Abstract
‘Hunger is the best spice’ is an old and wise saying that acknowledges the fact that almost any food tastes better when we are hungry. The neurobiological underpinnings of this lore include activation of the brain's reward system and the stimulation of this system by the hunger‐promoting hormone ghrelin. Ghrelin is produced largely from the stomach and levels are higher preprandially. The ghrelin receptor is expressed in many brain areas important for feeding control, including not only the hypothalamic nuclei involved in energy balance regulation, but also reward‐linked areas such as the ventral tegmental area. By targeting the mesoaccumbal dopamine neurones of the ventral tegmental area, ghrelin recruits pathways important for food reward‐related behaviours that show overlap with but are also distinct from those important for food intake. We review a variety of studies that support the notion that ghrelin signalling at the level of the mesolimbic system is one of the key molecular substrates that provides a physiological signal connecting gut and reward pathways.
Keywords: dopamine, ghrelin
Introduction
The present review is concerned with the actions of a circulating appetite‐promoting hormone ghrelin 1, produced mostly by the stomach, on the reward system of the brain. The idea that the gut utilises enteroendocrine hormones to communicate with pathways involved in rewarding aspects of food intake is a new and emerging field 2 suggesting the existence of an endocrine gut–brain reward axis that can modify reward signalling for food, a system important for survival. Ghrelin appears to have a physiological role in hunger and meal initiation 3, orchestrating a variety of behaviours that ensure animals go out into the environment to seek out and consume a variety and plentiful supply of nutrients. We focus especially on the effects of ghrelin on reward‐linked behaviour for food, including the mechanisms and pathways involved.
The ghrelin/growth hormone secretagogue receptor type 1A (GHSR‐1A) system
Ghrelin is a 28‐amino acid octanoylated peptide hormone 1 secreted predominantly from a specific type of endocrine cell of the stomach located within the gastric oxyntic mucosa and named P/D1‐type cells in humans 4 or A like‐type cells in rodents 5. Ghrelin mediates its actions via its unique specific receptor, the GHSR‐1A 6, which is a G protein‐coupled receptor highly expressed in the central nervous system 7, 8. Ghrelin is the only known naturally‐occurring peptide to be post‐translationally modified by a O‐octanoylation, a reaction catalysed by the enzyme ghrelin O‐acyl transferase (GOAT) 9. This modification is essential for ghrelin‐induced activation of GHSR‐1A. Ghrelin plays a well‐defined variety of physiological roles and is recognised for being the only known circulating peptide hormone that stimulates food intake 10, 11. As discussed below, ghrelin stimulates appetite via diverse mechanisms that promote food intake and that stimulate food reward‐related behaviours. In plasma, a non‐octanoylated form of ghrelin, named desacyl‐ghrelin that represents more than 90% of total ghrelin immunoreactivity, has been shown to exist 12. The GHSR‐1A‐independent effects of this peptide on food intake regulation have been described, although the physiological significance of desacyl‐ghrelin remains a matter of debate 13.
Plasma ghrelin levels fluctuate with meal cycle and energy status. One of the most striking features about the 24‐h secretory pattern of ghrelin in man is the large rise in plasma levels that occur just before mealtimes 3. The precise mechanism governing preprandial ghrelin release remains an open question 14. One possibility is that ghrelin release is controlled locally in the gastrointestinal tract 15. Some of the metabolic and hormonal factors able to directly regulate ghrelin secretion have been identified. Using ghrelin‐producing cell lines, it has been shown that low d‐glucose concentrations stimulate ghrelin release, whereas high d‐glucose and glucose metabolism block ghrelin release 16. Also, norepinephrine enhances ghrelin release by binding to β1‐adrenergic receptors on ghrelin cells; indeed, this mechanism has been proposed to modulate ghrelin secretion during fasting when the sympathetic tone is increased 17. It has also been shown that natural (i.e. α‐linolenic acid) or chemical agonist for the G protein‐coupled receptor 120 inhibit the secretion of ghrelin, suggesting that the decrease of ghrelin release after feeding is induced partially by long‐chain fatty acids acting directly on gastric cells 18. Importantly, ghrelin release is also coordinated by a descending pathway (e.g. via the vagus nerve) signalling either a food anticipatory signal 19 or a cry for nutrients by the brain. Anticipatory cues associated with the delivery of food have been shown to trigger ghrelin release in mice 20. Food anticipatory behaviour, including anticipation for palatable food, is decreased in models of suppressed ghrelin signalling, suggesting that ghrelin may, in turn, activate food anticipatory pathways 21, 22.
One of the key physiological targets of the ghrelin/GHSR‐1A system is the brain. The dedicated ghrelin receptor, GHSR‐1A, was identified in 1996 and was found to be expressed in many brain areas linked to feeding control, including hypothalamic, brainstem and mesolimbic pathways 6, 7, 8. Ghrelin is able to drive a feeding response when microinjected into many of these sites, including the arcuate nucleus of the hypothalamus (ARC), ventromedial nucleus 23, lateral hypothalamic area (LHA) 24, caudal brainstem 25, ventral tegmental area (VTA), nucleus accumbens (NAc) 26, 27, lateral amygdala 28 and ventral hippocampus 29. The orexigenic effects of ghrelin appear to be exclusively signalled through GHSR‐1A 6 because ghrelin fails to elicit a feeding response in mice lacking this receptor 30, as well as in rats pretreated with central injection with a GHSR‐1A antagonist 31. Food intake appears to be a common goal of the ghrelin‐appetitory networks, an endpoint achieved through integration with brain pathways involved in many diverse constructs and behaviours. Indeed, ghrelin signalling pathways are involved in hunger sensation 32, food anticipatory behaviour 21, 33, food reward 34, motivated (reward‐linked) behaviour for food 35, 36, memory 37, novelty‐seeking behaviour 38 and anxiety/stress‐like behaviour 28, 39, 40. In the present review, we focus especially on recent advances implicating the central ghrelin signalling system in food reward.
The first indication that central GHSR‐1A signalling could be implicated in food intake regulation was noted in 1993, when it was found that the growth hormone‐releasing peptide 6 (a growth hormone secretagogue that is now recognised to be a ghrelin mimetic) activates cells in the ARC, as reflected by an increase in the number of cells detected that express Fos protein 41. It emerged that approximately half of the ARC cells activated by this ghrelin mimetic expressed neuropeptide Y (NPY) mRNA, a potent orexigenic signal 42. These ghrelin‐sensitive NPY neurones, which co‐express another orexigenic peptide, agouti‐related protein (AgRP), are considered as a major node in the appetite‐regulatory circuitry. In line with this hypothesis, it was later found that NPY/AgRP neurones of the ARC express high levels of GHSR‐1A 43, 44. Moreover, ghrelin fails to increase food intake in mice lacking NPY and AgRP 45, 46, suggesting that these peptides play a pivotal role in the effects of ghrelin on food intake. Selective re‐expression of GHSR‐1A in AgRP neurones partially restored this phenotype 47.
Ghrelin signalling engages a complex network of neuronal circuitries to modulate feeding‐linked behaviour. For example, ghrelin‐induced food intake also appears to depend on orexin neurones of the LHA, where GHSR‐1A is expressed 24, 48 and the action of ghrelin on food reward requires intact orexin signalling 35. GHSR‐1A is expressed in vagal afferent neurones of the nodose ganglia and in the dorsal vagal complex 8, 49, providing an alternative route through which peripheral ghrelin could signal to appetitive neurocircuitry. Indeed, it has been suggested that vagus nerve integrity is required for ghrelin‐induced food intake 50, 51, although a subsequent study did not find this to be the case 52. The presence of GHSR‐1A in dopaminergic VTA neurones 8, 53, 54, as well as other cell types in this region, supports the possibility that ghrelin can regulate rewarding aspects of eating. Ghrelin may also regulate mesolimbic circuits indirectly via the cholinergic neurones of the laterodorsal tegmental area (LDTg), which also express GHSR‐1A 55, 56. The action of ghrelin at the level of the hippocampus, another brain area where GHSR‐1A is present in abundance 7, 8, also appears to be important for both motivational and learned aspects of feeding behaviour 29. Thus, the neuroanatomical distribution of the ghrelin receptor supports a role for the ghrelin/GHSR‐1A system in the regulation of both homeostatic and rewarding aspects of feeding.
In humans, the response of the brain's reward system to food cues, as measured using functional resonance imaging, is enhanced by fasting 57. The relevance of the ghrelin signalling in this observation was suggested by a functional magnetic resonance imaging study showing that ghrelin can mimic the effects of fasting on the reward networks 58 and was further confirmed by another imaging study in humans showing that ghrelin increases the neural response in brain centres implicated in rewarding aspects of feeding 59. In particular, ghrelin administration to human subjects increases the activation of some reward‐related brain centres, including the substance nigra and the VTA, in response to tempting food pictures 59. A more recent imaging study has not only confirmed previous observations, but also shown that subjects with polymorphisms in the fat mass and obesity‐associated gene genotype exhibited divergent neural responsiveness to peripheral ghrelin within brain regions that regulate rewarding aspects of appetite 60. Thus, several functional resonance imaging studies in healthy subjects strongly support a role for ghrelin in rewarding aspects of eating in human subjects.
Food reward: motivational and hedonic aspects of eating
All organisms have a capacity to seek out and consume food. In mammals, the neurobiological mechanisms sensing energy need, food availability and coordinating appetitive and food‐seeking behaviours are complex. Feeding control involves an integrated regulatory system of homeostatic brain circuits that drive food intake depending on energy store levels: processes for which the hypothalamus and brainstem have a primary role. Food intake is also regulated by reward pathways that process information about the pleasurable (hedonic) and incentive (motivational) aspects of food intake. Homeostatic and reward circuits mediating food intake are inter‐related: ‘Hunger is the best spice’, is a Swedish saying acknowledging that all foods have a rewarding value that is influenced by hunger and food availability. From an evolutionary perspective, food reward has a dual role: it not only promotes food seeking and eating when food is scarce, but also promotes over‐eating when food becomes available, aiming to establish sufficient energy stores for a future famine. If food is found rewarding, this will motivate animals to go out into the environment to seek it out, ensuring not only an adequate supply of calories, but also that consumed foods are of diverse nutritional composition. In the current obesogenic environment, however, food reward is no longer an evolutionary advantage for modern human beings. Instead, it contributes to the maladaptation to that environment, encouraging people to overeat calorie‐dense foods to a level far beyond metabolic need.
Neuronal circuits involved in food reward
A key feature of reward processing is the activation of a major dopaminergic cell group located in the VTA of the midbrain. These dopaminergic neurones project to the NAc and the prefrontal cortex, as well as to several other brain areas, including the hippocampus and the hypothalamus 61. The VTA receives projections from many brain nuclei, including the aforementioned areas that receive projections from the VTA, cholinergic neurones of the LDTg 55, as well as taste information via afferent sensory fibres 62, 63. Activation of dopaminergic VTA neurones occurs in response to both natural rewards (such as food or sex) and artificial rewards (such as alcohol or other addictive drugs of abuse). As is the case for addictive drugs, the consumption of a food reward has been found to trigger dopamine overflow/release in the NAc, as measured by microdialysis 64, and to increase phasic dopamine release in the striatum, as measured by fast‐scan cyclic voltammetry 65. Accumbal dopamine overflow is coupled to VTA dopamine neurone activity 66 and so it may be inferred that foods, especially palatable foods, have the capacity to activate the VTA‐NAc dopamine projection. Indeed, acute consumption of a high‐fat diet activates the mesolimbic circuit by neuronal pathways that require orosensory stimulation 67. Dopamine release in the NAc potently augments the drive to obtain food rewards 68. The shell part of the NAc is particularly important for eating behaviours engaging projections to the LHA neurones that control food intake. Orexigenic LHA neurones appear to be under a tonic inhibition that can be relieved by activation of reward pathways 69, 70. In addition, LHA orexin neurones send projections to the VTA, where they activate dopaminergic neurones 71, 72. Thus, LHA orexin neurones have been proposed as a potential link between homeostatic and reward circuits regulating food intake 73.
One of the primary roles attributed to the VTA‐NAc dopamine pathway is ‘wanting’ or motivational component of reward that is important for craving behaviour and that is linked to but distinct from the ‘liking’ or hedonic component of reward 74. As reviewed elsewhere 75, the mechanisms linking dopamine to rewarding aspects of eating are rather sophisticated. Novelty of the reward appears to be critical for achieving a maximal dopamine signal 76. With repeated exposure to that same reward (conditioning), the NAc dopamine response lessens (habituates) and transfers instead onto a predictive cue associated with its delivery 77, 78, 79. Thus, dopamine signalling is crucially important for the formation of associations between rewards and anticipatory cues 78, 80, 81. As a consequence of conditioning, the dopamine signal takes on a new role: as a predictor of reward; motivational behaviours are recruited as part of this mechanism ensuring that the expected reward is consumed. One hypothesis for the role of the dopamine signal in reward is that it serves as a ‘reward prediction error’ 82. It follows that NAc dopamine release could be important for assigning increasing reward value to food cues 83.
Multifaceted actions of ghrelin signalling on food reward
In 2006, it became apparent that ghrelin activates the dopamine system, triggering NAc dopamine release 84, dopamine turnover and VTA dopamine neurone activity 54. Consistent with this, a subpopulation of VTA dopamine neurones was found to express GHSR‐1A, although the receptor was also found to be located on other cell types within the VTA 54. The idea that ghrelin may provide a physiological signal connecting gut and reward pathways paved the way to studies exploring the effects of ghrelin on behaviours linked to dopamine signalling, including hedonic and motivational aspects of eating.
In rodents, reward can be assessed in the condition place preference (CPP) test in which the animals learn to associate the experience of reward with a particular environment/chamber. They will return to that chamber, spending more time there, even when the reward is no longer available. In satiated rats, the peripheral delivery of a GHSR‐1A antagonist has been shown to abolish CPP for chocolate 85. Similarly, in mice, the delivery of a GHSR‐1A antagonist suppresses/abolishes CPP for a high‐fat diet 35, and even for alcohol 86 or psychostimulant drugs such as cocaine or amphetamine 87. These data using GHSR‐1A antagonists, together with data from ghrelin receptor knockout mice 35, 85, 86, indicate that central ghrelin signalling is required for the rodents to experience reward from alcohol (an artificial reward) or food (a natural reward). Consistent with this, ghrelin appears to enhance CPP for a high‐fat diet 35 and psychostimulant drugs such as cocaine 88.
Craving‐like behaviour (i.e. ‘wanting’, motivated, goal‐direct behaviour) for food or other reward reinforcers can be explored in rodents using the ‘operant conditioning’ paradigm in which the animals have to progressively work harder (e.g. by pressing a lever) to obtain a reward. The animals are first trained on a fixed ratio schedule to associate the pressing of the lever a fixed number of times with the delivery of a reward. Subsequently, they are tested using a progressive ratio schedule in which the animal has to work increasingly hard for each reward obtained. The number of lever presses or the number of reward earned can be used to estimate motivation or goal‐directed behaviour. Motivated behaviour for sugar treats has been shown to be increased by ghrelin (administered peripherally or centrally to satiated rats) and decreased by a GHSR‐1A antagonist administered to fasted rats (also delivered both peripherally and centrally) 36. Central ghrelin administration increases operant lever‐pressing for sucrose in rats 36, 89. Similarly, operant behaviour for a high‐fat diet in mice, measured by nose pokes instead of lever presses, was increased by ghrelin 35. Also, GOAT‐deficient mice display an attenuated motivation for a high‐fat diet in an operant responding model and a decreased reward‐linked feeding response examined in a ‘dessert effect’ protocol, in which the intake of a palatable high‐fat diet pellet ‘dessert’ is assessed in calorically‐satiated mice 90.
It is clear that ghrelin enhances preference for pleasurable, sweet and fatty foods. In particular, ghrelin administration shifts food preference towards a high‐fat diet 91. Ghrelin administration also increases intake of palatable saccharin solution and preference for saccharin‐flavored foods in mice 92. Similarly, rats treated with a GHSR‐1A antagonist consume less peanut butter and the liquid nutritional supplement Ensure® (Abbott Laboratories, Chicago, IL, USA) but fail to change intake of regular chow in a free choice protocol 85. On the other hand, ghrelin also increases food anticipatory activity, which is characterised by increased arousal, increased locomotor activity and an elevated body temperature in anticipation of a predicted meal 21, 22. Ghrelin secreted in anticipation of a meal correlates with anticipatory locomotor activity and the administration of ghrelin increases locomotor activity and foraging‐like activities in rodents 93, 94, 95. Moreover, GHSR‐1A antagonists decrease anticipatory behaviour for a palatable meal 22. Interestingly, i.c.v. ghrelin fails to alter the avidity of licking, when lick motor patterns were recorded using lickometry, suggesting that ghrelin does not affect the hedonic valuation (i.e. ‘liking’) in rats 89. Odour also plays a role in conferring information about food availability, and ghrelin may have a beneficial role in to help animals seek out because it has been shown to stimulate sniffing and to increase olfactory sensitivity in mice 96. Overall, there is a great deal of evidence supporting a role for ghrelin in a variety of food reward‐related eating behaviours. It is perhaps not surprising therefore that ghrelin‐sensitive brain networks overlap considerably with those important for feeding control 97.
Neuronal circuits mediating the effects of ghrelin on food reward
The neurobiological substrates and circuits underpinning the effects of ghrelin on food‐motivated behaviour show some overlap with (but also divergence from) those involved in food intake 2, 97. Although food intake can be driven by ghrelin delivery to both the VTA 26, 54, 85 and the NAc 26, 85, food‐motivated behaviour occurs only after VTA (but not Nac) microinjection of ghrelin 27. VTA‐lesioned rats spend less time than control rats exploring tubes containing peanut butter in response to centrally‐administered ghrelin 85. Similar effects are observed in food‐restricted rats in which chronic intra‐VTA administration of ghrelin enhances, whereas chronic intra‐VTA delivery of a GHSR‐1A antagonist blunts, operant responding for chocolate‐flavored pellets 98. Collectively, these findings identify the VTA as a key target brain area for the effects of ghrelin on food‐motivated behaviour and suggest that direct action of ghrelin at the level of the VTA is sufficient to drive food‐motivated behaviour. Moreover, the uncoupling between food intake and food motivated behaviour elicited by intra‐VTA ghrelin resonates with the collective findings that NAc dopamine signalling is not coupled to normal feeding 99, 100 but rather in motivated behaviour towards palatable foods, independent of the calories consumed 101, 102. Importantly, only the effect of intra‐VTA ghrelin on food‐motivated behaviour was found to require NAc dopamine receptor 1 and 2 signalling, whereas food intake driven by VTA ghrelin was dopamine‐independent in this paradigm 103. Pretreatment of rats with a dopamine receptor 1 antagonist eliminates ghrelin‐induced increases in bar pressing, without compromising generalised licking motor control, supporting a role for dopamine receptor 1 signalling mainly in the motivational feeding effects of ghrelin 89. It has been shown that mice expressing GHSR‐1A selectively in tyrosine hydroxylase‐containing cells, including a subset of VTA dopaminergic neurones, display a significant, albeit reduced, response to the orexigenic effects of ghrelin and a full CPP for a high‐fat diet when treated with exogenous ghrelin or exposed to a chronic social defeat stress (CSDS) protocol. In the study, a nontraditional mouse model was used in which GHSR‐1A gene expression is disrupted by a transcriptional blocking cassette flanked by loxP sites that enable Cre recombinase‐mediated GHSR‐1A gene re‐expression 53. Thus, a variety of studies in rodents suggest that the action of ghrelin at the level of the dopaminergic neurones of the VTA is essential for the actions of ghrelin on both food intake and food reward.
Apart from the VTA‐NAc dopamine system, the rest of the neuronal circuitry engaged by ghrelin to modulate food reward‐related behaviours is quite unclear. For example, it has been suggested that ghrelin can regulate mesolimbic circuits indirectly via the cholinergic neurones of the LDTg, which express GHSR‐1A 55, 56. Also, the action of ghrelin on food reward appears to require intact orexin signalling, as indicated by the finding that the effects of ghrelin with respect to conditioning food CPP or operant conditioning for a food reward were blocked in orexin‐deficient mice and wild‐type mice given an orexin 1 receptor antagonist 35. A potential pathway would involve direct binding of ghrelin to GHSR‐1A present on orexin neurones of the LHA. This is supported by the finding that GHSR‐1A is expressed within the LHA of the rat 8, as well as by studies showing that ghrelin can induce action potentials and depolarisation in isolated orexin neurones 104. The ghrelin‐engaged orexin neurones would then project to the VTA, where orexin signalling is critical in the activation of mesolimbic dopaminergic circuit and reward‐seeking behaviours 105, 106. A recent study using fast‐scan cyclic voltammetry in awake rats to record dopamine spikes in the NAc core showed that the central infusion of ghrelin increases, whereas a GHSR‐1A antagonist suppresses, the magnitude of dopamine spikes evoked by food, respectively. In addition, potentiation of food‐evoked dopamine spikes was increased by intra‐LHA ghrelin and intra‐VTA blockade of orexin receptors attenuated food intake induced by central ghrelin 107. Thus, orexin signalling also appears as a key mediator of the actions of ghrelin on food reward.
Additionally, ghrelin might indirectly regulate food reward‐related behaviours by engaging central NPY/AgRP neurones of the ARC. In this regard, studies using DREADD technology (designer receptors exclusively activated by designer drugs) have revealed that selective activation of AgRP neurones is sufficient to drive food motivated behaviour in mice 108. NPY has been shown to be able to induce CPP when administered to the NAc 109. NPY also induces sucrose‐motivated behaviour when administered to the VTA or Nac, although it only increased sucrose consumption when administered to the NAc 110. Cross‐talk between these NPY‐ and ghrelin‐sensitive networks at the level of the VTA for food intake has been suggested by studies showing that the feeding effects, but not food‐motivated effects, of ghrelin were abolished by VTA pretreatment with a NPY receptor 1 antagonist. Interestingly, the converse was true for the VTA delivery of a mu‐preferring opioid receptor antagonist, which suppressed the effects of VTA ghrelin on food motivation but not food intake 111. Kappa opioid receptor pathways at the hypothalamic level also appear to be a component of the ghrelin sensitive circuitry that is important for feeding control 112. Collectively, these studies outline the divergence of behaviours (food motivation and food intake), involving overlapping but also distinct neurochemical pathways.
Physiological role of the ghrelin signalling system on food reward
Fifteen years after its discovery, the physiological relevance of the ghrelin signalling on food intake regulation, in general, and food reward pathways, in particular, is still a matter of discussion. Notably, some evidence strongly supports a role for ghrelin in short‐term food intake regulation. Ghrelin administration triggers eating in both human beings and rodents 10, 11. As described above, human plasma ghrelin levels decrease rapidly in response to nutrient ingestion, and 24‐h plasma profiles display marked preprandial increases and postprandial decreases associated with every meal 3. In rodents, ghrelin levels are suppressed within minutes by re‐feeding or enteral infusions of nutrients 113. Interestingly, blockade of ghrelin signalling in adult animals using anti‐ghrelin antibodies, GHSR‐1A antagonists or anti‐sense oligonucleotides report decreases in spontaneous food intake and body weight 114, 115, 116. By contrast, genetically modified mouse models, including mice overexpressing ghrelin or mice with genetic deletion of ghrelin, GHSR‐1A or GOAT, are almost indistinguishable from wild‐type mice in terms of ad lib. standard rodent chow diet feeding, food intake and body weights 117. The argument invariably put forward for the lack of a robust phenotype in these mutant mice is that compensatory physiological mechanisms arise during development. However, a recent study has reported that genetic ablation of ghrelin‐producing cells in adult mice fails to lead to a loss of appetite or body weight, or resistance to a high‐fat diet 118. Even a physiological role for plasma ghrelin concentrations in the regulation of basal food intake was questioned because the study found that only doses of ghrelin resulting in supraphysiological plasma levels of the hormone are able to increase food intake. In line with this possibility, no increase in appetite was observed in normal‐weight volunteers when plasma ghrelin is raised four‐fold above basal levels 119. By contrast to these data, ghrelin has been shown to increase hunger scores and food intake in a buffet test meal in both normal weight and obese subjects 120, 121. Thus, further studies are required to better clarify the role of basal ghrelin signalling on food intake and food reward‐linked behaviours.
The significance of ghrelin signalling likely becomes more evident in situations in which ghrelin signalling is physiologically more relevant, such as fasting, caloric restriction or stress 122. In this regard, GHSR‐1A deficient mice show important eating behaviour alterations under specific experimental conditions. For example, wild‐type mice subjected to prolonged caloric restriction show enhanced CPP for a high‐fat diet, whereas GHSR‐1A deficient mice lack such response 35, 92. Moreover, GHSR‐1A deficient mice in response to scheduled meals have both attenuated anticipatory hyper‐locomotion and reduced expression of the marker of cellular activation c‐Fos in the mesolimbic pathway 93, 123. Similarly, GHSR‐1A deficient mice do not anticipate food when exposed to an activity‐based anorexia model, in which mice are given free access to a running wheel and fed once per day for 2 h 21. Most humans change in their eating habits in response to stress, and an emerging literature has started to support the existence of a strong association between the ghrelin/GHSR‐1A system and stress. For example, the CSDS procedure, which subjects mice to daily bouts of social defeat by aggressive male mice, has been used to study the physiological effect of ghrelin on feeding behaviours 39, 53, 124. Wild‐type mice exposed to CSDS increase their plasma ghrelin levels and regular chow intake during and for at least 1 month after the defeat period. By contrast, GHSR‐1A deficient mice fail to show CSDS‐induced hyperphagia 39, 124. In wild‐type mice, CSDS also increases CPP for a high‐fat diet, whereas such a stress‐induced food reward response is not observed in CSDS‐exposed GHSR‐1A deficient mice 53. Thus, ghrelin signalling appears to be required for stress‐induced change in rewarding aspects of eating in mice, under these particular conditions. By contrast to these findings, wild‐type mice exposed to a chronic unpredictable stress procedure, which also elevates plasma ghrelin levels, decrease food intake and body weight gain, whereas similarly‐treated GHSR‐1A deficient mice lack these changes 124. Notably, elevations of plasma ghrelin are observed in several stress models and ghrelin administration to either humans or rodents has been shown to induce a strong activation of the hypothalamus‐pituitary‐adrenal neuroendocrine axis 125, 126. The physiological implications of ghrelin‐induced activation of stress responses or, conversely, the impact of stress on ghrelin responsiveness are currently unknown. Thus, further work is needed to clarify the inter‐relationship between of ghrelin, stress and food intake.
The ability of ghrelin to act in the brain to regulate food intake depends on the accessibility of circulating ghrelin to the above mentioned brain areas. Circulating ghrelin cannot freely cross the blood–brain barrier and it is currently unclear how this hormone enters the brain 127. In mice, ghrelin can be transported from the brain to the circulation via a saturable transport system; however, no such system has been identified for blood to brain transport 128. A recent study using ghrelin‐fluorescent tracer has shown that peripheral increases of plasma ghrelin mainly access to the ARC 127, 129. The ARC is a hypothalamic nucleus located in close apposition to the median eminence, a circumventricular organ with fenestrated capillaries that allows plasma ghrelin to diffuse to the brain parenchyma 130. The area postrema is another circumventricular organ also known to participate in food intake regulation and to express GHSR‐1A 8, 127. Early studies using GHSR‐1A agonists showed that these compounds activate cells in the area postrema and in some closely‐adjacent structures 131. In the study using the fluorescent ghrelin tracer, it was also found that high levels of circulating ghrelin can directly act on area postrema neurones, which then innervate several hypothalamic and brainstem feeding centres 127, 129. In line with this possibility, there are indications that long‐term effects of ghrelin on feeding depend on intact signalling at the area postrema 132, 133. The study using the fluorescent ghrelin tracer could not provide direct evidence for increases of plasma ghrelin reaching centres of the mesolimbic pathway, at least in an acute fashion. However, an acute effect of peripheral ghrelin on the mesolimbic pathways is supported by a study showing that intra‐VTA delivery of a selective GHSR‐1A antagonist blocked the orexigenic effect of peripherally‐administrated ghrelin 54. Also, it has been shown that peripheral administration of ghrelin in mice increases the extracellular concentration of dopamine in the NAc measured by in vivo microdialysis 134. However, the extent to which implantation of permanent cannulas in the brain affects the blood–brain barrier integrity in these studies is unclear. Interestingly, mice expressing GHSR‐1A selectively in tyrosine hydroxylase‐containing cells partially respond to ghrelin‐induced food intake and fully develop CPP for a high‐fat diet in response to either peripheral ghrelin administration during the conditioning sessions or after CSDS 53. Thus, future studies are required to clarify the physiological relevance of the action of peripheral ghrelin on the mesolimbic pathway.
The relevance of the expression of GHSR‐1A in brain areas without obvious access to circulating ghrelin is, in general, unclear. Although earlier studies suggested that ghrelin could be produced in the brain, more recent studies have clearly shown that ghrelin is not synthesised in the central nervous system 135, 136, 137. GHSR‐1A mainly signals through Gαq/11, phospholipase C, inositol phosphate and calcium mobilisation from intracellular stores; although it also activates other signalling pathways 138. An interesting feature of GHSR‐1A is its strong constitutive activity that makes it capable to signal in a ghrelin‐independent manner 139, 140. Thus, the increase of GHSR‐1A expression would accordingly increase activation of the downstream signalling pathways affecting, as a consequence, food intake and body weight regulation 124. Additionally, it has been proposed that an alternative mechanism by which GHSR‐1A regulates food intake involves its dimerisation with other G protein‐coupled receptors. GHSR‐1A has been shown to heterodimerise with the melanocortin 3 receptor, the serotonin 2C receptor and the dopamine receptors, which are all involved in food intake and food reward regulation. Heterodimerisation could serve to modulate specific functions of GHSR‐1A, such as signalling pathways, or to act as an allosteric mechanism to regulate signalling pathways of the other receptors, independently of ghrelin binding 141, 142, 143, 144.
Concluding remarks
The evidence reviewed here suggests that ghrelin/GHSR‐1A system is strongly linked to food reward‐related pathways in addition to and partially separate from those which drive food intake. Notably, the mechanisms by which ghrelin/GHSR‐1A system promotes food intake are multifaceted and are summarised in Fig. 1. The mesoaccumbal dopamine pathway appears to be a key target for ghrelin/GHSR‐1A system, opening the possibility that a primary role for ghrelin is to regulate rewarding aspects of eating. The ghrelin/GHSR‐1A system is not only up‐regulated by hunger and in anticipation to food, orchestrating a feeding response, but also by negative energy balance conditions, or psychological stress when the activation of the mesoaccumbal dopamine pathway helps animals cope with these detrimental conditions. Thus, the action of the ghrelin/GHSR‐1A system on the mesolimbic pathway is very advantageous for the survival of the animal in times of food scarcity. The constant abundance of palatable foods together to the excessive stress levels that we suffer in modern societies places the ghrelin/GHSR‐1A system in a new role in which it likely cause adverse consequences, including overeating beyond metabolic need and body weight gain. Therefore, the action of ghrelin on the mesolimbic system may have been a ‘great spice’ from an evolutionary perspective, although it no longer represents an advantage for modern human beings. Our knowledge of the neuronal circuits and molecular mechanisms mediating the actions of the ghrelin/GHSR‐1A system on the mesolimbic pathways has progressed considerably in recent years, yet still many novel and exciting aspects of this endocrine gut–brain reward axis likely remain to be discovered and will deserve intense research in the near future.
Figure 1.
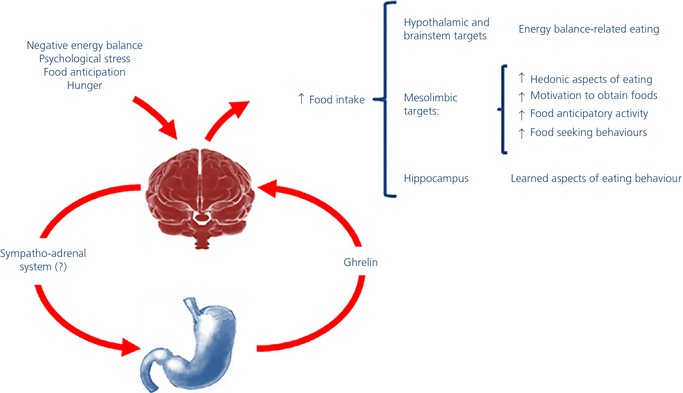
Endocrine gut–brain reward axis: a model of the effects of ghrelin on eating behaviour. Some specific conditions are known to influence eating regulation by affecting homeostatic brain circuits, which drive food intake depending on energy store levels, and/or reward brain circuits, which drive consumption based on the rewarding properties of foods. Several lines of evidence suggest a key role for the ghrelin/growth hormone secretagogue receptor type 1A system in mediating these eating behaviours. These specific conditions increase ghrelin, which in turn reaches the brain where, upon interaction with its receptor on dopaminergic neurones in the ventral tegmental area and likely in other brain nuclei, mediates an integrated and complex eating behavioural response.
Acknowledgements
SLD acknowledges support from the EC in the projects NeuroFAST, Full4Health and Nudge‐it (under grant agreement numbers 245009, 266408 and 607310, respectively), from the Swedish Research Council for Medicine (2012‐1758) and from Forskning ochUtvecklingsarbete/Avtal om Läkarutbildning och Forskning Göteborg (ALFGBG‐138741). MP acknowledges support from the grants of the National Agency of Scientific and Technological Promotion of Argentina (PICT2010‐1954 and PICT2011‐2142). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have no conflicts of interest to declare.
References
- 1. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth‐hormone‐releasing acylated peptide from stomach. Nature 1999; 402: 656–660. [DOI] [PubMed] [Google Scholar]
- 2. Skibicka KP, Dickson SL. Enteroendocrine hormones – central effects on behavior. Curr Opin Pharmacol 2013; 13: 977–982. [DOI] [PubMed] [Google Scholar]
- 3. Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001; 50: 1714–1719. [DOI] [PubMed] [Google Scholar]
- 4. Rindi G, Necchi V, Savio A, Torsello A, Zoli M, Locatelli V, Raimondo F, Cocchi D, Solcia E. Characterisation of gastric ghrelin cells in man and other mammals: studies in adult and fetal tissues. Histochem Cell Biol 2002; 117: 511–519. [DOI] [PubMed] [Google Scholar]
- 5. Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone‐releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000; 141: 4255–4261. [DOI] [PubMed] [Google Scholar]
- 6. Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 1996; 273: 974–977. [DOI] [PubMed] [Google Scholar]
- 7. Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res 1997; 48: 23–29. [DOI] [PubMed] [Google Scholar]
- 8. Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 2006; 494: 528–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA 2008; 105: 6320–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001; 86: 5992. [DOI] [PubMed] [Google Scholar]
- 11. Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR. Ghrelin causes hyperphagia and obesity in rats. Diabetes 2001; 50: 2540–2547. [DOI] [PubMed] [Google Scholar]
- 12. Patterson M, Murphy KG, le Roux CW, Ghatei MA, Bloom SR. Characterization of ghrelin‐like immunoreactivity in human plasma. J Clin Endocrinol Metab 2005; 90: 2205–2211. [DOI] [PubMed] [Google Scholar]
- 13. Delhanty PJ, Neggers SJ, van der Lely AJ. Des‐acyl ghrelin: a metabolically active peptide. Endocr Dev 2013; 25: 112–121. [DOI] [PubMed] [Google Scholar]
- 14. Yin X, Li Y, Xu G. Ghrelin fluctuation, what determines its production? Acta Biochim Biophys Sin (Shanghai) 2009; 41: 188–197. [DOI] [PubMed] [Google Scholar]
- 15. Dornonville de la Cour C, et al A‐like cells in the rat stomach contain ghrelin and do not operate under gastrin control. Regul Pept 2001; 99: 141–150. [DOI] [PubMed] [Google Scholar]
- 16. Sakata I, Park WM, Walker AK, Piper PK, Chuang JC, Osborne‐Lawrence S, Zigman JM. Glucose‐mediated control of ghrelin release from primary cultures of gastric mucosal cells. Am J Physiol Endocrinol Metab 2012; 302: E1300–E1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS. Ghrelin O‐acyltransferase (GOAT) is essential for growth hormone‐mediated survival of calorie‐restricted mice. Proc Natl Acad Sci USA 2010; 107: 7467–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gong Z, Yoshimura M, Aizawa S, Kurotani R, Zigman JM, Sakai T, Sakata I. G protein‐coupled receptor 120 signaling regulates ghrelin secretion in vivo and in vitro. Am J Physiol Endocrinol Metab 2014; 306: E28–E35. [DOI] [PubMed] [Google Scholar]
- 19. Natalucci G, Riedl S, Gleiss A, Zidek T, Frisch H. Spontaneous 24‐h ghrelin secretion pattern in fasting subjects: maintenance of a meal‐related pattern. Eur J Endocrinol 2005; 152: 845–850. [DOI] [PubMed] [Google Scholar]
- 20. Walker AK, Ibia IE, Zigman JM. Disruption of cue‐potentiated feeding in mice with blocked ghrelin signaling. Physiol Behav 2012; 108: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verhagen LA, Egecioglu E, Luijendijk MC, Hillebrand JJ, Adan RA, Dickson SL. Acute and chronic suppression of the central ghrelin signaling system reveals a role in food anticipatory activity. Eur Neuropsychopharmacol 2011; 21: 384–392. [DOI] [PubMed] [Google Scholar]
- 22. Merkestein M, Brans MA, Luijendijk MC, de Jong JW, Egecioglu E, Dickson SL, Adan RA. Ghrelin mediates anticipation to a palatable meal in rats. Obesity (Silver Spring) 2012; 20: 963–971. [DOI] [PubMed] [Google Scholar]
- 23. Currie PJ, Khelemsky R, Rigsbee EM, Dono LM, Coiro CD, Chapman CD, Hinchcliff K. Ghrelin is an orexigenic peptide and elicits anxiety‐like behaviors following administration into discrete regions of the hypothalamus. Behav Brain Res 2012; 226: 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Olszewski PK, Li D, Grace MK, Billington CJ, Kotz CM, Levine AS. Neural basis of orexigenic effects of ghrelin acting within lateral hypothalamus. Peptides 2003; 24: 597–602. [DOI] [PubMed] [Google Scholar]
- 25. Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes 2003; 52: 2260–2265. [DOI] [PubMed] [Google Scholar]
- 26. Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides 2005; 26: 2274–2279. [DOI] [PubMed] [Google Scholar]
- 27. Skibicka KP, Hansson C, Alvarez‐Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience 2011; 180: 129–137. [DOI] [PubMed] [Google Scholar]
- 28. Alvarez‐Crespo M, Skibicka KP, Farkas I, Molnar CS, Egecioglu E, Hrabovszky E, Liposits Z, Dickson SL. The amygdala as a neurobiological target for ghrelin in rats: neuroanatomical, electrophysiological and behavioral evidence. PLoS One 2012; 7: e46321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kanoski SE, Fortin SM, Ricks KM, Grill HJ. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K‐Akt signaling. Biol Psychiatry 2013; 73: 915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA 2004; 101: 4679–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salome N, Haage D, Perrissoud D, Moulin A, Demange L, Egecioglu E, Fehrentz JA, Martinez J, Dickson SL. Anorexigenic and electrophysiological actions of novel ghrelin receptor (GHS‐R1A) antagonists in rats. Eur J Pharmacol 2009; 612: 167–173. [DOI] [PubMed] [Google Scholar]
- 32. Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time‐ and food‐related cues. Am J Physiol Endocrinol Metab 2004; 287: E297–E304. [DOI] [PubMed] [Google Scholar]
- 33. Merkestein M, van Gestel MA, van der Zwaal EM, Brans MA, Luijendijk MC, van Rozen AJ, Hendriks J, Garner KM, Boender AJ, Pandit R, Adan R. GHS‐R1a signaling in the DMH and VMH contributes to food anticipatory activity. Int J Obes (Lond) 2014; 38: 610–618. [DOI] [PubMed] [Google Scholar]
- 34. Nutu M, Feng Y, Egecioglu E, Weijdegard B, Stener‐Victorin E, Shao R. Stromal cell‐specific apoptotic and antiestrogenic mechanisms may explain uterine defects in humans after clomiphene citrate therapy. Am J Obstet Gynecol, 2010; 203: 65 e1–65 e10. [DOI] [PubMed] [Google Scholar]
- 35. Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne‐Lawrence S, Rovinsky SA, Woloszyn J, Yanagisawa M, Lutter M, Zigman JM. Ghrelin increases the rewarding value of high‐fat diet in an orexin‐dependent manner. Biol Psychiatry 2010; 67: 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Skibicka KP, Hansson C, Egecioglu E, Dickson SL. Role of ghrelin in food reward: impact of ghrelin on sucrose self‐administration and mesolimbic dopamine and acetylcholine receptor gene expression. Addict Biol 2012; 17: 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschop MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci 2006; 9: 381–388. [DOI] [PubMed] [Google Scholar]
- 38. Hansson C, Shirazi RH, Naslund J, Vogel H, Neuber C, Holm G, Anckarsater H, Dickson SL, Eriksson E, Skibicka KP. Ghrelin influences novelty seeking behavior in rodents and men. PLoS One 2012; 7: e50409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lutter M, Sakata I, Osborne‐Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci 2008; 11: 752–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hansson C, Haage D, Taube M, Egecioglu E, Salome N, Dickson SL. Central administration of ghrelin alters emotional responses in rats: behavioural, electrophysiological and molecular evidence. Neuroscience 2011; 180: 201–211. [DOI] [PubMed] [Google Scholar]
- 41. Dickson SL, Leng G, Robinson IC. Systemic administration of growth hormone‐releasing peptide activates hypothalamic arcuate neurons. Neuroscience 1993; 53: 303–306. [DOI] [PubMed] [Google Scholar]
- 42. Dickson SL, Luckman SM. Induction of c‐fos messenger ribonucleic acid in neuropeptide Y and growth hormone (GH)‐releasing factor neurons in the rat arcuate nucleus following systemic injection of the GH secretagogue, GH‐releasing peptide‐6. Endocrinology 1997; 138: 771–777. [DOI] [PubMed] [Google Scholar]
- 43. Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature 2001; 409: 194–198. [DOI] [PubMed] [Google Scholar]
- 44. Willesen MG, Kristensen P, Romer J. Co‐localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology 1999; 70: 306–316. [DOI] [PubMed] [Google Scholar]
- 45. Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM, Ye Z, Nargund RP, Smith RG, Van der Ploeg LH, Howard AD, MacNeil DJ, Qian S. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti‐related protein. Endocrinology 2004; 145: 2607–2612. [DOI] [PubMed] [Google Scholar]
- 46. Luquet S, Phillips CT, Palmiter RD. NPY/AgRP neurons are not essential for feeding responses to glucoprivation. Peptides 2007; 28: 214–225. [DOI] [PubMed] [Google Scholar]
- 47. Wang Q, Liu C, Uchida A, Chuang JC, Walker A, Liu T, Osborne‐Lawrence S, Mason BL, Mosher C, Berglund ED, Elmquist JK, Zigman JM. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol Metab 2014; 3: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Toshinai K, Date Y, Murakami N, Shimada M, Mondal MS, Shimbara T, Guan JL, Wang QP, Funahashi H, Sakurai T, Shioda S, Matsukura S, Kangawa K, Nakazato M. Ghrelin‐induced food intake is mediated via the orexin pathway. Endocrinology 2003; 144: 1506–1512. [DOI] [PubMed] [Google Scholar]
- 49. Sakata I, Yamazaki M, Inoue K, Hayashi Y, Kangawa K, Sakai T. Growth hormone secretagogue receptor expression in the cells of the stomach‐projected afferent nerve in the rat nodose ganglion. Neurosci Lett 2003; 342: 183–186. [DOI] [PubMed] [Google Scholar]
- 50. Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin‐induced feeding and growth hormone secretion in rats. Gastroenterology 2002; 123: 1120–1128. [DOI] [PubMed] [Google Scholar]
- 51. Date Y. Ghrelin and the vagus nerve. Methods Enzymol 2012; 514: 261–269. [DOI] [PubMed] [Google Scholar]
- 52. Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating‐stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci 2006; 26: 11052–11060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chuang JC, Hrabovszky E, Hansson C, Jerlhag E, Alvarez‐Crespo M, Skibicka KP, Molnar CS, Liposits Z, Engel JA, Egecioglu E. Ghrelin mediates stress‐induced food‐reward behavior in mice. J Clin Invest 2011; 121: 2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest 2006; 116: 3229–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dickson SL, Hrabovszky E, Hansson C, Jerlhag E, Alvarez‐Crespo M, Skibicka KP, Molnar CS, Liposits Z, Engel JA, Egecioglu E. Blockade of central nicotine acetylcholine receptor signaling attenuate ghrelin‐induced food intake in rodents. Neuroscience 2010; 171: 1180–1186. [DOI] [PubMed] [Google Scholar]
- 56. Jerlhag E, Egecioglu E, Dickson SL, Svensson L, Engel JA. Alpha‐conotoxin MII‐sensitive nicotinic acetylcholine receptors are involved in mediating the ghrelin‐induced locomotor stimulation and dopamine overflow in nucleus accumbens. Eur Neuropsychopharmacol 2008; 18: 508–518. [DOI] [PubMed] [Google Scholar]
- 57. Goldstone AP, Prechtl de Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, Durighel G, Hughes E, Waldman AD, Frost G, Bell JD. Fasting biases brain reward systems towards high‐calorie foods. Eur J Neurosci 2009; 30: 1625–1635. [DOI] [PubMed] [Google Scholar]
- 58. Goldstone AP, Prechtl CG, Scholtz S, Miras AD, Chhina N, Durighel G, Deliran SS, Beckmann C, Ghatei MA, Ashby DR, Waldman AD, Gaylinn BD, Thorner MO, Frost GS, Bloom SR, Bell JD. Ghrelin mimics fasting to enhance human hedonic, orbitofrontal cortex, and hippocampal responses to food. Am J Clin Nutr 2014; 99: 1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab 2008; 7: 400–409. [DOI] [PubMed] [Google Scholar]
- 60. Karra E, O'Daly OG, Choudhury AI, Yousseif A, Millership S, Neary MT, Scott WR, Chandarana K, Manning S, Hess ME, Iwakura H, Akamizu T, Millet Q, Gelegen C, Drew ME, Rahman S, Emmanuel JJ, Williams SC, Ruther UU, Bruning JC, Withers DJ, Zelaya FO, Batterham RL. A link between FTO, ghrelin, and impaired brain food‐cue responsivity. J Clin Invest 2013; 123: 3539–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci 2007; 30: 194–202. [DOI] [PubMed] [Google Scholar]
- 62. Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward‐related learning and memory. Annu Rev Neurosci 2006; 29: 565–598. [DOI] [PubMed] [Google Scholar]
- 63. DiLeone RJ, Taylor JR, Picciotto MR. The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci 2012; 15: 1330–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci 1988; 42: 1705–1712. [DOI] [PubMed] [Google Scholar]
- 65. Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci 2007; 10: 1020–1028. [DOI] [PubMed] [Google Scholar]
- 66. Sombers LA, Beyene M, Carelli RM, Wightman RM. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci 2009; 29: 1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Valdivia S, Patrone A, Reynaldo M, Perello M. Acute high fat diet consumption activates the mesolimbic circuit and requires orexin signaling in a mouse model. PLoS One 2014; 9: e87478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci 2007; 30: 375–381. [DOI] [PubMed] [Google Scholar]
- 69. Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high‐fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci 2007; 27: 11075–11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci 1999; 19: 11040–11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci 2003; 23: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nakamura T, Uramura K, Nambu T, Yada T, Goto K, Yanagisawa M, Sakurai T. Orexin‐induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res 2000; 873: 181–187. [DOI] [PubMed] [Google Scholar]
- 73. Mahler SV, Smith RJ, Aston‐Jones G. Interactions between VTA orexin and glutamate in cue‐induced reinstatement of cocaine seeking in rats. Psychopharmacology 2013; 226: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev 1996; 20: 1–25. [DOI] [PubMed] [Google Scholar]
- 75. Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev 2013; 14: 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Norgren R, Hajnal A, Mungarndee SS. Gustatory reward and the nucleus accumbens. Physiol Behav 2006; 89: 531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Epstein LH, Temple JL, Roemmich JN, Bouton ME. Habituation as a determinant of human food intake. Psychol Rev 2009; 116: 384–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science 1997; 275: 1593–1599. [DOI] [PubMed] [Google Scholar]
- 79. Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct 2010; 6: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, Bonci A. Reward‐predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science 2008; 321: 1690–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron 2012; 76: 470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci 2013; 16: 966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci 2004; 24: 1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine‐overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol 2006; 11: 45–54. [DOI] [PubMed] [Google Scholar]
- 85. Egecioglu E, Jerlhag E, Salome N, Skibicka KP, Haage D, Bohlooly YM, Andersson D, Bjursell M, Perrissoud D, Engel JA, Dickson SL. Ghrelin increases intake of rewarding food in rodents. Addict Biol 2010; 15: 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, Datta R, Perrissoud D, Dickson SL, Engel JA Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci USA 2009; 106: 11318–11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jerlhag E, Egecioglu E, Dickson SL, Engel JA. Ghrelin receptor antagonism attenuates cocaine‐ and amphetamine‐induced locomotor stimulation, accumbal dopamine release, and conditioned place preference. Psychopharmacology 2010; 211: 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wellman PJ, Davis KW, Nation JR. Augmentation of cocaine hyperactivity in rats by systemic ghrelin. Regul Pept 2005; 125: 151–154. [DOI] [PubMed] [Google Scholar]
- 89. Overduin J, Figlewicz DP, Bennett‐Jay J, Kittleson S, Cummings DE. Ghrelin increases the motivation to eat, but does not alter food palatability. Am J Physiol Regul Integr Comp Physiol 2012; 303: R259–R269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Davis JF, Perello M, Choi DL, Magrisso IJ, Kirchner H, Pfluger PT, Tschoep M, Zigman JM, Benoit SC. GOAT induced ghrelin acylation regulates hedonic feeding. Horm Behav 2012; 62: 598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shimbara T, Mondal MS, Kawagoe T, Toshinai K, Koda S, Yamaguchi H, Date Y, Nakazato M. Central administration of ghrelin preferentially enhances fat ingestion. Neurosci Lett 2004; 369: 75–79. [DOI] [PubMed] [Google Scholar]
- 92. Disse E, Bussier AL, Veyrat‐Durebex C, Deblon N, Pfluger PT, Tschop MH, Laville M, Rohner‐Jeanrenaud F. Peripheral ghrelin enhances sweet taste food consumption and preference, regardless of its caloric content. Physiol Behav 2010; 101: 277–281. [DOI] [PubMed] [Google Scholar]
- 93. Blum ID, Patterson Z, Khazall R, Lamont EW, Sleeman MW, Horvath TL, Abizaid A. Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience 2009; 164: 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol 2007; 12: 6–16. [DOI] [PubMed] [Google Scholar]
- 95. Keen‐Rhinehart E, Bartness TJ. Peripheral ghrelin injections stimulate food intake, foraging, and food hoarding in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 2005; 288: R716–R722. [DOI] [PubMed] [Google Scholar]
- 96. Tong J, Mannea E, Aime P, Pfluger PT, Yi CX, Castaneda TR, Davis HW, Ren X, Pixley S, Benoit S, Julliard K, Woods SC, Horvath TL, Sleeman MM, D'Alessio D, Obici S, Frank R, Tschop MH. Ghrelin enhances olfactory sensitivity and exploratory sniffing in rodents and humans. J Neurosci 2011; 31: 5841–5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Skibicka KP, Dickson SL. Ghrelin and food reward: the story of potential underlying substrates. Peptides 2011; 32: 2265–2273. [DOI] [PubMed] [Google Scholar]
- 98. King SJ, Isaacs AM, O'Farrell E, Abizaid A. Motivation to obtain preferred foods is enhanced by ghrelin in the ventral tegmental area. Horm Behav 2011; 60: 572–580. [DOI] [PubMed] [Google Scholar]
- 99. Koob GF, Riley SJ, Smith SC, Robbins TW. Effects of 6‐hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat. J Comp Physiol Psychol 1978; 92: 917–927. [DOI] [PubMed] [Google Scholar]
- 100. Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res 2002; 137: 165–177. [DOI] [PubMed] [Google Scholar]
- 101. Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology 1991; 104: 515–521. [DOI] [PubMed] [Google Scholar]
- 102. Koch M, Schmid A, Schnitzler HU. Role of muscles accumbens dopamine D1 and D2 receptors in instrumental and Pavlovian paradigms of conditioned reward. Psychopharmacology 2000; 152: 67–73. [DOI] [PubMed] [Google Scholar]
- 103. Skibicka KP, Shirazi RH, Rabasa‐Papio C, Alvarez‐Crespo M, Neuber C, Vogel H, Dickson SL. Divergent circuitry underlying food reward and intake effects of ghrelin: dopaminergic VTA‐accumbens projection mediates ghrelin's effect on food reward but not food intake. Neuropharmacology 2013; 73: 274–283. [DOI] [PubMed] [Google Scholar]
- 104. Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 2003; 38: 701–713. [DOI] [PubMed] [Google Scholar]
- 105. Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci 2006; 26: 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Harris GC, Wimmer M, Aston‐Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature 2005; 437: 556–559. [DOI] [PubMed] [Google Scholar]
- 107. Cone JJ, McCutcheon JE, Roitman MF. Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neurosci 2014; 34: 4905–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos‐Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 2011; 121: 1424–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Josselyn SA, Beninger RJ. Neuropeptide Y: intraaccumbens injections produce a place preference that is blocked by cis‐flupenthixol. Pharmacol Biochem Behav 1993; 46: 543–552. [DOI] [PubMed] [Google Scholar]
- 110. Pandit R, Luijendijk MC, Vanderschuren LJ, la Fleur SE, Adan RA. Limbic substrates of the effects of neuropeptide Y on intake of and motivation for palatable food. Obesity (Silver Spring) 2014; 22: 1216–1219. [DOI] [PubMed] [Google Scholar]
- 111. Skibicka KP, Shirazi RH, Hansson C, Dickson SL. Ghrelin interacts with neuropeptide Y Y1 and opioid receptors to increase food reward. Endocrinology 2012; 153: 1194–1205. [DOI] [PubMed] [Google Scholar]
- 112. Romero‐Pico A, Vazquez MJ, Gonzalez‐Touceda D, Folgueira C, Skibicka KP, Alvarez‐Crespo M, Van Gestel MA, Velasquez DA, Schwarzer C, Herzog H, Lopez M, Adan RA, Dickson SL, Dieguez C, Nogueiras R. Hypothalamic kappa‐opioid receptor modulates the orexigenic effect of ghrelin. Neuropsychopharmacology 2013; 38: 1296–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Williams DL, Cummings DE, Grill HJ, Kaplan JM. Meal‐related ghrelin suppression requires postgastric feedback. Endocrinology 2003; 144: 2765–2767. [DOI] [PubMed] [Google Scholar]
- 114. Murakami N, Hayashida T, Kuroiwa T, Nakahara K, Ida T, Mondal MS, Nakazato M, Kojima M, Kangawa K. Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. J Endocrinol 2002; 174: 283–288. [DOI] [PubMed] [Google Scholar]
- 115. Bagnasco M, Tulipano G, Melis MR, Argiolas A, Cocchi D, Muller EE. Endogenous ghrelin is an orexigenic peptide acting in the arcuate nucleus in response to fasting. Regul Pept 2003; 111: 161–167. [DOI] [PubMed] [Google Scholar]
- 116. Asakawa A, Inui A, Kaga T, Katsuura G, Fujimiya M, Fujino MA, Kasuga M. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut 2003; 52: 947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Uchida A, Zigman JM, Perello M. Ghrelin and eating behavior: evidence and insights from genetically‐modified mouse models. Front Neurosci 2013; 7: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. McFarlane MR, Brown MS, Goldstein JL, Zhao TJ. Induced ablation of ghrelin cells in adult mice does not decrease food intake, body weight, or response to high‐fat diet. Cell Metab 2014; 20: 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lippl F, Erdmann J, Steiger A, Lichter N, Czogalla‐Peter C, Bidlingmaier M, Tholl S, Schusdziarra V. Low‐dose ghrelin infusion–evidence against a hormonal role in food intake. Regul Pept 2012; 174: 26–31. [DOI] [PubMed] [Google Scholar]
- 120. Druce MR, Neary NM, Small CJ, Milton J, Monteiro M, Patterson M, Ghatei MA, Bloom SR. Subcutaneous administration of ghrelin stimulates energy intake in healthy lean human volunteers. Int J Obes (Lond) 2006; 30: 293–296. [DOI] [PubMed] [Google Scholar]
- 121. Druce MR, Wren AM, Park AJ, Milton JE, Patterson M, Frost G, Ghatei MA, Small C, Bloom SR. Ghrelin increases food intake in obese as well as lean subjects. Int J Obes (Lond) 2005; 29: 1130–1136. [DOI] [PubMed] [Google Scholar]
- 122. Perello M, Zigman JM. The role of ghrelin in reward‐based eating. Biol Psychiatry 2012; 72: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lamont EW, Patterson Z, Rodrigues T, Vallejos O, Blum ID, Abizaid A. Ghrelin‐deficient mice have fewer orexin cells and reduced cFOS expression in the mesolimbic dopamine pathway under a restricted feeding paradigm. Neuroscience 2012; 218: 12–19. [DOI] [PubMed] [Google Scholar]
- 124. Patterson ZR, Khazall R, Mackay H, Anisman H, Abizaid A. Central ghrelin signaling mediates the metabolic response of C57BL/6 male mice to chronic social defeat stress. Endocrinology 2013; 154: 1080–1091. [DOI] [PubMed] [Google Scholar]
- 125. Cabral A, Suescun O, Zigman JM, Perello M. Ghrelin indirectly activates hypophysiotropic CRF neurons in rodents. PLoS One 2012; 7: e31462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, Mori K, Komatsu Y, Usui T, Shimatsu A, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K. Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab 2000; 85: 4908–4911. [DOI] [PubMed] [Google Scholar]
- 127. Fry M, Ferguson AV. Ghrelin: central nervous system sites of action in regulation of energy balance. Int J Pept 2010; 2010: 616757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Banks WA. The blood–brain barrier: connecting the gut and the brain. Regul Pept 2008; 149: 11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cabral A, Valdivia S, Fernandez G, Reynaldo M, Perello M. Divergent neuronal circuitries underlying acute orexigenic effects of peripheral or central ghrelin: critical role of brain accessibility. J Neuroendocrinol 2014; 26: 542–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Schaeffer M, Langlet F, Lafont C, Molino F, Hodson DJ, Roux T, Lamarque L, Verdie P, Bourrier E, Dehouck B, Baneres JL, Martinez J, Mery PF, Marie J, Trinquet E, Fehrentz JA, Prevot V, Mollard P. Rapid sensing of circulating ghrelin by hypothalamic appetite‐modifying neurons. Proc Natl Acad Sci USA 2013; 110: 1512–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Bailey AR, von Engelhardt N, Leng G, Smith RG, Dickson SL. Growth hormone secretagogue activation of the arcuate nucleus and brainstem occurs via a non‐noradrenergic pathway. J Neuroendocrinol 2000; 12: 191–197. [DOI] [PubMed] [Google Scholar]
- 132. Gilg S, Lutz TA. The orexigenic effect of peripheral ghrelin differs between rats of different age and with different baseline food intake, and it may in part be mediated by the area postrema. Physiol Behav 2006; 87: 353–359. [DOI] [PubMed] [Google Scholar]
- 133. Date Y, Shimbara T, Koda S, Toshinai K, Ida T, Murakami N, Miyazato M, Kokame K, Ishizuka Y, Ishida Y, Kageyama H, Shioda S, Kangawa K, Nakazato M. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metab 2006; 4: 323–331. [DOI] [PubMed] [Google Scholar]
- 134. Jerlhag E. Systemic administration of ghrelin induces conditioned place preference and stimulates accumbal dopamine. Addict Biol 2008; 13: 358–363. [DOI] [PubMed] [Google Scholar]
- 135. Furness JB, Hunne B, Matsuda N, Yin L, Russo D, Kato I, Fujimiya M, Patterson M, McLeod J, Andrews ZB, Bron R. Investigation of the presence of ghrelin in the central nervous system of the rat and mouse. Neuroscience 2011; 193: 1–9. [DOI] [PubMed] [Google Scholar]
- 136. Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia‐Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003; 37: 649–661. [DOI] [PubMed] [Google Scholar]
- 137. Sakata I, Nakano Y, Osborne‐Lawrence S, Rovinsky SA, Lee CE, Perello M, Anderson JG, Coppari R, Xiao G, Lowell BB, Elmquist JK, Zigman JM. Characterization of a novel ghrelin cell reporter mouse. Regul Pept 2009; 155: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Cong WN, Golden E, Pantaleo N, White CM, Maudsley S, Martin B. Ghrelin receptor signaling: a promising therapeutic target for metabolic syndrome and cognitive dysfunction. CNS Neurol Disord Drug Targets 2010; 9: 557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Mokrosinski J, Holst B. Modulation of the constitutive activity of the ghrelin receptor by use of pharmacological tools and mutagenesis. Methods Enzymol 2010; 484: 53–73. [DOI] [PubMed] [Google Scholar]
- 140. Damian M, Marie J, Leyris JP, Fehrentz JA, Verdie P, Martinez J, Baneres JL, Mary S. High constitutive activity is an intrinsic feature of ghrelin receptor protein: a study with a functional monomeric GHS‐R1a receptor reconstituted in lipid discs. J Biol Chem 2012; 287: 3630–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Schellekens H, van Oeffelen WE, Dinan TG, Cryan JF. Promiscuous dimerization of the growth hormone secretagogue receptor (GHS‐R1a) attenuates ghrelin‐mediated signaling. J Biol Chem 2013; 288: 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Rediger A, Piechowski CL, Yi CX, Tarnow P, Strotmann R, Gruters A, Krude H, Schoneberg T, Tschop MH, Kleinau G, Biebermann H. Mutually opposite signal modulation by hypothalamic heterodimerization of ghrelin and melanocortin‐3 receptors. J Biol Chem 2011; 286: 39623–39631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kern A, Albarran‐Zeckler R, Walsh HE, Smith RG. Apo‐ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron 2012; 73: 317–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Jiang H, Betancourt L, Smith RG. Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol Endocrinol 2006; 20: 1772–1785. [DOI] [PubMed] [Google Scholar]


