Abstract
Purpose
The generalisability of randomised controlled trials (RCTs) may be limited by restrictive entry criteria or by their experimental nature. Observational research can provide complementary findings but is prone to bias. Employing propensity score matching, to reduce such bias, we compared the real‐life effect of cinacalcet use on all‐cause mortality (ACM) with findings from the Evaluation of Cinacalcet Therapy to Lower Cardiovascular Events (EVOLVE) RCT in chronic haemodialysis patients.
Methods
Incident adult haemodialysis patients receiving cinacalcet, recruited in a prospective observational cohort from 2007–2009 (AROii; n = 10,488), were matched to non‐exposed patients regardless of future exposure status. The effect of treatment crossover was investigated with inverse probability of censoring weighted and lag‐censored analyses. EVOLVE ACM data were analysed largely as described for the primary composite endpoint.
Results
AROii patients receiving cinacalcet (n = 532) were matched to 1790 non‐exposed patients. The treatment effect of cinacalcet on ACM in the main AROii analysis (hazard ratio 1.03 [95% confidence interval (CI) 0.78–1.35]) was closer to the null than for the Intention to Treat (ITT) analysis of EVOLVE (0.94 [95%CI 0.85–1.04]). Adjusting for non‐persistence by 0‐ and 6‐month lag‐censoring and by inverse probability of censoring weight, the hazard ratios in AROii (0.76 [95%CI 0.51–1.15], 0.84 [95%CI 0.60–1.18] and 0.79 [95%CI 0.56–1.11], respectively) were comparable with those of EVOLVE (0.82 [95%CI 0.67–1.01], 0.83 [95%CI 0.73–0.96] and 0.87 [95%CI 0.71–1.06], respectively).
Conclusions
Correcting for treatment crossover, we observed results in the ‘real‐life’ setting of the AROii observational cohort that closely mirrored the results of the EVOLVE RCT. Persistence‐corrected analyses revealed a trend towards reduced ACM in haemodialysis patients receiving cinacalcet therapy. © 2015 The Authors. Pharmacoepidemiology and Drug Safety Published by John Wiley & Sons Ltd.
Keywords: cinacalcet, haemodialysis, mortality, persistence, bias, pharmacoepidemiology
Introduction
Although randomised controlled trials (RCTs) remain the ‘gold standard’ for assessing pharmaceutical interventions, their generalisability may be limited by restrictive entry criteria or their experimental nature; in some instances, they are unfeasible or unethical.1 Observational research studies yield important information about the effectiveness of treatment regimens and their use by clinicians in everyday ‘real world’ practice and thus complement the results of RCTs. They use less stringent inclusion/exclusion criteria and hence may closer reflect the overall patient population. They are prone to bias, however, especially confounding‐by‐indication, which may potentially distort estimated treatment effects.
Secondary hyperparathyroidism (sHPT) occurs early in the course of chronic kidney disease (CKD) and progresses with declining kidney function.2 The condition is largely defined by increased serum parathyroid hormone (PTH) levels accompanied by deregulated phosphate and calcium concentrations, and major complications include bone and cardiovascular diseases (CVD).3 These defining biochemical changes are independently and consistently associated with increased morbidity and mortality,4, 5, 6, 7, 8, 9 which is most likely in part mediated by cardiovascular calcification.10, 11, 12, 13 Effective sHPT control is therefore an important goal in the management of haemodialysis (HD) patients.14 The calcimimetic cinacalcet (Sensipar®/Mimpara®) is licenced for the treatment of sHPT in end‐stage renal disease patients on maintenance dialysis therapy.15 Cinacalcet effectively lowers serum PTH concentrations while concomitantly reducing serum calcium and, at least transiently, phosphorus concentrations.16, 17, 18, 19
Few observational studies have examined the effect of cinacalcet therapy on survival. Block and colleagues found a significant survival benefit associated with cinacalcet prescription, with reductions in all‐cause and cardiovascular mortality in almost 20 000 United States HD patients receiving intravenous vitamin D therapy.20 In a separate, smaller (n = 1184), post‐hoc study examining short‐term safety data pooled from four placebo‐controlled RCTs, Cunningham and colleagues reported that cinacalcet use was associated with a significantly reduced risk of parathyroidectomy, fracture and cardiovascular‐related hospitalisation, but not mortality.21
Propensity score matching (PSM) can reduce bias in observational research.22 PSM aims to achieve balance between treatment groups with regard to measured confounders by mimicking the randomisation used in clinical trials. In the current study, the effect of cinacalcet use on all‐cause mortality was investigated in HD patients using data obtained from Fresenius Medical Care (FMC) dialysis centres across Europe as part of the Analyzing data, Recognizing excellence, and Optimizing outcomes (ARO) CKD Research Initiative.23 The study used PSM to control for systematic differences between those receiving treatment for sHPT with cinacalcet and those who were not. We were more interested in the potential for treatment crossover to distort the estimated treatment effect of cinacalcet use on all‐cause mortality—and our ability to correct this form of bias—than estimation of the actual treatment effect itself. Findings were compared with treatment estimates for all‐cause mortality from the EVOLVE study24: an RCT designed to gain an insight into the long‐term clinical efficacy of cinacalcet.
Methods
(i) ARO data and analysis
Source data and study population
The AROii cohort comprised incident (<183 days dialysis vintage [the time since dialysis initiation]) adult HD patients presenting at over 300 FMC facilities in 14 European countries between January 2007 and December 2009, with no renal transplantation or peritoneal dialysis history. Raw, anonymised electronic patient‐level data,25 captured as part of normal clinical care, extracted and supplied quarterly, were limited to chronic HD patients (≥10 contiguous dialysis sessions) with accompanying laboratory data. Data were further restricted in this study to non‐parathyroidectomised, cinacalcet‐naïve (no cinacalcet use to the end of the first 90 days of follow‐up) patients who remained on study for ≥90 days. Patients from countries not prescribing cinacalcet were excluded.
Exposure and time period definitions
Patients' follow‐up time was divided into consecutive 90‐day dialysis vintage windows to maximise patient comparability with regard to CKD progression. Each interval included patients initiating cinacalcet and those who did not (cinacalcet/non‐cinacalcet patients, respectively). Baseline comprised the 90 days before treatment initiation for cinacalcet patients and the 90 days before the assessment interval for non‐cinacalcet patients. Patients accrued time‐at‐risk from the end of baseline until they experienced the event of interest (all‐cause mortality), underwent a parathyroidectomy, a kidney transplant, were lost to follow‐up (>45 days without continuous dialysis treatment) or the end of study follow‐up was reached (31 September 2012).
PSM approach
The PSM process resembled the principles of a sequential matching using time‐dependent covariates. However, unlike a time‐dependent PSM, where patients with similar time‐dependent covariates up to treatment initiation are matched,26 only baseline values were utilised.
The propensity for cinacalcet treatment was estimated using multivariate logistic regression, where exposure to cinacalcet treatment in each interval was fitted as the dependent variable and baseline covariates were fitted as independent variables (Supplementary Table A). The explanatory variables were chosen as potential risk factors for cinacalcet treatment and/or confounders of the relationship between treatment and mortality in this cohort.27 Analysis was restricted to patients with complete baseline data on PTH, serum calcium and serum phosphate, and interaction terms were included to represent the in vivo effect of active vitamin D (AVD) treatment on serum calcium and serum phosphate.
Matching was performed chronologically. Cinacalcet patients in the first interval were calliper‐matched by logit propensity score to up to four (‘ncontls’ option = 4) non‐cinacalcet patients using a greedy matching algorithm.28 A calliper width (‘dmax’ option) of 0.2 of the standard deviation of the logit of the propensity score was used.29 Logit propensity scores were unweighted (‘wts’ option = 1), and distances were calculated using weighted sums of absolute case‐control differences (‘dist’ option = 1). Matched patients and unmatched cinacalcet patients were removed from later intervals and the process repeated for subsequent intervals. The selection process employed in the PSM to create this population, where patients retain their matching exposure status regardless of their future exposure status, has parallels with the ITT analytical approach employed in RCTs.
The balance in baseline characteristics achieved by PSM was evaluated by calculating standardised differences29 between cinacalcet and non‐cinacalcet patients in the matched and overall ARO populations. As unmatched non‐exposed patients could be considered for matching in multiple exposure assessment windows, the characteristics of patients receiving cinacalcet, versus those who never received the drug, were examined in the overall ARO population by comparing data for the 3‐month period from recruitment in the AROii cohort. In the matched population, the balance was checked for each risk set separately and overall. Clinically significant differences aside, standardised differences of <10% were considered negligible.29
Time‐to‐event analysis
Following PSM, the association between cinacalcet exposure and all‐cause mortality, determined by patients' death dates, was estimated using matched Cox proportional hazards regression models, with hazard ratios (HR) and 95% confidence intervals (CI) calculated.29
Sensitivity analyses investigating non‐persistence
To account for non‐persistence to exposure status, both lag‐censoring analysis and inverse probability of censoring weights (IPCWs30) were applied.
Six‐month lag‐censoring was employed to match the pre‐specified lag‐censored analysis in the EVOLVE trial,24 but 0‐month lag‐censoring was also investigated. Patients who switched from non‐exposed to exposed were censored 0 or 6 months after cinacalcet initiation; cinacalcet patients with poorer persistence (defined as the first 90‐day period post‐initiation where prescriptions covered less than two‐thirds of the period) were censored 0 or 6 months after the time of reduced persistence. HRs with 95%CIs were calculated.
Inverse probability of censoring weights were derived based on baseline covariates, plus time‐dependent serum PTH, calcium and phosphate values, and applied in a pooled logistic regression model to estimate the effect of cinacalcet on mortality. The use of time‐dependent values here differed from implementation of the PSM procedure, where only baseline values serum PTH, calcium and phosphate were used. Cinacalcet patients with poorer persistence were censored at the time of reduced persistence while patients who switched from non‐exposed to exposed were censored at cinacalcet initiation. Consequently, the follow‐up time of persistent patients was weighted to compensate for those who did not persist. Weighted pooled odds ratios (ORs), approximating to HRs, were calculated with 95%CIs.
(ii) EVOLVE data and analysis
The EVOLVE trial was a large, multi‐centre, double‐blind, placebo‐controlled RCT where 3883 HD patients with sHPT were randomly assigned cinacalcet or placebo.24 Although there were numerically fewer primary composite endpoints (time to death or first non‐fatal cardiovascular event) in patients randomised to cinacalcet compared with those of placebo, this difference failed to reach statistical significance in unadjusted intention‐to‐treat analysis.24 Pre‐specified secondary and sensitivity analyses such as covariate adjustment and lag‐censoring, however, revealed a nominally significant 12–15% risk reduction with cinacalcet.24
All‐cause mortality was analysed as described previously for the primary composite endpoint. In the EVOLVE trial, the mortality endpoint was not part of the formal statistical testing strategy; associations are therefore considered ‘nominal’. For IPCW, data were censored at the time of study drug discontinuation in both treatment groups. For each interval, patients' weights were derived using baseline covariates, time‐dependent serum PTH, calcium and phosphate values, interaction terms with treatment and laboratory measures and the adverse event of hypocalcemia. The effect of cinacalcet on mortality was estimated using weighted pooled logistic regression.
Results
The ARO cohort and EVOLVE trial
The characteristics of the ARO cohort and the EVOLVE trial are summarised in Supplementary Table B. Aside from their observational and experimental nature, respectively, the major differences related to their geography, dialysis vintage, length of follow‐up and study selection criteria.
ARO study population
Of 11 190 patients recruited into the AROii cohort, 702 patients were excluded, because, alone or in combination, they were not receiving HD (n = 487), had a kidney transplant history (n = 86) or no laboratory data (n = 255), leaving 10 488 patients. When study‐specific selection criteria (no cinacalcet use in a country (n = 200), <90 days of follow‐up (n = 925), parathyroidectomy history (n = 12) and cinacalcet use up to and including the first 90 days of follow‐up (n = 275)) were applied alone or in combination, a further 1387 (13.2%) patients were excluded, leaving 9101 patients eligible for matching.
Baseline characteristics of the overall and matched ARO populations
Patients receiving cinacalcet during follow‐up (n = 1168; 12.8%) tended to be younger than those never exposed (n = 7933) and were healthier with regards to diabetes history, the need for catheterization or hospitalisation during the eligibility period and in terms of their inflammatory (C‐reactive protein) or nutritional (serum albumin) status but were unhealthier with regard to CVD and fracture history (Table 1). As expected, they had elevated serum calcium, phosphate and PTH levels and were more reliant on phosphate binders (especially non‐calcium‐based). They were more often prescribed CVD medications and AVD.
Table 1.
Characteristics of cinacalcet and non‐cinacalcet patients in the overall ARO and propensity score‐matched populations
| Parameter | ARO population* | Matched | ||||
|---|---|---|---|---|---|---|
| Non‐cinacalcet | Cinacalcet | Std diff.‡ | Non‐cinacalcet | Cinacalcet | Std diff. | |
| (n = 7933) | (n = 1168) | (n = 1790) | (n = 532) | |||
| Person time at risk (years) | ||||||
| Mean ± SD | 2.39 ± 1.44 | 3.44 ± 1.16 | 1.99 ± 1.25 | 1.94 ± 1.18 | ||
| Q1, Q3 | 1.01, 3.50 | 2.76, 4.32 | 0.94, 2.90 | 0.96, 2.82 | ||
| Patient attrition | ||||||
| Successful renal transplant | 771 (9.7) | 203 (17.4) | 263 (14.7) | 96 (18.0) | ||
| Parathyroidectomy | 13 (0.2) | 2 (0.2) | 6 (0.3) | 2 (0.4) | ||
| Lost to follow‐up | 1988 (25.1) | 146 (12.5) | 283 (15.8) | 80 (15.0) | ||
| Exposure period pre‐KDIGO | 6442 (81.2) | 987 (84.5) | 0.088 | 854 (47.7) | 245 (46.1) | 0.001 |
| Age group | ||||||
| <30 years | 165 (2.1) | 31 (2.7) | 0.038 | 46 (2.6) | 12 (2.3) | 0.040 |
| 30–49 years | 968 (12.2) | 218 (18.7) | 0.180 | 274 (15.3) | 79 (14.8) | 0.035 |
| 50–64 years | 2122 (26.7) | 338 (28.9) | 0.049 | 494 (27.6) | 146 (27.4) | 0.010 |
| ≥65 years | 4678 (59.0) | 581 (49.7) | 0.186 | 976 (54.5) | 295 (55.5) | 0.047 |
| Sex | ||||||
| Female | 3124 (39.4) | 494 (42.3) | 0.059 | 736 (41.1) | 218 (41.0) | 0.010 |
| Male | 4809 (60.6) | 674 (57.7) | 1054 (58.9) | 314 (59.0) | ||
| Baseline hospitalisation | 1780 (22.4) | 189 (16.2) | 0.159 | 261 (14.6) | 66 (12.4) | 0.052 |
| History of diabetes | 2874 (36.2) | 338 (28.9) | 0.156 | 539 (30.1) | 158 (29.7) | 0.000 |
| History of cancer | 659 (8.3) | 97 (8.3) | 0.000 | 185 (10.3) | 48 (9.0) | 0.047 |
| History of CVD | 1985 (25.0) | 345 (29.5) | 0.102 | 617 (34.5) | 190 (35.7) | 0.029 |
| History of fractures | 106 (1.3) | 33 (2.8) | 0.104 | 78 (4.4) | 18 (3.4) | 0.057 |
| Dialysis vintage (months) | ||||||
| Mean ± SD | 1.1 ± 2.1 | 1.0 ± 1.9 | 0.007 | 14.6 ± 11.3 | 16.3 ± 11.7 | 0.081 |
| Baseline vascular access | ||||||
| Catheter | 3712 (46.8) | 470 (40.2) | 0.132 | 553 (30.9) | 163 (30.6) | 0.004 |
| Non‐catheter only | 3064 (38.6) | 558 (47.8) | 0.186 | 1107 (61.8) | 338 (63.5) | 0.022 |
| Missing | 1157 (14.6) | 140 (12.0) | 0.077 | 130 (7.3) | 31 (5.8) | 0.036 |
| iPTH group [pg/mL] | ||||||
| <75 | 908 (11.4) | 52 (4.5) | 0.261 | 19 (1.1) | 1 (0.2) | 0.096 |
| ≥75–<150 | 1361 (17.2) | 106 (9.1) | 0.241 | 63 (3.5) | 17 (3.2) | 0.009 |
| ≥150–≤300 | 2138 (27.0) | 310 (26.5) | 0.009 | 258 (14.4) | 77 (14.5) | 0.053 |
| >300–≤600 | 1351 (17.0) | 350 (30.0) | 0.309 | 900 (50.3) | 260 (48.9) | 0.009 |
| >600 | 417 (5.3) | 194 (16.6) | 0.370 | 550 (30.7) | 177 (33.3) | 0.036 |
| Missing | 1758 (22.2) | 156 (13.4) | 0.232 | |||
| Total calcium [mmol/L] | ||||||
| <2.10 | 2298 (29.0) | 199 (17.0) | 0.286 | 345 (19.3) | 97 (18.2) | 0.008 |
| ≥2.10–≤2.37 | 4409 (55.6) | 700 (59.9) | 0.088 | 1064 (59.4) | 316 (59.4) | 0.017 |
| >2.37 | 797 (10.0) | 225 (19.3) | 0.263 | 381 (21.3) | 119 (22.4) | 0.027 |
| Missing | 429 (5.4) | 44 (3.8) | 0.078 | |||
| Phosphate [mmol/L] | ||||||
| <1.13 | 1360 (17.1) | 106 (9.1) | 0.241 | 184 (10.3) | 43 (8.1) | 0.045 |
| ≥1.13–≤1.78 | 4645 (58.6) | 639 (54.7) | 0.078 | 979 (54.7) | 311 (58.5) | 0.070 |
| >1.78 | 1644 (20.7) | 394 (33.7) | 0.295 | 627 (35.0) | 178 (33.5) | 0.046 |
| Missing | 284 (3.6) | 29 (2.5) | 0.064 | |||
| CRP [mg/L] | ||||||
| ≤ Q1§ | 1421 (17.9) | 285 (24.4) | 0.159 | 442 (24.7) | 135 (25.4) | 0.023 |
| > Q1–≤Q2 | 1461 (18.4) | 240 (20.5) | 0.054 | 440 (24.6) | 135 (25.4) | 0.004 |
| > Q2–≤Q3 | 1474 (18.6) | 230 (19.7) | 0.028 | 317 (17.7) | 89 (16.7) | 0.018 |
| > Q3 | 1565 (19.7) | 137 (11.7) | 0.221 | 194 (10.8) | 52 (9.8) | 0.018 |
| Missing | 2012 (25.4) | 276 (23.6) | 0.040 | 397 (22.2) | 121 (22.7) | 0.009 |
| Serum albumin [g/L] | ||||||
| ≤ Q1¦ | 1870 (23.6) | 143 (12.2) | 0.299 | 170 (9.5) | 50 (9.4) | 0.005 |
| > Q1–≤Q2 | 1756 (22.1) | 223 (19.1) | 0.075 | 290 (16.2) | 75 (14.1) | 0.048 |
| > Q2–≤Q3 | 1677 (21.1) | 298 (25.5) | 0.104 | 453 (25.3) | 141 (26.5) | 0.032 |
| > Q3 | 1581 (19.9) | 348 (29.8) | 0.230 | 718 (40.1) | 221 (41.5) | 0.022 |
| Missing | 1049 (13.2) | 156 (13.4) | 0.004 | 159 (8.9) | 45 (8.5) | 0.032 |
| Phosphate binder use | ||||||
| None | 4365 (55.0) | 462 (39.6) | 0.314 | 557 (31.1) | 156 (29.3) | 0.019 |
| Only calcium‐based | 2451 (30.9) | 274 (23.5) | 0.168 | 340 (19.0) | 96 (18.0) | 0.004 |
| Only non‐calcium‐based | 699 (8.8) | 266 (22.8) | 0.390 | 595 (33.2) | 189 (35.5) | 0.022 |
| Both calcium‐based and non‐calcium‐based | 418 (5.3) | 166 (14.2) | 0.305 | 298 (16.6) | 91 (17.1) | 0.002 |
| Cardiovascular medication use | 5047 (63.6) | 893 (76.5) | 0.283 | 1399 (78.2) | 427 (80.3) | 0.064 |
| Active vitamin D use | 3035 (38.3) | 598 (51.2) | 0.262 | 1039 (58.0) | 319 (60.0) | 0.031 |
ARO, Analyzing data, Recognizing excellence, and Optimizing outcomes; SD, standard deviation; KDIGO, Kidney Disease: Improving Global Outcomes; CVD, cardiovascular diseases; iPTH, intact parathyroid hormone; CRP, C‐reactive protein.
The standardised difference for categorical variables was based on weighted proportions to account for many‐to‐one matching. For dialysis vintage (the only continuous variable), standardised difference were based on weighted mean and variance.
Observations during eligibility period.
Observations during baseline period.
Standardised differences.
CRP quartiles (Q1 = 3.10 mg/L, Q2 = 8.08 mg/L, Q3 = 20.40 mg/L).
Serum albumin quartiles (Q1 = 34.0 g/L, Q2 = 37.5 g/L, Q3 = 40.5 g/L).
Five‐hundred and thirty‐two patients exposed to cinacalcet were matched to 1790 patients not exposed at the time of exposure assessment (Table 1). Of the latter, initially non‐exposed patients, 521 (29.1%) subsequently received cinacalcet in their follow‐up (henceforth termed ‘future cinacalcet’ patients). A further 115 patients receiving cinacalcet remained unmatched.
Matched cinacalcet patients (median [Q1,Q3] propensity score = 0.17 [0.07, 0.32]) contributed a median of 1.82 person‐years (PY) at risk to the study, while patients matched as non‐cinacalcet (median [Q1,Q3] propensity score = 0.13 [0.06, 0.22]) contributed a median of 1.94 PY at risk. Attrition due to a successful renal transplant, parathyroidectomy and lost to follow‐up was similar in the two groups. In the PSM population, the differences in baseline patient characteristics, so apparent in the overall ARO population, were negligible with the exception of the lowest stratum of PTH (Table 1). Few clinically relevant differences were observed at the individual risk‐set level (where sufficient data were available for comparison; data not shown).
Outcomes analyses in ARO and EVOLVE
The HR for all‐cause mortality in ARO, adjusted for the slight PTH imbalance described in the previous text, was 1.03 (95%CI 0.78–1.35). This estimate was closer to the null than that observed for EVOLVE (HR 0.94 [95%CI 0.85–1.04]; Supplementary Table C).
Persistence to patients' initial exposure status
Differential treatment crossover was observed for AROii patients matched as cinacalcet patients and those matched as non‐exposed (Figure 1). Ten percent of cinacalcet patients on study did not persist with therapy for the first 90 days post‐matching and this proportion increased to 24% for the first 180 days. The attrition rate slowed subsequently, with approximately 60% of cinacalcet patients persisting for the remainder of follow‐up. Treatment crossover was more gradual for non‐cinacalcet patients, probably reflecting sHPT disease progression over time. Overall, 845 patients (36.4%) did not persist to their initial matching exposure status, with cinacalcet patients less likely to persist in their exposure status than non‐cinacalcet patients (pooled OR 0.57 [95%CI 0.48–0.68]).
Figure 1.
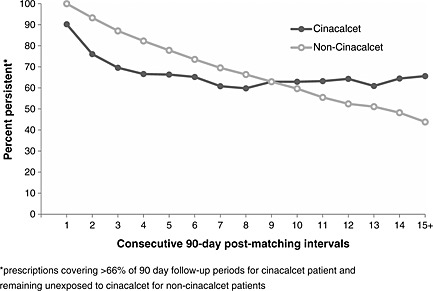
AROii patients' persistence to their exposure status according to their matched exposure status
Future AROii cinacalcet patients (n = 521) had higher serum PTH, calcium and phosphate than those never exposed (n = 1269; Supplementary Table D) and were more often prescribed phosphate binders. They tended to be younger, had higher serum albumin and were more often matched in periods prior to the publication of the Kidney Disease: Improving Global Outcomes (KDIGO™) guideline31 (which recommended a higher PTH treatment threshold). Of note, the all‐cause mortality rate was lower in future cinacalcet patients (3.4 deaths per 100 PY [95%CI 2.5–4.5]) than in those patients who remained never exposed (11.7 per 100 PY [95%CI 10.3–13.2]).
In EVOLVE, 1300 of the 1948 patients randomised to cinacalcet discontinued therapy (67%), while 1365 of the 1935 patients randomised to placebo discontinued study drug (71%).
Analytical correction for treatment crossover
When treatment crossover in AROii was corrected, by either 0‐ or 6‐month lag‐censoring or by IPCW, the estimated treatment effect moved away from the null (HRs 0.76 [95%CI 0.51–1.15], 0.84 [95%CI 0.60–1.18] and weighted pooled OR 0.79 [95%CI 0.56–1.11], respectively). These treatment effect estimates were more comparable to those observed in EVOLVE after treatment crossover correction (HRs 0.82 [95%CI 0.67–1.01], 0.83 [0.73–0.96] and weighted pooled OR 0.87 [0.71–1.06], respectively; Figure 2).
Figure 2.
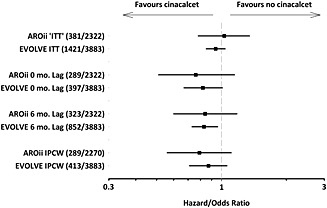
Graphical representation of the estimated treatment effects of cinacalcet on all‐cause mortality by the analytical approaches applied in AROii and Evaluation of Cinacalcet Therapy to Lower Cardiovascular Events (EVOLVE). IPCW, inverse probability of censoring weight
Discussion
It has been suggested that the analysis of an observational study should reflect that of a controlled experiment.32 With this in mind, we utilised PSM to mimic the randomisation used in clinical trials to reduce measured confounding when estimating the effect of cinacalcet therapy on all‐cause mortality in a cohort of European HD patients. Our PSM estimates were then presented alongside the most appropriate and readily available from experimental research: those obtained from the contemporaneous EVOLVE trial (conducted from August 2006 to January 2012 versus January 2007 to September 2012 for AROii). Not all the cinacalcet‐exposed patients were matched as such in our study, however, and some remained unmatched. We acknowledge at the outset, therefore, that the PSM‐estimated treatment effects correspond to the subset of the cinacalcet patients selected by the matching procedure and that this does not coincide with either the average treatment effect typically estimated by an unadjusted RCT analysis, or the average treatment of the treated estimated by other commonly applied propensity score methods.32 Our comparison with EVOLVE is warranted, however, as the estimates obtained represent the best available to assess the ability of the PSM to reduce the potential confounding arising from the prognostic differences between treated and untreated patients. It is impossible, however, to determine the extent to which discordant observational and experimental treatment effect estimates reflect, for example, unmeasured confounding in the PSM, differences in the study populations (Supplementary Table E), or are simply related but different estimands.
Both our observational and experimental research findings were influenced by treatment crossover‐introduced exposure misclassification. In our main PSM analysis not adjusting for non‐persistence, no difference was observed between cinacalcet‐treated and non‐treated patients with regard to all‐cause mortality. Importantly, future cinacalcet patients were generally healthier than those never exposed, with lower mortality observed in this group. Treatment crossover may have also influenced the current EVOLVE clinical trial findings: the potential for exposure misclassification increases in RCTs where the investigational product is commercially available or where routine laboratory data may unblind clinicians to patients' treatment assignation. Increased follow‐up time in event‐driven trials may also increase crossover potential. When non‐persistence was corrected in both studies—either through IPCW or by lag‐censoring—results suggestive of cinacalcet benefit were observed, but confidence intervals spanned 1 in both instances. It should be acknowledged, however, that these alternative analyses are not without limitations. Lag‐censoring non‐persistent patients reduced the sample size and/or follow‐up and hence the precision of the estimated treatment effects. Furthermore, this approach may be biased by informative censoring, as it assumes that non‐persistent patient are as likely to experience the study outcome as those who persisted. This is unlikely to be true, however, as prognostic differences may exist between persistent and non‐persistent patients. Similarly, unmeasured confounding might impact negatively on IPCW analyses.
At first sight, the main AROii treatment effect estimate (HR 1.03; 95%CI 0.78–1.35) differs substantially from those reported in the studies by Block et al. 20 and Cunningham et al. 21, but this might reflect differences in cinacalcet exposure classification. In the Block et al. study of US HD patients, cinacalcet prescription was treated as a time‐dependent exposure, with pre‐cinacalcet person time attributed to the control population. In contrast, we considered patients matched in the control population as remaining ‘never exposed to cinacalcet’ even if a fraction were treated later during follow‐up. Similarly, Cunningham et al. pooled data from ITT analyses of subjects randomised to cinacalcet or placebo in a time before cinacalcet was commercially available, removing the potential for crossover to the cinacalcet arm among placebo patients. A separate ‘non‐crossover’ analysis in AROii, where we excluded the potential for cinacalcet patients to match until treatment initiation suggested a similar treatment effect (HR 0.76 [95%CI 0.58–0.99]) when 681 cinacalcet patients were matched to 1779 non‐exposed patients. The most comparable EVOLVE analysis, where 0‐month lag‐censoring reflects the ITT population while on randomised treatment, revealed a treatment effect that paralleled the 0‐month lag‐censored analysis in AROii. When all of these findings are considered together (Figure 3), there is a trend towards a beneficial treatment effect of cinacalcet therapy on all‐cause mortality in HD patients. Figure 3 also illustrates the effect of sample size or follow‐up duration on estimated treatment effect precision: the HRs were broadly similar in the four studies, but only in the larger and/or longer studies did the narrower confidence intervals exclude 1.
Figure 3.
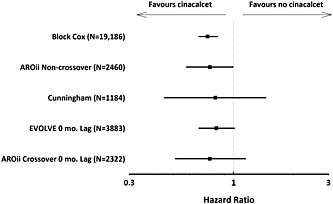
Estimated cinacalcet treatment effect on all‐cause mortality from different research studies. EVOLVE, Evaluation of Cinacalcet Therapy to Lower Cardiovascular Events
A number of additional benefits and limitations should be considered. As cinacalcet is indicated for use solely in the HD population in Europe, it is likely that incident HD patients exposed to cinacalcet in our study truly represent new users of the drug. ‘New user’ designs have been advocated as a way of minimising bias due to changing risk over time.33 Commencing time‐at‐risk from treatment initiation also eliminates immortal time bias (‘the misclassification, by treatment status, of follow‐up time during which, by definition, the study outcome cannot occur’34). Uncorrected immortal time bias can lead to the false impression of medical effectiveness. The number of patients in each analysis, however, was small in relation to the overall AROii cohort, and the time period available for observation was relatively short. In addition, the incident nature of the cohort implies less severe sHPT and hence less exposure to cinacalcet; in fact, in the EVOLVE study, the median (p10, p90) dialysis vintage of patients included in the trial was 45 (9, 146) months.35 The smaller sample size resulting from these factors was offset against the reduced bias gained through PSM.
While the matched study population was well balanced with regard to measured confounders, the potential for unmeasured confounding—inherent to PSMs and absent in RCTs with effective randomisation—still exists. High‐density PSM may, in addition to improving balance, reduce the potential for uncontrolled confounding by systematically assessing parameters for inclusion in PSM models.36 This technique is perhaps more applicable to broad‐ranging medical claims data rather than specific clinical data collected during routine care. Furthermore, high‐density PSM objectivity may diminish if optional a priori parameters are included. The list of parameters included in our PSM model was comprehensive but not exhaustive, making it conceivable that other confounding factors were not considered. Similarly, while AVD use was included as a covariate in our PSM model and treatment groups were well balanced with regard to this parameter, subsequent changes in patient management post‐matching, which might modify risk (e.g. in AVD use), are not captured and hence considered by the current study design.
In conclusion, treatment crossover can dilute treatment effect estimates in both observational research studies and RCTs. When corrected in the current study, a trend towards reduced all‐cause mortality in HD patients receiving cinacalcet therapy was observed, highlighting the need to report, quantify and correct this form of bias where possible. While only mimicking the randomisation aspect of RCTs, propensity score matching has the potential to generate treatment effects from ‘real‐life’ observational data that are comparable to those elicited from RCTs, especially when combined with techniques to correct exposure misclassification due to non‐persistence.
Study Collaborators
ARO Steering Committee members:
P. Aljama, Reina Sofia University Hospital, Cordoba, Spain
S.D. Anker, University Medical Centre Göttingen, Göttingen, Germany
B. Canaud, Lapeyronie University Hospital, Montpellier, France
T. B. Drueke, Inserm Unit 1088, Université de Picardie, Amiens, France
K.‐U. Eckardt (co‐chair), University of Erlangen‐Nuremberg, Germany
J. Floege (chair), RWTH University of Aachen, Aachen, Germany
A. de Francisco, Hospital Universitario Valdecilla, Universidad de Cantabria, Santander, Spain
F. Kronenberg, Medical University of Innsbruck, Innsbruck, Austria
I. C. Macdougall, King's College Hospital, London, UK
G. Schernthaner, Rudolfstiftung Hospital, Vienna, Austria
P. Stenvinkel, Karolinska Institutet, Stockholm, Sweden
D. C. Wheeler, University College London, London, UK
ARO project collaborators:
D Marcelli, Nephrocare, Fresenius Medical Care, Bad Homburg, Germany
B Molemans, Nephrology & General Medicine, Amgen (Europe) GmbH Zug
F Petavy, on behalf of Amgen Ltd.
EVOLVE Executive Committee members:
J. Floege, RWTH University of Aachen, Aachen, Germany
T. B. Drueke, Inserm Unit 1088, Université de Picardie, Amiens, France
D. C. Wheeler, University College London, London, UK
Conflict of Interest
JF reports having received advisor/consultant fees from Abbott, Amgen, Chugai, Genzyme and Vifor and speaker fees from Abbott, Amgen, FMC, Genzyme and Mitsubishi. TD reports having received advisor/consultant fees from Abbott, Amgen, Baxter, FMC, Genzyme, KAI Pharmaceuticals, Kirin, Theraclion and Vifor; speaker fees from Abbott, Amgen, Chugai, Genzyme, Kirin and Vifor; and grant/research support from Amgen, Baxter and Shire. AL de F reports having received advisor/consultant fees from Amgen and Fresenius and speaker fees from Abbott, Amgen and Fresenius. SA reports having received consultant fees from Amgen, Fresenius Kabi and Vifor; research support from Vifor; and speaker fees from Amgen and Vifor. DW reports having received research funding from Abbott, Genzyme and AstraZeneca and honoraria from Amgen, Abbott, Fresenius, Janssen, Otsuka, Shire and Vifor.
IG is a contractor for Amgen. IAG, YK and MF are full‐time employees of Amgen who may own stock and/or stock options in Amgen.
Key Points.
Observational research has the potential to complement randomised controlled trials but is prone to bias.
By combining propensity score matching with techniques to correct for treatment crossover, we were able to closely mirror the all‐cause mortality results of a randomised control trial in a ‘real‐life’ setting.
Persistence‐corrected analyses revealed a trend towards reduced all‐cause mortality in haemodialysis patients receiving cinacalcet therapy.
Ethics Statement
All ethical and regulatory obligations concerning the use of patient data were met at each participating FMC site.
Supporting information
Supporting info item
Acknowledgements
This study was funded by Amgen Europe GmbH, Zug, Switzerland.
Gillespie, I. A. , Floege, J. , Gioni, I. , Drüeke, T. B. , de Francisco, A. L. , Anker, S. D. , Kubo, Y. , Wheeler, D. C. , and Froissart, M. (2015) Propensity score matching and persistence correction to reduce bias in comparative effectiveness: the effect of cinacalcet use on all‐cause mortality. Pharmacoepidemiol Drug Saf, 24: 738–747. doi: 10.1002/pds.3789.
This study was presented in part at the European Renal Association/European Dialysis and Transplantation Association (ERA‐EDTA) 50th Congress, Istanbul 18–21 May 2013.
The copyright line for this article was changed on 19 August 2016 after original online publication.
References
- 1. Armstrong K. Methods in comparative effectiveness research. J Clin Oncol 2012; 30: 4208–4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gutierrez OM. Fibroblast growth factor 23 and disordered vitamin D metabolism in chronic kidney disease: updating the “trade‐off” hypothesis. Clin J Am Soc Nephrol 2010; 5: 1710–1716. [DOI] [PubMed] [Google Scholar]
- 3. Joy MS, Karagiannis PC, Peyerl FW. Outcomes of secondary hyperparathyroidism in chronic kidney disease and the direct costs of treatment. J Manag Care Pharm 2007; 13: 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Block GA, Hulbert‐Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998; 31: 607–617. [DOI] [PubMed] [Google Scholar]
- 5. Ganesh SK, Stack AG, Levin NW, Hulbert‐Shearon T, Port FK. Association of elevated serum PO4, Ca × PO4 product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 2001; 12: 2131–2138. [DOI] [PubMed] [Google Scholar]
- 6. Stevens LA, Djurdjev O, Cardew S, Cameron EC, Levin A. Calcium, phosphate, and parathyroid hormone levels in combination and as a function of dialysis duration predict mortality: evidence for the complexity of the association between mineral metabolism and outcomes. J Am Soc Nephrol 2004; 15: 770–779. [DOI] [PubMed] [Google Scholar]
- 7. Slinin Y, Foley RN, Collins AJ. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. J Am Soc Nephrol 2005; 16: 1788–1793. [DOI] [PubMed] [Google Scholar]
- 8. Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008; 52: 519–530. [DOI] [PubMed] [Google Scholar]
- 9. Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all‐cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant 2009; 24: 1506–1523. [DOI] [PubMed] [Google Scholar]
- 10. Goodman WG, Goldin J, Kuizon BD, et al. Coronary‐artery calcification in young adults with end‐stage renal disease who are undergoing dialysis. N Engl J Med 2000; 342: 1478–1483. [DOI] [PubMed] [Google Scholar]
- 11. Oh J, Wunsch R, Turzer M, et al. Advanced coronary and carotid arteriopathy in young adults with childhood‐onset chronic renal failure. Circulation 2002; 106: 100–105. [DOI] [PubMed] [Google Scholar]
- 12. Raggi P, Boulay A, Chasan‐Taber S, et al. Cardiac calcification in adult hemodialysis patients. A link between end‐stage renal disease and cardiovascular disease? J Am Coll Cardiol 2002; 39: 695–701. [DOI] [PubMed] [Google Scholar]
- 13. Moe SM, Drueke TB. Management of secondary hyperparathyroidism: the importance and the challenge of controlling parathyroid hormone levels without elevating calcium, phosphorus, and calcium‐phosphorus product. Am J Nephrol 2003; 23: 369–379. [DOI] [PubMed] [Google Scholar]
- 14. Danese MD, Belozeroff V, Smirnakis K, Rothman KJ. Consistent control of mineral and bone disorder in incident hemodialysis patients. Clin J Am Soc Nephrol 2008; 3: 1423–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. European Medicines Agency . Mimpara summary of product characteristics. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/000570/WC500028900.pdf (accessed: 05/02/2015).
- 16. Block GA, Martin KJ, de Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 2004; 350: 1516–1525. [DOI] [PubMed] [Google Scholar]
- 17. Lindberg JS, Culleton B, Wong G, et al. Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double‐blind, multicenter study. J Am Soc Nephrol 2005; 16: 800–807. [DOI] [PubMed] [Google Scholar]
- 18. Lindberg JS, Moe SM, Goodman WG, et al. The calcimimetic AMG 073 reduces parathyroid hormone and calcium x phosphorus in secondary hyperparathyroidism. Kidney Int 2003; 63: 248–254. [DOI] [PubMed] [Google Scholar]
- 19. Quarles LD, Sherrard DJ, Adler S, et al. The calcimimetic AMG 073 as a potential treatment for secondary hyperparathyroidism of end‐stage renal disease. J Am Soc Nephrol 2003; 14: 575–583. [DOI] [PubMed] [Google Scholar]
- 20. Block GA, Zaun D, Smits G, et al. Cinacalcet hydrochloride treatment significantly improves all‐cause and cardiovascular survival in a large cohort of hemodialysis patients. Kidney Int 2010; 78: 578–589. [DOI] [PubMed] [Google Scholar]
- 21. Cunningham J, Danese M, Olson K, Klassen P, Chertow GM. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health‐related quality of life in secondary hyperparathyroidism. Kidney Int 2005; 68: 1793–1800. [DOI] [PubMed] [Google Scholar]
- 22. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Francisco AL, Kim J, Anker SD, et al. An epidemiological study of hemodialysis patients based on the European Fresenius Medical Care hemodialysis network: results of the ARO study. Nephron Clin Pract 2011; 118: c143–154. [DOI] [PubMed] [Google Scholar]
- 24. Evolve Trial Investigators , Chertow GM, Block GA, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 2012; 367: 2482–2494. [DOI] [PubMed] [Google Scholar]
- 25. Steil H, Amato C, Carioni C, et al. EuCliD–a medical registry. Methods Inf Med 2004; 43: 83–88. [PubMed] [Google Scholar]
- 26. Lu B. Propensity score matching with time‐dependent covariates. Biometrics 2005; 61: 721–728. [DOI] [PubMed] [Google Scholar]
- 27. Floege J, Gillespie IA, Kronenberg F, et al. Development and validation of a predictive mortality risk score from a European hemodialysis cohort. Kidney Int 2015. doi:10.1038/ki.2014.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kosanke J, Bergstralh E. 2004. gmatch SAS Macro. http://www.mayo.edu/research/documents/gmatchsas/doc‐10027248 Accessed 17/03/2012.
- 29. Faries DE, Obenchain RL, Haro JM, Leon AC. Propensity score matching for estimating treatment effects In Analysis of Observational Health Care Data Using SAS. SAS Publishing: Cary, NC, 2010. [Google Scholar]
- 30. Hernan MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology 2008; 19: 766–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kidney Disease: Improving Global Outcomes CKD‐MBD Work Group . KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease‐mineral and bone disorder (CKD‐MBD). Kidney Int Suppl 2009: S1–130. [DOI] [PubMed] [Google Scholar]
- 32. Austin PC. The use of propensity score methods with survival or time‐to‐event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014; 33: 1242–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ray WA. Evaluating medication effects outside of clinical trials: new‐user designs. Am J Epidemiol 2003; 158: 915–920. [DOI] [PubMed] [Google Scholar]
- 34. Levesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 2010; 340: b5087. [DOI] [PubMed] [Google Scholar]
- 35. Chertow GM, Correa‐Rotter R, Block GA, et al. Baseline characteristics of subjects enrolled in the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) trial. Nephrol Dial Transplant 2012; 27: 2872–2879. [DOI] [PubMed] [Google Scholar]
- 36. Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High‐dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 2009; 20: 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


