Summary
The level of glycaemic control necessary to achieve optimal short‐term and long‐term outcomes in subjects with type 1 diabetes mellitus (T1DM) typically requires intensified insulin therapy using multiple daily injections or continuous subcutaneous insulin infusion. For continuous subcutaneous insulin infusion, the insulins of choice are the rapid‐acting insulin analogues, insulin aspart, insulin lispro and insulin glulisine. The advantages of continuous subcutaneous insulin infusion over multiple daily injections in adult and paediatric populations with T1DM include superior glycaemic control, lower insulin requirements and better health‐related quality of life/patient satisfaction. An association between continuous subcutaneous insulin infusion and reduced hypoglycaemic risk is more consistent in children/adolescents than in adults. The use of continuous subcutaneous insulin infusion is widely recommended in both adult and paediatric T1DM populations but is limited in pregnant patients and those with type 2 diabetes mellitus. All available rapid‐acting insulin analogues are approved for use in adult, paediatric and pregnant populations. However, minimum patient age varies (insulin lispro: no minimum; insulin aspart: ≥2 years; insulin glulisine: ≥6 years) and experience in pregnancy ranges from extensive (insulin aspart, insulin lispro) to limited (insulin glulisine). Although more expensive than multiple daily injections, continuous subcutaneous insulin infusion is cost‐effective in selected patient groups. This comprehensive review focuses on the European situation and summarises evidence for the efficacy and safety of continuous subcutaneous insulin infusion, particularly when used with rapid‐acting insulin analogues, in adult, paediatric and pregnant populations. The review also discusses relevant European guidelines; reviews issues that surround use of this technology; summarises the effects of continuous subcutaneous insulin infusion on patients' health‐related quality of life; reviews relevant pharmacoeconomic data; and discusses recent advances in pump technology, including the development of closed‐loop ‘artificial pancreas’ systems. © 2015 The Authors. Diabetes/Metabolism Research and Reviews Published by John Wiley & Sons Ltd.
Keywords: continuous subcutaneous insulin infusion, diabetes mellitus, paediatric, pregnancy, pharmacoeconomics, rapid‐acting insulin analogue
Introduction
Numerous clinical trials have demonstrated that near‐normal glucose control is associated with improved short‐term and long‐term outcomes in both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) 1, 2, 3, 4, 5, 6. For those patients who require insulin, the intensified regimens that are necessary to achieve this level of glycaemic control may be administered via multiple daily injections (MDI) 7 or by continuous subcutaneous insulin infusion (CSII) 8. CSII requires the patient to wear a portable electromechanical pump that infuses insulin at pre‐selected basal rates 9. The rate can be boosted by the patient as required for food intake 9. The pump itself comprises a battery‐operated motor, a computerized control mechanism, an insulin reservoir and an infusion set (subcutaneous cannula and tubing). Recent advances in pump technology include the development of sensor‐augmented pumps, in which the pump is integrated with a real‐time continuous glucose monitor (CGM) 10. Patch pumps (tubing‐free pumps in which the reservoir and integrated infusion set adhere to the skin) represent another recent development 9.
Whilst recent advances in pump technology allow patients a considerable choice of pump features, the development of modified insulin molecules has also allowed a choice of insulin. Regular human insulin (RHI) may be used in CSII 7, 11 but rapid‐acting insulin analogues (RAIAs) are now considered to be the insulins of choice for use in pumps 8, 12. Differences among RAIAs in physicochemical stability and pump compatibility are discussed later in this review.
The use of CSII is increasing in many European countries, particularly among paediatric patients 13, 14, 15. However, there is a wide variation in CSII usage among European countries (Figure 1), and Europe lags behind the United States in acceptance of this technology 9, 14, 16. Potential reasons for low CSII usage in Europe include the following: insufficient numbers of physicians and diabetes educators trained in the benefits of, indications for and use of pumps; absence of clear referral pathways from first‐opinion physicians to specialist pump centres; and inadequate or non‐existent funding of these devices by national healthcare systems/insurance companies 14, 16.
Figure 1.
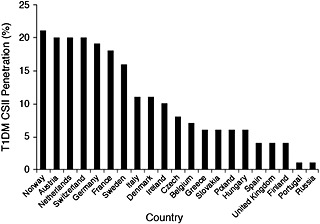
Continuous subcutaneous insulin infusion therapy penetration rates in patients with type 1 diabetes mellitus in European countries 14
This comprehensive review summarises evidence for the efficacy and safety of CSII in adult, paediatric and pregnant subjects with diabetes; discusses relevant clinical guidelines; reviews issues that surround use of this technology; summarises the effects of CSII on patients' quality of life (QOL); reviews relevant pharmacoeconomic data; and discusses recent advances in pump technology. The review, which includes expert opinion from leading European diabetologists, focuses primarily on the use of RAIAs in CSII and on European data.
Comparison of continuous subcutaneous insulin infusion and multiple daily injections: clinical data
The insulin used in CSII is typically an RAIA 5. For this reason, when evaluating the advantages and disadvantages of CSII, the ideal comparator is analogue‐based MDI. To date, however, few such trials have been reported and those that do exist are generally small 5. In the following summary, we have given preference to the strongest evidence available (i.e. the results of systematic reviews and meta‐analyses) and to guideline recommendations. We acknowledge, however, that the meta‐analyses cited were primarily published for the purposes of literature summary, rather than being ‘decision‐making meta‐analyses’, and may therefore give results that do not accurately reflect the relative merits of CSII and MDI in specific patient populations 17.
Use of continuous subcutaneous insulin infusion in adult patients
Systematic reviews, meta‐analyses, and guideline recommendations
In systematic reviews and meta‐analyses that considered studies in which a variety of insulins were used, no evidence was found for a difference between CSII and MDI in HbA1c control or risk of severe hypoglycaemia in adults with T2DM 5, 18, 19, 20, 21. This conclusion is reinforced by the results of a recent 12‐month randomized clinical trial involving older patients with T2DM (≥60 years) in which glucose variability was not different between CSII (insulin lispro)‐managed and MDI (insulin lispro and insulin glargine)‐managed groups 22. However, it is contradicted by an older study in which CSII (insulin lispro) provided better metabolic control than MDI (insulin lispro plus neutral protamine Hagedorn) in patients with T2DM who had failed to respond to conventional insulin therapy 23. A review published in 2010 concluded that although CSII has been shown to improve glucose control in patients with T2DM, the evidence to support its use in this population is inconsistent 24. However, expression of a preference for CSII among study subjects has been more consistent, and this mode of insulin delivery has been shown to improve QOL and treatment satisfaction in patients with T2DM 24. One gap in our understanding, identified by Bode 24, is whether CSII provides incremental clinical benefits for patients with T2DM after MDI has failed. This question was addressed by Reznik et al. 25 in a recent study in which 331 adult patients with T2DM who had poor glycaemic control despite MDI with insulin analogues were randomized to pump treatment (insulin lispro, aspart or glulisine) or to continue with MDI (insulin glargine or detemir, plus insulin lispro, aspart or glulisine). After 6 months, the pump therapy group had a significantly greater decrease in mean HbA1c (1.1 versus 0.4%; p < 0.0001) and were receiving a significantly lower mean total daily insulin dose (97 versus 122 units; p < 0.0001). There was no significant difference between groups in bodyweight change (1.5 [pump] versus 1.1 [MDI] kg; p = 0.322), and the amount of time spent with a blood glucose level <3.9 mmol/L during a 6‐day period of CGM was similar in the two groups (8.8 versus 5.1 min; p = 0.767). Reznik et al. concluded that, in patients whose T2DM is poorly controlled in spite of MDI, pump therapy is a safe and valuable treatment option 25.
The conclusions of studies relating to the use of CSII in adult patients with T1DM are much more consistent than those relating to patients with T2DM: numerous systematic reviews and meta‐analyses have concluded that this mode of insulin delivery – when used with a variety of insulins – is more effective than MDI at decreasing HbA1c in the T1DM population 5, 7, 18, 19, 20, 21, 26, 27. The magnitudes of the differences between CSII and MDI in end‐of‐treatment HbA1c in selected randomized controlled clinical trials are shown in Figure 2 7. Moreover, the improvements in glycaemic control that occur in this population after introduction of CSII – which are most marked in patients with poor control at baseline 5, 28, 29, 30 – are frequently associated with a lower daily insulin dose than is required with MDI 5, 26.
Figure 2.
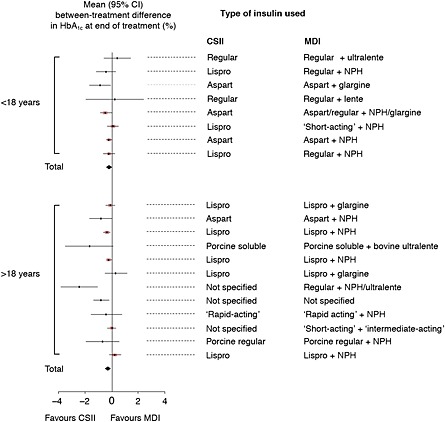
Meta‐analysis: effects of CSII and MDI on HbA1c in adult and paediatric patients with T1DM 7. A meta‐analysis of 12 randomised controlled trials involving a comparison of CSII and MDI in adult patients with T1DM demonstrated a statistically significant difference in HbA1c in favour of CSII of 0.29% (95% CI, 0.06% to 0.52%). In paediatric patients, meta‐analysis of eight trials also favoured CSII (0.22% [0.03% to 0.41%]). CI, confidence interval; CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injections; NPH, neutral protamine Hagedorn; T1DM, type 1 diabetes mellitus
The clear evidence for superior efficacy of CSII over MDI in adult patients with T1DM is not matched by superior safety, the majority of reviews having reported no clear evidence for a beneficial effect of CSII, compared with MDI, on the risk of mild or severe hypoglycaemia in this population 19, 20, 21, 31. CSII has not, however, been accompanied by an increase in hypoglycaemic risk 31, and two recent reviews have concluded that the risk of severe hypoglycaemia is lower in patients using CSII than in those using MDI 7, 28. Current clinical guidelines generally support these clinical findings, although none recommends the first‐line use of CSII in patients with T1DM (Table 1) 12, 32, 33, 34, 35.
Table 1.
Guideline recommendations pertaining to the use of continuous subcutaneous insulin infusion in patients with diabetes
| Patient population | Organization | Recommendations/statements |
|---|---|---|
| Adult | ||
| T1DM | NICE 32 | Use of CSII restricted to patients who have experienced poor glycaemic control or ‘disabling’ hypoglycaemia when using MDI |
| SFD 33 | CSII should be considered in patients who have: | |
| ●persistently elevated HbA1c despite intensified MDI | ||
| ●recurrent hypoglycaemia | ||
| ●marked glycaemic variability | ||
| ●variable insulin requirements | ||
| ●insulin allergy | ||
| ●experienced a negative impact of MDI on their social or professional life | ||
| T2DM | NICE 32 | CSII not recommended |
| SFD 33 | Use of CSII supported in patients who have: | |
| ●failed MDI | ||
| ●very high insulin requirements/insulin resistance | ||
| ●insulin allergy | ||
| Paediatric, T1DM | ||
| <12 years (no lower limit) | NICE 32 | CSII is a treatment option if MDI is impractical or inappropriate |
| 12–18 years | NICE 32 | Same criteria as adult patients for use of CSII |
| All patients in this age group should undergo a trial of MDI therapy | ||
| All ages (no lower limit) | SFD 33 | All indications for use of CSII in adults apply to children and adolescents. In addition, CSII is considered to be first‐line therapy in paediatric patients in whom MDI is not feasible for practical reasons and in those with: |
| ●neonatal/very early onset diabetes | ||
| ●glycaemic instability (very young children) | ||
| ●very low insulin requirements, especially at night (very young children) | ||
| ●nocturnal hypoglycaemia | ||
| ●pain and/or needle phobia | ||
| IDF/ISPAD 34 | ●CSII should be available and considered in paediatric/adolescent patients; when adequate education and support is provided, CSII is acceptable and successful even in young infants | |
| All ages (no lower limit) | Consensus statement* 12 | Criteria for consideration of CSII include: |
| ●recurrent severe hypoglycaemia | ||
| ●suboptimal glycaemic control | ||
| ●wide fluctuations in blood glucose levels (regardless of HbA1c) | ||
| ●microvascular complications | ||
| ●risk factors for macrovascular complications | ||
| ●lifestyle factors | ||
| ●eating disorders | ||
| ●pronounced dawn phenomenon | ||
| ●needle phobia | ||
| ●pregnancy/planned pregnancy | ||
| ●susceptibility to ketosis | ||
| ●competitive athletic endeavours | ||
| Pregnant | ||
| T1DM and T2DM | NICE 35 | No evidence of statistically significant differences between CSII and MDI therapy in maternal or foetal outcomes |
| T1DM | NICE 32, 35 | CSII is a treatment option for women with: |
| ●HbA1c ≥8.5% (≥69.4 mmol/mol) despite a high level of care on MDI therapy | ||
| ●significant disabling hypoglycaemia | ||
| SFD 33 | For patients who are planning a pregnancy/are currently pregnant, the mode of insulin administration should be subject to individualized risk/benefit analysis | |
| T2DM | SFD 33 | Therapeutic value of CSII not yet established |
CSII, continuous subcutaneous insulin infusion; IDF, International Diabetes Federation; ISPAD, International Society for Pediatric and Adolescent Diabetes; MDI, multiple daily injections; NICE, National Institute for Clinical Health and Excellence; QOL, quality of life; SFD, Société Francophone du Diabète; T1DM, type 2 diabetes mellitus; T2DM, type 2 diabetes mellitus
Consensus statement from the European Society for Paediatric Endocrinology, the Lawson Wilkins Pediatric Endocrine Society and the International Society for Pediatric and Adolescent Diabetes, endorsed by the American Diabetes Association and the European Association for the Study of Diabetes
Relative safety and efficacy of different insulins
The advent of modified insulin molecules has provided patients and physicians with substantial choice, although RAIAs are generally considered to be the preferred insulins for use in CSII 8. This is supported by the results of several meta‐analyses, the earliest of which concluded that, when used in CSII, RAIAs result in a modest but significant reduction in HbA1c when compared with soluble insulin 36. This meta‐analysis, which included data on insulin aspart and insulin lispro only, also found that patients prefer RAIAs to soluble insulin 36. A more recent meta‐analysis, conducted by the Cochrane Collaboration, concluded that, in patients with T1DM who are using CSII, RAIAs are associated with a small but significant benefit in long‐term glycaemic control when compared with RHI 37. These two analyses have been superseded by a 2009 meta‐analysis 31, which confirmed the findings of the earlier reports (improved glycaemic control with RAIAs [insulin aspart or insulin lispro] versus soluble insulin), as well as demonstrating lower hypoglycaemic risk when analogue insulins were used 31. This meta‐analysis was not, however, restricted to CSII.
Of the three RAIAs in current use, there are considerably more trial data relating to the use of insulin aspart and insulin lispro than to the use of insulin glulisine 37. The more widespread use of insulins aspart and lispro is supported by CSII studies that have demonstrated higher rates of occlusion and symptomatic hypoglycaemia with insulin glulisine than with either of the other RAIAs 38, 39, 40.
Data comparing insulin aspart and insulin lispro are less clear‐cut. For example, a small (n = 17) 3‐day randomized, crossover trial that compared the effects of CSII with insulin aspart and insulin lispro on glycaemic stability found that post‐prandial glucose levels were more stable with insulin aspart when the two preparations were infused as pre‐meal boluses 41. However, there were no differences in overall daily glucose stability when the two formulations were infused via CSII as basal insulins 41. Using a different study design (randomized, open‐label, parallel group), a larger patient group (n = 146), a considerably longer study duration (16 weeks), and different endpoints, Bode et al. 11 found no differences among buffered regular insulin, insulin aspart, and insulin lispro in mean change in HbA1c, or incidences of hypoglycaemic events or clogs/blockages in pumps or infusion sets 11.
The incidence of infusion set clogging or other infusion site/set complications was also similar in insulin lispro‐ and insulin aspart‐treated patients in two recent randomized, crossover, non‐inferiority studies in which the pump reservoir remained unchanged for 6 days 42. The overall rate of infusion site problems was low in both these studies and did not differ significantly between groups. Insulin lispro was non‐inferior to insulin aspart in terms of daily mean self‐monitored blood glucose during the entire treatment period (days 1 to 6) in both studies, and there was no significant difference between insulin lispro and insulin aspart in mean daily blood glucose levels on 5 of the 6 days in each study. However, on the final day of reservoir use (day 6), insulin lispro failed to demonstrate non‐inferiority to insulin aspart for mean blood glucose levels. The rates of hypoglycaemia (total and documented) were significantly lower in the insulin lispro‐treated patients. The difference between the two insulins in day 6 mean blood glucose levels is probably of limited clinical relevance because the majority of patients empty their reservoirs in less than 6 days in clinical practice.
Expert opinion
Although there is currently no clear evidence that the use of CSII is beneficial in adult patients with T2DM, long‐term studies are required, particularly regarding the effect of CSII on the prevention of long‐term complications. For patients with T1DM, CSII is more effective than MDI in reducing HbA1c, but its differential effect on safety – and on severe hypoglycaemic risk in particular – is minimal. RAIAs are preferred over other insulins for use in CSII but the improvements in glycaemic control and reductions in hypoglycaemic risk seen with these agents are small. Unfortunately, guidelines for the use of CSII differ considerably among European countries and this does not help to promote the rational use of this technology, which could be associated with cost savings for healthcare systems. Efforts should be made to harmonize clinical guidelines among countries, a process that could be facilitated by the European Association for the Study of Diabetes and the International Society for Pediatric and Adolescent Diabetes.
Use of continuous subcutaneous insulin infusion in paediatric patients
To our knowledge, no studies have been published concerning the use of CSII in paediatric patients with T2DM, and this population is not covered. Randomized clinical trials and long‐term studies regarding the use of CSII in paediatric patients with T1DM are also few 12. Exceptions include a case–control study designed to determine the long‐term safety and efficacy of CSII in children 43. The types of insulin used in CSII in this study were not specified. Compared with patients on MDI, those on CSII showed sustained improvements in glycaemic control over a 5‐year period 43. In a longer study that analysed data from a diabetes register, Dovc et al. 44 showed that metabolic control improved significantly over the period 2000 to 2011 in paediatric patients with T1DM in Slovenia and that, over this period, use of CSII was associated with significantly lower HbA1c values than MDI. These children were diagnosed with T1DM when they were between 0 and 17 years of age. In addition, Sulmont et al. 45 reported the results of 8 years of follow‐up in children in whom T1DM was diagnosed before 6 years of age. In this population, CSII was associated with better long‐term metabolic control and a lower risk of severe hypoglycaemia than MDI, especially when CSII was initiated at the time of diagnosis. Recently, a population‐based registry analysis also demonstrated a reduced risk of hypoglycaemia associated with the use of CSII in children 46. These recent data support the results of the systematic reviews and meta‐analyses summarized in the next section.
The challenges of treating diabetes in paediatric patients (neonates, young children and adolescents), the advantages of CSII over MDI in these age groups and issues surrounding the use of CSII during exercise are summarized in Table 2 5, 8, 12, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59.
Table 2.
Paediatric patients: challenges of treating diabetes and use of CSII
| Age group | Problem | Comments | Overview |
|---|---|---|---|
| Neonates | |||
| Unpredictable/variable feeding pattern | Compared with MDI, CSII better facilitates adaptation of insulin regimen to current feeding regimen (continuous enteral or parenteral feeding versus intermittent bottle feeding) 47, 48 | In neonates, CSII is safe, the subcutaneous infusion lines are well tolerated and CSII is more physiological, more accurate and easier to manage than MDI 47 | |
| Low insulin requirement | Accurate dosing of small amounts of insulin is easier with CSII than with MDI 47 | ||
| Little guidance is available regarding insulin dilution, but case reports describing successful dilution of insulin lispro with a compatible diluent or normal saline have been published 12, 49; insulin dilution is not necessary with the latest generation of insulin pumps (minimum infusion rate 0.025 U/h) | |||
| Young children | |||
| Glycaemic variability, risk of hypoglycaemia and DKA caused by erratic eating and exercise patterns | Compared with MDI, CSII improves control of blood glucose fluctuations and HbA1c; reduces risk of hypoglycaemia and dawn phenomenon 5 | In addition to its clinical advantages in young children, CSII provides improved lifestyle flexibility for both child and family 5 | |
| Reluctance of school to oversee and administer MDI; school not knowledgeable regarding use of MDI | CSII preferable to poorly managed MDI 5 | ||
| Adolescents | |||
| Poor adherence to CSII‐related tasks due to social and psychological factors 8, 50, 51, 52 | Problem is CSII‐specific; however, the same problem of poor adherence pertains to MDI in this population 8 | In adolescents, CSII is associated with high levels of satisfaction and, when compared with MDI, with a greater sense of control, increased independence and increased flexibility in diet and daily schedule 12, 58 | |
| Factitious manipulation of pump 53 | Problem is CSII‐specific | ||
| Missed meal‐time boluses 54 | Problem is CSII‐specific | ||
| Development of insulin resistance | May be managed more effectively with CSII than with MDI 8 | ||
| Changes in sleep and activity patterns | May be managed more effectively with CSII than with MDI 8 | ||
| All ages | Whether to leave CSII pump on, turn it off or reduce the infusion rate during exercise | Some studies conclude that it is preferable to discontinue basal insulin infusion during exercise (to reduce the risk of hypoglycaemia during or after exercise), 55, 56 and some that it is preferable to keep the pump on 57 | Advising patients is difficult because relevant data are sparse and, where they do exist, confusing 55, 56, 57, 59: studies performed in older children and adolescents have yielded conflicting results; use of CSII by young children during sporting activities has not been explored |
| In patients with T1DM who take regular moderate‐to‐heavy aerobic exercise, RAIA‐based CSII reduces post‐exercise hyperglycaemia and the risk of post‐exercise late‐onset hypoglycaemia, when compared with MDI 59 |
CSII, continuous subcutaneous insulin infusion; DKA, diabetic ketoacidosis; MDI, multiple daily injections; RAIA, rapid‐acting insulin analogue; T1DM, type 1 diabetes mellitus
Systematic reviews, meta‐analyses, and guideline recommendations
In systematic reviews and meta‐analyses that have considered studies in which a variety of insulins were used, the use of CSII in paediatric patients with T1DM has been associated with better glycaemic control (i.e. lower HbA1c; Figure 2) than MDI 5, 7, 21, 60, 61. As in adults, the magnitude of the improvement depends on the patient's HbA1c prior to CSII initiation 5, 62. These analyses also showed that the use of CSII in paediatric patients is associated with lower insulin requirements 21, 61 and lower hypoglycaemic risk 5 than MDI. However, these conclusions were not echoed by those of a recent systematic review of randomized controlled trials and selected observational studies carried out by the Agency for Healthcare Research and Quality in the United States, which found no difference between the effects of MDI and RAIA‐based CSII (with various insulins) on HbA1c or severe hypoglycaemia in children or adolescents with T1DM 19. However, the criteria used for selection of studies in this review may have introduced bias. Current clinical guidelines generally support the use of CSII in paediatric patients with T1DM, with no lower age limit. Moreover, in contrast to the situation in adults, it is generally recommended that CSII may be used as first‐line therapy in appropriately selected paediatric patients (Table 1) 12, 32, 33.
Relative safety and efficacy of different insulins
Although RAIAs are considered to be the insulins of choice for paediatric patients using CSII 8, 12, there are few studies on this topic. A 2004 study involving young children showed that when compared with RHI, insulin lispro was associated with improved post‐prandial glucose excursions and better parental satisfaction 63. The overall safety and efficacy of the two insulin preparations were, however, similar. A more recent comparison of insulin aspart and insulin lispro found that these two RAIAs were of similar safety and efficacy when used in CSII in children and adolescents with T1DM 64. It should be noted that in Europe, insulin glulisine is approved only for use in children ≥6 years of age 65. Insulin aspart may be used in children aged ≥2 years 66, and insulin lispro can be used in patients of any age 67. Insulin lispro was the first RAIA to be approved for clinical use and, as a result, has been used in CSII in numerous studies in paediatric patients 68.
Expert opinion
CSII with RAIA is becoming the treatment of choice in paediatric patients with T1DM, with a penetration of at least 50% in the United States 69 and in several European centres 44. There are obvious practical advantages associated with the flexibility of CSII, in that it allows individual age‐appropriate basal insulin infusion rates to be set 70 and erratic behaviour to be followed by multiple small prandial and/or correction boluses. When using CSII, age‐appropriate structured continuous education of the entire family – and possibly also of kindergarten/school personnel 71 – is of paramount importance. To be optimal, such education should be provided by a multidisciplinary team with a strong emphasis on psychosocial support and nutritional education, including the counting of carbohydrates. For patients who are using CSII, the time spent in hypoglycaemia, the frequency of hypoglycaemia 72 and the frequency of severe hypoglycaemia 73 can be reduced by using a sensor‐augmented insulin pump with an automated insulin‐suspend function, which suspends insulin delivery at a pre‐defined low glucose concentration. Sensor‐augmented pumps may eventually be superseded by artificial pancreas systems, in which CSII with RAIA are incorporated into closed‐loop insulin delivery systems. The success of such systems has already been demonstrated 74 and is discussed in a later section of this article.
Use of continuous subcutaneous insulin infusion in pregnancy
Pregnancy in patients with diabetes is associated with risks to both mother and foetus 35. The comparative safety and efficacy of CSII and MDI in pregnant women who have pre‐existing diabetes have been the subject of a number of studies 75. In contrast, the use of CSII in women with gestational diabetes has not been well explored 76 and is not covered in this review.
Systematic reviews, meta‐analyses and guideline recommendations
The strength of evidence comparing CSII and MDI in pregnant women with diabetes is currently insufficient 5, 19, 75, 76, 77, 78, the majority of the studies available to date being retrospective and all being observational 75. Only randomized controlled trials would allow a valid comparison of the effects of these two insulin regimens on relevant outcomes in this population 5, 19, 35, 75, 76, 77. This lack of data is reflected by current guideline recommendations (Table 1).
Relative safety and efficacy of different insulins
Of the three RAIAs currently available, all are approved for use in pregnancy 65, 66, 67. However, no clinical trials involving the use of insulin glulisine in pregnant patients have been published, and experience with this RAIA in pregnant women is limited 65. For this reason, caution is advised in the use of insulin glulisine in this population 65. In contrast, insulin lispro has been widely used in pregnant women, and insulin aspart has been compared with RHI in two randomized trials 66, 67. These studies have led regulatory authorities to conclude that there is no evidence of an adverse effect of insulin lispro or insulin aspart on pregnancy or on the health of the foetus/newborn 66, 67, a conclusion that is shared by the authors of several recent reviews 79, 80, 81. These findings are largely echoed by current guideline recommendations on the use of RAIAs in pregnant patients (Table 3) 35.
Table 3.
Guideline recommendations pertaining to the use of rapid‐acting insulin analogues in pregnant patients with diabetes 35
| Recommendations |
|---|
| ●There is no evidence that either insulin aspart or insulin lispro has adverse effects on pregnancy or on the foetus |
| ●Insulin lispro and insulin aspart have advantages over RHI during pregnancy |
| ●lower risk of hypoglycaemia |
| ●lower post‐prandial glucose excursions |
| ●improved overall glycaemic control |
| ●better patient satisfaction |
| ●Insulin lispro and insulin aspart may offer benefits over NPH |
| ●greater flexibility |
| ●improved glycaemic control |
| ●The use of insulin glulisine is not recommended because of a lack of relevant safety data |
NPH, neutral protamine Hagedorn; RHI, regular human insulin
The majority of studies that have investigated the use of insulin aspart and insulin lispro in pregnancy have not incorporated CSII, and the relative efficacy and safety of different insulin preparations when used in CSII to treat pregnant women have therefore not been well addressed in the scientific literature. One study that did compare the effects of insulin lispro and RHI when administered via both MDI and CSII found that insulin lispro was associated with fewer hypoglycaemic comas and a lower risk of pre‐term birth but a higher risk of the infant being small‐for‐gestational‐age 82. This latter finding contrasts with those of other studies in which no adverse effect of insulin lispro on perinatal outcomes has been found 80, 81. As pointed out by the authors of this study, the apparent associations between insulin lispro and foetal outcomes should be investigated further, not least because these findings are not supported by other reviews and studies, the majority of which have reported similar perinatal outcomes in RHI‐treated and insulin lispro‐treated women 67, 80, 81, 83.
Expert opinion
To date, there is no evidence for a benefit of CSII in women with pre‐gestational diabetes. However, the key objective in the management of diabetes in women with a planned or existing pregnancy is to reach the lowest possible level of HbA1c (thereby minimizing the risk of malformations and foetal complications related to hyperglycaemia [e.g. large‐for‐gestational‐age]) whilst minimizing the risk of hypoglycaemia (thereby improving maternal QOL and reducing the risk of the baby being small‐for‐gestational‐age). Among pregnant women and their caregivers, CSII is often preferred to MDI because of its greater flexibility for fine‐tuning insulin delivery and because it does not require MDI, which can become particularly onerous if the number of insulin injections increases because of a need to administer correction doses. CSII also allows caregivers to bypass existing uncertainty about the risk of long‐acting insulin analogues during pregnancy. It is acknowledged, however, that several case series involving the use of long‐acting insulin analogues during pregnancy have shown no evidence of increased maternal or neonatal risk. Similarly, observational reports that have compared the use of insulin lispro or insulin aspart with that of RHI during pregnancy have revealed no evidence of deleterious outcomes when RAIAs are used in either MDI or CSII regimens. In addition, because of their pharmacodynamic profiles, RAIAs are easier to manage than RHI in terms of reducing post‐meal glucose spikes without increasing late post‐meal hypoglycaemia. Overall, therefore, although no specific studies have assessed either the benefits of CSII or the choice of RAIAs during pregnancy in women with diabetes, accumulated experience supports the easier and more comfortable management of glucose control with CSII using RAIAs in this situation. Moreover, because there is evidence to show that CSII using RAIAs is more effective than MDI and/or RHI in achieving optimal glucose control outside pregnancy, there is no rationale for prohibiting the use of CSII and/or RAIAs before or during pregnancy. Conversely, there is no rationale for prescribing CSII and/or RAIAs to a woman with diabetes during pregnancy when she is reluctant to receive these regimens.
Issues related to the use of continuous subcutaneous insulin infusion in clinical practice
A number of issues surround the effective use of insulin pumps (summarized in Table 4) 11, 12, 38, 39, 40, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100. These include frequency of infusion set and injection site change, under‐delivery or over‐delivery of insulin and dermatological complications. Of these, under‐delivery of insulin and its consequences (hyperglycaemia and diabetic ketoacidosis 89) are potentially the most serious. The speed with which hyperglycaemia can develop in CSII‐treated patients is related to the small size of the subcutaneous insulin depot and the short duration of action of RAIAs 101. Occlusion within the pump or infusion set is one of the possible causes for insulin delivery failure in CSII‐treated patients. Controlled studies that examine the factors that may contribute to occlusion are therefore directly relevant to clinical practice.
Table 4.
Issues related to the use of CSII in clinical practice
| Issue/potential cause | Potential consequences | Incidence/prevalence | Preventive measures |
|---|---|---|---|
| Insufficiently frequent infusion set change | |||
| Non‐adherence to product registration information | Poor glycaemic control; increased day‐to‐day variability in plasma glucose levels; increased risk of infusion site problems (e.g. itching, bruising, swelling, pain) 84, 85, 86 | Incidence in vivo not documented | Change infusion set more frequently (every 48–72 h) 85, 86, 100 |
| Failure of insulin delivery | |||
| Occlusion within infusion set | Hyperglycaemia ± DKA | Incidence varies among studies; ≤1 clog/blockage per 4 weeks is typical 11 | Change catheter at least every 72 h 40, 87; use an RAIA with a relatively low risk of occlusion (insulin aspart or insulin lispro preferred to insulin glulisine) 39, 40, 87 |
| Excessive catheter wear time 40, 87 | — | Incidence in vivo not documented | — |
| Formation of insulin complexes (fibrils)/isoelectric precipitation of insulin (related to physicochemical stability of RAIA used) 38 | — | Incidence in vivo not documented | — |
| Pump failure/malfunction | Hyperglycaemia ± DKA | Malfunction rate: 25 per 100 pump‐years with complete failure in 44% of cases of malfunction (prospective study involving 640 consecutive new pumps manufactured by four different companies between 2001 and 2007 92 | Use pump with evidence of superior reliability |
| Planned pump disconnection | Glycaemic control unaffected by disconnection to change infusion set but for longer disconnection (30 min), blood glucose increases by approximately 1 mg/dL (0.056 mmol/L) for each minute of infusion interruption 88 | Incidence in vivo not documented | Minimize time for which pump disconnected |
| Unplanned pump disconnection | Hyperglycaemia ± DKA 89 | Incidence in vivo not documented | Use new‐generation, tubing‐free patch pumps |
| Hydrostatic effects | 25% decrease in insulin delivery when insulin pumped 80–100 cm upwards (bench‐based study with programmed infusion rate of 1 U/h; effect most evident at low basal infusion rates; effect demonstrated using insulin aspart) 90 | Incidence in vivo not documented | Wear pump horizontally and use short tubing or use new‐generation, tubing‐free patch pumps 99 |
| Over‐delivery of insulin | |||
| Reduced atmospheric pressure (as during air travel) leads to formation of bubbles/expansion of existing bubbles and displacement of insulin from cartridge (effect demonstrated using insulin aspart) 91 | Hypoglycaemia 91 | Incidence in vivo not documented | Use new‐generation, tubing‐free patch pumps |
| Hydrostatic effects | 23% increase in insulin delivery when insulin pumped 80–100 cm downwards (bench‐based study with programmed infusion rate of 1 U/h; effect most evident at low basal infusion rates; effect demonstrated using insulin aspart) 90 | Incidence in vivo not documented | Wear pump horizontally and use short tubing or use new‐generation, tubing‐free patch pumps 93, 99 |
| Dermatological complications | |||
| Allergy to bandage adhesive 93, 94 | Reduced patient comfort | Incidence in vivo not documented | Change adhesive type |
| Irritation, inflammation, and infection at infusion site 93, 94 | Reduced patient comfort; increased likelihood of CSII discontinuation 95 | Adults | Change infusion set at least every 48–72 h 85; consider changing catheter type (adverse event rate is influenced by catheter model) 96 |
| ●Irritation and/or infection: 0.06 to 12 per patient per year 12 | |||
| Children/adolescents (n = 50, insulin aspart or lispro only, cross‐sectional study) | |||
| ●Scar <3 mm diameter: 94% | |||
| ●Erythema not associated with nodules: 66% | |||
| ●Subcutaneous nodules: 62% 95 | |||
| Lipodystrophy (lipoatrophy and lipohypertrophy) 97 | Unpredictable insulin absorption; poor glycaemic control 97, 98 | Adults and children (n = 430, cross‐sectional study) | Rotate injection sites 97, 98 |
| ●Lipohypertrophy: 64% 98 | |||
| Children/adolescents (n = 50, insulin aspart or lispro only, cross‐sectional study) | |||
| ●Lipohypertrophy: 42% 95 |
CSII, continuous subcutaneous insulin infusion; DKA, diabetic ketoacidosis; RAIA, rapid‐acting insulin analogue
Occlusions
The incidence of occlusion is affected by catheter wear time 87 and by the specific RAIA used. For example, in a laboratory‐based study that involved pumps administering insulin aspart, insulin lispro or insulin glulisine for 5 days, occlusions were rare and independent of choice of RAIA in the first 72 h of the infusion 87. However, after this time, the incidence of occlusion increased substantially, particularly with insulin glulisine 87. These data emphasize the importance of changing catheters at least every 72 h, irrespective of the insulin used 40, 87. Differences among RAIAs in propensity for occlusion may be related to the physicochemical stability of the individual molecule 38. Occlusions may be caused by the formation of insulin complexes (fibrils) within the infusion set or by isoelectric precipitation of insulin 38. In vitro studies have shown that RAIAs differ in their propensity for fibril formation and in the pH at which they precipitate 38, 102. However, when attempting to compare the risk of occlusion of different RAIAs, the most relevant data are those generated in vivo. In a 13‐week, prospective, randomized, open‐label, crossover controlled clinical trial involving patients on CSII therapy, van Bon et al. 39 found that the monthly rate of unexplained hyperglycaemic episodes and/or perceived catheter set occlusions was significantly higher in insulin glulisine‐treated patients than in those who were receiving either insulin lispro or insulin aspart 39. A recent systematic review that aimed to determine the stability and performance of RAIAs used for CSII also concluded that the risk of occlusion is higher with insulin glulisine than with insulins aspart or lispro in vitro, and in vivo when the infusion duration extends beyond approximately 3 days 40. The conclusions made by van Bon et al. 39 relating to the primary endpoint of their study (no significant difference among insulin glulisine, insulin lispro and insulin aspart in CSII use with respect to the incidence of at least one unexplained hyperglycaemic event and/or perceived catheter set occlusion) have attracted some criticism 103. Other factors that had a significant effect on the risk of unexplained hyperglycaemia/infusion set occlusion in the study by van Bon et al. 39 included the time interval between infusion set changes (9% decrease in risk for each 6 h increase in interval; p < 0.0001) and body mass index (6% decrease for each 1 kg/m2 increase; p = 0.046). The authors concluded that these relationships occurred because patients who experienced few problems changed their catheters less frequently and because more obese patients had higher insulin infusion rates 39.
Expert opinion
For patients receiving CSII, it is desirable to change the infusion set every 48 to 72 h and to rotate the injection site. Indeed, it is of the utmost importance to regularly change the infusion set within the period recommended for the product(s) being used to achieve stable and optimal glycaemic control and to minimize the risk of adverse events. Regardless of the RAIA used, however, it is important that further improvements are made in the quality of catheters and infusion sets.
Quality of life and pharmacoeconomics
The potential impact on a patient's QOL and the financial implications of the mode of insulin administration form an important part of any decision about whether to embark on CSII 5. From a health service standpoint, these two factors are inextricably intertwined because a relatively expensive intervention may be deemed cost‐effective if it is associated with improved QOL.
Quality of life
Health‐related quality of life (HRQOL) in CSII‐treated patients with diabetes has been assessed using both generic and diabetes‐specific instruments 104. Although some HRQOL studies/instruments have shown that the QOL of patients using CSII is equivalent 105, 106 or inferior 106, 107 to that of those using MDI, the majority has found that CSII (when used with a variety of insulins) is associated with significant improvements in HRQOL/patient satisfaction 5, 19, 20, 104, 108, 109, 110, 111. This improved HRQOL/patient satisfaction has been demonstrated in adults with T1DM 5, 19, 20, 110 and T2DM 109, adolescents with T1DM 5, 19, 108, 111, children with T1DM 5, 19, 111 and parents of children with diabetes 5. In one study involving 577 adolescents, the favourable impact of CSII, when compared with MDI, on treatment satisfaction, perceived clinical efficacy, and reduction in treatment interference with daily activities was most evident in those patients with lower overall HRQOL 108, suggesting that the benefits of CSII may be most apparent in this group. CSII is also commonly associated with a lower fear of hypoglycaemia than MDI, a benefit that extends to both adult patients and caregivers of children with diabetes 5, 104.
Some of the most interesting – and most relevant – work relates to patients who have used CSII from the onset of diabetes. To date, such studies involve only paediatric patients. Skogsberg et al. 112 followed 72 children and adolescents with T1DM who were randomized to treatment with MDI (neutral protamine Hagedorn and insulin aspart) or CSII (insulin aspart) at the time of disease onset. Treatment satisfaction was significantly higher in the CSII group at all screening visits (1, 6, 12 and 24 months) (Figure 3) 112. Kordonouri et al. 113 also found a significant beneficial effect of CSII (with or without a CGM) on QOL in paediatric patients and the wellbeing of their primary caregivers when this therapy was instituted at diagnosis.
Figure 3.
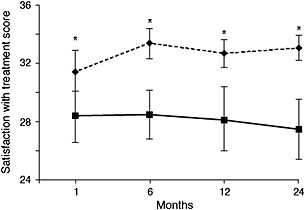
Treatment satisfaction in children treated with CSII (insulin aspart) or MDI (neutral protamine Hagedorn and insulin aspart) over a 2‐year period 112. Treatment satisfaction was measured using the Diabetes Treatment Satisfaction Questionnaire (DTSQ) in children with T1DM treated with CSII (n = 34; dotted lines, diamonds) or MDI (n = 38; straight lines, squares). The results are presented as mean ± 95% CI. CI, confidence interval; CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injections; T1DM, type 1 diabetes mellitus * p < 0.05
Expert opinion
Ultimately, the improved QOL experienced by patients and their families in association with the use of CSII may turn out to be the strongest argument in favour of this method of insulin administration. In addition, the flexibility that is inherent in CSII and the ability to administer multiple bolus doses of rapid‐acting insulin painlessly allow insulin therapy to be tailored to diverse challenges such as those posed by neonates with diabetes, patients attending kindergarten, fussy eaters and adults with shift‐work. Most diabetologists agree that psychosocial issues are extremely important in determining the long‐term prognosis of patients with diabetes. Thus, improved QOL may eventually be a critical factor in improving psychosocial outcomes and may outweigh the short‐term cost increases associated with CSII.
Pharmacoeconomics
The major costs associated with CSII are pump purchase/depreciation and consumables 5. Other costs include those associated with pump servicing and training in the use of CSII 5. These costs would be reduced by improvements in insulin stability within the pump and pump lifespan 5, 114, 115.
All current health‐economic comparisons of CSII and MDI have concluded that CSII (when used with a variety of insulins) is more expensive than MDI 5, 116, 117, 118. Recent data from a European country (United Kingdom), published in 2010, estimate the annual cost of consumables (e.g. tubing, cannulae) and pump (assuming a 4‐year lifespan) at about £1800–£2000 and £430–£720, respectively 5. This is approximately £1700 more than the annual cost of analogue‐based MDI 5. However, assuming that CSII is associated with a reduction in HbA1c of 1.2% compared with MDI, this mode of insulin delivery is deemed cost‐effective 5. Other groups have reached similar conclusions 117, citing incremental cost‐effectiveness ratios (ICERs) per quality‐adjusted life‐year gained with CSII compared with MDI of £25 648 (~€37 036) (2003 costs) 116 and £37 712 (~€46 235; 2008 publication) 32. These ICERs are sensitive to the patient's baseline HbA1c and to assumptions made regarding improvements in glycaemic control and adverse event rates. Such calculations underlie the recommendation of the National Institute for Health and Care Excellence (NICE) that, in adult patients with T1DM, CSII is a treatment option only in those whose HbA1c has remained ≥8.5% (69.4 mmol/mol) on MDI therapy despite a high level of care 32.
Expert opinion
A growing number of national and international registries that involve the collation of real‐world patient data are becoming available. In the future, these registries may allow more accurate pharmacoeconomic analyses. Currently, such analyses are driven by potential reductions in HbA1c or rates of severe hypoglycaemia requiring hospitalization. Management of diabetes is moving rapidly into the area of sensor‐augmented therapy and will eventually encompass closed‐loop approaches to insulin therapy (discussed in the next section). In conjunction with these advances, healthcare funders should be encouraged to support the delivery of well‐run registries. Such data will be of the utmost importance in the allocation of funds for modern insulins, pumps and technology, advances that may only become financially advantageous several years after implementation.
Recent advances in continuous subcutaneous insulin infusion technology
Conventional CSII delivers insulin at pre‐selected basal rates, with additional user‐initiated bolus infusions. In contrast, a closed‐loop insulin delivery system or ‘artificial pancreas’ delivers insulin at a rate that varies depending on the patient's interstitial glucose level 119. This is achieved by integrating the insulin pump with a CGM and a control algorithm that modulates the pump's insulin delivery rate in real time in response to the prevailing level of glycaemia (Figure 4) 119. Closed‐loop systems have demonstrated a number of advantages over sensor‐augmented CSII 119, and clinical trials involving their use are slowly moving from controlled laboratory conditions to transitional and home settings 72, 120, 121 as the technology and sophistication of the devices become more advanced. The complexity (and resultant cost) of such pumps may not be necessary for patients with T2DM, for whom simple disposable patch pumps may be more appropriate 24.
Figure 4.
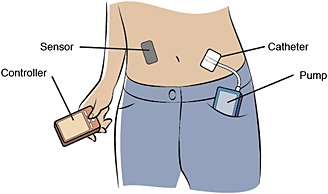
Components of a closed‐loop insulin delivery system 119. Interstitial glucose levels, measured by the sensor, are transmitted to the controller, which contains a control algorithm. This modulates the pump's insulin infusion rate in real time. All communication is wireless
Technological advances in continuous glucose monitors
The vast majority of glucose assays are electrochemical, utilizing glucose oxidase 122. Currently, CGMs predominantly use subcutaneously implanted amperometric sensors that provide continuous monitoring of glycaemia 122. The sensor is connected to a small on‐skin potentiostat, which may transmit information wirelessly to the computer within the pump or to a dedicated handheld device 122. Research is currently underway to develop a subcutaneously implanted glucose monitoring and transmitting device that includes an integrated miniature power source 122. Several initiatives in the development of fully implantable long‐term glucose sensors have also been reported 123.
The clinical effectiveness of real‐time continuous glucose monitoring was recently determined in a meta‐analysis, which showed that this technology is associated with significantly lower HbA1c and exposure to hypoglycaemia than self‐monitoring of blood glucose in patients with T1DM 124. The improvement in glycaemic control was greatest in patients who used the sensors most frequently and those who had the highest HbA1c at baseline 124.
Technological advances in pump technology
In addition to the development of bolus calculators and options for modulation of bolus shape 125, recent pump advances have allowed the incorporation of algorithms that suspend insulin delivery when glucose levels reach a certain threshold 72, 126 or when hypoglycaemia is predicted 127. Both strategies are effective in preventing overall and nocturnal hypoglycaemia 72, 73, 127. For example, a recent randomized study involving 247 patients showed that incorporation of the threshold‐suspend feature into sensor‐augmented pump therapy was associated with a significant 32% reduction in the incidence of nocturnal hypoglycaemic events 72.
Technological advances in closed‐loop systems
Closed‐loop systems that increase insulin delivery in a glucose‐responsive fashion are yet to be made commercially available. These artificial pancreas systems combine an external pump and sensor with a variable insulin infusion rate algorithm 128. The majority of current systems use a model predictive control (MPC) algorithm that links insulin delivery and meal ingestion to glucose excursions 119. Although capable of achieving near‐normal glucose concentrations overnight, artificial pancreas systems are challenged by meals and exercise. These problems can be ameliorated by announcing meals and exercise to the control algorithms using a bolus wizard, as is standard with conventional CSII, or by the addition of small manual pre‐meal ‘priming’ boluses 128. Initially studied under controlled laboratory conditions, closed‐loop systems are now undergoing trials in transitional and home settings 129.
Closed‐loop studies performed under controlled laboratory conditions
When used in adults with T1DM in an overnight study that compared closed‐loop delivery of insulin aspart (MPC‐based algorithm) with conventional CSII with insulin aspart, the closed‐loop approach was associated with significant improvements in glycaemic control and hypoglycaemic risk 130. In a more recent study involving both adults and adolescents receiving insulin lispro, Breton et al. 131 compared the performance of open‐loop CSII with that of two artificial pancreas systems, one designed to prevent extreme glucose excursions (standard control to range), the other to achieve near normoglycaemia (enhanced control to range [eCTR]). These options in tuning insulin delivery were enabled by the multi‐modular concept of MPC 132. Both artificial pancreas systems were superior to open‐loop CSII during a 22‐h hospitalization period that included meals and exercise. However, eCTR provided the most physiological control, with patients in near normoglycaemia 97% of the time and in tight glycaemic control 77% of the time 131. Closed‐loop systems have been similarly successful in paediatric patients. When compared with standard CSII in a population of children and adolescents, manual closed‐loop insulin delivery (infusion rate calculated by algorithm, delivery rate adjusted by nurse) was associated with reduced risk of nocturnal hypoglycaemia 133, and in children <7 years, a closed‐loop approach reduced the severity of overnight hyperglycaemia (versus standard CSII) without increasing hypoglycaemic risk 134. Insulin aspart was used in both these studies. Closed‐loop systems have also been used in pregnant women. Murphy et al. 135 investigated the performance of closed‐loop insulin delivery of insulin aspart using an MPC algorithm over a 24‐h period during both early (12–16 weeks) and late (28–32 weeks) gestation in ten women. The algorithm performed well at both time‐points, suggesting that overnight closed‐loop insulin delivery has the potential to be used safely during pregnancy.
Closed‐loop studies performed under transitional or home conditions
The success of laboratory studies has led researchers to investigate the performance of artificial pancreas systems in transitional and home situations. In an overnight study conducted in the setting of a diabetes camp, adolescent patients had less nocturnal hypoglycaemia and better overnight glycaemic control when treated with an artificial pancreas system than with a sensor‐augmented pump (Figure 5) 74. In a longer study (42 h), Kovatchev et al. 120 evaluated the performance of a wearable artificial pancreas system that used a smart phone as a closed‐loop control platform in patients who were maintained as outpatients or in a hybrid hospital–hotel setting. Patients operated the system via the user interface for the first 14 h of the study and then in closed‐loop mode for the remaining 28 h. System communication functioned correctly for 98% of the study duration, demonstrating that integration of smart phones into closed‐loop systems is worthy of further investigation 120.
Figure 5.
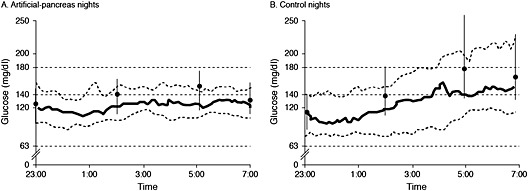
Nocturnal glycaemic control in adolescent patients with type 1 diabetes mellitus using an ‘artificial pancreas’ or a sensor‐augmented pump 74. Sensor glucose profiles obtained with an artificial pancreas (A) and a sensor‐augmented insulin pump (B). The type(s) of insulin used in this study was not specified. Solid black line and adjacent dashed lines: median glucose level and interquartile range. Circles and vertical lines: median capillary glucose measurements and interquartile range. The horizontal dashed lines indicate blood glucose levels of 180 mg/dL (10.0 mmol/L) and 63 mg/dL (3.5 mmol/L [the threshold for hypoglycaemia])
Continuous use of artificial pancreas systems in the patient's home is the ultimate goal. The feasibility of this approach has been demonstrated by Nimri et al. 121, who compared the safety and efficacy of closed‐loop and sensor‐augmented pump therapy in 15 patients over a total of 8 nights (crossover design). The closed‐loop system was associated with significant improvements in various measures of hypoglycaemia, and the study demonstrated the feasibility, safety and efficacy of the closed‐loop system under home conditions.
Bi‐hormonal systems
The insulin‐only systems described earlier may ultimately be superseded by bi‐hormonal devices that infuse both insulin and glucagon. Such systems have been tested under both closed‐loop 136 and semi‐automated hybrid control conditions 137, 138. The latter, which involves the addition of a partial meal‐priming insulin bolus, has achieved excellent glycaemic control when using insulin lispro, with minimal hypoglycaemia in trials of 51 h duration that involved the consumption of high‐carbohydrate meals and exercise 137. A closed‐loop system with full meal bolus using insulin aspart has demonstrated similar performance, albeit over a shorter time period 138.
Pump security
Recognition that closed‐loop systems are vulnerable to unauthorized access (‘hacking’) has led professional groups to publish design standards and governmental agencies to enact device regulations 139. As a result, hardware and software manufacturers have developed new products 139 that should improve the security of insulin pumps in general and of artificial pancreas systems in particular.
Expert opinion
Improved glycaemic control and reduced risk of hypoglycaemia appear achievable with closed‐loop systems that combine commercial sensors, pumps and RAIAs. Results from early transitional and short home studies are promising, demonstrating progression towards real‐life clinical use in T1DM. Closed‐loop systems continue to be limited by factors such as glucose sensor reliability, delays in insulin absorption and instrumentation/human factors, but these are gradually being addressed, underpinned by the development of new‐generation systems including, for example, glucagon co‐delivery. Performance over longer periods is being assessed in home studies that focus mainly, but not entirely, on overnight use.
Conclusions
This review has focused on the use of CSII in patients with diabetes and on the use of RAIAs as the insulins of choice in this delivery system. CSII has a number of advantages over MDI for patients with T1DM and, as a result, is now widely recommended for use in both adult and paediatric populations. These recommendations are based on widespread evidence of superior glycaemic control, lower daily insulin requirements, reduced risk of hypoglycaemia and better HRQOL/patient satisfaction. The reduction in hypoglycaemic risk has been demonstrated most consistently in paediatric patients. Among the three RAIAs currently available, substantial evidence has been provided regarding the use of insulin aspart and insulin lispro. Pharmacoeconomic data consistently show that CSII is more expensive than MDI. However, the efficacy, safety and HRQOL advantages associated with this technology have led many groups and organizations to conclude that CSII is cost‐effective in selected patient groups. Certainly, individual human factors are of utmost importance in the pathway leading to CSII use, and the search for a more flexible lifestyle has been a driving force toward CSII use in recent years 140.
The success of conventional ‘open‐loop’ CSII has led to the development of closed‐loop insulin delivery systems, which have now reached the stage of small‐scale, short‐duration clinical trials in patients' homes. If these systems fulfil their early promise and prove able to provide safe and effective glycaemic control in complex situations, they will transform the lives of people with insulin‐dependent diabetes.
Author contributions
All authors made substantial contributions to the conception and design of this article, and to the critical appraisal of content, and have read and approved the final version.
Conflicts of interest
Paolo Pozzilli reports having received speaker's honoraria from Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Merck, Novartis, Sanofi, and Takeda, and having received research grants to University Campus Bio‐Medico, Rome, from Boehringer Ingelheim, Eli Lilly, Medtronic, Novartis, Roche Diagnostics, and Sanofi.
Tadej Battelino reports having received research grants to the University of Ljubljana from GluSense, Medtronic, Novo Nordisk, and Sanofi, having served on advisory boards for Bayer, Eli Lilly, Medtronic, Novo Nordisk, and Sanofi, and having served on speaker panels for Bayer, Eli Lilly, Novo Nordisk, Medtronic, and Sanofi. He was supported in part by the Slovene National Research Agency ARRS grants P3‐0343 and J3‐4116.
Thomas Danne reports having received speaker's honoraria from A. Menarini Diagnostics and Dexcom, and having served as a consultant to Boehringer Ingelheim, Eli Lilly, Johnson & Johnson, Medtronic, Novo Nordisk, Roche, and Sanofi.
Roman Hovorka reports having received speaker's honoraria from B. Braun, Eli Lilly, Medtronic, Minimed Lifescan, and Novo Nordisk, having served on advisory panels for Animas, Eli Lilly, Medtronic, and Minimed, having received license fees from B. Braun and Beckton Dickinson, and having been involved in patent applications and having served as a consultant to B. Braun, Beckton Dickinson, Profil, and Sanofi.
Przemyslawa Jarosz‐Chobot reports having received speaker's honoraria from Bayer, Boehringer Ingelheim, Dexcom, Eli Lilly, Medtronic, Novo Nordisk, Roche, and Sanofi and having received consultant's honoraria from Eli Lilly, Medtronic, Roche, and Sanofi.
Eric Renard reports having served as a consultant/advisor to A. Menarini Diagnostics, Abbott, Cellnovo, Dexcom, Eli Lilly, Johnson & Johnson (Animas, LifeScan), Medtronic, Novo Nordisk, Roche Diagnostics, and Sanofi‐Aventis, and having received research grant or material support from Abbott, Dexcom, Insulet, and Roche Diagnostics.
Acknowledgements
The authors thank Drs Raffaella Gentilella and Adam Stefanski (Eli Lilly and Company) for their role in the preparation of this article, and Dr Janet Douglas and Caroline Spencer (Rx Communications, Mold, UK) for providing editorial assistance.
This work was supported by Eli Lilly and Company.
Pozzilli, P. , Battelino, T. , Danne, T. , Hovorka, R. , Jarosz‐Chobot, P. , and Renard, E. (2016) Continuous subcutaneous insulin infusion in diabetes: patient populations, safety, efficacy, and pharmacoeconomics. Diabetes Metab Res Rev, 32: 21–39. doi: 10.1002/dmrr.2653.
References
- 1. Nathan DM, Cleary PA, Backlund JY, et al. Diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study research group intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DCCT/EDIC Research Group , de Boer IH, Sun W, et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 2011; 365: 2366–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anonymous, the Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 4. Anonymous, the UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 5. Cummins E, Royle P, Snaith A, et al. Clinical effectiveness and cost‐effectiveness of continuous subcutaneous insulin infusion for diabetes: systematic review and economic evaluation. Health Technol Assess 2010; 14: iii–iv xi‐xvi, 1–181. [DOI] [PubMed] [Google Scholar]
- 6. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 7. Misso ML, Egberts KJ, Page M, O'Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev 2010; 1 CD005103. doi:10.1002/14651858.CD005103.pub2. [DOI] [PubMed] [Google Scholar]
- 8. Pickup JC. Insulin‐pump therapy for type 1 diabetes mellitus. N Engl J Med 2012; 366: 1616–1624. [DOI] [PubMed] [Google Scholar]
- 9. Thabit H, Hovorka R. Closed‐loop insulin delivery in type 1 diabetes. Endocrinol Metab Clin North Am 2012; 41: 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bergenstal RM, Tamborlane WV, Ahmann A, et al. STAR 3 study group. Effectiveness of sensor‐augmented insulin‐pump therapy in type 1 diabetes. N Engl J Med 2010; 363: 311–320. [DOI] [PubMed] [Google Scholar]
- 11. Bode B, Weinstein R, Bell D, et al. Comparison of insulin Aspart with buffered regular insulin and insulin lispro in continuous subcutaneous insulin infusion: a randomized study in type 1 diabetes. Diabetes Care 2002; 25: 439–444. [DOI] [PubMed] [Google Scholar]
- 12. Phillip M, Battelino T, Rodriguez H, et al. Use of insulin pump therapy in the pediatric age‐group: consensus statement from the European Society for Paediatric Endocrinology, the Lawson Wilkins Pediatric Endocrine Society, and the International Society for Pediatric and Adolescent Diabetes, endorsed by the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2007; 30: 1653–1662. [DOI] [PubMed] [Google Scholar]
- 13. Bergholtz CH, Olsen B, Johannesen J. Insulin pump therapy in children and teenagers. Ugeskr Laeger 2009; 171: 1913–1918[Article in Danish]. [PubMed] [Google Scholar]
- 14. Renard E. Insulin pump use in Europe. Diabetes Technol Ther 2010; 12(Suppl 1): S29–S32. [DOI] [PubMed] [Google Scholar]
- 15. Sulmont V, Lassmann‐Vague V, Guerci B, et al. French pediatric PUMP group. Access of children and adolescents with type 1 diabetes to insulin pump therapy has greatly increased in France since 2001. Diabetes Metab 2011; 37: 59–63. [DOI] [PubMed] [Google Scholar]
- 16. Pickup J. Insulin pumps. Int J Clin Pract Suppl 2011; (170): 16–19. [DOI] [PubMed] [Google Scholar]
- 17. Pickup JD. The evidence base for diabetes technology: appropriate and inappropriate meta‐analysis. J Diabetes Sci Technol 2013; 7: 1567–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fatourechi MM, Kudva YC, Murad MH, Elamin MB, Tabini CC, Montori VM. Clinical review: Hypoglycemia with intensive insulin therapy: a systematic review and meta‐analyses of randomized trials of continuous subcutaneous insulin infusion versus multiple daily injections. J Clin Endocrinol Metab 2009; 94: 729–740. [DOI] [PubMed] [Google Scholar]
- 19. Golden SH, Brown T, Yeh HC, et al Methods for insulin delivery and glucose monitoring: comparative effectiveness. Comparative Effectiveness Review No. 57. (Prepared by Johns Hopkins University Evidence‐based Practice Center under Contract No. 290‐2007‐10061‐I.) AHRQ Publication No. 12‐EHC036‐EF. Rockville, MD: Agency for Healthcare Research and Quality 2012. Available at: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0047870/. Accessed May 16, 2014.
- 20. Medical Advisory Secretariat . Continuous subcutaneous insulin infusion pumps for type 1 and type 2 adult diabetic populations: an evidence‐based analysis. Ontario Health Technology Assessment 2009; Series 9(20). [PMC free article] [PubMed]
- 21. Jeitler K, Horvath K, Berghold A, et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta‐analysis. Diabetologia 2008; 51: 941–951. [DOI] [PubMed] [Google Scholar]
- 22. Johnson SL, McEwen LN, Newton CA, et al. The impact of continuous subcutaneous insulin infusion and multiple daily injections of insulin on glucose variability in older adults with type 2 diabetes. J Diabetes Complications 2011; 25: 211–215. [DOI] [PubMed] [Google Scholar]
- 23. Berthe E, Lireux B, Coffin C, et al. Effectiveness of intensive insulin therapy by multiple daily injections and continuous subcutaneous infusion: a comparison study in type 2 diabetes with conventional insulin regimen failure. Horm Metab Res 2007; 39: 224–229. [DOI] [PubMed] [Google Scholar]
- 24. Bode BW. Insulin pump use in type 2 diabetes. Diabetes Technol Ther 2010; 12(Suppl 1): S17–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reznik Y, Cohen O, Aronson R, et al. OpT2mise Study Group. Insulin pump treatment compared with multiple daily injections for treatment of type 2 diabetes (OpT2mise): a randomised open‐label controlled trial. Lancet 2014; 384: 1265–1272. [DOI] [PubMed] [Google Scholar]
- 26. Pickup J, Mattock M, Kerry S. Glycaemic control with continuous subcutaneous insulin infusion compared with intensive insulin injections in patients with type 1 diabetes: meta‐analysis of randomised controlled trials. BMJ 2002; 324(7339): 705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N. Clinical and cost‐effectiveness of continuous subcutaneous insulin infusion for diabetes. Health Technol Assess 2004; 8: iii 1–171. [DOI] [PubMed] [Google Scholar]
- 28. Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta‐analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med 2008; 25: 765–774. [DOI] [PubMed] [Google Scholar]
- 29. Pickup JC, Kidd J, Burmiston S, Yemane N. Determinants of glycaemic control in type 1 diabetes during intensified therapy with multiple daily insulin injections or continuous subcutaneous insulin infusion: Importance of blood glucose variability. Diabetes Metab Res Rev 2006; 22: 232–237. [DOI] [PubMed] [Google Scholar]
- 30. Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care 2004; 27: 2590–2596. [DOI] [PubMed] [Google Scholar]
- 31. Jacobsen IB, Henriksen JE, Hother‐Nielsen O, Vach W, Beck‐Nielsen H. Evidence‐based insulin treatment in type 1 diabetes mellitus. Diabetes Res Clin Pract 2009; 86: 1–10. [DOI] [PubMed] [Google Scholar]
- 32. National Institute for Health and Care Excellence . Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus, TA151, 2008. Available at: http://guidance.nice.org.uk/TA151/Guidance/pdf/English. Accessed May 16, 2014.
- 33. Lassmann‐Vague V, Clavel S, Guerci B, et al. Société francophone du diabète (ex ALFEDIAM). When to treat a diabetic patient using an external insulin pump. Expert consensus. Société francophone du diabète (ex ALFEDIAM) 2009. Diabetes Metab 2010; 36: 79–85. [DOI] [PubMed] [Google Scholar]
- 34. International Diabetes Federation/International Society for Pediatric and Adolescent Diabetes . Global IDF/ISPAD Guideline for Diabetes in Childhood and Adolescence 2014. Available at: http://www.ispad.org/resource‐type/idfispad‐2011‐global‐guideline‐diabetes‐childhood‐and‐adolescence. Accessed April 14, 2014.
- 35. National Institute for Health and Care Excellence . Diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period (CG63) 2008. Available at: http://www.nice.org.uk/guidance/index.jsp?action=download&o=41320. Accessed May 16, 2014. [PubMed]
- 36. Colquitt J, Royle P, Waugh N. Are analogue insulins better than soluble in continuous subcutaneous insulin infusion? Results of a meta‐analysis. Diabet Med 2003; 20: 863–866. [DOI] [PubMed] [Google Scholar]
- 37. Siebenhofer A, Plank J, Berghold A, et al. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database Syst Rev 2006; 2 CD003287. [DOI] [PubMed] [Google Scholar]
- 38. Bode BW. Comparison of pharmacokinetic properties, physicochemical stability, and pump compatibility of 3 rapid‐acting insulin analogues‐aspart, lispro, and glulisine. Endocr Pract 2011; 17: 271–280. [DOI] [PubMed] [Google Scholar]
- 39. van Bon AC, Bode BW, Sert‐Langeron C, DeVries JH, Charpentier G. Insulin glulisine compared to insulin aspart and to insulin lispro administered by continuous subcutaneous insulin infusion in patients with type 1 diabetes: a randomized controlled trial. Diabetes Technol Ther 2011; 13: 607–614. [DOI] [PubMed] [Google Scholar]
- 40. Kerr D, Wizemann E, Senstius J, Zacho M, Ampudia‐Blasco FJ. Stability and performance of rapid‐acting insulin analogs used for continuous subcutaneous insulin infusion: a systematic review. J Diabetes Sci Technol 2013; 7: 1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Di Bartolo P, Pellicano F, Scaramuzza A, et al. Better postprandial glucose stability during continuous subcutaneous infusion with insulin aspart compared with insulin lispro in patients with type 1 diabetes. Diabetes Technol Ther 2008; 10: 495–498. [DOI] [PubMed] [Google Scholar]
- 42. Tamborlane WV, Renard E, Wadwa RP, et al Glycemic control after 6 days of insulin pump reservoir use in type 1 diabetes: Results of double‐blind and open‐label cross‐over trials of insulin lispro and insulin aspart. J Diabetes 2014. DOI: 10.1111/1753-0407.12162. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 43. Johnson SR, Cooper MN, Jones TW, Davis EA. Long‐term outcome of insulin pump therapy in children with type 1 diabetes assessed in a large population‐based case‐control study. Diabetologia 2013; 56: 2392–2400. [DOI] [PubMed] [Google Scholar]
- 44. Dovc K, Telic SS, Lusa L, et al. Improved metabolic control in pediatric patients with type 1 diabetes: a nationwide prospective 12‐year time trends analysis. Diabetes Technol Ther 2014; 16: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sulmont V, Souchon PF, Gouillard‐Darnaud C, et al. Metabolic control in children with diabetes mellitus who are younger than 6 years at diagnosis: continuous subcutaneous insulin infusion as a first line treatment? J Pediatr 2010; 157: 103–107. [DOI] [PubMed] [Google Scholar]
- 46. Cooper MN, O'Connell SM, Davis EA, Jones TW. A population‐based study of risk factors for severe hypoglycaemia in a contemporary cohort of childhood‐onset type 1 diabetes. Diabetologia 2013; 56: 2164–2170. [DOI] [PubMed] [Google Scholar]
- 47. Tubiana‐Rufi N. Insulin pump therapy in neonatal diabetes. Endocr Dev 2007; 12: 67–74. [DOI] [PubMed] [Google Scholar]
- 48. Beardsall K, Pesterfield CL, Acerini CL. Neonatal diabetes and insulin pump therapy. Arch Dis Child Fetal Neonatal Ed 2011; 96: F223–F224. [DOI] [PubMed] [Google Scholar]
- 49. Mianowska B, Szadkowska A, Fendler W, Mlynarski W. Use of lispro insulin diluted with normal saline to 10 U/ml in an insulin pump: case report. J Diabetes Sci Technol 2012; 6: 1238–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Seereiner S, Neeser K, Weber C, et al. Attitudes towards insulin pump therapy among adolescents and young people. Diabetes Technol Ther 2010; 12: 89–94. [DOI] [PubMed] [Google Scholar]
- 51. Olinder AL, Nyhlin KT, Smide B. Reasons for missed meal‐time insulin boluses from the perspective of adolescents using insulin pumps: ‘lost focus’. Pediatr Diabetes 2011; 12(4 Pt 2): 402–409. [DOI] [PubMed] [Google Scholar]
- 52. O'Connell MA, Donath S, Cameron FJ. Poor adherence to integral daily tasks limits the efficacy of CSII in youth. Pediatr Diabetes 2011; 12: 556–559. [DOI] [PubMed] [Google Scholar]
- 53. Moreau F, Spizzo H, Bursztejn C, et al. Factitious self‐manipulation of the external insulin pump in adolescents with Type 1 diabetes. Diabet Med 2011; 28: 623–624. [DOI] [PubMed] [Google Scholar]
- 54. Burdick J, Chase HP, Slover RH, et al. Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy. Pediatrics 2004; 113: e221–e224. [DOI] [PubMed] [Google Scholar]
- 55. Diabetes Research in Children Network (DirecNet) Study Group , Tsalikian E, Kollman C, et al. Prevention of hypoglycemia during exercise in children with type 1 diabetes by suspending basal insulin. Diabetes Care 2006; 29: 2200–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Admon G, Weinstein Y, Falk B, et al. Exercise with and without an insulin pump among children and adolescents with type 1 diabetes mellitus. Pediatrics 2005; 116: e348–e355. [DOI] [PubMed] [Google Scholar]
- 57. Delvecchio M, Zecchino C, Salzano G, et al. Effects of moderate‐severe exercise on blood glucose in Type 1 diabetic adolescents treated with insulin pump or glargine insulin. Endocrinol Invest 2009; 32: 519–524. [DOI] [PubMed] [Google Scholar]
- 58. Low KG, Massa L, Lehman D, Olshan JS. Insulin pump use in young adolescents with type 1 diabetes: a descriptive study. Pediatr Diabetes 2005; 6: 22–31. [DOI] [PubMed] [Google Scholar]
- 59. Yardley JE, Iscoe KE, Sigal RJ, Kenny GP, Perkins BA, Riddell MC. Insulin pump therapy is associated with less post‐exercise hyperglycemia than multiple daily injections: an observational study of physically active type 1 diabetes patients. Diabetes Technol Ther 2013; 15: 84–88. [DOI] [PubMed] [Google Scholar]
- 60. Churchill JN, Ruppe RL, Smaldone A. Use of continuous insulin infusion pumps in young children with type 1 diabetes: a systematic review. J Pediatr Health Care 2009; 23: 173–179. [DOI] [PubMed] [Google Scholar]
- 61. Pańkowska E, Błazik M, Dziechciarz P, Szypowska A, Szajewska H. Continuous subcutaneous insulin infusion vs. multiple daily injections in children with type 1 diabetes: a systematic review and meta‐analysis of randomized control trials. Pediatr Diabetes 2009; 10: 52–58. [DOI] [PubMed] [Google Scholar]
- 62. Nimri R, Weintrob N, Benzaquen H, Ofan R, Fayman G, Phillip M. Insulin pump therapy in youth with type 1 diabetes: a retrospective paired study. Pediatrics 2006; 117: 2126–2131. [DOI] [PubMed] [Google Scholar]
- 63. Tubiana‐Rufi N, Coutant R, Bloch J, et al. Special management of insulin lispro in continuous subcutaneous insulin infusion in young diabetic children: a randomized cross‐over study. Horm Res 2004; 62: 265–271. [DOI] [PubMed] [Google Scholar]
- 64. Weinzimer SA, Ternand C, Howard C, et al. Insulin Aspart Pediatric Pump Study Group. A randomized trial comparing continuous subcutaneous insulin infusion of insulin aspart versus insulin lispro in children and adolescents with type 1 diabetes. Diabetes Care 2008; 31: 210–215. [DOI] [PubMed] [Google Scholar]
- 65. Apidra SmPC 2013. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000557/human_med_000648.jsp&mid=WC0b01ac058001d124. Accessed May 16, 2014.
- 66. NovoRapid SmPC 2014. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000258/human_med_000935.jsp&mid=WC0b01ac058001d124. Accessed May 16, 2014.
- 67. Humalog SmPC 2011. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000088/human_med_000820.jsp&mid=WC0b01ac058001d124. Accessed May 16, 2014.
- 68. Kaiserman K, Rodriguez H, Stephenson A, Wolka L, Fahrbach JL. Continuous subcutaneous infusion of insulin lispro in children and adolescents with type 1 diabetes mellitus. Endocr Pract 2012; 18: 418–424. [DOI] [PubMed] [Google Scholar]
- 69. Beck RW, Tamborlane WV, Bergenstal RM, et al. T1D Exchange Clinic Network. The T1D Exchange clinic registry. J Clin Endocrinol Metab 2012; 97: 4383–4389. [DOI] [PubMed] [Google Scholar]
- 70. Holterhus PM, Bokelmann J, Riepe F, et al. German/Austrian DPV‐Initiative and the German Pediatric CSII Working Group. Predicting the optimal basal insulin infusion pattern in children and adolescents on insulin pumps. Diabetes Care 2013; 36: 1507–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bratina N, Battelino T. Insulin pumps and continuous glucose monitoring (CGM) in preschool and school‐age children: how schools can integrate technology. Pediatr Endocrinol Rev 2010; 7(Suppl 3): 417–421. [PubMed] [Google Scholar]
- 72. Bergenstal RM, Klonoff DC, Garg SK, et al. ASPIRE In‐Home Study Group. Threshold‐based insulin‐pump interruption for reduction of hypoglycemia. N Engl J Med 2013; 369: 224–232. [DOI] [PubMed] [Google Scholar]
- 73. Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor‐augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA 2013; 310: 1240–1247. [DOI] [PubMed] [Google Scholar]
- 74. Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med 2013; 368: 824–833. [DOI] [PubMed] [Google Scholar]
- 75. Golden SH, Sapir T. Methods for insulin delivery and glucose monitoring in diabetes: summary of a comparative effectiveness review. J Manag Care Pharm 2012; 18(6 Suppl): S1–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Farrar D, Tuffnell DJ, West J. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database Syst Rev 2007; 3 CD005542. [DOI] [PubMed] [Google Scholar]
- 77. Mukhopadhyay A, Farrell T, Fraser RB, Ola B. Continuous subcutaneous insulin infusion vs intensive conventional insulin therapy in pregnant diabetic women: A systematic review and metaanalysis of randomized, controlled trials. Am J Obstet Gynecol 2007; 197: 447–456. [DOI] [PubMed] [Google Scholar]
- 78. Kallas‐Koeman MM, Kong JM, Klinke JA, et al. Insulin pump use in pregnancy is associated with lower HbA1c without increasing the rate of severe hypoglycaemia or diabetic ketoacidosis in women with type 1 diabetes. Diabetologia 2014; 57: 681–689. [DOI] [PubMed] [Google Scholar]
- 79. Durnwald CP, Landon MB. Insulin analogues in the management of the pregnancy complicated by diabetes mellitus. Curr Diab Rep 2011; 11: 28–34. [DOI] [PubMed] [Google Scholar]
- 80. Edson EJ, Bracco OL, Vambergue A, Koivisto V. Managing diabetes during pregnancy with insulin lispro: a safe alternative to human insulin. Endocr Pract 2010; 16: 1020–1027. [DOI] [PubMed] [Google Scholar]
- 81. García‐Domínguez M, Herranz L, Hillman N, et al. Use of insulin lispro during pregnancy in women with pregestational diabetes mellitus. Med Clin(Barc) 2011; 137: 581–586. [DOI] [PubMed] [Google Scholar]
- 82. Chico A, Saigi I, García‐Patterson A, et al. Glycemic control and perinatal outcomes of pregnancies complicated by type 1 diabetes: influence of continuous subcutaneous insulin infusion and lispro insulin. Diabetes Technol Ther 2010; 12: 937–945. [DOI] [PubMed] [Google Scholar]
- 83. González Blanco C, Chyico Ballesteros A, Gich Saladich I, Corcoy Pla R. Glycemic control and pregnancy outcomes in women with type 1 diabetes mellitus using lispro versus regular insulin: a systematic review and meta‐analysis. Diabetes Technol Ther 2011; 13: 907–911. [DOI] [PubMed] [Google Scholar]
- 84. Luijf YM, Arnolds S, Avogaro A, et al. On behalf of the Ap@home consortium. Patch pump versus conventional pump: postprandial glycemic excursions and the influence of wear time. Diabetes Technol Ther 2013; 15: 575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schmid V, Hohberg C, Borchert M, Forst T, Pfützner A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy‐trouble starts on day 3. J Diabetes Sci Technol 2010; 4: 976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Thethi TK, Rao A, Kawji H, et al. Consequences of delayed pump infusion line change in patients with type 1 diabetes mellitus treated with continuous subcutaneous insulin infusion. J Diabetes Complications 2010; 24: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kerr D, Morton J, Whately‐Smith C, Everett J, Begley JP. Laboratory‐based non‐clinical comparison of occlusion rates using three rapid‐acting insulin analogs in continuous subcutaneous insulin infusion catheters using low flow rates. J Diabetes Sci Technol 2008; 2: 450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zisser H. Quantifying the impact of a short‐interval interruption of insulin‐pump infusion sets on glycemic excursions. Diabetes Care 2008; 31: 238–239. [DOI] [PubMed] [Google Scholar]
- 89. Realsen J, Goettle H, Chase HP. Morbidity and mortality of diabetic ketoacidosis with and without insulin pump care. Diabetes Technol Ther 2012; 14: 1149–1154. [DOI] [PubMed] [Google Scholar]
- 90. Zisser HC, Bevier W, Dassau E, Jovanovic L. Siphon effects on continuous subcutaneous insulin infusion pump delivery performance. J Diabetes Sci Technol 2010; 4: 98–10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. King BR, Goss PW, Paterson MA, Crock PA, Anderson DG. Changes in altitude cause unintended insulin delivery from insulin pumps: mechanisms and implications. Diabetes Care 2011; 34: 1932–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Guilhem I, Balkau B, Lecordier F, et al. Insulin pump failures are still frequent: a prospective study over 6 years from 2001 to 2007. Diabetologia 2009; 52: 2662–2664. [DOI] [PubMed] [Google Scholar]
- 93. Heinemann L, Krinelke L. Insulin infusion set: the Achilles heel of continuous subcutaneous insulin infusion. J Diabetes Sci Technol 2012; 6: 954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Riveline JP, Jollois FX, Messaoudi N, et al. Groupe Pompe Sud‐Francilien Insulin‐pump use in everyday practice: data from an exhaustive regional registry in France. Diabetes Metab 2008; 34: 132–139. [DOI] [PubMed] [Google Scholar]
- 95. Conwell LS, Pope E, Artiles AM, Mohanta A, Daneman A, Daneman D. Dermatological complications of continuous subcutaneous insulin infusion in children and adolescents. J Pediatr 2008; 152: 622–628. [DOI] [PubMed] [Google Scholar]
- 96. Renard E, Guerci B, Leguerrier AM, Boizel R, Accu‐Chek FlexLink Study Group . Lower rate of initial failures and reduced occurrence of adverse events with a new catheter model for continuous subcutaneous insulin infusion: prospective, two‐period, observational, multicenter study. Diabetes Technol Ther 2010; 12: 769–773. [DOI] [PubMed] [Google Scholar]
- 97. Richardson T, Kerr D. Skin‐related complications of insulin therapy: epidemiology and emerging management strategies. Am J Clin Dermatol 2003; 4: 661–667. [DOI] [PubMed] [Google Scholar]
- 98. Blanco M, Hernández MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin‐injecting patients with diabetes. Diabetes Metab 2013; 39: 445–453. [DOI] [PubMed] [Google Scholar]
- 99. Thabit H. Insulin pump therapy in type 1 diabetes‐merging technology with diabetes care. Am Med J 2012; 3: 93–99. [Google Scholar]
- 100. Medtronic Minimed Inc 2013. Safety first. Available at: http://www.medtronic‐diabetes.co.uk/product‐information/infusion‐sets/safety‐first.html. Accessed May 16, 2014.
- 101. Bruttomesso D, Costa S, Baritussio A. Continuous subcutaneous insulin infusion (CSII) 30 years later: still the best option for insulin therapy. Diabetes Metab Res Rev 2009; Rev 25: 99–111. [DOI] [PubMed] [Google Scholar]
- 102. Woods RJ, Alarcón J, McVey E, Pettis RJ. Intrinsic fibrillation of fast‐acting insulin analogs. J Diabetes Sci Technol 2012; 6: 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jovanovič L. Letter written in response to van bon et al. "Insulin glulisine compared to insulin aspart and to insulin lispro administered by continuous subcutaneous insulin infusion in patients with type 1 diabetes: a randomized controlled trial". Diabetes Technol Ther 2011; 13: 869–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rubin RR, Peyrot M, STAR 3 Study Group . Health‐related quality of life and treatment satisfaction in the Sensor‐Augmented Pump Therapy for A1C Reduction 3 (STAR 3) trial. Diabetes Technol Ther 2012; 14: 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Frøisland DH, Graue M, Markestad T, Skrivarhaug T, Wentzel‐Larsen T, Dahl‐Jørgensen K. Health related quality of life among Norwegian children and adolescents with type 1 diabetes on intensive insulin treatment: a population based study. Acta Paediatr 2013; 102: 889–895. [DOI] [PubMed] [Google Scholar]
- 106. Lozano‐Serrano M, García‐Seco JA, García‐Seco F, et al. Satisfaction and quality of life evaluation in patients with type 1 diabetes mellitus treated using continuous subcutaneous insulin infusion compared with multiple daily injections. Enferm Clin 2013; 23: 96–102[Article in Spanish]. [DOI] [PubMed] [Google Scholar]
- 107. Matziou V, Tsoumakas K, Vlahioti E, et al. Factors influencing the quality of life of young patients with diabetes. J Diabetes 2011; 3: 82–90. [DOI] [PubMed] [Google Scholar]
- 108. Cherubini V, Gesuita R, Bonfanti R, et al., VIPKIDS Study Group . Health‐related quality of life and treatment preferences in adolescents with type 1 diabetes. The VIPKIDS study. Acta Diabetol 2014; 51: 43–51. [DOI] [PubMed] [Google Scholar]
- 109. Rubin RR, Peyrot M, Chen X, Frias JP. Patient‐reported outcomes from a 16‐week open‐label, multicenter study of insulin pump therapy in patients with type 2 diabetes mellitus. Diabetes Technol Ther 2010; 12: 901–906. [DOI] [PubMed] [Google Scholar]
- 110. Marmolin ES, Brødsgaard J, Gjessing HJ, et al. Better treatment of outpatients with type 1 diabetes after introduction of continuous subcutaneous insulin infusion. Dan Med J 2012; 59: A4445. [PubMed] [Google Scholar]
- 111. Lukács A, Kiss‐Tóth E, Varga B, Soós A, Takác P, Barkai L. Benefits of continuous subcutaneous insulin infusion on quality of life. Int J Technol Assess Health Care 2013; 29: 48–52. [DOI] [PubMed] [Google Scholar]
- 112. Skogsberg L, Fors H, Hanas R, Chaplin JE, Lindman E, Skogsberg J. Improved treatment satisfaction but no difference in metabolic control when using continuous subcutaneous insulin infusion vs. multiple daily injections in children at onset of type 1 diabetes mellitus. Pediatr Diabetes 2008; 9: 472–479. [DOI] [PubMed] [Google Scholar]
- 113. Kordonouri O, Pankowska E, Rami B, et al. Sensor‐augmented pump therapy from the diagnosis of childhood type 1 diabetes: results of the Paediatric Onset Study (ONSET) after 12 months of treatment. Diabetologia 2010; 53: 2487–2495. [DOI] [PubMed] [Google Scholar]
- 114. Sharrow SD, Glass LC, Dobbins MA. 14‐day in vitro chemical stability of insulin lispro in the MiniMed paradigm pump. Diabetes Technol Ther 2012; 14: 264–270. [DOI] [PubMed] [Google Scholar]
- 115. Weiss RC, van Amerongen D, Bazalo G, Aagren M, Bouchard JR. Economic benefits of improved insulin stability in insulin pumps. Manag Care 2011; 20: 42–47. [PubMed] [Google Scholar]
- 116. Roze S, Valentine WJ, Zakrzewska KE, Palmer AJ. Health‐economic comparison of continuous subcutaneous insulin infusion with multiple daily injection for the treatment of type 1 diabetes in the UK. Diabet Med 2005; 22: 1239–1245. [DOI] [PubMed] [Google Scholar]
- 117. Nørgaard K, Sohlberg A, Goodall G. Cost‐effectiveness of continuous subcutaneous insulin infusion therapy for type 1 diabetes. Ugeskr Laeger 2010; 172: 2020–2025[Article in Danish]. [PubMed] [Google Scholar]
- 118. Bolli GB, Kerr D, Thomas R, et al. Comparison of a multiple daily insulin injection regimen (basal once‐daily glargine plus mealtime lispro) and continuous subcutaneous insulin infusion (lispro) in type 1 diabetes: a randomized open parallel multicenter study. Diabetes Care 2009; 32: 1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hovorka R. Closed‐loop insulin delivery: from bench to clinical practice. Nat Rev Endocrinol 2011; 7: 385–395. [DOI] [PubMed] [Google Scholar]
- 120. Kovatchev BP, Renard E, Cobelli C, et al. Feasibility of outpatient fully integrated closed‐loop control: first studies of wearable artificial pancreas. Diabetes Care 2013; 36: 1851–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Nimri R, Muller I, Atlas E, et al. Night glucose control with MD‐Logic artificial pancreas in home setting: a single blind, randomized crossover trial‐interim analysis. Pediatr Diabetes 2013; 15: 91–99. [DOI] [PubMed] [Google Scholar]
- 122. Heller A, Feldman B. Electrochemical glucose sensors and their applications in diabetes management. Chem Rev 2008; 108: 2482–2505. [DOI] [PubMed] [Google Scholar]
- 123. Renard E. Implantable continuous glucose sensors. Curr Diabetes Rev 2008; 4: 169–174. [DOI] [PubMed] [Google Scholar]
- 124. Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta‐analysis of randomised controlled trials using individual patient data. BMJ 2011; 343: d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zisser H, Robinson L, Bevier W, et al. Bolus calculator: a review of four "smart" insulin pumps. Diabetes Technol Ther 2008; 10: 441–444. [DOI] [PubMed] [Google Scholar]
- 126. Garg S, Brazg RL, Bailey TS, et al. Reduction in duration of hypoglycemia by automatic suspension of insulin delivery: the in‐clinic ASPIRE study. Diabetes Technol Ther 2012; 14: 205–209. [DOI] [PubMed] [Google Scholar]
- 127. Buckingham B, Chase HP, Dassau E, et al. Prevention of nocturnal hypoglycemia using predictive alarm algorithms and insulin pump suspension. Diabetes Care 2010; 33: 1013–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed‐loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care 2008; 31: 934–939. [DOI] [PubMed] [Google Scholar]
- 129. Renard E, Cobelli C, Kovatchev BP. Closed loop developments to improve glucose control at home. Diabetes Res Clin Pract 2013; 102: 79–85. [DOI] [PubMed] [Google Scholar]
- 130. Hovorka R, Kumareswaran K, Harris J, et al. Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ 2011; 342: d1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Breton M, Farret A, Bruttomesso D, et al. International Artificial Pancreas Study Group. Fully integrated artificial pancreas in type 1 diabetes: modular closed‐loop glucose control maintains near normoglycemia. Diabetes 2012; 61: 2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Patek SD, Magni L, Dassau E, et al. International artificial pancreas (iAP) study group. Modular closed‐loop control of diabetes. IEEE Trans Biomed Eng 2012; 59: 2986–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hovorka R, Allen JM, Elleri D, et al. Manual closed‐loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet 2010; 375: 743–751. [DOI] [PubMed] [Google Scholar]
- 134. Dauber A, Corcia L, Safer J, Agus MS, Einis S, Steil GM. Closed‐loop insulin therapy improves glycemic control in children aged <7 years: a randomized controlled trial. Diabetes Care 2013; 36: 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Murphy HR, Elleri D, Allen JM, et al. Closed‐loop insulin delivery during pregnancy complicated by type 1 diabetes. Diabetes Care 2011; 34: 406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. El‐Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed‐loop artificial pancreas for type 1 diabetes. Sci Transl Med 2010; 2 27ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Russell SJ, El‐Khatib FH, Nathan DM, Magyar KL, Jiang J, Damiano ER. Blood glucose control in type 1 diabetes with a bihormonal bionic endocrine pancreas. Diabetes Care 2012; 35: 2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Haidar A, Legault L, Dallaire M, et al. Glucose‐responsive insulin and glucagon delivery (dual‐hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. CMAJ 2013; 185: 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Frenger P. Hacking medical devices a review ‐ biomed 2013. Biomed Sci Instrum 2013; 49: 40–47. [PubMed] [Google Scholar]
- 140. Hammond P, Liebl A, Grunder S. International survey of insulin pump users: impact of continuous subcutaneous insulin infusion therapy on glucose control and quality of life. Prim Care Diabetes 2007; 1: 143–146. [DOI] [PubMed] [Google Scholar]


