Abstract
Aims
In chronic heart failure (CHF), changes in cardiac function define the course of the disease. The cardiac index (CI) is the most adequate indicator of cardiac function. Interpretation of serial CI measurements, however, requires knowledge of the biological variation of CI. Because measurements of CI can be confounded by the clinical situation or the method applied, biological variation might be subject to the same confounders.
Methods and results
We prospectively included 50 CHF patients who met rigid criteria for clinical stability. CI was measured by both inert gas rebreathing (IGR) and impedance cardiography (ICG) in weekly intervals over 3 weeks—each measurement performed at rest (IGRrest/ICGrest) and during low‐exercise 10 Watt pedalling (IGR10W/ICG10W). Intra‐class correlation coefficients (ICCs), reference change values, and minimal important differences of CI were determined for IGRrest, ICGrest, IGR10W, and ICG10W. Impedance cardiography and IGR showed moderate agreement at rest (20% (6–36)) and good agreement at 10 Watt (−4% (−23–16)). Depending on time interval, measurement modality for CI, and mode, ICC ranged between 0.42 and 0.78, ICC values for IGR were lower than those for ICG. Reference change value ranged between 3 and 15%, and minimal important difference ranged between 0.2 and 0.5 L/min/m2. Values for IGR were lower at rest and higher at 10 Watt than those for ICG.
Conclusion
Non‐invasive measurements of CI are stable over time. Measurement modalities for CI, however, are not interchangeable. Biological variation is less pronounced when obtained by ICG. The influence of low‐level exercise on stability of CI depends on the measurement modality.
Keywords: Cardiac index, Inert gas rebreathing, Impedance cardiography, Biological variation, Chronic heart failure
Introduction
Hemodynamic variables such as the cardiac index (CI) provide valuable diagnostic and prognostic information in the management of patients with chronic heart failure (CHF).1 The gold standard methods for measuring hemodynamic variables are thermodilution and the direct Fick's method. However, due to the invasive nature of these methods, they are only rarely used in the routine monitoring of CHF patients. The Fick method has been largely replaced by the inert gas rebreathing (IGR) and impedance cardiography (ICG) methods, which allow non‐invasive measurement of hemodynamic variables including the cardiac index.
Several studies have shown high precision and acceptable agreement between hemodynamic variables measured by IGR or ICG with those obtained by thermodilution,2, 3, 4 while other studies have suggested they are not sufficiently accurate.5, 6, 7, 8 However, many of these studies were conducted in unstable patients in a pre‐operative or critical care setting.4, 6, 7 Very few studies have investigated the precision and accuracy of non‐invasive methods for the measurement of hemodynamic variables in patients with stable CHF.2, 3, 8 Moreover, in some studies that used non‐invasive methods, their clinical applicability was based on single point measurements rather than time‐dependent trends in values for hemodynamic variables.7, 9 This may limit the applicability of the IGR and ICG methods in these studies, since they may be more accurate at measuring changes rather than absolute values in hemodynamic variables.8
The use of hemodynamic data for disease monitoring in CHF patients requires knowledge of the effect of biological variation on values for hemodynamic variables so that changes in these values during the course of the patient's treatment can be interpreted correctly as either a change due to worsening or improvement in the patient's disease or change due to inherent biological variation.
Biological variation can be regarded as the random variation that occurs around a homeostatic set point for a particular variable. Within‐subject biological variation refers to all biological (non‐disease‐related) sources of variation that can alter any individual's test results, including, but not limited to the following: seasonal and geographic variation, gender, and pulsatile and circadian biorhythms.10 It is inherent to any biological system and can only be measured in the strict absence of any change induced by disease instability or intervention.
Due to their close and immediate neurohormonal regulation, hemodynamic variables may be confounded by the clinical situation during measurement.11, 12, 13 The biological variation of hemodynamic variables might therefore be subject to the same confounders. As only little is known about the biological variation of values for CI and its potential confounders in CHF patients,14, 15 we determined the repeatability (intra‐class correlation coefficient (ICC)), reference change value (RCV), and minimal important difference (MID)—the most commonly accepted parameters for assessing biological variation— in a cohort of stable CHF patients whose CI was measured by IGR and ICG—both at rest and during low‐level exercise.
Methods
Patients
We prospectively recruited 50 patients with stable CHF from the heart failure outpatient clinic of the University Hospital, Heidelberg, Germany. The diagnosis of heart failure was established according to published guidelines.16 To be eligible for this study, patients had to meet all of the following 11 criteria: (i) CHF diagnosis and at least 1 year of follow‐up at our clinic; (ii) stable clinical condition with no hospitalization attributable to worsening heart failure within the previous 12 weeks; (iii) New York Heart Association (NYHA) functional class I–III; (iv) subjectively stable clinical condition since the last visit to the outpatient clinic, as judged both by the patient and the physician; (v) ischemic or idiopathic dilated cardiomyopathy as the aetiology of CHF; (vi) in case of ischemic origin, no revascularization planned during the next 8 weeks; (vii) age >18 years; (viii) complete adherence to guidelines of medical treatment regarding class of drugs; (ix) individually optimized doses of guideline‐recommended drugs for at least 4 weeks before study inclusion; (x) no recent involuntary change of weight exceeding 2 kg within 4 weeks before inclusion; and (xi) stability of rhythm between visits.
The study conformed to the principles of the declaration of Helsinki and was approved by the local ethics committee.
Study protocol
After the screening visit (V0), patients were seen in our clinic for three study visits (V1, V2, and V3) at weekly intervals (7 ± 1 day). All visits included performance of the following: patient history; physical examination; collection of blood samples; 12‐lead electrocardiogram (ECG); read‐out of external ECG event recorder (at follow‐up visits); and non‐invasive testing for CI using both the IGR and ICG methods at rest and during low (10 Watt) exercise pedalling. Altogether, 12 measurements of CI were performed in each of the 50 patients (four measurements at each visit).
Patients with sinus rhythm or a pacemaker rhythm at V1 were provided with an external tele‐ECG loop recorder (Vitaphone® Tele‐ECG‐Loop‐Recorder 3100 BT and Vitaphone® Tele‐ECG‐Loop‐Recorder 3300 BT, vitaphone GmbH, Mannheim, Germany) in order to exclude intermittent atrial fibrillation. Tele‐ECG loop recorder monitoring was continued until V3.
Hemodynamic measurements were performed in the mornings after overnight fasting using Innocor®, Innovision, Odense, Denmark for IGR and CardioScreen 2000, Medis GmbH, Ilmenau, Germany for ICG. After 10 min of rest in a semi‐recumbent position on an ergometer, CI was measured by both IGR and ICG (IGRrest and ICGrest). Subsequently, patients started low‐exercise pedalling at 10 Watt with a pedalling rate of 50–60/min. After 3.5 min of pedalling, IGR and ICG measurements were repeated (IGR10W and ICG10W).
Inert gas rebreathing
Rebreathings were performed in a closed system, which consisted of a three‐way respiratory valve connecting a facemask, an anti‐static rubber bag, and an infrared photo‐acoustic gas analyser. Patients rebreathed a gas mixture of nitrous oxide (N2O) (0.5%) and sulphur hexafluoride (0.1%) in oxygen, diluted with atmospheric air, from an anaesthesia bag of size 3–6 L, depending on patient's sex, height, and age. The rebreathing manoeuvre was started after a normal expiration at a breathing rate of 20/min. A constant ventilation rate was ensured by having the patient breathe in synchrony with a graphical tachometer on the computer screen, and a constant ventilation volume was ensured by requesting the patient to empty the rebreathing bag completely with each breath. Rebreathing was typically performed over 5–8 breaths, of which the last 2–3 breaths were used for the calculation of pulmonary blood flow and CI. Between the measurements, an interval of 5 min was strictly adhered in order to guarantee the complete elimination of N2O. The details of CI calculations using Innocor® IGR (Innovision) have been described previously.9
Impedance cardiography
According to the manufacturer's guidelines, four pairs of standard electrocardiographic electrodes were placed at both sides of the neck and both sides of the inferior aspect of the thorax at the level of the xiphoid process with an inter‐electrode gap of 5 cm. Verification of the correct signal quality was accomplished by visualization of the ECG, the impedance waveform, and its first derivative. Detailed descriptions of ICG parameters and their calculations have been published elsewhere.17
Statistics
Agreement between impedance cardiography and inert gas rebreathing
The agreement of CI measurements between IGR and ICG was analysed by calculating both Pearson's correlation coefficients and standardized mean differences (SMDs) of CI values obtained by IGR and ICG. The SMD is used for contrasting two groups on a continuous dependent variable. It is defined as SMD = (difference in mean outcome/variable of interest between groups)/(standard deviation of outcome/variable of interest among participants).18
The pooled standard deviation adjusts the differences between groups for both the scale and precision of measurement and the size of the population sample used.19 Cohen offered the following guidelines for interpreting the magnitude of the SMD: small, SMD <0.2; medium, SMD ≈0.5; and large, SMD ≈0.8.20
Coefficient of variation and reference change value
The coefficient of variation (CV) was calculated as follows: CV = 100 × (standard deviation/mean). The total CV (CVt) and the analytical CV (CVa) provided the basis for calculating the individual biological CV (CVi), where
Values for CVa were taken from the technical manuals of the IGR and ICG devices. Values for CV considered indicative of low, moderate, and high imprecision were <6%, 6–10%, and >10%, respectively.15
Reference change values were calculated from median CVt values, according to the formula:
where Z = 1.96 (i.e. the Z‐score for 95% confidence level with a 2‐tailed P <0.05), na is the number of replicate assays, and ns is the number of patient samples.
Within‐subject variation was calculated and reported using an intra‐class correlation. The ICC was determined using CI measurements at V1 and V2, and it was calculated separately for IGR and ICG measurements. Following Rosner,21 we suggest that ICC <0.4 indicates poor reliability, 0.4 ≤ICC <0.75 as fair to good reliability and ICC ≥0.75 as excellent reliability.
Minimal important difference
The MID was determined using the one‐standard error of measurement (one‐SEM)‐based approach developed by Wyrwich et al. 22 following the equation:
where SD is the population standard deviation, SQRT is the abbreviation for square‐root, and r is the reliability coefficient (i.e. the degree of absolute agreement among measurements). We selected the ICC as the reliability coefficient used in the earlier equation because it accounts for the proportion of variance in test values due to between‐subject variation; it is simple to calculate; and it provides an estimate of MID that is in good agreement with other methods.22, 23
The relationship between relative changes in CI and the respective CI value at V1 was assessed following the method proposed by Bland and Altman.24 Analyses were carried out separately for measurements at rest and at low‐exercise pedalling. Calculations were obtained using MedCalc software version 12.7 (Ostend, Belgium) and results were displayed using GraphPad Prism version 6.02 for Windows (GraphPad Software, La Jolla, CA, USA). A P‐value of 0.05 was regarded as statistically significant.
Subgroup analysis
All analyses were repeated in the subgroup of patients with atrial fibrillation at baseline. In addition, heart rate (HR) measurements obtained by either IGR or ICG were analysed in this subgroup.
Results
Patient characteristics
The clinical characteristics for all patients are shown in Table 1. Although the mean left ventricular ejection fraction was significantly reduced, the majority of patients were in NYHA class I and II. Correspondingly, the mean 6 min walking distance was mildly reduced. The majority of patients were in sinus rhythm.
Table 1.
Baseline characteristics
| Characteristica | Value (n (%)) |
|---|---|
| Men | 42 (84) |
| Age (years) | 63 ± 11 |
| Aetiology | |
| Ischemic | 27 (54) |
| Dilated cardiomyopathy | 23 (46) |
| Co‐morbidity | |
| Hypertension | 49 (98) |
| Diabetes | 17 (34) |
| Dyslipidaemia | 34 (68) |
| COPD | 2 (4) |
| Systolic BP (mmHg) | 125 ± 18 |
| Diastolic BP (mmHg) | 76 ± 10 |
| Heart rate (1/min) | 66 ± 8 |
| Heart rhythm | |
| Sinus rhythm | 41 (82) |
| Atrial fibrillation | 8 (16) |
| Pacemaker | 1 (2) |
| BMI (kg/m2) | 29 ± 5 |
| NYHA | |
| I | 17 (35) |
| II | 28 (57) |
| III | 4 (8) |
| LVEF | 35 ± 7 |
| 6 MWT (m) | 491 ± 91 |
| Sodium (mmol/L (135–145)) | 140 ± 3 |
| Potassium (mmol/L (3.5–4.8)) | 4.4 ± 0.5 |
| eGFR (mL/min/1.73m2 (90–150)) | 79 ± 22 |
| Urea (mmol/L (<7.515)) | 6.85 ± 2.17 |
| Hemoglobin (mmol/L (♂: 8.073–10.557; ♀: 7.452–9.315)) | 8.82 ± 0.75 |
| Treatment | |
| Beta blocker | 49 (98) |
| ACE inhibitor | 32 (64) |
| ARB | 21 (42) |
| Aldosterone Antagonist | 25 (50) |
| Ivabradine | 3 (6) |
| Aspirine | 16 (32) |
| ICD | 14 (28) |
| Pacemaker | 1 (2) |
| CRT | 3 (6) |
Values in parentheses represent normal range values in the local laboratory.
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate using the Modification of Diet in Renal Disease equation; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association functional class; 6 MWT, 6 minute walk test;.
Tele‐electrocardiogram‐loop‐recorder
Tele‐ECG‐loop‐recorder monitoring was provided in 42 patients with sinus rhythm or pacemaker activity at V1. No episode of atrial fibrillation was detected in any of these patients during 2 weeks of monitoring.
Measurements of cardiac index
Overall, 575 CI measurements were performed in 50 patients. There was one missing CI measurement in 25 patients. Results of serial CI measurements with respect to the method applied (IGRrest/ ICGrest/ IGR10 W/ ICG10 W) are shown in Table 2. CI measured at rest was lower as compared with measurements at 10 Watt pedalling irrespective of the method of determination applied (P <0.001 for IGRrest vs. IGR10W and P <0.001 for ICGrest vs. ICG10W).
Table 2.
Measurements of cardiac index at the respective visit, performed at rest and at 10 Watt pedalling, separate for method of determination (inert gas rebreathing and impedance cardiography)
| CI (V1) (L/min/m2) | CI (V2) (L/min/m2) | CI (V3) (L/min/m2) | CImean (L/min/m2) | P‐value | |
|---|---|---|---|---|---|
| IGRrest | 2.19 ± 0.55 | 2.33 ± 0.67 | 2.40 ± 0.89 | 2.30 ± 0.71 | 0.36 |
| ICGrest | 2.89 ± 0.41 | 2.86 ± 0.48 | 2.82 ± 0.41 | 2.86 ± 0.43 | 0.76 |
| IGR10W | 3.18 ± 0.73 | 3.07 ± 0.72 | 3.14 ± 0.77 | 3.13 ± 0.74 | 0.73 |
| ICG10W | 2.98 ± 0.61 | 3.01 ± 0.51 | 3.01 ± 0.49 | 3.00 ± 0.54 | 0.96 |
CI, cardiac index; V1, visit 1; V2, visit 2 (1 week after visit 1); V3, visit 3 (2 weeks after visit 1); IGRrest, inert gas rebreathing measured at rest; IGR10W, inert gas rebreathing measured at 10 Watt pedalling; ICGrest, impedance cardiography measured at rest; ICG10W, impedance cardiography measured at 10 Watt pedalling.
Values are presented as mean ± standard deviation. The P‐value corresponds to the analysis of covariance of CI at V1, V2, and V3.
Agreement of inert gas rebreathing and impedance cardiography
The Pearson's correlation coefficients were r = 0.10 for ICGrest vs. IGRrest (P = 0.23) and r = 0.24 for ICG10W vs. IGR10W (P < 0.01). At rest, mean CI derived by ICG was significantly higher as compared with IGR (2.86 ± 0.43 vs. 2.30 ± 0.71 L/min/m2; P < 0.001). The SMD of ICGrest vs. IGRrest was 20.27%. In contrast to rest measurements, ICG measurements at 10 Watt pedalling resulted in significantly lower CI values as compared with IGR (3.00 ± 0.54 vs. 3.13 ± 0.74 L/min/m2; P = 0.02). The SMD of ICG10W vs. IGR10W was −7.45%.
Stability of measurements, reference change values and minimal important differences
The CV was <6% with all measurement strategies, indicating low biological variability. The respective ICCs ranged between 0.416 and 0.780, showing acceptable reproducibility of measurements. The Bland–Altman plots of the individual absolute differences of CI measurements between V1 and V3 vs. the respective mean values are depicted in Figure 1. CVs, RCVs, ICCs, and MIDs of CI with respect to the measurement strategy applied are shown in Tables 3 (V1 vs. V2) and 4 (V1 vs. V3). Overall, the biological variation of CI was lowest when measured by ICG at rest, while it was highest for IGR measurements at rest.
Figure 1.
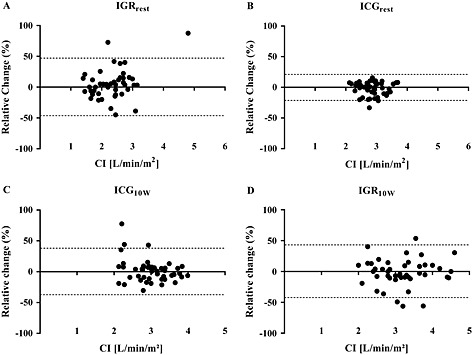
Bland–Altman plot for CI values between V1 and V3 for IGRrest (A), ICGrest (B), IGR10W (C), or ICG10W (D). CI, cardiac index; V1, first study visit; V3, third study visit; IGRrest, inert gas rebreathing measured at rest; ICGrest, impedance cardiography measured at rest; IGR10W, inert gas rebreathing measured at low‐exercise pedalling; ICG10W, impedance cardiography measured at low‐exercise pedalling.
Table 3.
Coefficients of variation, reference change values and minimal important differences of cardiac index measurements performed at rest and at 10 Watt pedalling using inert gas rebreathing and impedance cardiography (visit 1 vs. visit 2)
| IGRrest (%) | ICGrest (%) | IGR10W (%) | ICG10W (%) | |
|---|---|---|---|---|
| CVa (%) | 2 | 2 | 2 | 2 |
| CVi (%) | 5.44 (2.82–9.78) | 1.12 (0.65–4.26) | 3.80 (0.86–8.48) | 3.34 (1.76–6.56) |
| CVt (%) | 5.80 (2.99–9.98) | 2.29 (1.19–4.71) | 4.29 (1.32–8.71) | 3.89 (2.02–6.86) |
| RCV (L/min/m2) | 0.33 | 0.10 | 0.34 | 0.28 |
| RCV (% of CI) | 15.14 | 3.32 | 10.59 | 9.33 |
| ICC | 0.532 | 0.689 | 0.780 | 0.665 |
| MID (L/min/m2) | 0.37 | 0.22 | 0.34 | 0.35 |
| MID (% of CI) | 15.87 | 7.76 | 11.02 | 11.78 |
CI, cardiac index; CV, coefficient of variation; CVa, analytical CV; CVi, individual CV; CVt; total CV; ICC, intraclass correlation coefficient; ICGrest, impedance cardiography measured at rest; ICG10W, impedance cardiography measured at 10 Watt pedalling; IGRrest, inert gas rebreathing measured at rest; IGR10W, inert gas rebreathing measured at 10 Watt pedalling; RCV, reference change value; MID, minimal important difference.
Values are represented as median (interquartile range).
Table 4.
Coefficients of variation, reference change values and minimal important differences of cardiac index measurements performed at rest and at 10 Watt pedalling using inert gas rebreathing and impedance cardiography (visit 1 vs. visit 3)
| IGRrest (%) | ICGrest (%) | IGR10W (%) | ICG10W (%) | |
|---|---|---|---|---|
| CVa (%) | 2 | 2 | 2 | 2 |
| CVi (%) | 5.53 (2.32–9.89) | 2.99 (1.70–4.87) | 4.63 (3.02–9.38) | 3.71 (2.58–6.96) |
| CVt (%) | 5.88 (2.53–10.09) | 3.60 (1.97–5.26) | 5.04 (3.18–9.59) | 4.21 (2.77–7.24) |
| RCV (L/min/m2) | 0.37 | 0.24 | 0.41 | 0.31 |
| RCV (% of CI) | 15.37 | 8.39 | 12.88 | 10.34 |
| ICC | 0.416 | 0.713 | 0.544 | 0.622 |
| MID (L/min/m2) | 0.41 | 0.22 | 0.49 | 0.37 |
| MID (% of CI) | 17.73 | 7.46 | 15.86 | 12.51 |
CI, cardiac index; CV, coefficient of variation; CVa, analytical CV; CVi, individual CV; CVt; total CV; ICC, intraclass correlation coefficient; ICGrest, impedance cardiography measured at rest; ICG10W, impedance cardiography measured at 10 Watt pedalling; IGRrest, inert gas rebreathing measured at rest; IGR10W, inert gas rebreathing measured at 10 Watt pedalling; RCV, reference change value; MID, minimal important difference.
Values are represented as median (interquartile range).
Subgroup analysis
A total of eight patients (16%) had atrial fibrillation in the baseline ECG. Mean resting HR at V1 was 73 ± 7/min as compared with 64 ± 7/min in patients with sinus rhythm (P <0.01). HR measured by IGRrest did not differ from HR measurement obtained by ICGrest (P = 0.56). In contrast, HR at low exercise pedalling obtained by IGR10W was significantly higher when compared with measurements using ICG10W (P = 0.03). HR measurements were stable for each method over time (Supporting information, Table S1).
Analyses of CI measurements in patients with atrial fibrillation mainly confirmed the results from the general cohort. Again, CI measurements were stable over time for each method (Table S2). The agreement of ICG and IGR measurements, however, was poor, while CI measured by IGRrest was lower as compared with IGR10W (P <0.0001), this was not true for ICG measurements (P = 0.62 for ICGrest vs. ICG10W). ICG measurements both at rest and at low‐exercise pedalling resulted in higher CI values as compared with IGR measurements (P <0.001 for ICGrest vs. IGRrest and P = 0.02 for ICG10W vs. IGR10W, respectively). The Pearson's correlation coefficients were r = 0.01 for ICGrest vs. IGRrest (P = 0.96) and r = 0.11 for ICG10W vs. IGR10W (P = 0.65). The SMD of ICGrest vs. IGRrest was 37.43%, while it was 8.65% for ICG10W vs. IGR10W.
In patients with atrial fibrillation, the CV ranged between 1 and 8%, indicating low to moderate biological variability. The respective ICCs were >0.70 in the majority of cases, showing good reproducibility of measurements. The 2 week reproducibility of ICG measurements at low‐exercise pedalling, however, was poor. CVs, RCVs, ICCs, and MIDs of CI in patients with atrial fibrillation with respect to the measurement strategy applied are shown in Tables S3 (V1 vs. V2) and S4 (V1 vs. V3). As in the general cohort, the biological variation of CI was lowest when measured by ICG at rest.
Discussion
Non‐invasive measurement of hemodynamic variables, especially CI, is a promising tool in the monitoring of CHF patients. Interpretation of change in values for these variables, however, requires knowledge of the biological variation of the respective hemodynamic variables in the population of interest. We determined RCV and MID for CI in CHF patients while accounting both for the method of determination and the possibly confounding effect of the measurement setting. Our main results are as follows:
In stable CHF patients, intermediate‐term reproducibility of non‐invasive CI measurements is acceptable for all measurement strategies investigated.
The agreement of IGR and ICG measurements at rest is moderate. Although it improves when measurements are performed during low‐exercise pedalling, CI values obtained by IGR and ICG were still significantly different and may therefore not be used interchangeably.
The biological variation of CI is lower when obtained by ICG than by IGR.
Low‐level exercise (10 Watt) lowers biological variation for IGR but increases it for ICG, resulting in similar biological variation for both methods during 10 Watt.
These results were essentially unchanged in the subgroup of patients with atrial fibrillation at baseline.
We chose IGR and ICG to determine CI because of their non‐invasive nature, ease of use, reproducibility, and accuracy, making them ideally suited for routine clinical use.2, 3, 4 In addition, several studies have reported that these methods are also useful in the non‐invasive measurement of cardiac output (CO) levels in cardiac patients.4, 9, 17, 25 In addition, studies have shown that IGR provides reliable measurements of CO during cardiopulmonary exercise in healthy subjects.26
Our study is the first to describe the biological variation of CI expressed as either RCV or MID in stable CHF patients. We prefer to use CI over CO measurement as it facilitates between‐subject comparison of cardiac function. In addition, CI, but not CO, is accepted as a major criterion for high‐urgency heart transplantation in the Eurotransplant.27
There are little data on the biological variability of CO measurements.14, 15, 26, 28, 29 Moreover, studies addressing this issue differ significantly in design, patient selection, and methods for measuring CO.26, 28, 29 We are aware of only two studies on the variation of values for CO in CHF patients: Moser et al. performed thermodilution over 2 hours in 39 patients with advanced CHF,14 while Jakovljevic et al. reported the reproducibility of CO over a 1 week interval in 19 CHF patients who underwent IGR at rest and during near‐maximal exercise.15 The CVs ranged from 3.4 to 7.2% at rest and 5.4% during exercise. These values correspond well to those obtained in our study.
Our study contributes significantly to both the understanding of measurement principles and the concept of biological variation in the measurement of CI for the following reasons: we assessed the variation of values for CI over longer intervals than previous studies; our patient cohort consisted of carefully selected CHF patients who met rigid criteria for heart failure stability; we accounted for the influence of both measurement technique and physical activity on values for CI; and we determined values for biological variation using two different approaches—RCV and MID. In addition, our study is the first to provide a separate subgroup analysis of stable CHF patients with atrial fibrillation.
In our study, CI values obtained by ICGrest were significantly higher than values obtained by IGRrest irrespective of heart rhythm, and agreement between values for ICGrest and IGRrest was moderate. This latter finding corresponds to the findings in the study by Trinkmann et al. who also reported acceptable agreement between ICG and IGR methods in the determination of CO.25 Here, in the subset of patients with low CO (<4.1 L/min), ICG also resulted in significantly higher CO values as compared with IGR. Thus, in patients with systolic CHF, ICGrest seems to overestimate cardiac function as compared with IGRrest. This is of clinical importance, as values obtained by either method at rest may not be interchangeable.
Lastly, we found that agreement between CI values obtained by IGR and ICG improved when performed in CHF patients undergoing low‐exercise pedalling. This may be due to the improved standardization of test conditions (e.g. ambient conditions, body posture, and muscle tension) when performing IGR or ICG in these patients.11, 12, 13 Nevertheless, mean CI values determined by ICG10W were significantly lower as compared with IGR10W. Therefore, for measurements of CI at rest as for measurements during low‐exercise pedalling, values obtained by either method should not be used interchangeably in the monitoring of in patients with CHF.
Limitations
As we did not perform invasive verification of our values for CI, we cannot comment on the accuracy of our IGR and ICG methods. However, both methods have previously shown both acceptable agreement with CI values obtained by thermodilution2, 3, 4 and high precision3, 4, 9, 17, 25—a prerequisite for the interpretation of serial measurements of hemodynamic variables.
We cannot completely exclude a pre‐selection bias in the selection of our patients, as presentation to our outpatient CHF clinic depends on referring physicians. Also, since measures of biological variation are context dependent, our results may not be applicable to other settings. In addition, because our patient cohort was comprised mostly of Caucasians, applying the results obtained from our study to populations from different ethnic/racial backgrounds may not be reliable.
Patients included in this study mostly suffered from mild CHF symptoms (NYHA I/II). Serial CI measurements in patients with severe heart failure symptoms (NYHA III/IV) may show a higher variation of CI. Such an analysis, however, would also include patients with relevant minor clinical changes and/or instabilities and therefore determine the clinical variation rather than the biological variation of CI in CHF, as clinical stability is the prerequisite for the determination of biological variation. We calculated RCVs and MIDs using well‐established formulas; however, values for RCVs and MIDs may not only depend on the population of individuals studied and the clinical procedures used but also on the time interval between successive measurements of hemodynamic variables. RCVs and MIDs provided in our study were higher when calculated from data obtained in CHF patients over a two week interval as compared with a one week interval. Therefore, our RCV and MID values for CI by IGR or IGC methods may not be transferable to serial CI measurements over longer time intervals. However, differences between 1 week and 2 week RCVs and MIDs were small, and most studies on biological variation have considered the bias of different sampling intervals to be negligible.30
Subgroup analyses of CI measurements in patients with atrial fibrillation mainly confirmed the results of the general cohort. Due to the limited number of patients included into the subgroup analysis, however, the results should be interpreted with caution.
Conclusion
In our prospectively recruited cohort of 50 patients with stable systolic CHF, values for CI by IGR or ICG methods are stable over time. In addition, values for CI obtained by either IGR or ICG show moderate agreement that improves when these values are determined during low‐level exercise. Therefore, values for CI from IGR and ICG are method dependent and not interchangeable. Lastly, biological variation is lower for CI values obtained by ICG than by IGR.
Conflict of Interest
None declared.
Funding
This study was supported by Roche Diagnostics GmbH, Germany. We acknowledge financial support by Deutsche Forschungsgemeinschaft and Ruprecht‐Karls‐Universität Heidelberg within the funding programme Open Access Publishing.
Supporting information
Supporting info item
Täger, T. , Fröhlich, H. , Franke, J. , Slottje, K. , Horsch, A. , Zdunek, D. , Hess, G. , Dösch, A. , Katus, H. A. , Wians, F. H. , and Frankenstein, L. (2015) Biological variation of the cardiac index in patients with stable chronic heart failure: inert gas rebreathing compared with impedance cardiography. ESC Heart Failure, 2: 112–120. doi: 10.1002/ehf2.12040.
Institution where work was performed: University of Heidelberg
The copyright line for this article was changed on 6 October 2016 after original online publication.
References
- 1. Mullens W, Abrahams Z, Skouri HN, Taylor DO, Starling RC, Francis GS, Young JB, Tang WH. Prognostic evaluation of ambulatory patients with advanced heart failure. Am J Cardiol 2008; 101: 1297–1302. [DOI] [PubMed] [Google Scholar]
- 2. Sobanski P, Sinkiewicz W, Kubica J, Blazejewski J, Bujak R. The reliability of noninvasive cardiac output measurement using the inert gas rebreathing method in patients with advanced heart failure. Cardiol J 2008; 15: 63–70. [PubMed] [Google Scholar]
- 3. Agostoni P, Cattadori G, Apostolo A, Contini M, Palermo P, Marenzi G, Wasserman K. Noninvasive measurement of cardiac output during exercise by inert gas rebreathing technique: a new tool for heart failure evaluation. J Am Coll Cardiol 2005; 46: 1779–1781. [DOI] [PubMed] [Google Scholar]
- 4. Koobi T, Kaukinen S, Ahola T, Turjanmaa VM. Non‐invasive measurement of cardiac output: whole‐body impedance cardiography in simultaneous comparison with thermodilution and direct oxygen Fick methods. Intensive Care Med 1997; 23: 1132–1137. [DOI] [PubMed] [Google Scholar]
- 5. Peyton PJ, Chong SW. Minimally invasive measurement of cardiac output during surgery and critical care: a meta‐analysis of accuracy and precision. Anesthesiology 2010; 113: 1220–1235. [DOI] [PubMed] [Google Scholar]
- 6. Doering L, Lum E, Dracup K, Friedman A. Predictors of between‐method differences in cardiac output measurement using thoracic electrical bioimpedance and thermodilution. Crit Care Med 1995; 23: 1667–1673. [DOI] [PubMed] [Google Scholar]
- 7. Fuller HD. Improving the accuracy of impedance cardiac output in the intensive care unit: comparison with thermodilution cardiac output. Congest Heart Fail 2006; 12: 271–276. [DOI] [PubMed] [Google Scholar]
- 8. Leslie SJ, McKee S, Newby DE, Webb DJ, Denvir MA. Non‐invasive measurement of cardiac output in patients with chronic heart failure. Blood Press Monit 2004; 9: 277–280. [DOI] [PubMed] [Google Scholar]
- 9. Saur J, Fluechter S, Trinkmann F, Papavassiliu T, Schoenberg S, Weissmann J, Haghi D, Borggrefe M, Kaden JJ. Noninvasive determination of cardiac output by the inert‐gas‐rebreathing method‐‐comparison with cardiovascular magnetic resonance imaging. Cardiology 2009; 114: 247–254. [DOI] [PubMed] [Google Scholar]
- 10. Ricós C PC, Minchinela J, Álvarez V, Simón M, Biosca C, Doménech M, Fernández P, Jiménez CV, Garcia‐Lario JV, Cava F. Application of biological variation – a review. Biochem Med 2009; 19: 250–259. [Google Scholar]
- 11. Doering L, Dracup K. Comparisons of cardiac output in supine and lateral positions. Nurs Res 1988; 37: 114–118. [PubMed] [Google Scholar]
- 12. Loeber CP, Goldberg SJ, Donnerstein RL, Butler MA. Time variability of cardiac output and stroke volume in persons without cardiac disease. Am J Cardiol 1987; 59: 714–716. [DOI] [PubMed] [Google Scholar]
- 13. van Dijk N, de Bruin IG, Gisolf J, de Bruin‐Bon HA, Linzer M, van Lieshout JJ, Wieling W. Hemodynamic effects of leg crossing and skeletal muscle tensing during free standing in patients with vasovagal syncope. J Appl Physiol 2005; 98: 584–590. [DOI] [PubMed] [Google Scholar]
- 14. Moser DK, Frazier SK, Woo MA, Daley LK. Normal fluctuations in pulmonary artery pressures and cardiac output in patients with severe left ventricular dysfunction. European Journal of Cardiovascular Nursing: Journal of the Working Group on Cardiovascular Nursing of the European Society of Cardiology 2002; 1: 131–137. [DOI] [PubMed] [Google Scholar]
- 15. Jakovljevic DG, Seferovic PM, Nunan D, Donovan G, Trenell MI, Grocott‐Mason R, Brodie DA. Reproducibility of cardiac power output and other cardiopulmonary exercise indices in patients with chronic heart failure. Clin Sci 2012; 122: 175–181. [DOI] [PubMed] [Google Scholar]
- 16. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Task Force for the D , Treatment of A , Chronic Heart Failure of the European Society of C , Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P, Guidelines ESCCfP . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the hHeart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012; 14: 803–869. [DOI] [PubMed] [Google Scholar]
- 17. Yancy C, Abraham WT. Noninvasive hemodynamic monitoring in heart failure: utilization of impedance cardiography. Congest Heart Fail 2003; 9: 241–250. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org.: The Cochrane Collaboration, 2011.
- 19. Faraone SV. Interpreting estimates of treatment effects: implications for managed care. P and T: A Peer‐reviewed Journal for Formulary Management 2008; 33: 700–711. [PMC free article] [PubMed] [Google Scholar]
- 20. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed Hillsdale, Ney Jersey: Routledge; 1988. [Google Scholar]
- 21. Rosner B. Fundementals of Biostatistics. 6th ed Duxbury: Thomson Brooks/Cole; 2006. [Google Scholar]
- 22. Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM‐based criterion for identifying meaningful intra‐individual changes in health‐related quality of life. J Clin Epidemiol 1999; 52: 861–873. [DOI] [PubMed] [Google Scholar]
- 23. Wells G, Beaton D, Shea B, Boers M, Simon L, Strand V, Brooks P, Tugwell P. Minimal clinically important differences: review of methods. J Rheumatol 2001; 28: 406–412. [PubMed] [Google Scholar]
- 24. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–310. [PubMed] [Google Scholar]
- 25. Trinkmann F, Berger M, Hoffmann U, Borggrefe M, Kaden JJ, Saur J. A comparative evaluation of electrical velocimetry and inert gas rebreathing for the non‐invasive assessment of cardiac output. Clinical Research in Cardiology: Official Journal of the German Cardiac Society 2011; 100: 935–943. [DOI] [PubMed] [Google Scholar]
- 26. Fontana P, Boutellier U, Toigo M. Reliability of measurements with Innocor during exercise. Int J Sports Med 2009; 30: 747–753. [DOI] [PubMed] [Google Scholar]
- 27. Foundation EI . ET thoracic allocation system (EThAS). Eurotransplant Manual ‐ Version 3, 2013. 1–53.
- 28. Huang CC, Tsai YH, Chen NH, Lin MC, Tsao TC, Lee CH, Hsu KH. Spontaneous variability of cardiac output in ventilated critically ill patients. Crit Care Med 2000; 28: 941–946. [DOI] [PubMed] [Google Scholar]
- 29. Sasse SA, Chen PA, Berry RB, Sassoon CS, Mahutte CK. Variability of cardiac output over time in medical intensive care unit patients. Crit Care Med 1994; 22: 225–232. [DOI] [PubMed] [Google Scholar]
- 30. Iglesias Canadell N, Hyltoft Petersen P, Jensen E, Ricos C, Jorgensen PE. Reference change values and power functions. Clinical Chemistry and Laboratory Medicine: CCLM / FESCC 2004; 42: 415–422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


