Abstract
The aim of the present study was to determine the effects of luseogliflozin on 24‐h glucose levels, assessed by continuous glucose monitoring, and on pharmacodynamic variables measured throughout the day. In this double‐blind, placebo‐controlled, crossover study, 37 patients with type 2 diabetes mellitus inadequately controlled with diet and exercise were randomized into two groups. Patients in each group first received luseogliflozin then placebo for 7 days each, or vice versa. After 7 days of treatment, the mean 24‐h glucose level was significantly lower with luseogliflozin than with placebo [mean (95% confidence interval) 145.9 (134.4–157.5) mg/dl vs 168.5 (156.9–180.0) mg/dl; p < 0.001]. The proportion of time spent with glucose levels ≥70 to ≤180 mg/dl was significantly greater with luseogliflozin than with placebo [median (interquartile range) 83.2 (67.7–96.5)% vs 71.9 (46.9–83.3)%; p < 0.001] without inducing hypoglycaemia. The decrease in glucose levels was accompanied by reductions in serum insulin levels throughout the day.
Keywords: 24‐h glucose variability, continuous glucose monitoring, luseogliflozin, SGLT2 inhibitor, type 2 diabetes
Introduction
Luseogliflozin is a sodium–glucose co‐transporter 2 (SGLT2) inhibitor that was recently approved in Japan for the treatment of type 2 diabetes mellitus (T2DM) 1. In a recent phase III study, luseogliflozin monotherapy significantly reduced glycated haemoglobin (HbA1c) and body weight with a low risk of hypoglycaemia over 24 weeks of treatment in Japanese patients with T2DM 2. Few studies have examined the effect of SGLT2 inhibitors on 24‐h glycaemic variability or the risk of asymptomatic hypoglycaemia 3. Moreover, no studies have described the effects of SGLT2 inhibitors on the changes in serum insulin or plasma glucagon concentrations throughout the day; these data are important in terms of understanding the effects of these drugs on pancreatic α and β cells 4, 5. We conducted a randomized, double‐blind, placebo‐controlled, crossover study to evaluate 24‐h glucose variability, as measured by continuous glucose monitoring (CGM), and the pharmacodynamics of 2.5 mg luseogliflozin administered once daily for 7 days in Japanese patients with T2DM.
Methods
In this double‐blind, placebo‐controlled, crossover study, Japanese patients with T2DM inadequately controlled with diet and exercise (HbA1c 7.0–10.0%) were randomized into two groups. The patients in these groups received luseogliflozin followed by placebo for 7 days each (L/P group), or vice versa (P/L group). Each treatment period was separated by a washout period of 7–14 days. Patients were hospitalized on day 7 and consumed a standardized meal (536 kcal, with ∼20% protein, 25% fat and 55% carbohydrate) at breakfast, lunch and dinner.
The primary endpoints were indices derived from CGM measured on day 7. Glucose concentrations are presented in mg/dl (1 mg/dL = 0.0556 mmol/L). Other efficacy endpoints were pharmacodynamic variables, including serum insulin, plasma glucagon and urinary glucose excretion. Pharmacodynamic variables were measured at 0 (before drug administration), 1, 2, 5 (before lunch), 6, 7, 11 (before dinner), 12, 13, 15 (before bedtime) and 24 h on day 7. Major safety variables were adverse events (AEs), adverse drug reactions, abnormal or unexpected changes in laboratory test values, body weight, vital signs and 12‐lead ECG.
Written informed consent was obtained from all patients before enrolment. The study was approved by the institutional review board of each centre and was conducted in accordance with the ethical principles of the Declaration of Helsinki. The study was registered with the Japan Pharmaceutical Information Centre (identifier: JapicCTI‐142548).
Statistical analyses included calculation of least‐squares mean differences between placebo and luseogliflozin with 95% confidence intervals, which were estimated for each variable in the mixed‐effects model. Non‐normally distributed variables were analysed using the paired non‐parametric Wilcoxon test. The median and interquartile range were estimated for each variable using the Wilcoxon test.
Results
Participants and Baseline Demographics
A total of 37 patients were randomly allocated to either the L/P group or the P/L group, of whom 35 completed the study. Baseline demographics were similar between the groups (Table S1). The safety and the pharmacodynamics analysis set were identical, and both included all 37 allocated patients. Data for 3 patients who did not complete the 24‐h CGM were excluded from all pharmacodynamic analyses.
Pharmacodynamics
The variations in 24‐h glucose levels measured by CGM after 7 days of treatment with luseogliflozin and placebo are shown in Figure 1 and the CGM‐derived indices are shown in Table 1. The glucose levels were consistently lower with luseogliflozin than with placebo throughout the day. The mean 24‐h glucose level and the proportion of time with a glucose level ≥181 mg/dl over 24 h were significantly lower with luseogliflozin than with placebo (both p < 0.001; Figure S1). The area over the curve and the proportion of time spent during the 24 h with a glucose level <70 mg/dl were 0 mg/dl·h and 0%, respectively, with both treatments. Although the M‐value was significantly lower with luseogliflozin, there was no difference in the standard deviation around the mean glucose concentration or mean amplitude of glycaemic excursion values between the two treatments.
Figure 1.
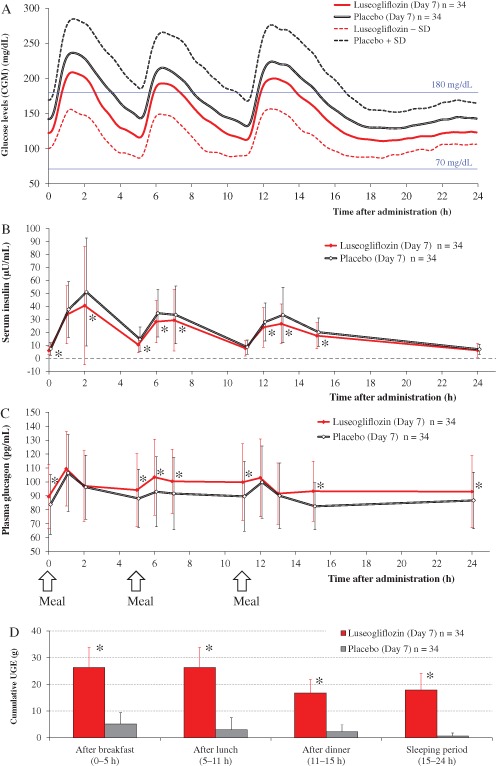
(A) Twenty‐four‐hour plasma glucose levels, as measured by continuous glucose monitoring after 7 days of once‐daily administration of 2.5 mg luseogliflozin or placebo. Values are means ± standard deviation (s.d.). Glucose: 1 mg/dl = 0.0556 mmol/l. (B) Twenty‐four‐hour serum insulin levels after 7 days of once‐daily administration of 2.5 mg luseogliflozin or placebo. Values are means ± s.d. *p < 0.05 for luseogliflozin versus placebo. Insulin: 1 µU/ml = 6.945 pmol/l. (C) Twenty‐four‐hour plasma glucagon levels after 7 days of once‐daily administration of 2.5 mg luseogliflozin or placebo. Values are means ± s.d. *p < 0.05 for luseogliflozin versus placebo. Glucagon: 1 pg/ml = 1 ng/l. (D) Cumulative urinary glucose excretion after 7 days of once‐daily administration of 2.5 mg luseogliflozin or placebo. Values are means ± s.d. *p < 0.05 for luseogliflozin versus placebo.
Table 1.
Twenty‐four‐hour glucose variables
| Variable | n | Placebo | Luseogliflozin | Difference vs placebo | p |
|---|---|---|---|---|---|
| Normally distributed variables | |||||
| 24‐h mean glucose level, mg/dl | 34 | 168.5 (156.9, 180.0) | 145.9 (134.4, 157.5) | −22.5 (−28.2, −16.8) | <0.001 |
| SD over 24 h, mg/dl | 34 | 37.1 (32.8, 41.4) | 35.3 (31.0, 39.6) | −1.8 (−5.5, 1.9) | 0.330 |
| AUC for glycaemic variability, mg/dl·h | |||||
| Throughout the day (0–24 h) | 34 | 4031.9 (3755.7, 4308.1) | 3492.6 (3216.4, 3768.8) | −539.3 (−675.9, −402.7) | <0.001 |
| After breakfast (0–5 h) | 34 | 959.8 (890.2, 1029.4) | 813.8 (744.2, 883.3) | −146.0 (−183.9, −108.1) | <0.001 |
| After lunch (5–11 h) | 34 | 1034.8 (949.5, 1120.1) | 905.8 (820.6, 991.1) | −129.0 (−176.2, −81.8) | <0.001 |
| After dinner (11–15 h) | 34 | 789.0 (729.7, 848.3) | 696.7 (637.4, 756.0) | −92.3 (−128.6, −56.0) | <0.001 |
| Sleeping period (15–24 h) | 34 | 1248.3 (1174.1, 1322.6) | 1076.3 (1002.1, 1150.6) | −172.0 (−215.6, −128.4) | <0.001 |
| Peak glucose level, mg/dl | |||||
| Throughout the day, 0–24 h | 34 | 249.4 (231.7, 267.1) | 226.3 (208.6, 243.9) | −23.1 (−35.6, −10.7) | 0.001 |
| After breakfast, 0–5 h | 34 | 244.9 (227.6, 262.1) | 218.9 (201.7, 236.2) | −25.9 (−39.1, −12.7) | <0.001 |
| After lunch, 5–11 h | 34 | 221.2 (204.3, 238.2) | 199.4 (182.5, 216.4) | −21.8 (−33.4, −10.2) | 0.001 |
| After dinner, 15–24 h | 34 | 230.2 (213.4, 247.0) | 206.7 (190.0, 223.5) | −23.5 (−35.2, −11.8) | <0.001 |
| Lowest glucose level, mg/dl | 34 | 114.8 (106.9, 122.6) | 95.0 (87.1, 102.8) | −19.8 (−26.4, −13.2) | <0.001 |
| Mean amplitude of glycaemic excursions, mg/dl | 34 | 92.6 (82.1, 103.2) | 90.7 (80.2, 101.3) | −1.9 (−12.6, 8.8) | 0.719 |
| Non‐normally distributed variables | |||||
| Proportion of time over 24 h with glucose levels in the following ranges, % | |||||
| ≥181 mg/dl | 34 | 28.1 (16.7, 53.1) | 16.3 (2.8, 32.3) | −14.8 (−20.1, −3.8) | <0.001 |
| ≥70 to ≤180 mg/dl | 34 | 71.9 (46.9, 83.3) | 83.2 (67.7, 96.5) | 14.4 (1.4, 20.1) | <0.001 |
| <70 mg/dl | 34 | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.125 |
| AUC for glucose levels ≥181 mg/dl, mg/dl·h | 34 | 149.2 (66.9, 561.7) | 80.2 (1.5, 209.0) | −77.3 (−308.5, −6.4) | <0.001 |
| AOC for glucose levels <70 mg/dl, mg/dl·h | 34 | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.125 |
| M‐value | 34 | 12.5 (6.2, 27.5) | 6.9 (3.1, 13.9) | −5.1 (−12.9, −1.8) | <0.001 |
Values are derived from 24‐h continuous glucose monitoring performed after 7 days of once‐daily administration of 2.5 mg luseogliflozin or placebo. Normally distributed variables are presented as means and 95% confidence intervals, and the differences between the two treatments were analysed using a mixed‐effects model. Non‐normally distributed variables are presented as medians (interquartile range), and differences between the two treatments were determined using the paired Wilcoxon test. Data are shown for the pharmacodynamic analysis set. Glucose: 1 mg/dl = 0.0556 mmol/l. AOC, area over the curve; AUC, area under the curve; SD, standard deviation around the mean glucose concentration.
In all periods, luseogliflozin significantly increased cumulative urinary glucose excretion compared with placebo (all p < 0.05; Figure 1, Table S2).
The serum insulin and plasma glucagon levels on day 7 in each treatment period are shown in Figure 1. Pharmacodynamic variables [serum insulin, plasma glucagon and ketone bodies (acetoacetic acid and β‐hydroxybutanoic acid)] are summarized in Table S2. Serum insulin levels were lower throughout the 24‐h measurement period with luseogliflozin than with placebo, and the area under the curve (AUC) over 24 h was significantly smaller with luseogliflozin than with placebo (p < 0.001). Plasma glucagon levels were higher throughout the 24‐h measurement period with luseogliflozin than with placebo. The serum ketone body levels were higher throughout the day with luseogliflozin than with placebo. The serum ketone body levels decreased after each meal during the luseogliflozin administration period (Figure S2).
Safety
Nine AEs occurred in 6 patients during administration of luseogliflozin, and 11 AEs occurred in 7 patients during administration of placebo (Table S3). One patient died from malignant lymphoma, which was judged to be unrelated to the study drug because relevant clinical variables were elevated before taking the study drug. All of the other AEs were mild in severity and there were no serious AEs leading to study discontinuation. No episodes of hypoglycaemia were reported. Table S4 shows the clinical laboratory variables and Table S5 shows urine volume and water intake.
Discussion
In the present study, luseogliflozin increased cumulative urinary glucose excretion and lowered glucose levels at all measured time points over the 24‐h period, and significantly reduced the AUCs for glucose compared with placebo. These results indicate that once‐daily administration of luseogliflozin reduces glucose levels throughout the day, including overnight. Moreover, luseogliflozin reduced the peak glucose levels, lowest glucose levels and 24‐h mean glucose levels almost equally. The mean amplitude of glycaemic excursions value was not significantly different between the two treatments. It seems that luseogliflozin caused a downward shift in the 24‐h glucose profile by reducing fasting and postprandial glucose levels; however, as variable responses in FPG and PPG have been observed with luseogliflozin in other clinical trials in Japanese patients with T2DM 2, 6, 7, further CGM studies of SGLT2 inhibitors in patients with T2DM are necessary to clarify the effects of SGLT2 inhibitors on mean amplitude of glycaemic excursions. The present results also indicate that luseogliflozin monotherapy is associated with a low risk of hypoglycaemia because the proportion of time spent with glucose levels of 70–180 mg/dl was increased without an increase in the proportion of time spent with glucose levels <70 mg/dl. Furthermore, no episodes of hypoglycaemia were observed in this study. Notably, the reduction in glucose levels was accompanied by reductions in serum insulin levels throughout the day, and the AUCs for serum insulin were significantly lower with luseogliflozin than with placebo. As we observed in previous clinical pharmacology studies 8, luseogliflozin inhibited glucose reuptake via the renal proximal tubules, increased urinary glucose excretion, decreased glucose levels, and reduced insulin requirements 9, 10; therefore, luseogliflozin has a potential advantage over other therapies because it may improve glucose levels and reduce insulin secretion. The reduction in the insulin requirement in luseogliflozin‐treated patients suggests that it may be possible to reduce the insulin doses in patients who need insulin therapy when using luseogliflozin.
In conclusion, the 24‐h glucose levels, measured by CGM, and pharmacodynamic variables throughout the day in the present study indicate that luseogliflozin could lead to favourable improvements in glycaemic control without inducing hypoglycaemia or increasing the burden on pancreatic β cells, and that luseogliflozin could be a valuable treatment option for T2DM.
Conflict of Interest
R. N. has received consultancy fees or lecture fees from Abbott Diabetes Care, Inc., Astellas Pharma US, Inc., AstraZeneca Pharmaceuticals LP, Boehringer Ingelheim Pharmaceuticals, Inc., Eli Lilly Japan K.K., Kissei Pharmaceutical Co., Ltd., Novartis Corporation, Novo Nordisk Pharma Ltd, Sanofi K.K., Taisho Pharmaceutical Co., Ltd, Taisho Toyama Pharmaceutical Co., Ltd and Takeda Pharmaceutical Company Limited. T. O. has received research support fees or lecture fees from AbbVie GK., Astellas Pharma US, Inc., Bayer HealthCare, Eli Lilly Japan K.K., GlaxoSmithKline, Kowa Pharmaceuticals, Mitsubishi Tanabe Pharma Corporation, Novo Nordisk Pharma Ltd, Sanwa Kagaku Kenkyusho Co., Ltd, Sumitomo Dainippon Pharma Co., Ltd, Taisho Pharmaceutical Co., Ltd and Takeda Pharmaceutical Company Limited. S. K. has received research support fees from Daiichi‐Sankyo Co., Ltd., Kissei Pharmaceutical Co., Ltd., MSD, K.K., Novo Nordisk Pharma Ltd., Sanofi K.K. and Taisho Pharmaceutical Co., Ltd. H. J. has received consultancy fees, research support fees or lecture fees from Astellas Pharma US, Inc., AstraZeneca Pharmaceuticals LP, Boehringer Ingelheim Pharmaceuticals, Inc., Daiichi Sankyo Co., Ltd., Eli Lilly Japan K.K., GlaxoSmithKline, Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Novo Nordisk Pharma Ltd., Sanofi K.K., Kowa Pharmaceuticals, Sumitomo Dainippon Pharma Co., Ltd., Mochida Pharmaceutical Co., Ltd., Novartis Pharmaceuticals Corporation, MSD, K.K., Taisho Pharmaceutical Co., Ltd and Takeda Pharmaceutical Company Limited. K. S., H. O., M. U., S. S. and Y. S. are employees of Taisho Pharmaceutical Co., Ltd., which is developing luseogliflozin.
R. N., K. S., H. O., M. U., S. S. and Y. S. designed the study and wrote the manuscript. T. O., S. K. and H. J. participated in data collection. K. S., H. O., M. U., S. S. and Y. S. secured the research funding. All authors have read and approved the final manuscript.
Supporting information
Figure S1 . Proportion of time over 24 h with glucose levels ≥181, ≥70 to ≤180 or <70 mg/dl, as measured by continuous glucose monitoring.
Figure S2. Twenty‐four‐hour serum acetoacetic acid (A) and serum β‐hydroxybutanoic acid (B) levels after 7 days of once‐daily administration of 2.5 mg luseogliflozin or placebo.
Table S1. Patient characteristics.
Table S2. Pharmacodynamic variables.
Table S3. Summary of adverse events.
Table S4. Clinical laboratory variables.
Table S5. Urine volume and water intake on day 7.
Acknowledgements
This study was supported by Taisho Pharmaceutical Co., Ltd, Tokyo, Japan. The authors wish to thank Nicholas D. Smith, PhD, of Edanz Group Ltd., for providing editorial support, which was funded by Taisho Pharmaceutical Co., Ltd.
References
- 1. Markham A, Elkinson S. Luseogliflozin: first global approval. Drugs 2014; 74: 945–950. [DOI] [PubMed] [Google Scholar]
- 2. Seino Y, Sasaki T, Fukatsu A, Ubukata M, Sakai S, Samukawa Y. Efficacy and safety of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, double‐blind, placebo‐controlled, phase 3 study. Curr Med Res Opin 2014; 30: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 3. Nishimura R, Tanaka Y, Koiwai K et al. Effect of empagliflozin monotherapy on postprandial glucose and 24‐hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double‐blind, placebo‐controlled, 4‐week study. Cardiovasc Diabetol 2015; 14: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol 2012; 8: 495–502. [DOI] [PubMed] [Google Scholar]
- 5. Jurczak MJ, Lee HY, Birkenfeld AL et al. SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta‐cell function. Diabetes 2011; 60: 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seino Y, Sasaki T, Fukatsu A, Sakai S, Samukawa Y. Efficacy and safety of luseogliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a 12‐week, randomized, placebo‐controlled, phase II study. Curr Med Res Opin 2014; 30: 1219–1230. [DOI] [PubMed] [Google Scholar]
- 7. Seino Y, Sasaki T, Fukatsu A, Ubukata M, Sakai S, Samukawa Y. Dose‐finding study of luseogliflozin in Japanese patients with type 2 diabetes mellitus: a 12‐week, randomized, double‐blind, placebo‐controlled, phase II study. Curr Med Res Opin 2014; 30: 1231–1244. [DOI] [PubMed] [Google Scholar]
- 8. Sasaki T, Seino Y, Fukatsu A, Samukawa Y, Sakai S, Watanabe T. TS‐071, a novel potent and highly selective renal sodium‐glucose co‐transporter 2 (SGLT2) inhibitor, increases urinary glucose excretion and reduces plasma glucose levels in Japanese patients with type 2 diabetes mellitus. Diabetologia 2011; 54: S345–S346. [Google Scholar]
- 9. Yamamoto K, Uchida S, Kitano K et al. TS‐071 is a novel, potent and selective renal sodium‐glucose cotransporter 2 (SGLT2) inhibitor with anti‐hyperglycaemic activity. Br J Pharmacol 2011; 164: 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Musso G, Gambino R, Cassader M, Pagano G. A novel approach to control hyperglycemia in type 2 diabetes: sodium glucose co‐transport (SGLT) inhibitors: systematic review and meta‐analysis of randomized trials. Ann Med 2012; 44: 375–393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 . Proportion of time over 24 h with glucose levels ≥181, ≥70 to ≤180 or <70 mg/dl, as measured by continuous glucose monitoring.
Figure S2. Twenty‐four‐hour serum acetoacetic acid (A) and serum β‐hydroxybutanoic acid (B) levels after 7 days of once‐daily administration of 2.5 mg luseogliflozin or placebo.
Table S1. Patient characteristics.
Table S2. Pharmacodynamic variables.
Table S3. Summary of adverse events.
Table S4. Clinical laboratory variables.
Table S5. Urine volume and water intake on day 7.


