Abstract
HPV infection is an important public health problem in developing countries. We investigated HPV genotypes in the Uyghur female population of Karasay Township, Hotan region. A population‐based cervical cancer screening was conducted for 4,500 women in Karasay Township, Xinjiang Hotan, China. A total of 900 women were selected by systematic sampling with a 5:1 proportion (ages 20–69). The subjects completed a questionnaire and consented to HPV typing and Pap smear examination. Colposcopic biopsies were performed for patients with cytological abnormalities (≥ASCUS). A total of 117 of the 900 women (13%) assessed were infected with HPV. The most common subtype was HPV‐16, and other common high‐risk types included HPV‐58 and HPV‐39. A total of 40 women (4.44%) were identified with abnormal cytology (≥ASCUS) by Pap smear. A significant link was found between HPV prevalence and cytological diagnosis. The HPV infection rates for the patients with cervical inflammation, CIN, and cancer were 18.18%, 64.71%, and 100%, respectively. Significant differences in HPV infection rates were found among the patients with the three groups of pathological results. In Karasay, the HPV infection rate in Uyghur women is lower than previously reported; however, the proportion infected with HR‐HPV is higher. HPV‐16, HPV‐58, and HPV‐39 are the most prevalent genotypes. J. Med. Virol. 87:1960–1965, 2015. © 2015 The Authors. Journal of Medical Virology Published by Wiley Periodicals, Inc.
Keywords: HPV, Muslim women, cervical cancer, China
INTRODUCTION
Cervical cancer is the second most common cancer worldwide. According to Globecan data in 2012, there were over 528,000 new cases of cervical cancer, nearly 85% of which occurred in less developed countries [Globecan, 2012]. The death rate from cervical cancer has fallen in many countries since the introduction of cervical screening programs and systematic cytological screening [Levi et al., 2000; Peto et al., 2004]. In 2012, the cervical cancer incidence in China was 62/100,000, while the mortality rate was 30/100,000 [Globecan, 2012]. While cervical cancer mortality appears to have declined considerably in urban China in recent years [Li et al., 2000], this decrease is less marked in rural areas. Screening results have indicated that the incidence and mortality rate of cervical cancer are 459‐590/100,000 and 17.78/100,000, respectively, for Uyghur women residing in Xinjiang. These numbers are obviously higher than those reported for other ethnic groups. Moreover, the age of patients from Xinjiang is younger compared with that of patients from other regions [Suzuk et al., 1997; Abliz et al., 2001].
A number of molecular and epidemiologic studies have demonstrated a strong relationship between human papillomavirus (HPV) infection and cervical cancer, as HPV DNA is detectable in virtually all cervical cancers [Walboomers et al., 1999]. HPV‐16 is the most prevalent HPV type worldwide. In Asia, HPV‐58 and HPV‐52 are the next most common types after HPV‐16 and HPV‐18 [Villiers et al., 2004; Meijer et al., 2006; Matsumoto, 2007]. These epidemiologic findings, in combination with follow‐up studies, demonstrate the roles of certain HPV types in cervical cancer development [Kulasingam et al., 2002].
To understand the potential impact of HPV vaccination and HPV‐based screening, the collection of epidemiological data on HPV type‐specific prevalences among women from different populations is important. The use of HPV DNA testing as an adjunct to Pap smear cytology testing for the diagnosis of cervical cancer to improve screening sensitivity and negative predictive values, especially for high‐risk types, is becoming increasingly attractive as a cost‐effective primary screening method [Clifford et al., 2005]. Therefore, the genotyping of HPV in clinical settings is regarded as an important diagnostic tool for cervical cancer, and also as a means of providing valuable information necessary for HPV prevention, treatment and vaccination. The Xinjiang Uyghur Autonomous Region, located in western China, has a population of 20 million Uyghur (the main ethnic group). The Karasay township is a high‐risk region in Xinjiang. However, the HPV genotype distribution in Uyghur women has not been examined to date. In this study, we investigated HPV infections in Uyghur women, determined the prevalences of various HPV genotypes among Uyghur women receiving a routine Pap smear in the Karasay township of Hotan, and elucidated the relationship between HPV infections and squamous intraepithelial lesions.
MATERIALS AND METHODS
Study Population
A total of 4,500 women were recruited to participate in this study from June to September, 2008 in the Karasay village of Hotan city, Xinjiang, China. The women ranged in age from 20 to 69 years. Those who were married, not pregnant or lactating, and had no history of cervix therapy were considered to be eligible. A total of 900 cases were selected by random sampling with a 5:1 proportion. The 4,500 participants were assigned numbers from 1 to 4,500, and every 5th patient was chosen for this study. Informed consent was obtained from all participants. This study was approved by the Ethical Committee of The Affiliated Tumor Hospital of Xinjiang Medical University.
Questionnaire
Each woman was interviewed and filled out a structured questionnaire containing demographic information, knowledge about HPV infections and cervical cancer, relevant medical history, sexual history, pregnancy history, contraception use, smoking history, and family history. Then, gynecological examination was conducted by a physician in a separate room for confidentiality and to ensure the reliability of the information provided by the participants.
(See Fig. 1: Flow chart of study samples).
Figure 1.
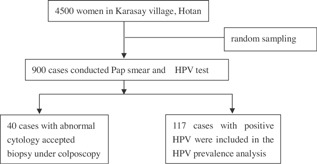
Flow chart of study sample.
Specimen Collection
Each enrolled woman underwent gynecological examination. A Pap smear was obtained, fixed in 95% ethyl alcohol and air‐dried. A cytobrush was used for HPV detection. The head of the brush was placed in small bottle containing Cytorich preserving fluid. All specimens were stored at 4°C until analysis. Participants with abnormal cytology results were referred for colposcopy and biopsy.
Cytology
Pap smears were interpreted by experienced pathologists at Xinjiang Tumor Hospital, Urumqi. Cytological diagnoses were evaluated according to the Bethesda system criteria. Terminology used in this study are as follows: atypical squamous cells of undetermined significance (ASC‐US), atypical squamous cells‐cannot exclude HSIL (ASC‐H), low‐grade squamous intraepithelial lesions (LSIL), high‐grade squamous intraepithelial lesions (HSIL), and squamous cell carcinoma (CC).
HPV Genotyping
HPV genotyping was performed using the flow‐through hybridization and gene chip (HybriMax) method (Hybribio Company Ltd. HongKong, China) according to the manufacturer's protocol. The types of HPV detected included 13 high‐risk types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68), 5 low‐risk types (6, 11, 42, 43, and 44) and 3 common Chinese types (53, 66, and CP8304).
Colposcopy
Colposcopic biopsy was performed for participants with abnormal cytological results. Direct biopsy was conducted for visible lesions; otherwise, biopsies were collected from four random quadrants at the three, six, nine, and twelve o' clock positions in the conjunction belt of the cervix. All specimens were interpreted by the senior pathologist of Xinjiang Tumor Hospital according to the pathological diagnostic standard criteria. The results of this study were classified as normal, CIN1, CIN2, CIN3, and cervical cancer.
Statistical Analysis
Statistical analysis was performed using SPSS version 13.0 for Windows. The χ2 test was performed to assess the statistical significance of differences in the prevalence of HPV infection. P‐values of less than 0.05 were considered to be significant.
RESULTS
General Status of the Population
A total of 900 women were enrolled in this study, with a mean age of 40.91 ± 8.9 years. A total of 75 (8.33%) of the participants were illiterate. The number of women with a primary school degree, middle school degree, and higher education were 679 (75.44%), 122 (13.57%), and 24 (2.66%), respectively. The majority of the participants were farmers (95.35% of the total); other occupations included workers (0.58%), cadres (2.33%), and service employees (0.97%). None of the 900 women smoked or drank alcohol.
HPV Prevalence and Genotyping
A total of 117 (13%) out of the 900 were determined to be infected with HPV. The infection frequencies of high‐risk HPV (HR‐HPV) and low‐risk HPV (LR‐HPV) were 11.78% and 1.22%, respectively, and a total of 90.62% of the HPV‐positive women were infected with HR‐HPV. Three participants had multiple infections, including one with HPV‐16; HPV‐58, and two with HPV‐16; HPV‐33. The most frequent genotypes were HPV‐16 (70.94%, 83/117), followed by HPV‐58 (7.69%, 9/117), HPV‐39 (6.84%, 8/117), HPV‐6 (5.98%, 7/117), HPV‐33 (2.56%, 3/117), HPV‐18 (1.71%, 2/117), HPV‐11 (1.71%, 2/117), HPV‐43 (1.71%, 2/117), HPV‐52 (1.71%, 2/117), and HPV‐62 (1.17%, 2/117). The age‐specific HPV prevalence is shown in Figure 2. Women aged 35–40 and 40–45 had the highest HPV prevalences (24.79% and 23.08%, respectively), while the lowest rate of HPV infection was observed in women aged 25–30 (1.71%). Women older than 65 years also had a low HPV infection rate (2.56%).
Figure 2.
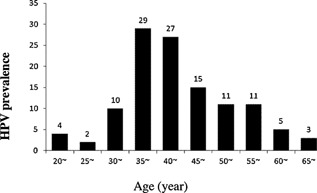
Age‐specific prevalence of Human Papillomavirus (HPV).
(See Fig. 2 : Age‐specific Prevalences of Human Papillomavirus [HPV]).
HPV Prevalence in Patients with Different Cytological Results
The conventional cervical pap smear results were abnormal for 40 samples out of the 900 tested (4.44%), while 860 were identified as normal or inflamed. A total of 25 participants had abnormal cytological results out of the 117 HPV‐positive women (21.37%) compared with 15 out of the 783 HPV‐negative women (1.92%). The differences between these two groups were statistically significant (x2 = 90.688, P = 0.000). The HPV infection rate increased concomitantly with the severity of cytological diagnosis. A total of 3 women were infected with HPV, out of 10 cases with ASC‐US (30%), and the HPV infection rates were 75% in women with HSIL and 100% in women with cervical cancer. These links between HPV infection and the cytological results were significantly different (x2 = 115.470, P = 0.000).
(See Table I: HPV Prevalences according to Different Cytological Results).
Table I.
HPV Prevalence According to Different Cytological Results
| HPV | Cytological | Result | |||||
|---|---|---|---|---|---|---|---|
| Infection | Normal | ASCUS | ASCH | LSIL | HSIL | CC | Total |
| HPV positive | 92 (10.7) | 3 (30.0) | 4 (44.4) | 3 (75.0) | 6 (75.0) | 9 (100.0) | 17 (13.0) |
| High risk | 60 (6.9) | 1(10.0) | 2 (22.2) | 2 (50.0) | 5 (62.5) | 9 (100.0) | 79 (8.8) |
| Low risk | 32 (3.7) | 2 (20.0) | 2 (22.2) | 1 (25.0) | 1 (12.5) | 0 (0.0) | 38 (4.2) |
| HPVnegative | 768(89.3) | 7 (70.0) | 5 (55.6) | 1 (25.0) | 2 (25.0) | 0 (0.0) | 783 (87.0) |
| Total | 860 | 10 | 9 | 4 | 8 | 9 | 900 |
HPV Prevalences in Women with Different Pathological Diagnosis
Befitting the process of the study, colposcopic biopsy was performed for 40 women with positive cytological results. A total of 11 women were diagnosed with inflammation, 17 with cervical intraepithelial neopalsia and 12 with cervical invasive cancer. Similar to the cytological results, the HPV infection rate significantly increased with the severity of cervical lesion (x2 = 16.453, P = 0.000). The prevalence of each HPV genotype detected in the cervical lesions is reported in Table II.
Table II.
HPV Prevalence According to Different Pathological Result
| HPV | Pathological | Result | ||||
|---|---|---|---|---|---|---|
| Infection | Normal | CIN1 | CIN2 | CIN3 | Invasive cancer | Total |
| HPV positive | 2 (18.2) | 2 (28.6) | 3 (75.0) | 6 (100.0) | 12 (100.0) | 25 (62.5) |
| HPV 16 | 2 (100.0) | 1 (50.0) | 3 (100.0) | 5 (83.3) | 11 (91.7) | 22 (88.0) |
| HPV 39 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 1 (4.0) |
| HPV 58 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 1 (4.0) |
| HPV 62 | 0 (0.0) | 1 (50.0) | 0 (0.0) | 0 (16.7) | 0 (0.0) | 1 (4.0) |
| HPV negative | 9 (81.8) | 5 (71.4) | 1 (25.0) | 0 (000) | 0 (0.0) | 15 (37.5) |
| Total | 11 | 7 | 4 | 6 | 12 | 40 |
(See Table II: HPV Prevalences according to Different Pathological Results).
DISCUSSION
This population‐based study was conducted in Xinjiang to assess HPV type‐specific prevalence amongst Uyghur women across a broad age‐range. Overall, 13% of the women were found to be HPV‐positive in this study. Among them, the HPV infection rate was 10.70% in those with normal cytological results. This rate is higher than that reported for HPV distribution in Uyghur women by MAYINEUR Niyazi et al. [2011]. In this study, the HPV infection rate was 8.27%. This discrepancy in rates among studies may be related to differences in resources, the ages of study subjects and the methods used for HPV detection. HPV infection in Chinese women has been reported to be higher in a nationwide study 12.1% [Li and Dai, 2008], and it has been determined to be even higher in some provinces, such as Shanxi (20.8%) [Zhao et al., 2001] and Jiangxi (20.63%) [Cao et al., 2006]. The HPV prevalence determined in the present study is lower than those reported by some studies conducted in other countries [Zhuan‐ping et al., 2004; Baseman and Koutsky, 2005; Ledy OliveiraI et al., 2006; Cotton et al., 2007], but is higher than those calculated in others [Pavani Sowjanya et al., 2005]. The HR‐HPV infection rates for the 900 women evaluated in the present study were 11.78%, higher than MAYINEUR Niyazi et al. [2011] reported as 7.25% in Uyghur women, but lower than 17.7% of Fang‐Hui Zhao et al. [2012] reported for crude HR‐HPV prevalence in their pooled analysis of 17 population‐based studies in China. This discrepancy was considered to be related to different sources of population of the studies.
The total HPV rate was unexpectedly low, since Xinjiang Hotan is considered to be a high‐risk region for cervical cancer [Suzuk et al., 1997; Abliz et al., 2001]. These results indicate that the population may have been less exposed to HPV; thus, their development of cervical cancer is most likely attributed to the involvement of other risk factors including lifestyle, sexual behaviors, and genetic background [Abliz et al., 2007; Abliz et al., 2008].
This study reported an HR‐HPV infection rate of 90.62% for the HPV‐positive women, and HPV‐16 was found to be the most frequent type, followed by HPV‐58 and HPV‐39. HPV‐18 was detected in two participants. These results differ from those of studies conducted elsewhere in China and world‐wide, and also differ from a hospital‐based study of Uyghur women that was conducted at Xinjiang Tumor Hospital [Abliz et al., 2007]. The findings of this study require further confirmation, and the question of whether HPV‐58 and HPV‐39 are the common subtypes in other high‐risk regions of Xinjiang still needs to be addressed.
Studies performed by the World Cancer Organization have demonstrated that the prevalence of cervical HPV infection greatly varies worldwide (comparisons have been made between ethnic groups and regions), but that its prevalence is consistent with the distribution of cervical cancer [Bosch et al., 2002; Bhatla et al., 2006; Ragin et al., 2009]. A majority of cervical cancer cases have been associated with HPV‐16 or HPV‐18 in all regions; however, approximately one‐quarter of all cervical cancer cases have been associated with a genotype other than HPV‐16, and the distribution of these subtypes has been demonstrated to vary by region [Deluca et al., 2004; Clifford et al., 2005]. There is a great deal of variation among published reports about the distribution of the HPV subtypes in China and worldwide. The results of many of these studies have little coherence because few have investigated the correlation between HPV infection and race [Pavani Sowjanya et al., 2005].
In Germany, the prevalence of HR‐HPV detected by the HC2 method is 6.4% and that of the carcinogenic types is 4.3%, HPV‐16, HPV‐31, HPV‐52, HPV‐51, HPV‐18, and HPV‐45 were the most common carcinogenic types detected in the study population. Among women with histologically confirmed high‐grade lesions, HPV‐16, HPV‐45, HPV‐58, HPV‐18, HPV‐31, HPV‐33, and HPV‐52 are the predominant types. HPV‐16 is the most frequently detected type reported in other studies, followed by HPV‐33, HPV‐18, and HPV ‐31 [Klug et al., 2007]. In Asian countries, the proportions of HPV‐52 and ‐58 are higher than those of HPV‐45, HPV‐31, and HPV‐33. In Mainland China, the second most common type is HPV‐18, followed by types HPV‐58 and HPV‐52, the infection rates of which are higher than those of HPV‐16 and HPV‐18 in some regions [Lo et al., 2002; Masumoto et al., 2003]. The predominant HPV subtype of cervical cancer patients from 14 cities in China has been determined to be HPV‐16 and ‐58, further confirming that HPV‐58 is one of the most common types in China [Li et al., 1996]. ZHENG Yi et al. [2009] have reported that the HPV‐16, HPV‐59, HPV‐56, HPV‐18, HPV‐33, and HPV‐58 are the common HPV types in Uyghur women from Payzawat Kashgar, which is another high‐risk area in the Xinjiang Uyghur Autonomous Region. In this study, the distribution of HPV subtypes detected in Uyghur women in Karasay, Xinjiang Hotan, was different from those reported in other regions of China and in other countries worldwide. The most frequent HPV subtype was HPV‐16, following by HPV‐58, and HPV‐39. The distributions and frequencies of HPV subtypes reflect their peculiarities. Thus, our data requires confirmation by further studies, and the question of whether HPV‐58 and HPV‐39 are the most common subtypes in other high‐risk regions in Xinjiang remains to be determined.
Many studies conducted in Xinjiang have demonstrated that essentially most women with CIN1‐3 have detectable HPV‐DNA. A total of 70–80% of patients with low‐grade lesions tested positive for HPV. The HPV‐positive rate in patients with high‐grade lesions is 80–90%, and that in patients with invasive cervical cancer exceeds 95% [Lo et al., 2002; ShQ et al., 2006; Abliz et al., 2007; Dutra et al., 2008]. In our study, CIN and cervical cancer were found in 3.22% of the study population, resulting in a lower rate compared with those reported by Xiangyuan (4.31%) and Yangcheng (4.35%) [Shen et al., 2003]. The relatively low rate of CIN and cervical cancer in this study was possibly due to the screening procedure of only using a pap smear for colposcopy, and cervical cytology is inherently prone to sample‐quality variation, subjective interpretation error, and false‐negative results. In this study, the detection rates of HPV in patients with ASCUS, ASC‐H, LSIL, HSIL, and cancer were 30%, 44.44%, 75%, 75%, and 100%, respectively. These finding are in accordance with relevant previous reports [Ledy OliveiraI et al., 2006; Peedicayil, et al., 2006; Cotton et al., 2007]. HPV was detected in 10.70% of women with negative cytology, 88.04% (81/92) of whom were infected with a HR‐HPV subtype. A total of 11 patients with cervical inflammation were confirmed by histology, 2 of whom were infected with HPV‐16. Therefore, although the HPV infection rates were unexpectedly low and the ratio of HR‐HPV subtypes were high (especially the HPV‐16 subtype, which may have increased the incidence of cervical cancer in this population), other factors may play a role in this type of carcinogenesis of Uyghur women, such as early marriage, early pregnancy, education level, and socioeconomic status [Abliz et al., 2008].
The distribution of HPV in Uyghur women will play a guiding role in vaccine research and immunization of this population. Follow‐up visits and proper intervention for HPV infection could reduce the occurrence of cervical carcinoma and precancerous changes in this region. However, the findings and conclusions of this study are limited by the small sample size studied. Therefore, a much larger population‐based multi‐center study is required to validate our findings.
ACKNOWLEDGEMENT
We would like to thank the doctors at Karasay County Hospital for their cooperation.
The copyright line for this article was changed on 17 November 2015 after original online publication.
REFERENCES
- Abliz G, Peng ZhL, Liu SHL. 2001. Detection of Human papillomavirus DNA in cervical cancer tissues from Xinjiang Hotan Uyhur Women. Sichuan J Cancer Control 14:77–81. [Google Scholar]
- Abliz Guzalnur, Abduxkur Guzalnur, Mamat Aynur. 2007. The relationships between cervical cancer of Uighur women and sexual behaviour. Matern Child Health Care Chin 22:4224–4226. [Google Scholar]
- Abliz Guzalnur, Lin Lu, Anaytul Mihrgul. 2008. The relationship between race and susceptibility to cervical cancer in Xinjiang uighur women. Chin J Clin Oncol 35:629–632. [Google Scholar]
- Baseman JG, Koutsky LA. 2005. The epidemiology of human papillomavirus infections. J Clin Virol 32:16–24. [DOI] [PubMed] [Google Scholar]
- Bhatla N, Dar L, Patro AR, Kriplani A, Gulati A, Broor S, Iyer VK, Mathur S, Shah KV, Gravitt PE. 2006. Human papillomavirus type distribution in cervical cancer in Delhi, India. Int J Gynecol Pathol 25:398–402. [DOI] [PubMed] [Google Scholar]
- Bosch FX, Lorincz A, Munoz N, Meijer CJ. 2002. The causal relationship between human papillomavirus and cervical cancer. J Clin Pathol 55:244–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Xiqing, Du Haijun, Zhou Ling. 2006. Effecting factors of HPV infection. J Int Viorol 13:104–107. [Google Scholar]
- Clifford GM, Gallus S, Herrero R, Muñoz N, Snijders PJ, Vaccarella S, Anh PT, Ferreccio C, Hieu NT, Matos E, Molano M, Rajkumar R, Ronco G, de Sanjosé S, Shin HR, Sukvirach S, Thomas JO, Tunsakul S, Meijer CJ, Franceschi S. 2005. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: A pooled analysis. Lancet 366:991–998. [DOI] [PubMed] [Google Scholar]
- Cotton SC, Sharp L, Seth R, Masson LF, Little J, Cruickshank ME, Neal K, Waugh N; TOMBOLA Group. 2007. Lifestyle and socio‐demographic factors associated with high‐risk HPV infection in UK women. Br J Cancer 97:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluca GD, Lucero RH, Martin de Civetta MT, Vicente L, de Gorodner OL, Schelover E, Alonso JM. 2004. Human papillomavirus genotypes in women with cervical cytological abnormalities from an area with high incidence of cervical cancer. Rev Inst Med Trop Sao Paulo 46:9–12. [DOI] [PubMed] [Google Scholar]
- De Villiers EM, Gunst K, Stein H, Scherübl H. 2004. Esophageal squamous cell cancer in patients with head and neck cancer: Prevalence of Human Papillomavirus DNA sequences. Int J Cancer 109:253–258. [DOI] [PubMed] [Google Scholar]
- Dutra I, Santos MR, Soares M, Couto AR, Bruges‐Armas M, Teixeira F, Monjardino L, Hodgson S, Bruges‐Armas J. 2008. Characterisation of human papillomavirus (HPV) genotypes in the Azorean population, Terceira island. Infect Agent Cancer 21;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Globecan fn. 2012. Estimated cancer Incidence, Mortality and Prevalence in 2012 worldwide, web site: http://globocan.iarc.fr/Default.aspx
- Klug SJ, Hukelmann M, Hollwitz B, Düzenli N, Schopp B, Petry KU, Iftner T. 2007. Prevalence of human papillomavirus types in women screened by cytology in Germany. J Med Virol 79:616–625. [DOI] [PubMed] [Google Scholar]
- Kulasingam SL, Hughes JP, Kiviat NB, Mao C, Weiss NS, Kuypers JM, Koutsky LA. 2002. Evaluation of Human Papillomavirus testing in primary screening for cervical abnormalities. JAMA 288:14. [DOI] [PubMed] [Google Scholar]
- Levi F, Lucchini F, Negri E, Franceschi S, la Vecchia C. 2000. Cervical cancer mortality in young women in Europe: Patterns and trends. Eur J Cancer 36:2266–2271. [DOI] [PubMed] [Google Scholar]
- Li Ni, Dai Min. 2008. Multi central cross study of Chinese women HPV infection. J Chin Dis Control 12:411–415. [Google Scholar]
- Li J, Liu BY, Hausen Zur, et al. 1996. The investigation of HPV infection and geographic distribution of cervical cancer tissue of in China. J Exp Clin VIR 10:50–55. [Google Scholar]
- Li HQ, Jin SQ, Xu HX, David B. 2000. The decline in the mortality rates of cervical cancer and a plausible explanation in Shandong, China. Int J Epidemiol 29:398–404. [PubMed] [Google Scholar]
- Lo WK, Wong YF, Chan KM, Li JC, Poon JS, Wang VW, Zhu SN, Zhang TM, He ZG, Wu QL, Li GD, Tam JS, Kahn T, Lam P, Cheung TH, Chung TK. 2002. Prevalence of human papillomavirus in cervical cancer: A multicenter study in China. Int J Cancer 100:327–331. [DOI] [PubMed] [Google Scholar]
- Masumoto N, Fujii T, Ishikawa M, Mukai M, Saito M, Iwata T, Fukuchi T, Kubushiro K, Tsukazaki K, Nozawa S. 2003. Papanicolaou tests and molecular analyses using new fluid‐based specimen collection technology in 3000 Japanese women. Br J Cancer 88:1883–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K. 2007. Human papillomavirus and cervical cancer. Nippon Rinsho 65:2113–2124. [PubMed] [Google Scholar]
- Meijer CJ, Snijders PJ, Castle PE. 2006. Clinical utility of HPV genotyping. Gynecol Oncol 103:12–17. [DOI] [PubMed] [Google Scholar]
- Niyazi MAYINEUR, Li LI, Feng CHEN, Wen‐hua ZHANG. 2011. Epidemilological study on HPV infection of Xinjiang Uyghur women related to cervical cancer. Chin Clin Oncol 04:322–325. [Google Scholar]
- Oliveira LH, Rosa ML, Pereira CR, Vasconcelos GA, Silva RA, Barrese TZ, Carvalho MO, Abi GM, Rodrigues EM, Cavalcanti SM. 2006. Human papillomavirus status and cervical abnormalities in women from public and private health care in Rio de Janeiro State. Rev Inst Med Trop Sao Paulo 48:279–285. [DOI] [PubMed] [Google Scholar]
- Peto J, Gilham C, Fletcher O, Matthews FE. 2004. The cervical cancer epidemic that screening has prevented in the UK. Lancet 364:249–256. [DOI] [PubMed] [Google Scholar]
- Peedicayil A, Abraham P, Sathish N, John S, Shah K, Sridharan G, Gravitt P. 2006. Human papillomavirus genotypes associated with cervical neoplasia in India. Int J Gynecol Cancer. 16:1591–1595. [DOI] [PubMed] [Google Scholar]
- Ragin CC, Watt A, Markovic N, Bunker CH, Edwards RP, Eckstein S, Fletcher H, Garwood D, Gollin SM, Jackson M, Patrick AL, Smikle M, Taioli E, Wheeler VW, Wilson JB, Younger N, McFarlane‐Anderson N. 2009. Comparisons of high‐risk cervical HPV infections in Caribbean and US populations. Infect Agent Cancer. 10; 41:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ShQ Jiang, Song‐Ai TU, ZHOU JL. 2006. Aimurehan Investigation and analysis of gynecopathy in Cele county of Xinjiang, China. Matern Child Health Care Chin 21:524–526. [Google Scholar]
- Shen YH, Chen F, Huang MN, Liu B, Wang XX, Zhao FH, Li SM, Li N, Wu LY, Rong SD, Zhang WH, Ren SD, Huang RD, Qiao YL. 2003. Human papillomavirus infection in Shanxi Province, People's Republic of China: The high incidence region of cervical cancer. Chin Acad Med Sci 25:381–385. [PubMed] [Google Scholar]
- Suzuk L, Noffsinger AE, Aili M. 1997. Detection of human papillomavirus DNA in cervical squamous cell carcinoma in Southern Xinjiang Uygur women. Zhonghua Fu Chan Ke Za Zhi 32:405–408. [PubMed] [Google Scholar]
- Sowjanya AP, Jain M, Poli UR, Padma S, Das M, Shah KV, Rao BN, Devi RR, Gravitt PE, Ramakrishna G 2005. Prevalence and distribution of high‐risk human papilloma virus (HPV) types in invasive squamous cell carcinoma of the cervix and in normal women in Andhra Pradesh India. BMC Infect Dis 5:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189:12–19. [DOI] [PubMed] [Google Scholar]
- Zheng Yi, et al. 2009. Study on HPV type prevelance in Xinjiang Uyghur women. Mod Biomed Dev 07:67–71. [Google Scholar]
- Zhao FH, Lewkowitz AK, Hu SY, Chen F, Li LY, Zhang QM, Wu RF, Li CQ, Wei LH, Xu AD, Zhang WH, Pan QJ, Zhang X, Belinson JL, Sellors JW, Smith JS, Qiao YL, Franceschi S. 2012. Prevalence of human papillomavirus and cervical intraepithelial neoplasia in China: A pooled analysis of 17 population‐based studies. Int J Cancer 131:2929–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Fang Hui, Li Nan, Ma Junfei. 2001. Relation study of HPV infection and cervical cancer in Shanxi Xiangyuan. J Chin Epidemiol 22:375–378. [PubMed] [Google Scholar]
- Zhuan‐ping ZENG, Feng CHEN, Bin LIUH. 2004. Analysis of risk factors for cervical cancer in Yangcheng county. Cancer Res Prev Treat 31:178–181. [Google Scholar]


