Abstract
Aim
To assess time to insulin initiation among patients with type 2 diabetes mellitus (T2DM) treated with sitagliptin versus sulphonylurea as add‐on to metformin.
Methods
This retrospective cohort study used GE Centricity electronic medical records and included patients aged ≥18 years with continuous medical records and an initial prescription of sitagliptin or sulphonylurea (index date) with metformin for ≥90 days during 2006–2013. Sitagliptin and sulphonylurea users were matched 1 : 1 using propensity score matching, and differences in insulin initiation were assessed using Kaplan–Meier curves and Cox regression. We used conditional logistic regression to examine the likelihood of insulin use 1–6 years after the index date for each year.
Results
Propensity score matching produced 3864 matched pairs. Kaplan–Meier analysis showed that sitagliptin users had a lower risk of insulin initiation compared with sulphonylurea users (p = 0.003), with 26.6% of sitagliptin users initiating insulin versus 34.1% of sulphonylurea users over 6 years. This finding remained significant after adjusting for baseline characteristics (hazard ratio 0.76, 95% confidence interval 0.65–0.90). Conditional logistic regression analyses confirmed that sitagliptin users were less likely to initiate insulin compared with sulphonylurea users [odds ratios for years 1–6: 0.77, 0.79, 0.81, 0.57, 0.29 and 0.75, respectively (p < 0.05 for years 4 and 5)].
Conclusions
In this real‐world matched cohort study, patients with T2DM treated with sitagliptin had a significantly lower risk of insulin initiation compared with patients treated with sulphonylurea, both as add‐on to metformin.
Keywords: database research, DPP‐IV inhibitor, insulin therapy, sulphonylureas, type 2 diabetes
Introduction
Because of the progressive nature of type 2 diabetes mellitus (T2DM), there is a gradual decline in the effectiveness of oral antihyperglycaemic drugs (OADs) over time, reflecting an ongoing diminution in insulin secretory function 1. As a result, long‐term glycaemic control may be difficult to maintain with OADs, and many patients ultimately advance to insulin therapy 2, 3. Insulin, while effective, complicates the management of T2DM, leads to hypoglycaemia risk and weight gain, and increases a patient's overall treatment burden. A number of observational studies have shown that OADs vary in the rate of treatment failure and time to insulin initiation. This difference may reflect the mechanism of action of each individual drug class, which has varying effects on β‐cell function over time. For example, a retrospective study of patients with T2DM in the USA found that patients treated with sulphonylureas had a higher probability of progression to insulin compared with patients treated with metformin and/or thiazolidinedione, even after adjusting for patient demographics, comorbidities and propensity scores 4. Sulphonylurea therapy was also associated with earlier onset of insulin use versus metformin in a Canadian study 5, and a study from Germany showed that patients who began treatment with sulphonylureas had three times the risk of insulin initiation compared with those who started on metformin 6. In another study, conducted in several European countries, patients treated with sulphonylureas were significantly more likely to start insulin compared with patients treated with metformin 7.
As well as the type of OAD prescribed, the previous literature has described other factors associated with insulin initiation. These include patient demographics, such as younger age 8, 9, 10, 11, 12, 13, lower income 13 and non‐Hispanic race/ethnicity 9, as well as higher glycated haemoglobin (HbA1c) 8, 9, 10, 11, 12, 13, 14, 15, fasting plasma glucose 11, serum creatinine 14, duration of T2DM 9, 13 and presence of comorbidities and diabetes‐related complications, including depression, lipid disorders, micro‐ and macrovascular complications and overall chronic disease score 4, 7, 9, 11, 13. Patients who are treated with higher doses of OADs 16, initiate with more than one agent 13, have greater concomitant medication use 11, 16, and who have a history of hospitalization are also at increased risk 16. Furthermore, being treated by a specialist has been shown to be positively associated with insulin use 9, 13, 16. Some of these factors may affect treatment patterns more than the underlying biology of disease.
Before insulin treatment, clinical guidelines generally recommend metformin as first‐line therapy for patients with T2DM, but the ideal drug sequence after metformin failure remains unclear 17, 18. Both sulphonylureas and dipeptidyl peptidase‐4 (DPP‐4) inhibitors are commonly prescribed with metformin as dual therapy for the treatment of T2DM.
Although there have been several investigations regarding insulin initiation in patients treated with sulphonylureas versus insulin‐sensitizing drugs, there is a paucity of data assessing DPP‐4 inhibitors in this regard. We therefore decided to compare time to insulin initiation, as well as the likelihood of insulin initiation, between patients treated with sitagliptin and those treated with a sulphonylurea, both added to metformin.
Materials and Methods
Study Design and Subjects
This was a retrospective, matched cohort study using data from the GE Centricity electronic medical records (EMR) database. GE Centricity is used in the USA by >20 000 clinicians to manage 30 million patients in 49 states. More than 5000 providers also contribute data to the Medical Quality Improvement Consortium to create a research database. The Medical Quality Improvement Consortium represents a variety of practice types, including solo practitioners, community clinics, academic medical centres and large integrated delivery networks, and approximately two‐thirds of participating clinicians are primary care physicians. The de‐identified database contains longitudinal patient data, including demographic information, vital signs, laboratory results, medication list entries, prescription orders, diagnoses and problem lists. Compared with national averages, the GE EMR population is older, predominantly has commercial insurance, and has a higher proportion of patients residing in northeastern and mid‐western states 19. Approximately 10.2% of patients in the database have a diagnosis of diabetes, slightly more than the 9.3% recently reported in the general US population 20. At the time of the present analysis, data were available up to 31 August 2013.
The index period of the study was between 17 October 2006 (date of sitagliptin's approval by the US Food and Drug Administration) and 31 May 2013. The study population of interest comprised patients aged ≥18 years who initiated a sulphonylurea or sitagliptin as dual therapy with metformin during the index period. The index date for each patient was set as the date of the initiation of either a sulphonylurea or sitagliptin during the index period, whichever occurred earlier. The 1 year preceding the index date was defined as the baseline period. To be included, eligible patients had to have: continuous medical records during the baseline period and 90 days after the index date; used metformin on or within 1 year of the index date; continuously used a sulphonylurea plus metformin or sitagliptin plus metformin for at least 90 days after the index date; no history of type 1 diabetes mellitus any time before the index date; no history of gestational or secondary diabetes in the baseline period and any time after the index date; no history of non‐metformin OAD use in the baseline period; no prescription for other OADs in the first 90 days after the index date; and no missing days of supply for sulphonylureas, sitagliptin or metformin within the first 90 days after the index date. All drugs in the sulphonylurea class were considered, including chlorpropamide, tolazamide, tolbutamide, glipizide, glyburide, micronized glyburide and glimepiride. Patients included in the study were followed until insulin initiation or until the end of data collection.
Measures and Outcomes
Patients were divided into two cohorts determined by treatment exposure: the sulphonylurea cohort consisted of those who received a sulphonylurea plus metformin on the index date, while the sitagliptin cohort included patients who received sitagliptin plus metformin on the index date. The outcome measures were: (i) time to insulin initiation, and (ii) insulin initiation within the follow‐up periods 1, 2, 3, 4, 5 and 6 years from the index date.
To compare differences in cohort characteristics, a number of measures were assessed during the baseline period. Measures evaluated included: index year; patient demographics; prescribing physician specialty; health plan type; baseline metformin use; and days on metformin in the baseline period. Laboratory and clinical assessments included HbA1c, total cholesterol, LDL, HDL, triglycerides, serum creatinine, estimated glomerular filtration rate, alanine transaminase, aspartate transaminase, body mass index (BMI) and blood pressure. Other measures evaluated included previous diagnoses of hypoglycaemia, microvascular complications, macrovascular complications, kidney disease, liver disease, pancreatitis, gallstones, depression, hypertension, obesity (also defined as BMI ≥30 kg/m2), hyperlipidaemia and malignant neoplasms.
Statistical Analysis
Before any matching, baseline measures were analysed descriptively for all patients and compared between the sulphonylurea and sitagliptin cohorts, with between‐cohort differences assessed using Wilcoxon rank‐sum tests for continuous variables and chi‐squared tests for categorical variables. Descriptive analysis assessed the percent of patients initiating insulin during the study period, and the average time to insulin among patients who initiated in the follow‐up.
Propensity score matching was then used to mitigate underlying differences in covariates between the cohorts, and a multivariable logistic regression was used to build the propensity score model. The independent variables included in the logistic regression model were all of the aforementioned covariates, and propensity scores were estimated with the stratification of missing laboratory patterns to account for missing values. Upon the estimation of propensity scores, a greedy matching algorithm matched the sitagliptin cohort to the sulphonylurea cohort 1 : 1. Matching used a caliper size equal to 0.2 times the standard deviation of the estimated log propensity scores. To ensure pairs were precisely matched on important covariates, the following variables were exact‐matched: the duration of follow‐up period (in years), previous metformin use, age group (<65 vs ≥65 years), HbA1c group (<8% vs 8–9% vs ≥9%), prescribing physician (primary care physicians vs specialists) and missing patterns of the laboratory assessments 21, 22.
Using the matched‐pair sample, descriptive analysis reviewed any remaining differences in baseline characteristics of the sitagliptin cohort versus the sulphonylurea cohort. Post‐matching between‐cohort covariate differences were assessed using Wilcoxon rank sum tests for continuous variables and chi‐squared tests for categorical variables, as well as standard differences 23.
Kaplan–Meier analysis was then used to estimate time to insulin initiation in the sitagliptin cohort versus the sulphonylurea cohort. To account for pairing, between‐cohort differences were assessed using partial likelihood ratio tests. Next, the number and proportion of patients who initiated insulin within 1, 2, 3, 4, 5 and 6 years from the index date was estimated, and between‐cohort differences were calculated using McNemar's tests. Only patients with sufficient follow‐up (i.e. continuous recording) over each different follow‐up period were included in each Kaplan–Meier analysis.
Next, multivariable Cox proportional hazard regression analysis was used to quantify the relative risk of insulin initiation in the sitagliptin cohort versus the sulphonylurea cohort, controlling for covariates and stratified by matched pairs. The relative risk was quantified using hazard ratios (HRs), where a value of <1 indicated that sitagliptin was associated with a lower risk of insulin initiation compared with sulphonylurea. Next, conditional logistic regression was used to analyse insulin initiation separately within 1, 2, 3, 4, 5 and 6 years, with the relative risk quantified using odds ratios (ORs). In all multivariable analyses, 95% confidence intervals (CIs) and p values were evaluated using Wald's statistics.
To explore the effect of baseline HbA1c on insulin use, subgroup analyses repeated all analyses among patients with baseline HbA1c <9 and ≥9%. All analyses were conducted in sas version 9 using a threshold of 5% for all tests of statistical significance.
Results
Sample Selection and Characteristics
A total of 528 902 patients had at least one prescription for a sulphonylurea or sitagliptin during the index period (Table S1). After applying all inclusion and exclusion criteria, 20 529 patients remained in the sample, of whom 14 425 (70.3%) initiated dual therapy with a sulphonylurea plus metformin and 6104 (29.7%) initiated dual therapy with sitagliptin plus metformin. Table 1 shows the baseline patient characteristics before and after propensity score matching. In the overall, unmatched sample, 52% of patients were male, the mean age was 58 years, and average HbA1c was 8.3%. Compared with the sulphonylurea cohort, the sitagliptin cohort had significantly lower HbA1c levels (sitagliptin: 7.9% vs sulphonylurea: 8.5%; p < 0.01), more days on metformin during baseline (224.8 vs 205.6 days; p < 0.01) and a lower incidence of comorbidities.
Table 1.
Baseline patient characteristics before and after propensity score matching
| Patient Characteristic | Pre‐matching | Post‐matching | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sulphonylurea | Sitagliptin | p | Sulphonylurea | Sitagliptin | Stand. diff. | p | |||||
| (N = 14 425) | (N = 6104) | (N = 3864) | (N = 3864) | ||||||||
| n | % | n | % | n | % | n | % | ||||
| Male | 7504 | 52.0 | 3074 | 50.4 | 0.029 | 1945 | 50 | 1947 | 50.4 | −0.001 | 0.964 |
| Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | ||||
| Index age, years | 58.0 | 12.5 | 57.4 | 11.8 | 0.001 | 57.4 | 11.7 | 57.3 | 11.7 | 0.010 | 0.596 |
| n | % | n | % | n | % | n | % | ||||
| Race | |||||||||||
| White | 8714 | 60.4 | 3951 | 64.7 | <0.0001 | 2502 | 64.8 | 2491 | 64.5 | 0.006 | 0.979 |
| Black | 1504 | 10.4 | 577 | 9.5 | 379 | 9.8 | 390 | 10.1 | −0.010 | ||
| Hispanic | 510 | 3.5 | 126 | 2.1 | 88 | 2.3 | 79 | 2.0 | 0.014 | ||
| Asian | 290 | 2.0 | 94 | 1.5 | 57 | 1.5 | 60 | 1.6 | −0.006 | ||
| Other race | 278 | 1.9 | 150 | 2.5 | 93 | 2.4 | 95 | 2.5 | −0.004 | ||
| Unknown/undetermined | 3129 | 21.7 | 1206 | 19.8 | 745 | 19.3 | 749 | 19.4 | −0.003 | ||
| Residential location | |||||||||||
| Midwest | 3554 | 24.6 | 978 | 16.0 | <0.0001 | 720 | 18.6 | 698 | 18.1 | 0.014 | 0.577 |
| Northeast | 4072 | 28.2 | 2323 | 38.1 | 1293 | 33.5 | 1350 | 34.9 | −0.032 | ||
| South | 4661 | 32.3 | 2270 | 37.2 | 1463 | 37.9 | 1442 | 37.3 | 0.011 | ||
| West | 2138 | 14.8 | 533 | 8.7 | 388 | 10.0 | 374 | 9.7 | 0.011 | ||
| Prescribing physician specialty | |||||||||||
| Primary care | 11 498 | 79.7 | 4657 | 76.3 | <0.0001 | 3262 | 84.4 | 3262 | 84.4 | 0.000 | 1.000 |
| Specialist | 718 | 5.0 | 618 | 10.1 | 170 | 4.4 | 170 | 4.4 | 0.000 | ||
| Other | 2 | 0.0 | 1 | 0.0 | — | — | — | — | — | ||
| Unknown | 2207 | 15.3 | 828 | 13.6 | 432 | 11.2 | 432 | 11.2 | 0.000 | ||
| Private or commercial health plan | 3519 | 24.4 | 1974 | 32.3 | <0.0001 | 1151 | 29.8 | 1153 | 29.8 | −0.001 | 0.960 |
| Metformin use pre‐index date | 9232 | 64.0 | 4138 | 67.8 | <0.0001 | 2721 | 70.4 | 2721 | 70.4 | 0.000 | 1.000 |
| Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | ||||
| Days on metformin pre‐index date | 205.6 | 133.6 | 224.8 | 128.1 | <0.0001 | 222.1 | 130.5 | 221.8 | 127.9 | 0.002 | 0.994 |
| Diabetes‐related comorbidities | |||||||||||
| n | % | n | % | n | % | n | % | ||||
| Hypoglycaemia | 38 | 0.3 | 19 | 0.3 | 0.552 | 11 | 0.3 | 11 | 0.3 | 0.000 | 1.000 |
| Microvascular complications | 513 | 3.6 | 240 | 3.9 | 0.191 | 163 | 4.2 | 159 | 4.1 | 0.005 | 0.820 |
| Retinopathy | 86 | 0.6 | 42 | 0.7 | 0.445 | 36 | 0.9 | 31 | 0.8 | 0.016 | 0.540 |
| Neuropathy | 64 | 0.4 | 21 | 0.3 | 0.310 | 16 | 0.4 | 13 | 0.3 | 0.012 | 0.577 |
| Nephropathy | 384 | 2.7 | 184 | 3.0 | 0.159 | 120 | 3.1 | 118 | 3.1 | 0.003 | 0.895 |
| Macrovascular diseases | 762 | 5.3 | 295 | 4.8 | 0.183 | 199 | 5.2 | 183 | 4.7 | 0.019 | 0.401 |
| Stroke | 52 | 0.4 | 18 | 0.3 | 0.461 | 13 | 0.3 | 10 | 0.3 | 0.014 | 0.531 |
| Transient ischaemic attack | 48 | 0.3 | 10 | 0.2 | 0.037 | 7 | 0.2 | 5 | 0.1 | 0.010 | 0.563 |
| Congestive heart failure | 109 | 0.8 | 30 | 0.5 | 0.035 | 21 | 0.5 | 18 | 0.5 | 0.010 | 0.630 |
| Myocardial infarction | 49 | 0.3 | 9 | 0.1 | 0.018 | 7 | 0.2 | 7 | 0.2 | 0.000 | 1.000 |
| Ischaemic heart disease, including angina | 331 | 2.3 | 127 | 2.1 | 0.343 | 82 | 2.1 | 81 | 2.1 | 0.002 | 0.937 |
| Arrhythmia | 189 | 1.3 | 91 | 1.5 | 0.308 | 58 | 1.5 | 52 | 1.3 | 0.013 | 0.564 |
| Peripheral arterial diseases | 160 | 1.1 | 61 | 1.0 | 0.486 | 42 | 1.1 | 40 | 1.0 | 0.005 | 0.824 |
| Other kidney diseases | 352 | 2.4 | 144 | 2.4 | 0.729 | 103 | 2.7 | 92 | 2.4 | 0.019 | 0.425 |
| Liver disease | 94 | 0.7 | 62 | 1.0 | 0.006 | 36 | 0.9 | 35 | 0.9 | 0.003 | 0.905 |
| Pancreatitis | 12 | 0.1 | 8 | 0.1 | 0.315 | 3 | 0.1 | 7 | 0.2 | −0.032 | 0.206 |
| Gallstone | 34 | 0.2 | 18 | 0.3 | 0.441 | 11 | 0.3 | 12 | 0.3 | −0.005 | 0.835 |
| Depression | 58 | 0.4 | 20 | 0.3 | 0.428 | 14 | 0.4 | 15 | 0.4 | −0.004 | 0.852 |
| Hypertension | 1244 | 8.6 | 513 | 8.4 | 0.607 | 321 | 8.3 | 340 | 8.8 | −0.018 | 0.440 |
| Obesity | 607 | 4.2 | 231 | 3.8 | 0.161 | 170 | 4.4 | 150 | 3.9 | 0.026 | 0.253 |
| Hyperlipidaemia | 1452 | 10.1 | 649 | 10.6 | 0.221 | 392 | 10.1 | 401 | 10.4 | −0.008 | 0.736 |
| Malignant neoplasms | 312 | 2.2 | 140 | 2.3 | 0.560 | 84 | 2.2 | 93 | 2.4 | −0.016 | 0.494 |
| Laboratory assessments | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | |||
| HbA1c, % | 8.4 | 2.0 | 7.9 | 1.6 | <0.0001 | 8.0 | 1.6 | 8.0 | 1.7 | 0.005 | 0.439 |
| Body mass index, kg/m2 | 34.3 | 7.7 | 34.4 | 7.4 | 0.042 | 34.5 | 7.5 | 34.6 | 7.5 | −0.010 | 0.740 |
| Total cholesterol, mmol/l | 4.7 | 1.2 | 4.6 | 1.1 | <0.0001 | 178.0 | 44.5 | 177.7 | 43.9 | 0.006 | 0.708 |
| Diastolic blood pressure, mmHg | 78.2 | 10.4 | 77.9 | 9.4 | 0.178 | 77.9 | 9.9 | 78.0 | 9.4 | −0.007 | 0.599 |
| Systolic blood pressure, mmHg | 131.4 | 17.1 | 129.1 | 15.2 | <0.0001 | 129.3 | 15.4 | 129.4 | 15.0 | −0.012 | 0.329 |
| Serum creatinine, µmol/l | 81.2 | 23.8 | 79.5 | 20.4 | 0.006 | 0.9 | 0.3 | 0.9 | 0.2 | 0.017 | 0.982 |
| Alanine transaminase, U/l | 35.0 | 23.8 | 35.7 | 22.7 | 0.000 | 35.7 | 23.9 | 35.7 | 22.2 | 0.000 | 0.221 |
| Aspartate transaminase, U/l | 27.6 | 21.0 | 27.6 | 17.9 | 0.005 | 27.6 | 17.9 | 27.6 | 17.7 | 0.001 | 0.222 |
Statistical differences were assessed using Wilcoxon rank‐sum tests for continuous variables, and chi‐squared test for categorical variables. Post‐matching differences were also assessed using standard differences. All drugs in the sulphonylurea class were considered, including chlorpropamide, tolazamide, tolbutamide, glipizide, glyburide, micronized glyburide and glimepiride. For each laboratory test, the most recent test result during the pre‐index period was used. If a test was not conducted within this period, the test result was set to missing. Assessments with missing values for >30% of the sample are not shown. HbA1c, glycated haemoglobin; s.d., standard deviation; stand. diff., standard difference.
After applying propensity score matching, the matched‐pair sample consisted of 7728 patients (3864 sulphonylurea users and 3864 sitagliptin users). As expected, after propensity score matching, any differences between the sulphonylurea and sitagliptin cohorts were substantially reduced and no longer statistically significant (Table 1).
Time to Insulin Analysis
Table 2 outlines the average time to insulin of unmatched patients who initiated insulin during the follow‐up. In all, 9.9% of the sitagliptin cohort and 14.1% of the sulphonylurea cohort initiated insulin during the study period. Among the sitagliptin users who initiated, the mean time to insulin initiation was 1.94 years. Among the sulphonylurea users who initiated, the mean time to insulin initiation was modestly longer, at 2.07 years. Figure 1 shows the Kaplan–Meier results using the entire matched sample and showed that the sitagliptin cohort had a significantly lower risk of insulin initiation compared with the sulphonylurea cohort (p < 0.01). Six years after the index date, 26.6% of the sitagliptin cohort and 34.1% of the sulphonylurea cohort had initiated insulin therapy.
Table 2.
Time to insulin initiation among patients initiating insulin in the study period
| Variable | All unmatched patients | Patients with baseline HbA1c <9% | Patients with baseline HbA1c ≥9% | |||
|---|---|---|---|---|---|---|
| Sitagliptin | Sulphonylurea | Sitagliptin | Sulphonylurea | Sitagliptin | Sulphonylurea | |
| Number of observations | 6104 | 14 425 | 4281 | 8650 | 1031 | 3789 |
| Patients initiating insulin in the study period, n (%) | 607 (9.9) | 2033 (14.1) | 374 (8.7) | 1060 (12.3) | 155 (15.0) | 664 (17.5) |
| Mean (s.d.) time to insulin among patients initiating insulin in study period, years | 1.94 (1.36) | 2.07 (1.42) | 2.07 (1.40) | 2.18 (1.46) | 1.69 (1.29) | 1.90 (1.37) |
Seven hundred and ninety‐two patients in the sitagliptin cohort and 1986 in the sulphonylurea cohort had missing baseline HbA1c data. HbA1c, glycated haemoglobin; s.d., standard deviation.
Figure 1.
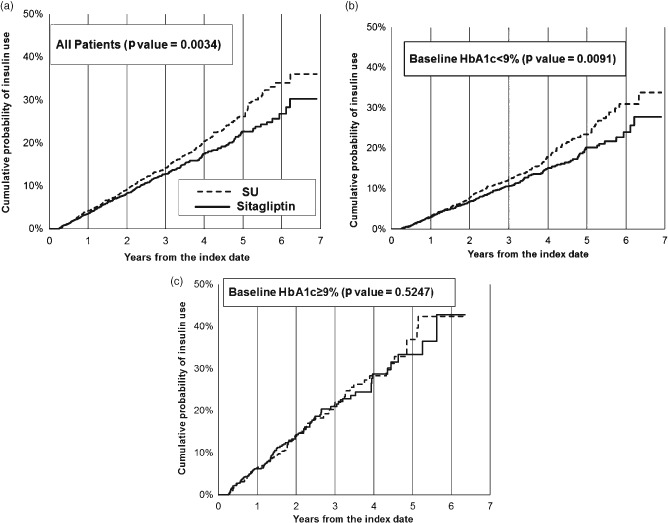
Kaplan–Meier cumulative distribution function for insulin initiation. (a) All patients; (b) Patients with baseline HbA1c < 9%; (c) Patients with baseline HbA1c >= 9%. SU, sulphonylurea; HbA1c, glycated haemoglobin.
After covariate adjustment, the risk of initiating insulin therapy was 24% lower for the sitagliptin cohort compared with the sulphonylurea cohort (HR 0.76; 95% CI 0.65–0.90; Table 3). The Cox model also showed that a 1% increase in HbA1c level was associated with a 20% increase in the risk of insulin initiation (HR 1.20; 95% CI 1.00–1.42). Other significant associations with insulin initiation included age and obesity.
Table 3.
Cox proportional hazards model: insulin initiation in matched sample
| Variable | HR | (95% CI) | p |
|---|---|---|---|
| Sitagliptin vs sulphonylurea | 0.761 | (0.646, 0.897) | 0.0011 |
| Male | 0.827 | (0.639, 1.072) | 0.1521 |
| White | 1.000 | (0.769, 1.300) | 0.9971 |
| Commercial or private health plan | 0.680 | (0.513, 0.901) | 0.0072 |
| Residential location | |||
| West | 1.000 | — | 0.6135 |
| Midwest | 0.917 | (0.592, 1.421) | |
| Northeast | 0.805 | (0.498, 1.299) | |
| South | 0.749 | (0.472, 1.188) | |
| Age group, years | |||
| 18–35 years | 1.000 | — | 0.0485 |
| 36–45 years | 1.133 | (0.628, 2.047) | |
| 46–55 years | 0.843 | (0.496, 1.435) | |
| 56–65 years | 0.675 | (0.387, 1.178) | |
| 66–75 years | 1.757 | (0.535, 5.766) | |
| ≥76 years | 1.894 | (0.551, 6.517) | |
| Index year | |||
| 2006 and 2007 | 1.000 | — | 0.4027 |
| 2008 | 0.794 | (0.310, 2.032) | |
| 2009 | 1.372 | (0.360, 5.220) | |
| 2010 | 1.015 | (0.210, 4.915) | |
| 2011 | 2.308 | (0.367, 14.528) | |
| 2012 and 2013 | 1.769 | (0.215, 14.561) | |
| Days of metformin use during baseline period | 0.996 | (0.983, 1.010) | 0.5858 |
| Baseline diabetes‐related complications | |||
| Microvascular complications | 0.975 | (0.545, 1.743) | 0.9313 |
| Macrovascular diseases | 1.569 | (0.961, 2.562) | 0.0719 |
| Other kidney diseases | 0.475 | (0.220, 1.026) | 0.0581 |
| Liver disease | 0.735 | (0.253, 2.137) | 0.5718 |
| Hypoglycaemia | 0.674 | (0.137, 3.309) | 0.6269 |
| Gallstone | 0.594 | (0.049, 7.261) | 0.6832 |
| Depression | 2.719 | (0.240, 30.750) | 0.4189 |
| Hypertension | 1.208 | (0.766, 1.906) | 0.4160 |
| Obesity | 1.361 | (1.017, 1.823) | 0.0384 |
| Hyperlipidaemia | 1.121 | (0.753, 1.667) | 0.5743 |
| Malignant neoplasms | 0.871 | (0.367, 2.066) | 0.7544 |
| Laboratory assessments | |||
| HbA1c, % | 1.195 | (1.004, 1.422) | 0.0446 |
| Total cholesterol, mmol/l | 1.000 | (0.796, 1.255) | 0.8544 |
| Diastolic blood pressure, mmHg | 0.991 | (0.976, 1.007) | 0.2817 |
| Systolic blood pressure, mmHg | 1.001 | (0.991, 1.011) | 0.7869 |
| Serum creatinine, µmol/l | 0.857 | (0.984, 1.009) | 0.5941 |
| Alanine transaminase, U/l | 0.999 | (0.991, 1.006) | 0.7235 |
| Aspartate transaminase, U/l | 0.998 | (0.990, 1.007) | 0.7325 |
95% CIs and p values assessed using Type 3 Wald's statistics. Mean imputation by index drug was applied to fill missing laboratory assessments. As a result, all patients (n = 7728) were used in the regression model. CI, confidence interval; HbA1c, glycated haemoglobin; s.d., standard deviation.
Insulin Initiation Analysis
Table 4 summarizes the results from six multivariable conditional logistic regression models that estimated the likelihood of insulin initiation from 1 to 6 years after index date. Conditional logistic regression analyses supported previous trends, and after adjusting for baseline characteristics, the sitagliptin cohort was significantly less likely to initiate insulin within 4 (OR 0.57, 95% CI 0.39–0.84) and 5 years (OR 0.29, 95% CI 0.11–0.75).
Table 4.
Summary of multivariable conditional logistic regressions: likelihood of insulin use in matched sample
| Outcome | Sample | N | OR | 95% CI | p |
|---|---|---|---|---|---|
| Insulin use within 1 year post‐index | At least 1‐year post‐index eligibility | 5744 | 0.770 | (0.54, 1.09) | 0.1412 |
| Insulin use within 2 years post‐index | At least 2‐years post‐index eligibility | 3686 | 0.790 | (0.60, 1.04) | 0.0951 |
| Insulin use within 3 years post‐index | At least 3‐years post‐index eligibility | 2214 | 0.813 | (0.60, 1.10) | 0.1746 |
| Insulin use within 4 years post‐index | At least 4‐years post‐index eligibility | 1286 | 0.570 | (0.39, 0.84) | 0.0048 |
| Insulin use within 5 years post‐index | At least 5‐years post‐index eligibility | 596 | 0.288 | (0.11, 0.75) | 0.0104 |
| Insulin use within 6 years post‐index | At least 6‐years post‐index eligibility | 140 | 0.750 | (0.24, 2.32) | 0.6175 |
95% CIs and p values assessed using Type 3 Wald's statistics. Conditional logistic regression was used to separately analyse insulin initiation within 1, 2, 3, 4, 5 and 6 years post‐index. An OR of <1 indicated a reduced risk of insulin initiation among sitagliptin users compared with sulphonylurea users. Covariates included in each model were index year, demographic information (age, gender and ethnicity), specialty of prescribing physician, health plan type, residential location, pre‐index metformin use, days on metformin use within 1 year before the index date and comorbid conditions. Laboratory assessments included HbA1c, body mass index, fasting blood glucose, LDL, HDL, triglycerides, total cholesterol, blood pressure, serum creatinine, estimated glomerular filtration rate, alanine transaminase and aspartate transaminase. CI, confidence interval; OR, odds ratio.
Subgroup Analysis
All analyses were conducted in subgroups defined by baseline HbA1c <9 and ≥9%. Among patients with HbA1c <9%, Kaplan–Meier analysis showed that the sitagliptin cohort had a lower risk of insulin initiation compared with the sulphonylurea cohort (p < 0.01; Figure 1). Six years after the index date, it was estimated that 24.0% of the sitagliptin cohort and 30.9% of the sulphonylurea cohort initiated insulin therapy. Multivariable analyses were consistent with these results. Among patients with HbA1c levels <9%, the sitagliptin cohort had 23% lower risk of initiating insulin compared with the sulphonylurea cohort (HR 0.77, 95% CI 0.62–0.95). By contrast, among patients with baseline HbA1c ≥9%, Kaplan–Meier analysis found no significant difference between the sulphonylurea and sitagliptin cohorts (p = 0.52; Figure 1). While results from the Cox model showed a lower risk of insulin initiation in the sitagliptin cohort compared with the sulphonylurea cohort, results were not statistically significant (HR 0.75; 95% CI 0.49–1.15). Conditional logistic regression models were also used to explore the association between insulin initiation and treatment cohort in each subgroup; however, because of a lack of discordant pairs, the models were not reliable (results not shown).
Discussion
The present analysis showed that patients with T2DM on dual therapy with sitagliptin plus metformin had a lower risk of insulin initiation compared with patients on dual therapy with sulphonylureas and metformin. This trend remained significant after controlling for patient characteristics, and was mainly driven by a lower risk among patients with a baseline HbA1c <9%.
Findings from the present study were strengthened by the use of propensity score matching, which mitigated underlying differences in baseline characteristics between the two groups, which may have otherwise influenced the outcomes. The use of propensity score matching has been used successfully by others to explore time to insulin among patients with T2DM who initiated metformin or sulphonylureas as monotherapy 15.
Results were in line with previous literature that found that patients treated with sulphonylureas had a higher likelihood of insulin use, and earlier insulin initiation, compared with patients treated with other OADs such as metformin 4, 5, 6, 7, 14, 16. Only one previous study has assessed the risk of insulin initiation among patients treated with DPP‐4 inhibitors specifically, reporting similar findings. Also using Cox regression, Kostev and Rathmann 7 found that, compared with patients treated with metformin, patients treated with DPP‐4 inhibitors had a lower risk of insulin initiation, while patients treated with sulphonylureas had a higher risk. Our data extend this observation with the use of propensity score matching, which adds robustness to the analysis.
Insulin is an invaluable treatment option to help achieve glycaemic control in patients with T2DM. Nonetheless, most patients and clinicians prefer delaying its initiation until necessary because it is associated with hypoglycaemia and weight gain, and is also viewed as increasing the complexity of care, including an increased need for self‐monitoring of blood glucose. Accordingly, there remains a need for effective oral agents that can maintain durable glycaemic control and delay insulin when metformin monotherapy is unable to attain or maintain glycaemic targets.
Sitagliptin, a DPP‐4 inhibitor, reduces blood glucose levels through the modulation of the incretin system. Its main effects are to increase insulin secretion and decrease glucagon secretion in a glucose‐dependent fashion 24. In T2DM, there is progressive loss of the sensitivity of islet cell function to ambient glycaemia 25. DPP‐4 inhibitors appear to restore the appropriate response of both the α and β‐cells to glucose concentrations. This, in turn, increases insulin and decreases glucagon secretion during hyperglycaemia, while decreasing insulin and increasing glucagon secretion during hypoglycaemia 26. Sulphonylureas, by contrast, work solely through the stimulation of insulin secretion, which is not substantially modulated by the absence of hyperglycaemia or even by the presence of hypoglycaemia. That is, these agents increase insulin secretion even at very low glucose levels, which is in contrast to DPP‐4 inhibitors. Secondary failure rates have long been considered another disadvantage of this class 27. In one large, randomized clinical trial, the sulphonylurea glyburide was associated with greater loss of efficacy than either metformin or the thiazolidinedione, rosiglitazone 28; however, there are no long‐term clinical trial data to support the proposed durability of effectiveness of sitagliptin versus sulphonylurea therapy.
In the present study we assessed other factors associated with insulin initiation besides type of treatment. The finding that HbA1c was significantly associated with insulin initiation is not surprising and is consistent with a number of previous studies 2, 8, 9, 10, 11, 12, 13, 14, 15. After controlling for treatment type, patient characteristics and laboratory assessments, the only significant comorbid condition associated with earlier insulin use was obesity. This is actually contrary to other literature that did find a positive association between insulin use and a range of other comorbidities 4, 7, 9, 11, 13; however, none of these previous studies used propensity score matching. Notably, Fu et al. 14 also used propensity score matching and also could not find a significant association between a variety of comorbid conditions and insulin initiation, but did find a significant association with BMI. Variance in significant factors associated with insulin initiation across studies may be attributable to differences in patient characteristics and disease progression, the type of therapy, and length of time exposed to OADs, as well as the statistical methods applied.
Previous real‐world observational studies suggest that after 6 years of treatment ∼15–25% of sulphonylurea users had initiated insulin, compared with the 28.7% figure in our study 6, 10. The higher incidence of insulin use reported here may be attributable to the fact that, by study design, all patients were on dual therapy, representing a cohort of patients with more advanced disease. Differences in insulin initiation may also be attributable to varying types of sulphonylurea drugs across studies, as there may be some variation in treatment failure within the class 29.
The present study has several limitations, mainly attributable to the data source. First, insulin initiation was primarily estimated based on prescription data; however, the days‐of‐supply and end dates for prescriptions were unavailable for a considerable number of patients, resulting in a drop in sample size. Without days‐of‐supply, the study could not distinguish between short‐term and regular insulin users. Second, while the database captured prescription and drug information, it could not ascertain whether patients adequately followed physicians' instructions when taking medications. Likewise, prescription data alone cannot fully assess therapy patterns, and the study could not ascertain if a patient was continuously treated with the index therapy beyond 90 days, as required by the study design, or if they discontinued or switched therapy. Third, EMR data are mostly collected from primary care physicians, and, therefore, do not always capture diagnoses and treatments during hospitalizations or by specialists. As a result, serious comorbid conditions and severe hypoglycaemic events that require inpatient services or specialist care, insulin use during hospitalization, or prescriptions written by specialists may be under‐recorded in the present study. Furthermore, hypoglycaemic events that are potentially mild or moderate and where a patient does not seek treatment are not captured by EMR data, nor is any care that has been received outside of the healthcare network.
There were also methodological limitations. Although propensity score matching creates balanced treatment groups based on observed baseline characteristics, the possibility of potential imbalances between matched groups attributable to unobserved characteristics cannot be excluded. Second, more than half of the study patients were right censored at the end of continuous medical recording; therefore, average time to insulin was only calculated among those who initiated insulin during the follow up. Because of the exclusion of censored patients, the average time to insulin between the two groups was not statistically comparable. Hence, the Kaplan–Meier comparison was more appropriate. Furthermore, logistic regression cannot fully account for the timing of insulin use, which may be right censored. The time period during which an outcome can be observed must be prespecified, and patients without complete data must be excluded; therefore, as the follow‐up period increased, the number of matched patients included in each conditional logistic regression model decreased. In addition to the inability to account for censoring, the conditional logistic regression models were less reliable because the majority of matched‐pairs were concordant (i.e. both case and control had the same response). Finally, it should be noted that a lower risk of insulin initiation may not necessarily indicate better glucose control in patients, as patients and clinicians are often reluctant to initiate insulin, even when indicated.
Future research may build on this study by using more granular prescription data that are able to measure the use of index therapy beyond 90 days, and whether patients added to or switched from the index therapy. This would provide more information about how the length of time exposed to each type of therapy was associated with insulin use. Further analysis may also investigate treatment patterns of insulin use beyond initiation.
In conclusion, in this real‐world study, patients in the USA with T2DM treated with a combination of sitagliptin and metformin had a significantly lower risk of initiating insulin therapy compared with patients treated with a combination of sulphonylurea and metformin, driven mainly by the subgroup of patients with lower HbA1c levels. Although the differences appeared modest in terms of actual time to insulin therapy, these differences may be accentuated over time with longer follow‐up. Whether our results represent a more advantageous effect on β cell function of sitagliptin over sulphonylureas is not clear. Nonetheless, physicians and patients should consider this information when deciding the optimum dual therapy for the effective management of T2DM. Further studies will be needed to determine whether incretin‐based therapy, such as with DPP‐4 inhibitors, might potentially change the natural history of T2DM. Such studies would assess whether early treatment with incretin‐related agents could alter the progressive decline in β‐cell function that characterizes the disease.
Conflict of Interest
J. T., C. P. S. F., Z. L. and B. H. from Asclepius Analytics LLC have received fees for consulting from Merck & Co., Inc. L. B reports grants from Eli Lilly and Co., Novo Nordisk, and Sanofi US, Inc. L. B has also received personal fees from Sanofi, AstraZeneca, Janssen Pharmaceuticals, Merck & Co, Inc., GlaxoSmithKline, Intarcia Therapeutics Inc. and Quest Diagnostics. S. E. I. reports personal fees from Merck & Co., Boehringer Ingelheim, AstraZeneca, Novo Nordisk, Amgen, Lexicon, Intarcia, Gilead, and Sanofi/Regeneron. S. E. I. also reports non‐financial support from Takeda. K. G. B. was an employee of Merck & Co., Inc. at the time of the study, and is a current employee of Boehringer Ingelheim Pharmaceuticals Inc. Y. Q. was an employee of Merck & Co., Inc. at the time of the study, and is a current employee of Novartis. K. T., S. R., S. S. E., P. M., L. R. and P. B. are employees of Merck & Co., Inc.
K. T., K. G. B, S. S. E., P. M., L. R., J. T. and L. B. planned the study design, interpreted the results and reviewed/edited the manuscript. Z. L. and Y. Q. planned the study design, performed analyses, interpreted results and reviewed/edited the manuscript. S. E. I. supervised analyses, interpreted results and reviewed/edited the manuscript. C. P. S. F. performed analyses, interpreted results and reviewed/edited the manuscript. S. R., P. B. and B. H. interpreted results and reviewed/edited the manuscript.
Supporting information
Table S1. Sample selection flowchart.
Acknowledgements
The authors received writing assistance from Arielle Bensimon of Asclepius Analytics LLC, who received fees from Merck & Co., Inc. These data were previously published in abstract form at the 74th Scientific Sessions of the American Diabetes Association, 13–17 June 2014 and the 50th Annual Meeting of the European Association for the Study of Diabetes, 15–19 September 2014, Vienna, Austria.
References
- 1. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA. Beta‐cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia 2004; 47: 31–39. [DOI] [PubMed] [Google Scholar]
- 2. Petznick A. Insulin management of type 2 diabetes mellitus. Am Fam Physician 2011; 84: 183–190. [PubMed] [Google Scholar]
- 3. Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 1999; 281: 2005–2012 [Epub 8 June 1999]. [DOI] [PubMed] [Google Scholar]
- 4. Rascati KL, Richards KM, Lopez D, Cheng LI, Wilson JP. Progression to insulin for patients with diabetes mellitus using the Texas Medicaid database. Clin Ther 2011; 33: 2016–2020. [DOI] [PubMed] [Google Scholar]
- 5. Eurich DT, Simpson SH, Majumdar SR, Johnson JA. Secondary failure rates associated with metformin and sulfonylurea therapy for type 2 diabetes mellitus. Pharmacotherapy 2005; 25: 810–816. [DOI] [PubMed] [Google Scholar]
- 6. Kostev K, Dippel FW, Rathmann W. Predictors of insulin initiation in metformin and sulfonylurea users in primary care practices: the role of kidney function. J Diabetes Sci Technol 2014; 8: 1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kostev K, Rathmann W. Influence of macro‐ and microvascular comorbidity on time to insulin initiation in type 2 diabetes patients: a retrospective database analysis in Germany, France, and UK. Prim Care Diabetes 2013; 7: 167–171. [DOI] [PubMed] [Google Scholar]
- 8. Balkau B, Bouee S, Avignon A et al. Type 2 diabetes treatment intensification in general practice in France in 2008–2009: the DIAttitude Study. Diabetes Metab 2012; 38(Suppl. 3): S29–35. [DOI] [PubMed] [Google Scholar]
- 9. Parchman ML, Wang CP. Initiation of insulin among veterans with type 2 diabetes and sustained elevation of A1c. Prim Care Diabetes 2012; 6: 19–25. [DOI] [PubMed] [Google Scholar]
- 10. Ringborg A, Lindgren P, Yin DD, Martinell M, Stalhammar J. Time to insulin treatment and factors associated with insulin prescription in Swedish patients with type 2 diabetes. Diabetes Metab 2010; 36: 198–203. [DOI] [PubMed] [Google Scholar]
- 11. Spoelstra JA, Stol RP, de Bruyne MC et al. Factors associated with switching from oral hypoglycaemic agents to insulin therapy. Neth J Med 2002; 60: 243–248. [PubMed] [Google Scholar]
- 12. Donnan PT, Steinke DT, Newton RW, Morris AD. Changes in treatment after the start of oral hypoglycaemic therapy in Type 2 diabetes: a population‐based study. Diabet Med 2002; 19: 606–610. [DOI] [PubMed] [Google Scholar]
- 13. Reach G, Le Pautremat V, Gupta S. Determinants and consequences of insulin initiation for type 2 diabetes in France: analysis of the National Health and Wellness Survey. Patient Prefer Adherence 2013; 7: 1007–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fu AZ, Qiu Y, Davies MJ, Engel SS. Initial sulfonylurea use and subsequent insulin therapy in older subjects with type 2 diabetes mellitus. Diabetes Ther 2012; 3: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rubino A, McQuay LJ, Gough SC, Kvasz M, Tennis P. Delayed initiation of subcutaneous insulin therapy after failure of oral glucose‐lowering agents in patients with Type 2 diabetes: a population‐based analysis in the UK. Diabet Med 2007; 24: 1412–1418. [DOI] [PubMed] [Google Scholar]
- 16. Perez N, Moisan J, Sirois C, Poirier P, Gregoire JP. Initiation of insulin therapy in elderly patients taking oral antidiabetes drugs. CMAJ 2009; 180: 1310–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013; 36: 1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garber AJ, Abrahamson MJ, Barzilay JI et al. American Association of Clinical Endocrinologists' comprehensive diabetes management algorithm 2013 consensus statement–executive summary. Endocr Pract 2013; 19: 536–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brixner DI, McAdam‐Marx C, Ye X et al. Six‐month outcomes on A1C and cardiovascular risk factors in patients with type 2 diabetes treated with exenatide in an ambulatory care setting. Diabetes Obes Metab 2009; 11: 1122–1130. [DOI] [PubMed] [Google Scholar]
- 20. Crawford AG, Cote C, Couto J et al. Comparison of GE Centricity Electronic Medical Record database and National Ambulatory Medical Care Survey findings on the prevalence of major conditions in the United States. Popul Health Manag 2010; 13: 139–150. [DOI] [PubMed] [Google Scholar]
- 21. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 1985; 39: 33–38. [Google Scholar]
- 22. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med 1998; 17: 2265–2281. [DOI] [PubMed] [Google Scholar]
- 24. Nauck MA. Incretin‐based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications. Am J Med 2011; 124(Suppl. 1): S3–18. [DOI] [PubMed] [Google Scholar]
- 25. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care 2009; 32(Suppl. 2): S151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ahrén B, Schweizer A, Dejager S, Villhauer EB, Dunning BE, Foley JE. Mechanisms of action of the dipeptidyl peptidase‐4 inhibitor vildagliptin in humans. Diabetes Obes Metab 2011;13:775‐‐783. [DOI] [PubMed] [Google Scholar]
- 27. Lebovitz HE, Feinglos MN. Sulfonylurea drugs: mechanism of antidiabetic action and therapeutic usefulness. Diabetes Care 1978; 1 : 189‐‐198. [DOI] [PubMed] [Google Scholar]
- 28. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355: 2427‐‐2443. [DOI] [PubMed] [Google Scholar]
- 29. Satoh J, Takahashi K, Takizawa Y, et al. Secondary sulfonylurea failure: comparison of period until insulin treatment between diabetic patients treated with gliclazide and glibenclamide. Diabetes Res Clin Pract 2005; 70: 291‐‐297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Sample selection flowchart.


