Summary
Background
Aspergillus niger prolyl endoprotease (AN‐PEP) efficiently degrades gluten molecules into non‐immunogenic peptides in vitro.
Aim
To assess the efficacy of AN‐PEP on gluten degradation in a low and high calorie meal in healthy subjects.
Methods
In this randomised, double‐blind, placebo‐controlled, cross‐over study 12 healthy volunteers attended to four test days. A liquid low or high calorie meal (4 g gluten) with AN‐PEP or placebo was administered into the stomach. Via a triple‐lumen catheter gastric and duodenal aspirates were sampled, and polyethylene glycol (PEG)‐3350 was continuously infused. Acetaminophen in the meals tracked gastric emptying time. Gastric and duodenal samples were used to calculate 240‐min area under the curve (AUC 0–240 min) of ?‐gliadin concentrations. Absolute ?‐gliadin AUC 0–240 min was calculated using duodenal PEG‐3350 concentrations.
Results
AN‐PEP lowered α‐gliadin concentration AUC 0–240 min, compared to placebo, from low and high calorie meals in stomach (low: 35 vs. 389 μg × min/mL; high: 53 vs. 386 μg × min/mL; P < 0.001) and duodenum (low: 7 vs. 168 μg × min/mL; high: 4 vs. 32 μg × min/mL; P < 0.001) and absolute α‐gliadin AUC 0–240 min in the duodenum from low (2813 vs. 31 952 μg × min; P < 0.001) and high (2553 vs. 13 095 μg × min; P = 0.013) calorie meals. In the placebo group, the high compared to low calorie meal slowed gastric emptying and lowered the duodenal α‐gliadin concentration AUC 0–240 min (32 vs. 168 μg × min/mL; P = 0.001).
Conclusions
AN‐PEP significantly enhanced gluten digestion in the stomach of healthy volunteers. Increasing caloric density prolonged gastric residence time of the meal. Since AN‐PEP already degraded most gluten from low calorie meals, no incremental effect was observed by increasing meal caloric density. ClinicalTrials.gov, Number: NCT01335503; www.trialregister.nl, Number: NTR2780.
Introduction
Gluten is a storage protein present in wheat, barley and rye and is exceptionally rich in proline, rendering gluten peptides resistant to gastrointestinal digestion. Proline‐cleaving proteases are absent in the human gastrointestinal tract. Therefore, long proline‐rich gluten peptides reach the small intestine intact after ingestion.1 About 1% of the western population is suffering from coeliac disease.2, 3, 4, 5 In these patients, exposure of duodenum and proximal small intestine to the specific amino acid sequences of such poorly digested proline‐rich gluten peptides triggers an abnormal immune response. This causes inflammation with infiltration of lymphocytes in the intestinal mucosa and ultimately villous atrophy and crypt hyperplasia.6 Adverse reactions to gluten consumption are not limited to subjects suffering from coeliac disease. Presently non‐coeliac gluten sensitivity has been clinically recognised as a separate condition in which neither allergic nor autoimmune mechanisms are involved.7, 8 The symptoms experienced by these subjects are often identical to those seen in coeliac disease.9, 10
A lifelong gluten‐free diet is the only treatment for individuals who cannot tolerate gluten. However, a gluten‐free diet is hard to comply with as gluten‐free products may not always be labelled, and may not always be at hand during social events or travelling.11, 12, 13
Prolyl endopeptidases belong to a family of enzymes with the ability to cleave at internal proline residues within a peptide.14 Early investigations on oral protease therapy as an approach to degrade gluten have focused on bacterial prolyl oligopeptidases.15, 16, 17, 18 However, several in vitro studies conducted with such enzymes revealed only low enzymatic activity at acidic stomach pH and rapid degradation of these enzymes by pepsin.16 Moreover, these enzymes were not able to prevent passage of potentially harmful gluten fragments into the small intestine.16, 19 But, other enzymatic preparations have shown to be capable of degrading complex gluten proteins both in vitro and in vivo.20, 21, 22, 23
In this respect, the Aspergillus niger‐derived prolyl endoprotease (AN‐PEP) also presents a promising option to degrade inadvertent dietary gluten. The use of the enzyme as food supplement has undergone successful evaluation by the French Agency for Food, Environmental and Occupational Health & Safety.24 AN‐PEP is active between pH 2 and 6, with optimum activity at gastric pH between 3 and 5.19 In a dynamic, multi‐compartmental gastrointestinal in vitro model, AN‐PEP was shown to degrade almost all immunogenic gluten epitopes from gluten‐containing meals into non‐immunogenic fragments during passage through the stomach compartment.25 A pilot study in coeliac patients showed that a combination of AN‐PEP and gluten was safe and well tolerated.26 Before AN‐PEP can be used as a future digestive aid for subjects intolerant to gluten, it is essential that the promising in vitro results are confirmed in a human in vivo study, focussing on duodenal gluten delivery after intake of gluten‐containing meals with and without AN‐PEP. Thereafter, the efficacy and safety of AN‐PEP should be evaluated in target populations.
Our aim was to investigate the efficacy of AN‐PEP on gluten degradation in an intragastrically delivered gluten‐containing meal in healthy volunteers. To standardise meal intake, we administrated the meal intragastrically at a fixed rate instead of by oral ingestion. Secondly, we hypothesised that increasing the caloric density of a meal enhances gluten degradation by delaying gastric emptying and thereby prolonging gastric residence time of the meal.
Materials and methods
The study was approved by the Medical Ethics Committee of the Maastricht University Medical Center (MUMC+) and conducted in full accordance with the principles of the Declaration of Helsinki of 1975 as revised in 2008 and with the Dutch Regulations on Medical Research involving Human Subjects (WMO, 1998). The study was performed at the MUMC+ from December 2011 to May 2012. All participants gave their written informed consent before participation. The trial has been registered in the Clinical Trials register (NCT01335503) and the Dutch trial register (NTR2780).
Subjects
Healthy volunteers aged 18–45 years were recruited by advertisement. All subjects were screened by means of a standardised general physical examination. Reasons for exclusion included: history of gastrointestinal disorders or gastrointestinal surgery interfering with gastrointestinal function; history of major disease, use of medication (except oral contraceptives) within 14 days before testing; dieting; pregnancy; lactation; excessive alcohol consumption (>20 alcoholic consumptions/week) and smoking.
Design and intervention
In this double‐blind, randomised, placebo‐controlled, crossover study, participants attended to four test days with at least 1 week washout period between two test days. At test days, participants were randomised in a double‐blind fashion to 1 of the 24 possible orders of the four interventions; a low calorie gluten meal with AN‐PEP or placebo, or a high calorie gluten meal with AN‐PEP or placebo. The randomisation list was generated by an independent and blinded statistician using a computerised procedure. All participants and investigators remained blinded to treatment until the analyses were completed. After an overnight fast, a triple lumen catheter (adapted from Freka Trelumina, Fresenius Kabi Nederland B.V., Zeist, The Netherlands) was introduced trans‐nasally via the stomach into the duodenum, under fluoroscopic guidance. The proximal lumen, with an infusion port positioned in the stomach, was used for administration of the test meal and aspiration of gastric contents. The second lumen, positioned 5 cm distal to the pylorus, was used for continuous perfusion of the inert dilution marker polyethylene glycol (PEG)‐3350. The third and distal lumen (positioned at the tip and located 15 cm distal to the pylorus) was used for aspiration of duodenal contents. The catheter position was secured by radiology and regularly checked during the tests by measuring pH of each aspirate. Meals, mixed with acetaminophen (Centrafarm B.V., Etten‐Leur, The Netherlands), and either AN‐PEP or placebo, were infused into the stomach over a 5‐min period, at a rate of 80 mL/min. A PEG‐3350 (Norgine B.V., Amsterdam, The Netherlands) solution (15 mg/mL in 0.9% saline) was continuously infused into the proximal part of the duodenum at 3 mL/min to calculate the gluten amount corrected for dilution of duodenal content by endogenous secretions.27, 28, 29, 30 Infusion started 60 min prior to meal infusion, to achieve steady‐state conditions in fluid secretion and absorption at the start of meal infusion, and continued till 240 min. Gastric and duodenal content was sampled at baseline (t = 0 min), and after start of meal infusion at t = 15, 30, 45, 60, 75, 90, 120, 150, 180, 210 and 240 min. Gastric content was also aspirated at t = 5 and t = 10 min. Mixing of the meal with AN‐PEP or placebo and acetaminophen was performed immediately prior to the start of infusion. Approximately 3 mL and 2 mL were aspirated from the gastric and duodenal port, respectively, for pH and gluten epitope measurements. Also, acetaminophen concentrations in gastric samples were measured. The samples were immediately frozen in liquid nitrogen to stop enzyme activity and were subsequently stored at −80 °C until analysis.
Gluten meals
Dry meals were packaged in sachets of airtight tinfoil and stored at room temperature. On test days, meals were prepared at a food‐grade kitchen facility. During preparation of the test meal, 1 g acetaminophen was added. Table 1 shows the composition of the meals. All test meals contained 5.2 g of gluten powder, of which 4.0 g was gluten protein (Syral, Aalst, Belgium). Encapsulated refined olive oil powder (VanaGrasa 80B; FrieslandCampina, Kievit B.V., Meppel, The Netherlands) was used as fat source for the meals and together with maltodextrin as additional energy source for the high calorie meal, and sodium caseinate (DMV, Veghel, The Netherlands) was used to match both meals for protein content. The dry meal powders were dissolved in a total volume of 300 mL tap water of 40 °C by stirring with a spoon and subsequently mixing with a Turrax (Ultra Turrax T25; IKA, Staufen, Germany). Low and high caloric meals were not blinded to the investigator.
Table 1.
Recipe and nutritional composition of the low and high calorie test meal per 300 g portion
| Low calorie meal | High calorie meal | |
|---|---|---|
| Vana Grasa (g) | 7.0 | 22.0 |
| Sucrose (g) | 17.0 | 16.9 |
| Maltodextrin (g) | 0.0 | 36.3 |
| Caseinate (g) | 0.7 | 0.3 |
| Gluten powder (g) | 5.2 | 5.2 |
| Citric acid (g) | 0.1 | 0.1 |
| Water (g) | 270.9 | 245.1 |
| Protein (g) | 4.9 | 4.9 |
| Fat (g) | 5.7 | 17.7 |
| Carbohydrate (g) | 18.0 | 56.5 |
| Ash (g) | 0.2 | 0.5 |
| Protein (kcal) | 19.6 | 19.6 |
| Fat (kcal) | 51.6 | 159.6 |
| Carbohydrate (kcal) | 71.9 | 225.8 |
| Total (kcal) | 143.1 | 405.1 |
| Caloric density (kcal/g) | 0.5 | 1.4 |
| Osmolarity (mOsm/kg) | 194.8 | 373.6 |
| pH | 6.0 | 6.0 |
AN‐PEP enzyme and placebo
The AN‐PEP enzyme was obtained from DSM Food Specialties (Delft, The Netherlands). A total of 6.1 mL of AN‐PEP corresponding with 1.600.000 Protease Picomol International (1 Protease Picomol International is the amount of enzyme that releases one picomole of p‐nitroaniline per second under defined assay conditions) in a total of 100 mL water was added to the 300 mL test meals. A 6.1 mL solution consisting of 4.8 g water, 1.3 g maltodextrin, 0.01 g caramel liquid (Brenntag, Deerlijk, Belgium), 0.03 g citric acid and 0.02 g sodium benzoate (Wuhan Youji, Wuhan, China) at pH 4.2, with a similar appearance to AN‐PEP, served as placebo.
Sample pre‐treatment
Upon thawing, the enzyme in the gastric and duodenal samples was immediately inactivated by increasing the pH of the sample to 11–12 using 1 mol/L NaOH, heating at 85 °C for 10 min and neutralising the pH with 1 mol/L HCl. For the gluten content analysis, 100 μL from each sample was frozen again at −80 °C for further analysis by ELISA, or mixed with loading buffer (60% glycerol, 300 mmol/L Tris, pH 6.8, 12 mmol/L EDTA, pH 8.0, 12% sodium dodecylsulphate, 864 mmol/L 2‐mercaptoethanol, 0.05% bromophenol blue), boiled for 5 min for further analysis by Coomassie Blue staining and western blot.
Gluten monitoring by ELISA analyses
The sample was diluted 40–5000 times in phosphate‐buffered saline and the presence of the DQ2.5‐glia‐α3 epitope was quantified using the Gluten‐Tec ELISA assay (EuroProxima B.V., Arnhem, The Netherlands) according to the manufacturer's instructions.31 The DQ2.5‐glia‐α3 epitope is directly adjacent to the 33‐mer that contains the immunodominant DQ2.5‐glia‐α1 and DQ2.5‐glia‐α2 epitopes. As the α‐gliadins contain only a single copy of the DQ2.5‐glia‐α3 epitope, the measurement of this epitope provides an accurate estimate of the actual α‐gliadin content of the samples.32
Volume marker
PEG‐3350 concentrations were determined in samples obtained from the distal aspiration port in the duodenum, using reversed‐phase HPLC in combination with evaporative light scatter detection. The analysis was based on PEG analysis as described by van Wijck et al.33 PEG‐3350 concentrations were used to calculate the dilution of duodenal samples by endogenous secretions, using the formula described by Beglinger et al.27
V represents the calculated duodenal volume (mL per 15 or 30 min); F the flow rate of PEG solution perfused (3 mL/min); [PEG]perfused the concentration of PEG in the perfusate; PEGmeasured the concentration of PEG in the duodenal juice collected for 15 or 30 min. The number ‘15’ has to be replaced by ‘30’ if the time interval between two samples was 30 min.
To calculate the absolute duodenal gluten amount at a certain time point, the calculated duodenal volume was multiplied with the gluten concentration at that time point.
Gluten monitoring by western blot
Measurement of gluten epitopes by ELISA is an indirect method of gluten analysis. To confirm the presence of intact gluten proteins and relatively large fragments thereof we also performed western blot analysis. The proteins present in samples isolated from the stomach were separated on SDS‐PAGE, blotted onto polyvinylidene fluoride membrane and the gliadin proteins were visualised with a monoclonal antibody specific for the immunodominant DQ2.5‐glia‐α1 epitope.34
Presence of AN‐PEP in gastric and duodenal samples
To assess the presence of AN‐PEP protein in gastric and duodenal samples, the protein in the samples was separated on SDS‐PAGE followed by Coomassie Blue staining.
Assessment of gastric emptying
Gastric emptying rate was measured at all test days of 6 of the 12 participants who completed the study, randomly chosen. Gastric emptying rate was determined according to the changes of the acetaminophen concentration over time in the stomach, with stomach samples taken at pre‐determined time points. Gastric fluid samples were first deproteinated by adding a 10% solution of trichloroacetic acid. After centrifugation (800g, 5 min) the supernatant was injected into HPLC using a reversed‐phase method with UV detection at 250 nm (Agilent 1100 series HPLC Value System, Waldbronn, Germany). A composition of MilliQ and acetonitrile (97: 3%v/v) was used to elute the samples. As the total gastric volume changes constantly after meal ingestion, it was difficult to calculate the concentration via a formula. A calibration curve of acetaminophen was used to calculate the acetaminophen concentration in the samples. As a pragmatic approach, the total gastric emptying time (in minutes) was derived from this acetaminophen concentration‐time curve. The time point when the acetaminophen concentration in a gastric sample is zero indicates the complete passage of the test meal into the duodenum and is thus considered to represent total gastric emptying time.
Gastrointestinal symptoms questionnaire
At the end of each test day, participants were requested to complete a ‘Symptoms Diary’, to ensure that all gastrointestinal complaints of the test day, caused by the intervention, were reported to the investigator. This questionnaire included eight items, each rated on a five‐point Likert scale. The lowest score, 1, denotes no symptoms and 5 denotes the most pronounced symptoms. Items that were included in the ‘Symptoms Diary’ were: abdominal discomfort, abdominal pain, abdominal distension, constipation, diarrhoea, flatus, eructation and nausea.
Statistics
The primary outcome of this study was the effect of AN‐PEP on gluten degradation, measured by the difference in the 240‐min AUC (AUC 0–240 min) of duodenal gluten epitope concentration between AN‐PEP and placebo. Secondly, we investigated the effect of AN‐PEP on gluten degradation, measured by the difference in AUC 0–240 min of absolute amounts of gluten epitopes in the duodenum between both interventions. The AUC 0–240 min was calculated by using the trapezoidal equation. We calculated that a sample size of 12 subjects would be required based on a standardised effect size of 1.3, a power of 90% and α = 0.05 (one sided). Seventeen subjects were recruited taking into account a drop out of five subjects. Baseline characteristics are presented as mean (s.d.) for numerical variables and number (%) for categorical variables. Differences in AUCs, gastric emptying rate and ‘Symptom Diary’ scores between combinations of treatment (AN‐PEP or placebo) and meal (high or low calorie) were assessed using linear mixed models based on restricted maximum likelihood, where the natural logarithm of the AUCs was taken into account for the expected non‐normality. The linear mixed model accounts for the correlation between repeated measures within a person (cross‐over design) and missing data, where a likelihood approach was used assuming missing data at random. Fixed factors were treatment, meal, treatment × meal and test day. The best fitting covariance structure, i.e. structure of variances over different test days and correlations between test days, was based on Akaike's Information Criterion. All statistical analyses were performed using ibm spss Statistics for Windows (Version 20.0; IBM Corp., Armonk, NY, USA). Two‐sided P ≤ 0.05 were considered statistically significant.
Results
Study subjects
A total of 12 healthy volunteers [67% male; age 26 ± 6 years; BMI (in kg/m2) = 22 ± 3] were included in the study. One of these subjects did not complete the fourth test day and only the results of the two high calorie meal test days were available for inclusion in the analyses. In two other subjects the catheter progressed more distally into the small intestine on one occasion, causing administration of (part of) the meal directly into the duodenum. Data of these experiments were omitted, but data from their remaining test days were still included in the analysis as the linear mixed model accounts for missing data. Initially 17 subjects were enrolled in the study, but five subjects dropped out due to discomfort related to the nasoduodenal tube. Data of drop outs were omitted from analyses.
pH of gastric samples
The mean gastric pH of gastric samples, taken during test days when low and high calorie meals combined with AN‐PEP were infused, ranged between 2.3 and 5.3 and was similar to the pH range of the samples of placebo‐containing meals (data not shown).
DQ2.5‐glia‐α3 concentrations in stomach and duodenum and absolute amount in duodenum
The mean DQ2.5‐glia‐α3 concentrations in stomach and duodenum samples after ingestion of low and high calorie meals with and without AN‐PEP are shown in Figure 1 and the AUC 0–240 min in Table 2. The mean duodenal DQ2.5‐glia‐α3 concentrations per participant are shown in Figure 2. Over a 240‐min period, AN‐PEP reduced the gastric DQ2.5‐glia‐α3 concentrations, compared to placebo, both after ingestion of the low (35 vs. 389 μg × min/mL; P < 0.001) and the high (53 vs. 386 μg × min/mL; P < 0.001; Figure 1a,b; Table 2) calorie meals. This was also observed for duodenal DQ2.5‐glia‐α3 concentrations (low calorie: 7 vs. 168 μg × min/mL; high calorie: 4 vs. 32 μg × min/mL; P < 0.001; Figure 1c,d; Table 2). In the placebo intervention, the duodenal DQ2.5‐glia‐α3 concentrations were significantly lower after intake of a high compared to a low calorie meal (32 vs. 168 μg × min/mL; P = 0.001; Figure 1c,d; Table 2). In the presence of AN‐PEP this difference was not present (4 vs. 7 μg × min/mL; P > 0.05; Figure 1c,d; Table 2) and low duodenal DQ2.5‐glia‐α3 concentrations were observed after intake of both a high and low calorie meal. The AUC 0–240 min of DQ2.5‐glia‐α3 concentrations in the duodenum of AN‐PEP‐receiving subjects was around or lower than the limit of detection (26.7 μg/L × 240 min = 7 μg × min/mL) and lower than the limit of quantification (89 μg/L × 240 min = 21 mg × min/L) of the ELISA assay. The pattern for the duodenal DQ2.5‐glia‐α3 concentrations corresponds with the data for absolute duodenal DQ2.5‐glia‐α3 amount, which is corrected for the dilution during the digestion process. AN‐PEP lowered the calculated absolute duodenal α‐gliadin compared to placebo in both low (2813 vs. 31 952 μg × min; P < 0.001) and high (2553 vs. 13 095 μg × min; P = 0.013; Figure 1e,f; Table 2) calorie meals.
Figure 1.
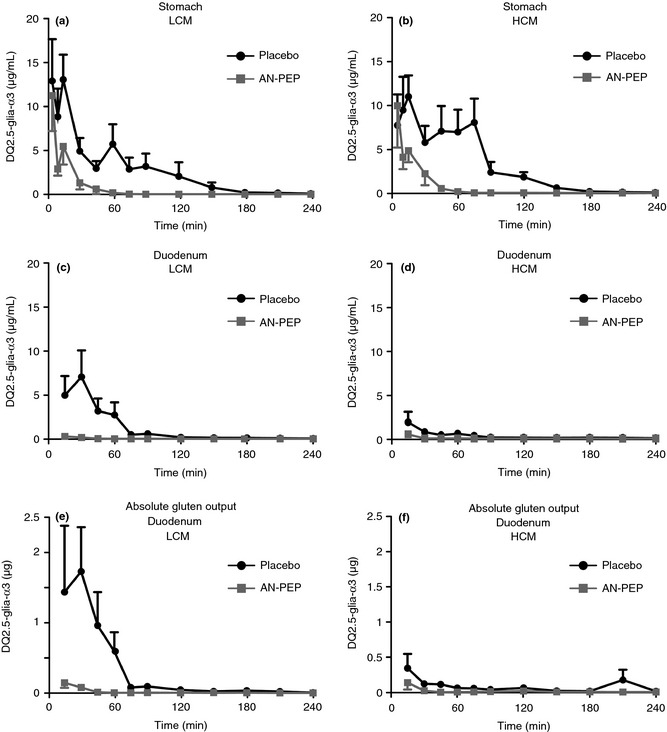
(a) DQ2.5‐glia‐α3 concentration (mean ± SEM) over time in the stomach in low calorie meals. (b) DQ2.5‐glia‐α3 concentration (mean ± S.E.M.) over time in the stomach in high calorie meals. (c) DQ2.5‐glia‐α3 concentration (mean ± S.E.M.) over time in the duodenum in low calorie meals. (d) DQ2.5‐glia‐α3 concentration (mean ± S.E.M.) over time in the duodenum in high calorie meals. (e) Absolute DQ2.5‐glia‐α3 output (mean ± S.E.M.) over time in the duodenum in low calorie meals. (f) Absolute DQ2.5‐glia‐α3 output (mean ± S.E.M.) over time in the duodenum in high calorie meals.
Table 2.
AUC0–240 min of DQ2.5‐glia‐α3 concentrations in stomach and duodenum and the AUC0–240 min of absolute DQ2.5‐glia‐α3 amount in duodenum
| Low calorie meal | High calorie meal | |||
|---|---|---|---|---|
| Placebo | AN‐PEP | Placebo | AN‐PEP | |
| AUC 0–240 min DQ2.5‐glia‐α3, μg × min/mL | ||||
| Stomach | ||||
| Mean | 389 | 35a | 386 | 53a |
| 95% CI | 180–840 | 17–73 | 192–775 | 25–113 |
| Duodenum | ||||
| Mean | 168 | 7a , d | 32b | 4a , e |
| 95% CI | 80–352 | 3–14 | 16–63 | 2–9 |
| AUC 0–240 min DQ2.5‐glia‐α3, μg × min | ||||
| Duodenum | ||||
| Mean | 31 952 | 2813a | 13 095 | 2553c |
| 95% CI | 12 670–80 579 | 1206–6555 | 5967–28 730 | 884–7369 |
P < 0.001 compared to placebo.
P = 0.001 compared to low calorie placebo meal.
P < 0.05 compared to placebo.
Below the level of quantitation 21 μg × min/mL.
Below the level of detection 7 μg × min/mL.
Figure 2.
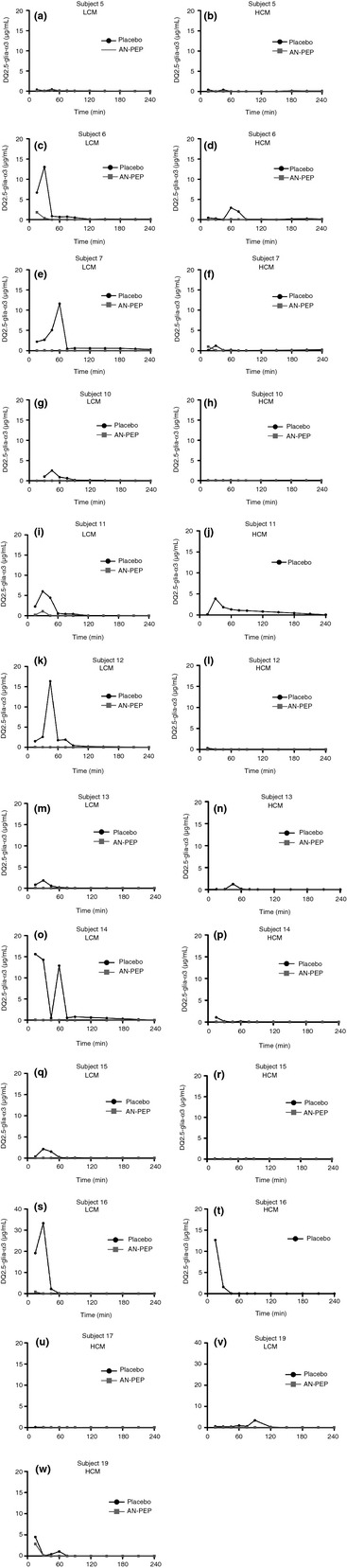
DQ2.5‐glia‐α3 concentration over time in the duodenum of the individual subjects in low calorie meals (LCM) and high calorie meals (HCM). (a) + (b): subject 5. (c) + (d): subject 6. (e) + (f): subject 7. (g) + (h): subject 10. (i) + (j): subject 11. (k) + (l): subject 12. (m) + (n): subject 13. (o) + (p): subject 14. (q) + (r): subject 15. (s) + (t): subject 16. (u): subject 17. (v) + (w): subject 19.
Gluten monitoring by western blot
Western blot analysis of stomach samples indicated that gluten degradation was accelerated by the addition of AN‐PEP. Compared to samples of placebo‐containing meals, samples of AN‐PEP‐containing meals showed generally a markedly faster degradation of DQ2.5‐glia‐α1. In many cases, very little or even no gluten protein could be detected when AN‐PEP was taken with the meal. In contrast, in gastric samples of placebo‐containing meals, in some cases DQ2.5‐glia‐α1 was still detectable up to 2 h after meal infusion. In duodenal samples, the western blots were unable to detect significant amounts of intact gluten proteins, neither with AN‐PEP, nor with placebo (Figure 3). This finding suggests that in both cases little or no intact water‐insoluble gluten protein reaches the duodenum.
Figure 3.
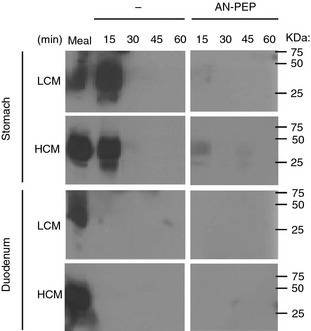
Representative western blot showing degradation of water‐insoluble DQ2.5‐glia‐α1 over time in the stomach and duodenum in low and high calorie meals with and without AN‐PEP.
Overall, for the majority of the meals analysed, the pattern on gluten degradation by both ELISA and western blot showed a correlation, demonstrating the robustness of the study results.
Presence of AN‐PEP in gastric and duodenal samples
After administration of AN‐PEP‐containing meals, a band with AN‐PEP's characteristic molecular weight (66 kD) was visible in the gastric samples of 14 different test days (Figure 4). In the other test days, the AN‐PEP signal was either too weak to be detected or masked by other proteins with a similar electrophoretic mobility. In the placebo‐containing meals, a band with this particular molecular weight was always absent (data not shown). With high calorie meals, AN‐PEP was detectable for a longer period than with low calorie meals. AN‐PEP was not found in the duodenal samples of any test day (Figure 4), possibly due to degradation of the enzyme by trypsin or chymotrypsin under conditions of high pH in the duodenum.
Figure 4.
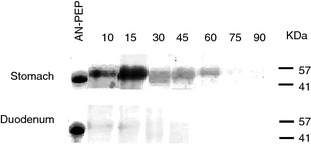
Representative SDS‐PAGE gel showing the presence of AN‐PEP protein in gastric and duodenal aspirates.
Gastric emptying
The mean of total gastric emptying time of the high calorie meals was approximately twice as long as compared to the low calorie meals, being significantly different in the presence of placebo (172 vs. 88 min; P = 0.014) but not in the presence of AN‐PEP (154 vs. 100 min; P = 0.100).
Gastrointestinal symptoms questionnaire
Mild gastrointestinal symptoms were reported on some occasions during low and high calorie meal intervention. Overall, discomfort was low for each different intervention. No significant differences were observed in reported gastrointestinal symptoms between meal types in combination with AN‐PEP or placebo (data not shown).
Discussion
In the current placebo‐controlled intervention study, AN‐PEP‐mediated gluten digestion was studied in the stomach and duodenum of healthy volunteers. This is the first study showing that the AN‐PEP enzyme efficiently degrades gluten from a meal in the stomach of human subjects.
Considering the enzyme's optimum pH range between 3 and 5, and the mean pH of the gastric samples ranged between 2.3 and 5.3 with both low and high calorie meals, this points to optimal enzyme activity during the entire digestive process in the stomach.19 Irrespective of the caloric density of the meal, the enzyme degraded almost all the gluten present in the stomach within a period of 1 h, whereas with placebo, gluten were present for 3 h. Furthermore, the addition of AN‐PEP did not result in differences in gastrointestinal‐related symptoms compared to placebo, confirming that intake of the enzyme is safe and well tolerated by human subjects.26
We also tested whether increasing meal caloric density would improve gluten degradation by delaying gastric emptying and thus prolonging exposure time of gluten proteins to AN‐PEP and endogenous gastric proteases. Although the low calorie meal in the stomach was emptied within about 1.5 h, the gastric emptying of the high calorie meal took about twice as long. Delayed gastric emptying also resulted in longer gastric residence time of AN‐PEP. These findings are consistent with a previously reported delay in gastric emptying rate with increased meal caloric density in human subjects.35 Within 1 h, AN‐PEP degraded gluten to concentrations around or below reliably detectable levels, irrespective of meal caloric density. Without AN‐PEP enzyme, high gluten concentrations were present in the stomach when given with a low or high calorie meal, supporting the notion that pepsin exerts only minimal proteolytic action on dietary gluten.36 Interestingly, in absence of the enzyme, less gluten reached the duodenum with high than with low calorie meals. Possibly, the fat content of the high calorie meals supported gluten digestion in the duodenum by increasing pancreatic enzyme outputs which has been described for high‐fat as compared to low‐fat diets.37
We used a marker infusion technique to correct for dilutions resulting from biliary and pancreatic secretions that might have influenced the gluten concentrations measured in the duodenum. The absolute gluten values obtained, therefore, represent a better measure of true gluten exposure than the gluten concentrations. The absolute amount of gluten reaching the duodenum was significantly reduced with AN‐PEP irrespective of meal caloric density, consistent with the findings for gluten concentrations. The duodenal gluten degradation pattern was comparable between concentrations and absolute amounts suggesting little influence of duodenal dilutions. Insoluble gluten measurements confirmed that AN‐PEP is able to significantly reduce gluten before entering the duodenum.
A band on SDS‐PAGE with AN‐PEPs characteristic molecular weight was observed in gastric, but not in duodenal aspirates, indicating AN‐PEP is present and active in the stomach but not in the duodenum. Possibly, under duodenal neutral pH conditions, bile and pancreatic enzymes may have degraded the enzyme.
Apart from AN‐PEP, other enzymes detoxifying gluten are currently under investigation. A mixture of two proteases, namely PEP derived from Sphingomonas capsulate and a barley protease (EP‐B2), termed ALV003, has previously been shown to be capable of degrading complex gluten proteins in vitro.20, 23 In a human setting, ALV003 was well tolerated and effective in detoxifying 1 g of gluten.21 A recent study in coeliac patients showed that ALV003 attenuated small intestinal mucosal injury induced by 6‐week ingestion of 2 g gluten daily.22 Another protease mix, STAN‐1, showed effective in vitro gluten‐degrading properties.38 These enzymes have been investigated for their applicability as a future coeliac disease drug therapy.
This study made use of a triple lumen nasogastroduodenal catheter. This enabled the simultaneous administration of a test meal, infusion of a dilution marker, and aspiration of gastric and duodenal contents. Clear benefit of this approach is that it allowed us to measure the actual gluten concentration present in duodenum samples. This information is important for safe use in subjects intolerant to gluten. Further, by infusion of the dilution marker we could calculate the absolute intraduodenal gluten appearance. In none of previous mentioned studies, investigating other gluten‐detoxifying enzymes, true gluten presence in the duodenum has been measured. To standardise each meal intake, AN‐PEP was added to the meal and thereafter immediately infused intragastrically at a standardised rate, to avoid differences in gluten degradation during meal consumption between interventions, caused by variable meal ingestion rates. We acknowledge that this does not represent a fully physiological meal setting, in which solid food and AN‐PEP are ingested separately and undergo the normal physiological processes of mixing in the stomach. A randomised placebo‐controlled trial is underway in which the efficacy of AN‐PEP in an actual meal setting will be investigated. Furthermore, this technique also has another drawback. Migration of the catheter, due to gastrointestinal peristalsis, caused erroneous infusion of the meal into the duodenum in two subjects at one occasion, as was noted based on pH profiles. These data were excluded from analysis.
The AN‐PEP enzyme has been developed as a dietary supplement that in conjunction with a gluten‐free diet may help subjects intolerant to gluten to digest unintended dietary gluten. Despite these promising results, the data do not prove that AN‐PEP allows subjects intolerant to gluten to ingest gluten safely. Oral enzymes cannot replace a gluten‐free diet, yet our observations suggest that AN‐PEP may be useful as a digestive aid to help digest hidden gluten. The enzyme may protect against unintentional ingestion of gluten on a daily basis, during social events or travelling. A randomised placebo‐controlled trial is underway to address AN‐PEP's efficacy in the target population which is necessary prior to AN‐PEP's use to be considered safe and effective.
In conclusion, AN‐PEP efficiently degrades gluten from a meal in the stomach of healthy volunteers before entering the duodenum. Increasing the caloric density of a gluten meal slowed gastric emptying rate and prolonged gastric residence time of the enzyme. Since AN‐PEP with a low calorie meal already degraded almost all gluten, a high calorie meal could not further increase the efficacy of the enzyme to digest gluten.
Authorship
Guarantor of the article: Bouke N. Salden.
Author contributions: Bouke N. Salden: study concept and design; acquisition of data; interpretation of data; drafting of the manuscript; statistical analysis. Veronica Monserrat: analysis and interpretation of data; drafting of the manuscript. Freddy J. Troost: study concept and design; acquisition of data; interpretation of data; critical revision of the manuscript for important intellectual content; study supervision. Maaike J Bruins: study concept and design; critical revision of the manuscript for important intellectual content; obtained funding; technical and material support; study supervision. Luppo Edens: study concept and design; critical revision of the manuscript for important intellectual content; obtained funding; technical and material support. Roger Bartholomé: analysis and interpretation of data; drafting of the manuscript. Guido R Haenen: analysis and interpretation of data; drafting of the manuscript. Bjorn Winkens: statistical analysis; critical revision of the manuscript for important intellectual content. Frits Koning: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. Ad A Masclee: study concept and design; interpretation of data; critical revision of the manuscript for important intellectual content; study supervision.
All authors approved the final version of the manuscript.
Acknowledgments
Declaration of personal interests: None.
Declaration of funding interests: This study was funded in full by DSM Food Specialties, Delft, The Netherlands. The new scientific data included in this publication are considered proprietary to DSM Nutritional Products AG, in particular, but not limited according to, art. 21 of the Regulation EC No 1924/2006 on nutrition and health claims made on foods and other pertaining provisions of the EC General Food Law.’ AN‐PEP is not intended to treat or prevent coeliac disease.
B. N. Salden and V. Monserrat share co‐first authorship.
This article was accepted for publication after full peer‐review.
The copyright line for this article was changed on 31 July 2015 after original online publication.
References
- 1. Piper JL, Gray GM, Khosla C. Effect of prolyl endopeptidase on digestive‐resistant gliadin peptides in vivo. J Pharmacol Exp Ther 2004; 311: 213–9. [DOI] [PubMed] [Google Scholar]
- 2. West J, Logan RF, Hill PG, et al Seroprevalence, correlates, and characteristics of undetected coeliac disease in England. Gut 2003; 52: 960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maki M, Mustalahti K, Kokkonen J, et al Prevalence of Celiac disease among children in Finland. N Engl J Med 2003; 348: 2517–24. [DOI] [PubMed] [Google Scholar]
- 4. Fasano A, Berti I, Gerarduzzi T, et al Prevalence of celiac disease in at‐risk and not‐at‐risk groups in the United States: a large multicenter study. Arch Intern Med 2003; 163: 286–92. [DOI] [PubMed] [Google Scholar]
- 5. Bingley PJ, Williams AJ, Norcross AJ, et al Undiagnosed coeliac disease at age seven: population based prospective birth cohort study. BMJ 2004; 328: 322–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Green PH, Cellier C. Celiac disease. N Engl J Med 2007; 357: 1731–43. [DOI] [PubMed] [Google Scholar]
- 7. Sapone A, Bai JC, Ciacci C, et al Spectrum of gluten‐related disorders: consensus on new nomenclature and classification. BMC Med 2012; 10: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sapone A, Lammers KM, Casolaro V, et al Divergence of gut permeability and mucosal immune gene expression in two gluten‐associated conditions: celiac disease and gluten sensitivity. BMC Med 2011; 9: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sapone A, Lammers KM, Mazzarella G, et al Differential mucosal IL‐17 expression in two gliadin‐induced disorders: gluten sensitivity and the autoimmune enteropathy celiac disease. Int Arch Allergy Immunol 2010; 152: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferguson A, Gillett H, Humphreys K, Kingstone K. Heterogeneity of celiac disease: clinical, pathological, immunological, and genetic. Ann N Y Acad Sci 1998; 859: 112–20. [DOI] [PubMed] [Google Scholar]
- 11. Lerner A. New therapeutic strategies for celiac disease. Autoimmun Rev 2010; 9: 144–7. [DOI] [PubMed] [Google Scholar]
- 12. Sollid LM, Khosla C. Future therapeutic options for celiac disease. Nat Clin Pract Gastroenterol Hepatol 2005; 2: 140–7. [DOI] [PubMed] [Google Scholar]
- 13. Sollid LM, Khosla C. Novel therapies for coeliac disease. J Intern Med 2011; 269: 604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gass J, Khosla C. Prolyl endopeptidases. Cell Mol Life Sci 2007; 64: 345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matysiak‐Budnik T, Candalh C, Cellier C, et al Limited efficiency of prolyl‐endopeptidase in the detoxification of gliadin peptides in celiac disease. Gastroenterology 2005; 129: 786–96. [DOI] [PubMed] [Google Scholar]
- 16. Shan L, Marti T, Sollid LM, Gray GM, Khosla C. Comparative biochemical analysis of three bacterial prolyl endopeptidases: implications for coeliac sprue. Biochem J 2004; 383(Pt 2): 311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shan L, Molberg O, Parrot I, et al Structural basis for gluten intolerance in celiac sprue. Science 2002; 297: 2275–9. [DOI] [PubMed] [Google Scholar]
- 18. Gass J, Ehren J, Strohmeier G, Isaacs I, Khosla C. Fermentation, purification, formulation, and pharmacological evaluation of a prolyl endopeptidase from Myxococcus xanthus: implications for Celiac Sprue therapy. Biotechnol Bioeng 2005; 92: 674–84. [DOI] [PubMed] [Google Scholar]
- 19. Stepniak D, Spaenij‐Dekking L, Mitea C, et al Highly efficient gluten degradation with a newly identified prolyl endoprotease: implications for celiac disease. Am J Physiol Gastrointest Liver Physiol 2006; 291: G621–9. [DOI] [PubMed] [Google Scholar]
- 20. Gass J, Vora H, Bethune MT, Gray GM, Khosla C. Effect of barley endoprotease EP‐B2 on gluten digestion in the intact rat. J Pharmacol Exp Ther 2006; 318: 1178–86. [DOI] [PubMed] [Google Scholar]
- 21. Siegel M, Garber ME, Spencer AG, et al Safety, tolerability, and activity of ALV003: results from two phase 1 single, escalating‐dose clinical trials. Dig Dis Sci 2012; 57: 440–50. [DOI] [PubMed] [Google Scholar]
- 22. Lahdeaho ML, Kaukinen K, Laurila K, et al Glutenase ALV003 attenuates gluten‐induced mucosal injury in patients with celiac disease. Gastroenterology 2014; 146: 1649–58. [DOI] [PubMed] [Google Scholar]
- 23. Gass J, Bethune MT, Siegel M, Spencer A, Khosla C. Combination enzyme therapy for gastric digestion of dietary gluten in patients with celiac sprue. Gastroenterology 2007; 133: 472–80. [DOI] [PubMed] [Google Scholar]
- 24. ANSES ‐ French Agency for Food, Environmental and Occupational Health & Safety . https://www.anses.fr/AVIS de l?Anses relatif à une demande d'autorisation de mise sur le marché d'un nouvel aliment ou d'un ingrédient alimentaire: préparation enzymatique contenant une activité protéase pour une utilisation dans les compléments alimentaires. 31 July 2014.
- 25. Mitea C, Havenaar R, Drijfhout JW, Edens L, Dekking L, Koning F. Efficient degradation of gluten by a prolyl endoprotease in a gastrointestinal model: implications for coeliac disease. Gut 2008; 57: 25–32. [DOI] [PubMed] [Google Scholar]
- 26. Tack GJ, van de Water JM, Bruins MJ, et al Consumption of gluten with gluten‐degrading enzyme by celiac patients: a pilot‐study. World J Gastroenterol 2013; 19: 5837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beglinger C, Fried M, Whitehouse I, Jansen JB, Lamers CB, Gyr K. Pancreatic enzyme response to a liquid meal and to hormonal stimulation. Correlation with plasma secretin and cholecystokinin levels. J Clin Invest 1985; 75: 1471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vu MK, van der Veek PP, Frolich M, et al Does jejunal feeding activate exocrine pancreatic secretion? Eur J Clin Invest 1999; 29: 1053–9. [DOI] [PubMed] [Google Scholar]
- 29. Lam WF, Gielkens HA, Coenraad M, Souverijn JH, Lamers CB, Masclee AA. Effect of insulin and glucose on basal and cholecystokinin‐stimulated exocrine pancreatic secretion in humans. Pancreas 1999; 18: 252–8. [DOI] [PubMed] [Google Scholar]
- 30. Symersky T, Vu MK, Frolich M, Biemond I, Masclee AA. The effect of equicaloric medium‐chain and long‐chain triglycerides on pancreas enzyme secretion. Clin Physiol Funct Imaging 2002; 22: 307–11. [DOI] [PubMed] [Google Scholar]
- 31. Mujico JR, Dekking L, Kooy‐Winkelaar Y, et al Validation of a new enzyme‐linked immunosorbent assay to detect the triggering proteins and peptides for celiac disease: interlaboratory study. J AOAC Int 2012; 95: 206–15. [DOI] [PubMed] [Google Scholar]
- 32. Sollid LM, Qiao SW, Anderson RP, Gianfrani C, Koning F. Nomenclature and listing of celiac disease relevant gluten T‐cell epitopes restricted by HLA‐DQ molecules. Immunogenetics 2012; 64: 455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Wijck K, Bessems BA, van Eijk HM, Buurman WA, Dejong CH, Lenaerts K. Polyethylene glycol versus dual sugar assay for gastrointestinal permeability analysis: is it time to choose? Clin Exp Gastroenterol 2012; 5: 139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spaenij‐Dekking EH, Kooy‐Winkelaar EM, Nieuwenhuizen WF, Drijfhout JW, Koning F. A novel and sensitive method for the detection of T cell stimulatory epitopes of alpha/beta‐ and gamma‐gliadin. Gut 2004; 53: 1267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kwiatek MA, Menne D, Steingoetter A, et al Effect of meal volume and calorie load on postprandial gastric function and emptying: studies under physiological conditions by combined fiber‐optic pressure measurement and MRI. Am J Physiol Gastrointest Liver Physiol 2009; 297: G894–901. [DOI] [PubMed] [Google Scholar]
- 36. Hausch F, Shan L, Santiago NA, Gray GM, Khosla C. Intestinal digestive resistance of immunodominant gliadin peptides. Am J Physiol Gastrointest Liver Physiol 2002; 283: G996–1003. [DOI] [PubMed] [Google Scholar]
- 37. Boivin M, Lanspa SJ, Zinsmeister AR, Go VL, DiMagno EP. Are diets associated with different rates of human interdigestive and postprandial pancreatic enzyme secretion? Gastroenterology 1990; 99: 1763–71. [DOI] [PubMed] [Google Scholar]
- 38. Lahdeaho ML, Lindfors K, Airaksinen L, Kaukinen K, Maki M. Recent advances in the development of new treatments for celiac disease. Expert Opin Biol Ther 2012; 12: 1589–600. [DOI] [PubMed] [Google Scholar]


