindlimb asymmetries were (on average) of comparable magnitude but of opposite sign between lungeing directions (Table 1). However, within each horse and for fore‐ and hindlimbs separately, less than half of the study population had equivalent asymmetric movement when lungeing in opposite directions. While this is in accordance with previous work 8 on a much smaller sample, at this stage we do not know whether these small asymmetries between reins are related to pain or to biological variation due to handedness of the horse or to asymmetrical handling/riding 22, 23, 24, 25.
Straight line thresholds cannot be used when lungeing
Similar to previous studies 8, 9, in all but one horse (data not shown), at least one head or pelvic symmetry variable exceeded the straight trot threshold values in at least one direction. Possibly in contradiction to clinical experience, we conclude that small amounts of measured head and pelvic asymmetry should not automatically be regarded as indicative of lameness.
From the threshold analysis, a subset of these horses had apparent forelimb lameness of the same character (HDmin and HDmax) on both reins and some had a bilateral inside forelimb involvement. One explanation of the head movement asymmetries mimicking inside forelimb HDmin type lameness seen in this study, is that these horses had true bilateral forelimb lameness not detected before lungeing. However, sharp turning while lungeing in a tight circle, especially on harder ground, may be a difficult task for some horses and evidence of lameness on the inside limb during such a manoeuvre may not be indicative of a clinical problem 9. Thresholds for HDmax, HDmin, PDmax and PDmin measures established for straight line trot are not appropriate when evaluating horses during lungeing.
Objective symmetry measurements in relation to visual assessment of lameness
We used PDmin and PDmax to measure pelvic movement symmetry, suggesting that a horse can move with 2 types of hindlimb asymmetries in one or in both hindlimbs 26. It is unknown whether this differentiation can be made by ‘subjective’ visual evaluation of the horse or how vertical pelvic asymmetry equates to the often mentioned ‘hip hike’ and ‘hip dip’ observation when using pelvic rotation to detect hindlimb lameness in horses. Parkes et al. 21 suggested that around 20% relative asymmetry of movement is needed for consistent visual detection. Vertical pelvic movement is normally asymmetric between right and left parts of the stride as the horse is moving in a circle, most likely due to torso tilt 5, 9. Whether this natural asymmetry is taken into consideration by clinicians evaluating lameness during lungeing is unknown and requires further study. There is no gold standard to evaluate lameness during lungeing. Keegan et al. 17 suggested that evaluators look at different kinematic variables since different combinations of objective measures of movement of the head, limb and foot correlated with the different examiners' visual lameness evaluations. Our results indicate that, if during lungeing evaluators use only vertical head or pelvic asymmetry without adapting the intrinsic thresholds that may be applied during straight line assessments, this could lead to over diagnosis of lameness. Agreement between experienced evaluators grading and deciding on the affected limb in horses with mild lameness is low and a full lameness evaluation, including evaluating the horse on the lunge, does not improve agreement 17. Circle size and speed 9, the horse's ability to lunge or its prior lungeing training and sidedness or laterality, all contribute to increased variability and likelihood of disagreement. Further, compensatory movements in the other half of the body with primary lameness are often present 27, 28 and this could confound lungeing evaluation 12.
Benefit of this study
The practical strength of this study, with a number of uncontrolled variables (age, sex, breed, country, circle size or speed) but large number of horses investigated, is the potential for clinical generalisation. Systematic results were not masked by confounding case selection and environmental measures. The results can be extrapolated to lungeing horses in general but not to specific subpopulations or evaluation conditions. The horse population evaluated is quite representative of the riding horse population, albeit from 2 countries, perceived as generally sound by owners.
Conclusions
When horses with symmetrical motion pattern on a straight line were lunged, vertical head and pelvic movement became asymmetric, often mimicking motion that would normally be graded as lame using thresholds determined for straight line trot. Circle‐dependent asymmetry must be taken into account when assessing lameness during lungeing. The most common asymmetric motion of the head is less downward movement during stance of the inner forelimb and more upward movement of the head after stance of the outer forelimb. The most common asymmetric motion of the pelvis is less downward movement during stance of the inner hindlimb and less upward movement of the pelvis after stance of the outer hindlimb. We suggest that perfect symmetry between reins cannot be expected and in order to investigate whether comparison of movement symmetry between reins has high enough specificity to detect lameness further studies with horses with confirmed lameness are required.
The high proportion of horses that had movement asymmetries in this study, either in a straight line and hence excluded from analysis after the initial screening phase or on the lunge, raises highly important research questions. It is not known to what extent these asymmetries are related to pain, mechanical abnormalities or normal variation. Further studies are required to resolve this uncertainty and may have important welfare implications.
Authors' declaration of interest
K. G. Keegan is a faculty member of the University of Missouri and owner of a US patent for the equipment used in this study. K. G. Keegan is an unpaid shareholder of Equinosis, manufacturer of the equipment used in this study. None of the other authors has any financial or personal relationship that could inappropriately influence or bias the contents of the paper.
Ethical animal research
This study was conducted within guidelines of the participating sites institutional animal care and use procedures (C 206/10, 2010‐08‐27) and informed consent for data collection obtained from the horse‐owners.
Source of funding
Swedish‐Norwegian Foundation for Equine Research funded the study.
Authorship
The study was designed by M. Rhodin, A. French, K. G. Keegan and A. Egenvall. Data collection and study execution were done by M. Rhodin, A. French, K. Keegan and A. Egenvall. All authors contributed to data analysis and interpretation, preparation of the manuscript and approved the final version of the manuscript.
Supporting information
Supplementary Item 1: Multivariable modelling of the association between kinematic data and horse and surface
Acknowledgements
Part of the results were presented as a poster at the 8th International Conference on Equine Exercise Physiology, South Africa, 7–11 November 2010.
Manufacturer's address
Equinosis, St Louis, Missouri, USA.
References
- 1. Egenvall, A. , Penell, J.C. , Bonnett, B.N. , Olson, P. and Pringle, J. (2006) Mortality of Swedish horses with complete life insurance between 1997 and 2000: variations with sex, age, breed and diagnosis. Vet. Rec. 158, 397‐406. [DOI] [PubMed] [Google Scholar]
- 2. Keegan, K.G. (2007) Evidence‐based lameness detection and quantification. Vet. Clin. North Am. Equine Pract. 23, 403‐423. [DOI] [PubMed] [Google Scholar]
- 3. Weishaupt, M.A. , Wiestner, T. , Hogg, H.P. , Jordan, P. , Auer, J.A. and Barrey, E. (2001) Assessment of gait irregularities in the horse: eye vs. gait analysis. Equine Vet. J. 33, Suppl. 33, 135‐140. [DOI] [PubMed] [Google Scholar]
- 4. Baxter, G.M. , Stashak, T.S. and Adams, O.R. (2011) Examination for lameness In: Lameness in Horses, 6th edn Ed: Baxter G.M., Wiley‐Blackwell, Chichester, West Sussex, England: pp. 115. [Google Scholar]
- 5. Clayton, H.M. and Sha, D.H. (2006) Head and body centre of mass movement in horses trotting on a circular path. Equine Vet. J. 36, Suppl. 36, 462‐467. [DOI] [PubMed] [Google Scholar]
- 6. Walker, A.M. , Wilson, A.M. and Pfau, T. (2010) Comparison of kinematic symmetry index calculations and the effects of straight and circular trotting. Equine Vet. J. 42, Suppl. 38, 482‐487. [DOI] [PubMed] [Google Scholar]
- 7. Hobbs, S.J. , Licka, T. and Polman, R. (2011) The difference in kinematics of horses walking, trotting and cantering on a flat and banked 10 m circle. Equine Vet. J. 43, 686‐694. [DOI] [PubMed] [Google Scholar]
- 8. Starke, S.D. , Willems, E. , May, S.A. and Pfau, T. (2012) Vertical head and trunk movement adaptations of sound horses trotting in a circle on a hard surface. Vet. J. 193, 73‐80. [DOI] [PubMed] [Google Scholar]
- 9. Pfau, T. , Stubbs, N.C. , Kaiser, L.J. , Brown, L.E.A. and Clayton, H.M. (2012) Effect of trotting speed and circle radius on movement symmetry in horses during lunging on a soft surface. Am. J. Vet. Res. 73, 1890‐1899. [DOI] [PubMed] [Google Scholar]
- 10. Pfau, T. , Jennings, C. , Mitchell, H. , Olsen, E. , Walker, A. , Egenvall, A. , Tröster, S. , Weller, R. and Rhodin, M. (2016) Lungeing on hard and soft surfaces: Movement symmetry of trotting horses considered sound by their owners. Equine Vet. J. 48, 83‐89. [DOI] [PubMed] [Google Scholar]
- 11. Chateau, H. , Camus, M. , Holden‐Douilly, L. , Falala, S. , Ravary, B. , Vergari, C. , Lepley, J. , Denoix, J.‐M. , Pourcelot, P. and Crevier‐Denoix, N. (2013) Kinetics of the forelimb in horses circling on different ground surfaces at the trot. Vet. J. 198, Suppl. 1, e20‐e26. [DOI] [PubMed] [Google Scholar]
- 12. Rhodin, M. , Pfau, T. , Roepstorff, L. and Egenvall, A. (2013) Effect of lungeing on head and pelvic movement asymmetry in horses with induced lameness. Vet. J. 198, Suppl. 1, e39‐e45. [DOI] [PubMed] [Google Scholar]
- 13. Keegan, K.G. , Kramer, J. , Yonezawa, Y. , Maki, H. , Pai, P.F. , Dent, E.V. , Kellerman, T.E. , Wilson, D.A. and Reed, S.K. (2011) Assessment of repeatability of a wireless inertial sensor‐based lameness evaluation system for horses. Am. J. Vet. Res. 72, 1156‐1163. [DOI] [PubMed] [Google Scholar]
- 14. Buchner, H.H.F. , Savelberg, H.H.C.M. , Schamhardt, H.C. and Barneveld, A. (1996) Head and trunk movement adaptations in horses with experimentally induced fore‐ or hindlimb lameness. Equine Vet. J. 28, 71‐76. [DOI] [PubMed] [Google Scholar]
- 15. SAS Institute Inc. (2014) SAS/STAT 13.2 User's Guide, SAS Institute Inc, Cary, North Carolina. [Google Scholar]
- 16. Keegan, K.G. , Wilson, D.A. , Wilson, D.J. , Smith, B. , Gaughan, E.M. , Pleasant, R.S. , Lillich, J.D. , Kramer, J. , Howard, R.D. , Bacon‐Miller, C. , Davis, E.G. , May, K.A. , Cheramie, H.S. , Valentino, W.L. and van Harreveld, P.D. (1998) Evaluation of mild lameness in horses trotting on a treadmill by clinicians and interns or residents and correlation of their assessments with kinematic gait analysis. Am. J. Vet. Res. 59, 1370‐1377. [PubMed] [Google Scholar]
- 17. Keegan, K.G. , Dent, E.V. , Wilson, D.A. , Janicek, J. , Kramer, J. , Lacarrubba, A. , Walsh, D.M. , Cassells, M.W. , Esther, T.M. , Schiltz, P. , Frees, K.E. , Wilhite, C.L. , Clark, J.M. , Pollitt, C.C. , Shaw, R. and Norris, T. (2010) Repeatability of subjective evaluation of lameness in horses. Equine Vet. J. 42, 92‐97. [DOI] [PubMed] [Google Scholar]
- 18. Hammarberg, M. , Egenvall, A. , Pfau, T. and Rhodin, M. (2016) Rater agreement of visual lameness assessment in horses during lungeing. Equine Vet. J. 48, 78‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keegan, K.G. , Macallister, C.G. , Wilson, D.A. , Gedon, C.A. , Kramer, J. , Yonezawa, Y. , Maki, H. and Pai, P.F. (2012) Comparison of an inertial sensor system with a stationary force plate for evaluation of horses with bilateral forelimb lameness. Am. J. Vet. Res. 73, 368‐374. [DOI] [PubMed] [Google Scholar]
- 20. Greve, L. and Dyson, S.J. (2013) An investigation of the relationship between hindlimb lameness and saddle slip. Equine Vet. J. 45, 570‐577. [DOI] [PubMed] [Google Scholar]
- 21. Parkes, R.S.V. , Weller, R. , Groth, A.M. , May, S.A. and Pfau, T. (2009) Evidence of the development of “domain‐restricted” expertise in the recognition of asymmetric motion characteristics of hindlimb lameness in the horse. Equine Vet. J. 41, 112‐117. [DOI] [PubMed] [Google Scholar]
- 22. Farmer, K. , Krueger, K. and Byrne, R.W. (2010) Visual laterality in the domestic horse (Equus caballus) interacting with humans. Anim. Cogn. 13, 229‐238. [DOI] [PubMed] [Google Scholar]
- 23. Licka, T. , Kapaun, M. and Peham, C. (2004) Influence of rider on lameness in trotting horses. Equine Vet. J. 36, 734‐736. [DOI] [PubMed] [Google Scholar]
- 24. Robartes, H. , Fairhurst, H. and Pfau, T. (2013) Head and pelvic movement symmetry in horses during circular motion and in rising trot. Vet. J. 198,Suppl. 1, e52‐e58. [DOI] [PubMed] [Google Scholar]
- 25. Symes, D. and Ellis, R. (2009) A preliminary study into rider asymmetry within equitation. Vet. J. 181, 34‐37. [DOI] [PubMed] [Google Scholar]
- 26. Kramer, J. , Keegan, K.G. , Kelmer, G. and Wilson, D.A. (2004) Objective determination of pelvic movement during hind limb lameness and pelvic height differences. Am. J. Vet. Res. 65, 741‐747. [DOI] [PubMed] [Google Scholar]
- 27. Weishaupt, M.A. , Wiestner, T. , Hogg, H.P. , Jordan, P. and Auer, J.A. (2006) Compensatory load redistribution of horses with induced weight‐bearing forelimb lameness trotting on a treadmill. Vet. J. 171, 135‐146. [DOI] [PubMed] [Google Scholar]
- 28. Kelmer, G. , Keegan, K.G. , Kramer, J. , Wilson, D.A. , Pai, F.P. and Singh, P. (2005) Computer‐assisted kinematic evaluation of induced compensatory movements resembling lameness in horses trotting on a treadmill. Am. J. Vet. Res. 66, 646‐655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Item 1: Multivariable modelling of the association between kinematic data and horse and surface


