Abstract
Extracts of Salacia reticulata Wight (Hypocrataceae) roots, stems, and leaves have been used in Asia for hundreds of years for the folkloric treatment of diabetes and other health problems. Constituents that have been identified as exhibiting anti‐diabetic effects include salacinol, kotalanol, ponkorinol, salaprinol, and their corresponding de‐0‐sulfonated compounds. Mangiferin, kotalagenin 16‐acetate and various proanthocyanidin oligomers have also been isolated. Studies indicate that Salacia extracts modulate multiple targets that influence carbohydrate and lipid metabolism including α‐glucosidase, aldose reductase, pancreatic lipase, peroxisomal proliferator‐activated receptor‐α, glucose transporter‐4 mediated glucose uptake, and angiotensin II type 1 receptor. Furthermore, Salacia extracts exhibit free radical scavenging, antioxidant and hepatoprotectant activities. In human studies, Salacia extracts have been shown to decrease plasma glucose and insulin levels, decrease HbA1c, and modulate serum lipid levels with no adverse effects being reported. Similar results have been demonstrated in rat and mouse models as well as in vitro systems. Safety of S. reticulata and other Salacia species as S. oblonga and S. chinensis in rats and mice indicate that extracts are exceedingly safe. No clinical studies have examined the effects of Salacia extracts on human weight loss, although weight loss and decreases in weight gain have been demonstrated in animal models. Because of the large number of pharmacologically active compounds, it is difficult to establish standards for extracts. © 2015 The Authors. Phytotheraphy Research published by John Wiley & Sons Ltd.
Keywords: Salacia reticulata, antidiabetic, anti‐hyperlipidemic, antioxidant, hepatoprotectant
Introduction
Salacia reticulata Wight (Hypocrataceae) is a woody climbing shrub with greenish‐brown bark that is indigenous to India and Sri Lanka, while other species as S. chinensis and S. oblonga are also found in Asia and other parts of the world. In addition to treating diabetes, decoctions (aqueous extracts) of S. reticulata and extracts of other Salacia species have been used for hundreds of years for the treatment of asthma, rheumatism, hemorrhoids, itching and swelling, gonorrhea, skin diseases, and amenorrhea (Arunakumara and Subasinghe, 2010). S. reticulata has been used also as a supplementary food in Japan to prevent diabetes and obesity (Kishino et al., 2006; Li et al., 2008).
Interest in the use of Salacia extracts have risen in recent years for a number of reasons, including the rapid increase in the incidence of diabetes and pre‐diabetes, the need for safe and effective drugs and medicinal foods that can assist in the control of blood sugar as well as lipid levels, and the fact that Salacia extracts exhibit multiple mechanisms of action with respect to carbohydrate and lipid metabolism (Li et al., 2008). In addition, extracts of Salacia appear to be very safe, based on animal and in vitro studies as well as human use.
Because S. reticulata has been the most widely studied of the Salacia species, this review focuses primarily on the human, animal, and in vitro studies conducted with this species. Information is also provided with respect to effects of aqueous extracts of S. oblonga and S. chinensis. Among the reported beneficial effects, constituents from Salacia species that have been identified as exhibiting anti‐diabetic effects include salacinol, kotalanol, ponkorinol, salaprinol, and their corresponding de‐0‐sulfonated compounds. The molecular structure of salacinol is shown in Fig. 1. In addition, mangiferin, kotalagenin 16‐acetate, and various proanthocyanidin oligomers have also been isolated. Antidiabetic, anti‐hyperlipidemic, antioxidant, and hepatoprotective effects as well as safety, chemical constituents, and mechanistic studies are considered. No direct comparisons between S. oblonga and S. chineisis with S. reticulata with regard to effects on carbohydrate and lipid metabolism have been made.
Figure 1.
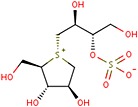
Molecular structure of salacinol.
Carbohydrate Metabolism
A number of human clinical studies have assessed the ability of aqueous S. reticulata extracts to modulate carbohydrate metabolism. Key studies are summarized in Table 1. Shimoda et al. (1998) conducted one of the earliest clinical studies on the hypoglycemic effects of an aqueous extract of S. reticulata, demonstrating that the extract was beneficial in controlling postprandial hyperglycemia. Kajimoto et al. (2000) conducted a double‐blind placebo‐controlled study with borderline type II diabetics and observed that S. reticulata extract significantly decreased blood glucose levels relative to the control group.
Table 1.
Key studies involving Salacia species
| Plant species | Model system | Compound used | Parameters examined | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S. chinensis | KK‐Ay mice | S. chinensis stem extract | Glucose lowering | HBA1c | — | — | — | — | — | Morikawa et al., 2015 |
| S. oblonga | Human | N/A | Glucose lowering | LDL | HDL | Triglycerides | Total cholesterol | ‐ | Singh et al., Saudi J. Kid Dis. Transpla. 2015 | |
| S. oblonga, T. cordifolia, E. offinalis, C. longa, and G. sylvestre | Human | Polyherbal combo with Salacia (G‐400) | Glucose lowering | HBA1c | LDL | HDL | Triglycerides | Total cholesterol | Liver enzymes | Kurian et al., Nutrition, 2014 |
| S. chinensis | Rats | S. chinensis | Glucose lowering | — | — | — | — | — | Liver enzymes | Sellamuthu et al., Pak. J. Pharm. Sci., 2014 |
| S. chinensis | Rats | S. chinensis | Glucose lowering | — | — | — | — | — | Liver enzymes | Sellamuthu et al., J. Med. Food. 2013 |
| S. chinensis | Human | S. chinensis extract | Glucose lowering | — | — | — | — | — | — | Koteshwar et al. Pharmacog. Mag. 2013 |
| S. reticulata | Human | S. reticulate extract | Glucose lowering | — | LDL | — | — | Cholesterol | — | Shivaprasad et al., J. Med. Food, 2013 |
| S. oblonga | Rats | S. oblonga extract | Glucose lowering | HBA1c | LDL | HDL | Triglycerides | Cholesterol | — | Bhat et al., J Clin Diagn Res. 2012 |
| S. oblonga | KK‐Ay/TaJcl type 2 | S. oblonga | Glucose lowering | HBA1c | LDL | HDL | Triglycerides | — | — | Nakata et al., Nutr Res Pract. 2011 |
| S. reticulata | Human | S. reticulate bark | Glucose lowering | HBA1c | LDL | HDL | Triglyceride | Cholesterol | — | Radha and Amrithaveni 2009 |
| S. reticulata | KK‐Ay Mouse | S. reticulata | Glucose lowering | — | — | — | — | — | Hepatic gene expression | Im et al., J. Ethnopharmacol., 2009. |
| S. reticulata | Wistar Rats | S. reticulate extract | Glucose lowering | — | — | — | ‐ | ‐ | ‐ | Oe and Ozaki 2008 Biochem., 2008. |
| S. oblonga | Human | S. oblonga extract | Glucose lowering | — | — | — | ‐ | ‐ | ‐ | Williams et al., Am J Clin Nutr. 2007 |
| S. oblonga | Human | S. oblonga extract | Glucose lowering | — | — | — | — | — | — | A. L. Collene et al., Nutrition. 2005 |
| S. reticulata | Human | S. reticulate extract | Glucose lowering | HBA1c | — | — | — | — | — | Jayawardena et al., J Ethnopharmacol. 2005 |
| S. oblonga | Human | S. oblonga extract | Glucose lowering | — | — | — | — | — | — | Heacock et al., J. Am. Diet Assoc., 2005 |
| S. oblonga | Zucker Rats | S. oblonga extract | Glucose lowering | — | — | — | — | — | — | Y. Li et al., Life Sci. 2004 |
| S. oblonga | Rats | S. oblonga root extract | Glucose lowering | — | — | — | — | — | Liver enzymes | Krishnakumar et al., Indian J Physiol Pharmacol., 1999 |
| S. macrosperma | Rats | S. macrosperma extract | Glucose lowering | — | LDL | HDL | — | Cholesterol | — | Venkateswarlu et al., Planta Med., 1993 |
HBA1c, hemoglobin A1c; LDL, low density lipoprotein; HDL, high density lipoprotein.
Jayawardena et al. (2005) administered an S. reticulata tea to 51 type II diabetic subjects in a double‐blind, randomized placebo controlled cross‐over study. The subjects received the tea or placebo for 3 months each. Glycosylated hemoglobin, hemoglobin A1c (HbA1c), at the end of treatment was significantly lower as compared with the placebo, and the dose of glibenclamide being used by some subjects was decreased. The tea had no effect on liver or kidney function, and no significant adverse effects were reported. It is not clear how much of the product was actually consumed by the subjects.
Tanimura et al. (2005) examined the effects of a mixture of an aqueous extract of S. reticulata and cyclodextrin on serum glucose and insulin levels in response to a sucrose tolerance test and on serum glucose levels over time in human subjects. Pretreatment with the aqueous extract prior to the sucrose loading significantly suppressed postprandial hyperglycemia. An S. reticulata bark powder (2 g in capsule form per day for 90 days) was given to 30 type II diabetic patients (Radha and Amrithaveni, 2009). Significant decreases in fasting blood glucose, HbA1c, and lipids were reported.
A randomized, double‐blind, placebo‐controlled study involving 29 prediabetic and mildly hyperlipidemic human subjects evaluated the safety and efficacy of S. reticulata leaf and bark aqueous extracts (Shivaprasad et al., 2013). The subjects received either one of the extracts (500 mg/day) or placebo orally for 6 weeks. The root extract showed statistically significant reductions in blood sugar levels and lipid profiles at 3 and 6 weeks, while the subjects on the leaf extract exhibited significantly lower fasting blood sugar levels only at 6 weeks.
Several human studies involving extracts of S. oblonga and S. chinensis have also been reported. In a randomized double blind cross‐over study involving 39 healthy non‐diabetic subjects, Heathcock et al. (2005) observed that administration of 1000 mg of an aqueous extract of S. oblonga in conjunction with a test meal resulted in a 23% reduction in plasma glucose and a 29% decrease in serum insulin 2 hours postprandially. Expired hydrogen was shown to significantly increase as compared with the placebo group, suggesting a decrease in absorption of sugars with an increase in hydrogen production due to sugar fermentation by intestinal microorganisms.
In a follow‐up study by these same investigators (Collene et al., 2005) involving 43 healthy human subjects, 1000 mg of an aqueous extract of S. oblonga was shown to decrease plasma glucose by 27% and serum insulin by 35% over a 2‐h time span, while increasing exhaled hydrogen by 60%. Thus, the extract exhibited promising benefits with respect to carbohydrate metabolism. No adverse effects were reported, although this was a single‐dose study.
Williams et al. (2007) examined the ability of an aqueous extract of S. oblonga to lower blood glucose levels in 66 borderline diabetic human subjects in a double blind cross‐over placebo controlled study when 240 and 480 mg of the extract were consumed with a high carbohydrate control meal. The high dose lowered the peak glucose response by 27%, and both doses significantly lowered serum insulin levels, indicating the S. oblonga extract may be useful in this population for postprandial glucose control. No adverse effects were reported.
Nataka et al. (2011) administered two S. oblonga tea products to 41 human subjects for 60 days. One product consisted of 200 mg of S. oblonga leaves in 100 mL of water, and the other product contained 300 mg of leaves plus a vitamin mixture plus 20 mg of a lipopolysaccharide derived from a Gram‐negative bacterium (IP‐PA1). Unfortunately, the authors did not use a placebo group but merely compared with two groups. Therefore, the effects of the S. oblonga itself were not determined. The authors reported that the combination product lowered fasting blood glucose and HbA1c more rapidly than the S. oblonga alone and also significantly improved high density lipoprotein (HDL) and low density lipoprotein (LDL). No adverse effects were reported and no information was provided with respect to body weight changes.
Kurian et al. (2014) used a polyherbal product containing S. oblonga to manage hyperglycemia in patients with type II diabetes at a dose of 1000 mg per day for 8 weeks to 89 patients. Additional plant components included Tinospora cordifolia, Emblica officinalis, Curcuma longa, and Gymnema sylvestre. Fasting and postprandial blood glucose levels as well as glycosylated hemoglobin (HbA1c) were significantly decreased. No information was provided regarding the plant parts used or how the product was prepared.
In a cross‐over designed study, 30 healthy human subjects were given placebo or 1000 mg of an S. chinensis hydroalcoholic extract as a one‐time dose (Koteshwar et al., 2013). The extract decreased postprandial plasma glucose levels after a carbohydrate‐rich meal by about 13% at 90 min, while the plasma glucose area under the curve was decreased by about 34%.
Various studies have examined the effects of extracts of S. reticulata on carbohydrate metabolism in rats and mice. One of the earliest studies involved a screening of about 40 plants aqueous extracts in these animals for their oral hypoglycemic activity (Karunanayake et al., 1984). The extract of S. reticulata was shown to have a significant effect on carbohydrate metabolism. Venkateswarlu et al. (1993) demonstrated that various fractions of an alcoholic extract of roots of S. macrosperma administered orally to alloxan‐induced diabetic rats for 8 days alleviated diabetic symptoms including elevated blood glucose levels.
Ruvin Kumara et al. (2005) examined the hypoglycemic activity of an aqueous extract of root bark from S. reticulata in alloxan‐induced diabetic rats. The extract was further fractionated. The precipitate from a methanol fraction was administered for 120 days, which improved glucose tolerance. Furthermore, the extract significantly reduced fasting blood glucose levels, fructosemia and HbA1c, polydypsia and hyperphagia while weight loss was modulated. No adverse effects were reported.
The anti‐diabetic properties of various extracts of S. reticulata were examined by Chandrashekar et al. (2009). Water, ethanol, petroleum ether, ethyl acetate, and diethyl ether extracts of bark and root were all shown to exhibit time‐dependent decreases in blood glucose levels of rats over 30 days. Giron et al. (2009) demonstrated that an aqueous extract of S. oblonga increases glucose transporter‐4 (GLUT4)‐mediated glucose uptake in L6 rat myotubes by activating GLUT4 promotor transcription. Mangiferin was identified as the bioactive compound.
Yoshino et al. (2009) examined the anti‐diabetic activity of an aqueous extract of the leaves of S. reticulata in mice. The simultaneous oral administration of the extract with sucrose or maltose inhibited the postprandial elevation of plasma glucose and insulin levels and also intestinal α‐glucosidase activity. These same effects were observed when the extract was added to the drinking water as compared with the control group of diabetic mice. In addition, the treatment prevented the elevation of pancreatic, plasma, and kidney lipid peroxide levels, lowered plasma insulin levels, and prevented the elevation of kidney aldose reductase activities in diabetic mice. Thus, multiple beneficial effects were observed with respect to carbohydrate metabolism. No adverse effects were reported.
The mechanisms of blood glucose lowering by an aqueous extract of stems of S. reticulata were studied in KK‐Ay genetically diabetic mice (Im et al., 2009). After treating the mice for 4 weeks with the extract, various assays demonstrated that the hepatic gluconeogenic enzyme fructose‐1,6‐diphosphatase was significantly decreased relative to the controls. Studies in cultured liver cells demonstrated the extract decreased the mRNA levels for this enzyme. Furthermore, mangiferin dose‐dependently down‐regulated the mRNA for this enzyme, providing an explanation for the ability of the extract to decrease fasting blood glucose levels in mice, although other compounds in the extract were most probably also involved.
Shimada et al. (2010, 2014) examined the anti‐obesity effects and safety of an aqueous extract of S. reticulata. Spontaneously obese and diabetic mice were fed the extract ad libitum for 2 months. The mice receiving the extract showed suppression of body weight gain and alleviation of abnormal glucose tolerance.
Sim et al., 2010) examined the inhibitory activities of the isolated S. reticulata constituents salacinol, kotalanol, and de‐0‐sulfonated kotalanol against human intestinal maltase‐glucoamylase using X‐ray crystallography. The de‐0‐sulfonated kotalanol was shown to be the most potent inhibitor known to date for this enzyme, being some 2000‐fold more potent than drugs commonly used in clinical practice.
Rajashree et al. (2011) examined the effects of a mixture of S. reticulata and Catheranthus roseus L. extracts on streptozotocin‐diabetic rats. The extract combination was administered intragastrically daily for 30 days and resulted in significantly reduced blood glucose levels. The relative beneficial effects of the two components are not known.
In a study involving spontaneously obese diabetic mice, Akase et al. (2011) demonstrated that an aqueous extract of S. reticulata produced dose‐dependent decreases in body weight gain and improved glucose tolerance, hypertension, and peripheral neuropathy. Mechanistically, it appears that the glucosidase inhibitory effect of salacinol and kotalanol is similar to acarbose that is clinically used for the inhibition of postprandial hyperglycemia. In addition, a 13‐membered ring thiocyclitol, found in Salacia, also exhibited similar glucosidase inhibitory activity. Compared with non‐obese mice, improved insulin resistance and blood glucose‐lowering effects observed in spontaneously obese diabetic mice were attributed to this mechanism. Similarly, Kataoka (2006) have reported that S. reticulata extract improved HbA1c in patients with type II diabetes. In addition, hepatocellular swelling, fatty degeneration of hepatocytes, inflammatory cell infiltration, and single‐cell necrosis were markedly improved in mice receiving the extract. The authors concluded that Salacia extract has the ability to prevent obesity and associated metabolic disorders including the development of metabolic syndrome.
An aqueous methanol extract of S. oblonga inhibited the increase in serum glucose levels in sucrose and maltose‐loaded rats (Matsuda et al., 1999). An ethyl acetate extract of the aqueous methanol extract exhibited inhibitory activity against α‐glucosidase and aldose reductase. The α‐glucosidase inhibitors salicinol and kotalanol were isolated from the water soluble portion, while kotalagenin‐16‐acetate was isolated from the ethyl acetate soluble portion. An aqueous extract of S. oblonga was shown to markedly improve interstitial and perivascular fibrosis, as well as postprandial hyperglycemia in obese Zucker rats (Li et al., 2004).
A hydroalcoholic extract of S. oblonga was administered to streptozotocin‐diabetic Wistar rats for 16 weeks at doses of 50 and 100 mg/kg (Bhat et al., 2012). Both doses significantly decreased random blood glucose levels (approximately 45%) as well as HbA1c, while increasing serum insulin levels.
Mangiferin is another common constituent present in Salacia species. Muruganandan et al. (2005a) administered mangiferin at doses of 10 and 20 mg/kg per day intraperitoneally to streptozotocin–induced diabetic rats for 28 days. Both doses significantly lowered blood glucose levels. In another study, the administration of mangiferin isolated from S. chinensis at a dose of 40 mg/kg body weight per day for 30 days to streptozotocin‐induced diabetic rats resulted in a significant lowering of blood glucose levels (Sellamuthu et al., 2014). These studies clearly demonstrated the role of mangiferin in the antidiabetic properties of various Salacia extracts and preparations.
In summary, human and animal studies with aqueous extracts of S. reticulata and as well as S. oblonga have been shown to decrease blood sugar, insulin, and HbA1c levels and do so without the report of adverse events. The longest study involved the treatment of subjects for 3 months.
Lipid Metabolism
As noted previously, several studies have demonstrated the beneficial effects of Salacia preparations with respect to lipid metabolism in humans (Radha and Amrithaveni, 2009; Shivaprasad et al., 2013; Nataka et al., 2011). The effects of S. oblonga on triglyceride and cholesterol levels were assessed in diabetic and non‐diabetic patients with chronic kidney disease for 6 months (Singh et al., 2015). As compared with patients receiving a placebo, the treated patients experienced significantly reduced serum triglyceride as well as cholesterol levels with greater reductions in diabetic patients. In addition, significant reductions were also seen in C‐reactive protein and interleukin‐6, markers of inflammation. The authors indicated that the treated patients received 1000‐mg S. oblonga twice daily, but failed to note the plant part used and whether this was an extract or a plant powder. As a consequence, the study is not reproducible.
A polyherbal product containing S. oblonga was used to treat hyperlipidemic conditions in patients with type II diabetes (Kurian et al., 2014). The product was given at a dose of 1000 mg per day for 8 weeks. What plant parts, how the combination was prepared, and the relative proportions of each constituent were not disclosed. Significant decreases were observed with respect to triglycerides, total cholesterol, and low‐density lipoprotein cholesterol with an increase in HDL cholesterol.
A number of studies have examined the effects of extracts of S. reticulata on lipid metabolism in rats and mice. In a screening of about 40 plants, an extract of S. reticulata was shown to have a significant effect on lipid metabolism (Karunanayake et al., 1984).
The lipase inhibitory properties of an aqueous extract of S. reticulata and some of its constituents were determined in obese rats and in vitro systems (Yoshikawa et al., 2002a). The extract was administered orally for 27 days and was shown to inhibit pancreatic lipase, rat adipocyte tissue‐derived lipoprotein lipase, and glycerophosphate dehydrogenase activities. Furthermore, polyphenols, diterpenes and triterpenes, salicinol, and various catechins were shown to exhibit inhibitory activity against pancreatic lipase.
Muruganandan et al. (2005a) administered mangiferin, a prominent constituent in Salacia species, to streptozotocin‐diabetic rats intraperitoneally at doses of 10 mg/kg and 20 mg/kg per day for 28 days. Both doses resulted in significant decreases in plasma total cholesterol, triglycerides, and LDL cholesterol with elevations in HDL cholesterol. These results demonstrated the antihyperlipidemic and antiatherogenic activities of the mangiferin.
Kishino et al. (2006, 2009) have conducted several studies involving an aqueous extract of S. reticulata and cyclodextrin. In the first study (Kishino et al., 2006), mice that received the mixture for 8 weeks showed a decrease in body weight gain and visceral fat as compared with the control group. In Sprague–Dawley rats fed a high fat diet for 5 weeks, adding the mixture to the diet resulted in decreases in body weight, visceral fat, and plasma leptin and adiponectin concentrations.
In the second study, Kishino et al. (2009) examined the effects of a mixture of an aqueous extract of S. reticulata and cyclodextrin added to the diet of fatty Wistar rats. After 6 weeks body weight gain, food intake, visceral fat mass, liver triglyceride content, and serum insulin were significantly lower, indicating that the mixture influenced lipid metabolism.
Shimada et al. (2010, 2014) examined the anti‐obesity effects of an aqueous extract of S. reticulata fed ad libitum to spontaneously obese and diabetic mice for 2 months. The mice receiving the extract showed suppression of body weight gain and fat accumulation, alleviation of abnormal lipid metabolism, and suppression of intrahepatic fat accumulation as well as mesenteric adipocyte hypertrophy. In an in vitro experiment using mouse‐derived adipocyte precursor 3 T3‐L1 cells, these investigators showed that the extract significantly suppressed fat accumulation in the differentiation induction and maturation phases.
Shimada et al. (2011) also conducted studies with mouse‐derived precursor 3 T3‐L1 cells exposed to an aqueous extract of S. reticulata. The extract exhibited significant inhibition of differentiation of mature adipocytes and decreased the expression of genes and proteins of peroxisome proliferator‐activated receptor (PPAR)γ and CCAA‐enhancer binding protein α, and the activity of glycerophosphate dehydrogenase, as well as caused a decrease in serum adiponectin, all of which are associated with lipid metabolism. Mangiferin did not suppress fat accumulation in these cells, indicating that this component of the extract was not involved in the inhibition of adipocyte differentiation.
Akase et al. (2011) demonstrated that an aqueous extract of S. reticulata administered to spontaneously obese diabetic mice produced dose‐dependent decreases in body weight gain and decreased the accumulation of visceral and subcutaneous fat. Rajashree et al. (2011) administered an aqueous extract of a mixture of S. reticulata and Catheranthus roseus L. to streptozotocin‐diabetic rats intragastrically for 30 days. The extract resulted in significant reductions in serum cholesterol and serum triglycerides.
Bhat et al. (2012) not only examined the antidiabetic effects of a hydroalcoholic extract of S. olbonga in streptozotocin‐diabetic rats, but also the anti‐hyperlipidemic effects. The rats were treated with 50 or 100 mg/kg of the extract for 16 weeks. Both doses significantly decreased serum triglyceride levels, while the higher dose also produced a significant increase in HDL‐cholesterol, a very desirable effect.
In summary, various human and animal studies have demonstrated the beneficial effects of Salacia extracts with respect to decreases in plasma total cholesterol, triglycerides, and LDL cholesterol with elevations in HDL cholesterol.
Chemoprotective and Antioxidant Effects
Various studies have examined the antioxidant, chemoprotective and antiproliferative effects of extracts of S. reticula and mangiferin. Yoshikawa et al. (2002b) demonstrated that aqueous and methanolic extracts of roots and stems of S. reticulata prevented carbon tetrachloride‐induced hepatotoxicity in rats and also blocked the formation of thiobarbituric acid reactive substances in liver, an indicator of oxidative stress‐mediated lipid peroxidation. Isolated mangiferin as well as several catechins were also shown to be potent scavengers of a variety of free radicals.
Muruganandan et al. (2005b) observed that the administration of mangiferin at doses of 10 and 20 mg/kg per day intraperitoneally for 28 days to rats exhibited a immunoprotective role through the inhibition of induced oxidative stress in lymphocytes, neutrophils, and macrophages. Mangiferin inhibited induced increases in lipid peroxidation and decreases in catalase and superoxide dismutase activities in these cells. These same authors also demonstrated that mangiferin administration at these same doses for 28 days protected against oxidative damage to cardiac and renal tissues in streptozotocin‐induced diabetic rats (Muruganandan et al., 2002).
The free radical scavenging properties of various extracts of S. reticulata were examined by Chandrashekar et al. (2009). Water, ethanol, petroleum ether, ethyl acetate, and diethyl ether extracts of bark and root were prepared. In vitro studies demonstrated that all extracts exhibited the ability to scavenge superoxide and hydrogen peroxide, with the petroleum ether and diethyl acetate extracts showing greatest activity. The active constituents were not identified.
The anti‐proliferative effects of a hot‐water extract of leaves of S. reticulata were demonstrated on interleukin‐1β‐activated cells obtained from the synovium of type II collagen antibody‐induced arthritic mice (Sekiguchi et al., 2012). Various polyphenolic fractions did not affect cell proliferation. Protease digestion indicated that low‐molecular weight proteins present in the aqueous extract were responsible for the anti‐proliferative activity.
The effects of an aqueous extract of S. reticulata on hepatic cytochrome P450 activity in vivo in mice and in liver microsomes in vitro were determined (Yokotani et al., 2013). The extract was fed orally at doses of 0%, 0.5%, 1.5%, and 4.5%. The Salacia at the highest dose suppressed body weight, decreased hepatic cytochrome P450 content, but increased the activities of CYP1A1, CYP2B, and CYP2C. It had an insignificant effect relative to the control group with respect to CYP3A, the most important drug metabolizing enzyme system. The extract also had no effect at any dose on the hepatic enzymes alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase, indicating no hepatotoxicity. The extract inhibited CYP1A2 activity in mouse and human liver microsomes at the highest concentration used a dose that is over 125 times an equivalent typical human dose. Based on this study, it is doubtful that the S. reticulata extract would have significant effects on the cytochrome P450 drug metabolizing enzyme system at doses usually consumed because effects were seen only at the highest dose.
The administration of 40 mg mangiferin per kilogram per day for 30 days to streptozotocin‐induced diabetes significantly reduced tissue markers of oxidative stress including manoldialdehyde, hydroperoxides, and reduced glutathione levels (Sellamuthu et al., 2013).
A root extract of S. reticulata was shown to produce a dose‐dependent inhibition of melanin synthesis and antioxidant activity in UV‐irradiated or MSH‐induced B16 melanoma cells (Suwannalert et al., 2014). The authors suggested that the root extract may be useful in the treatment of hyperpigmentation disorders.
Huang et al. (2006, 2008) have conducted several mechanistic studies in obese Zucker diabetic rats and have demonstrated that an aqueous extract of S. oblonga inhibits the cardiac expression of angiotensin II type 1 receptor and modulates cardiac PPAR‐α‐mediated transcription of fatty acid metabolism, thus protecting against cardiac hypertrophy, lipid accumulation, and increasing fatty acid oxidation. An extract of S. oblonga was shown to exhibit anti‐oxidative, antiinflammatory, anti‐proliferative, and lysosomal membrane stabilizing activity in rats (Ismail et al., 1997).
He et al. (2011) have shown that an extract of S. oblonga as well as its major component mangiferin attenuate diabetes‐induced renal fibrosis in rats by suppressing the stimulatory effect of angiotensin II on proliferation and increased mRNA expression and/or activities of collagens I and IV, fibronectin, AT1, TGF‐β1, and PAI‐1. The hepatoprotective properties of a methanolic extract of S. chinensis in primary cultured mouse hepatocytes (Nakamura et al., 2011) has been demonstrated.
Safety
As previously noted, herbal preparations of S. reticulata and other Salacia species have been used for many years for the folkloric treatment of various diseases without reference to adverse effects. In none of the human studies reviewed previously were any adverse effects described or reported, with studies varying from a single dose to daily dosing for up to 3 months with aqueous extracts of S. reticulata and S. oblonga (Jayawardena et al., 2005; Collene et al., 2005; Williams et al., 2007; Nataka et al., 2011; Shivaprasad et al., 2013). For example, in a randomized, double‐blind, placebo‐controlled study involving 29 pre‐diabetic and mildly hyperlipidemic human subjects treated with 500 mg/day of aqueous extracts from S. reticulata leaf and bark (Shivaprasad et al., 2013), no adverse events were observed based on physical examination and clinical laboratory evaluations.
Several studies have addressed the safety of Salacia extracts in animals. Shimoda et al. (1999) examined the safety of a single 5000 mg/kg dose of an extract of S. reticulata trunk and concluded that the extract had no serious acute toxicity or mutagenicity. The most serious effect was diarrhea because of this enormously high dose. In a subsequent study, Shimoda et al. (2001) demonstrated that an aqueous extract of S. reticulata exhibited no antigenicity or phototoxic effects when given orally at up to 320 mg/kg five times a week for 3 weeks.
Wolf and Weisbrode (2003) demonstrated that the administration of an aqueous extract of S. oblonga for 14 days to Sprague–Dawley rats did not result in clinical chemistry or histopathologic indications of toxic effects. The treated rats did exhibit lower body weights and feed intake.
Im et al. (2008) conducted a safety evaluation of an aqueous extract of S. reticulata stem in mice using a gene expression DNA microarray. The extract was given daily for 3 weeks. The assay assessed expression of genes for stress response, ribosomal proteins, transcription, cell function, inflammatory/immune response, and metabolism. The authors concluded that the extract was non‐toxic based on a lack of effect on these parameters.
In a toxicological study by Flammang et al. (2007), an aqueous S. oblonga root extract was administered daily for 90 days via oral gavage to rats. The authors concluded that the no observable adverse effect level (NUAEL) was 2500 mg/kg per day and that no chromosomal aberrations were observed in peripheral blood lymphocytes after 90 days of treatment. On the other hand, Rong et al. (2008) demonstrated hepatic hypertrophy when S. oblonga extract doses of 300 and 900 mg/kg per day were administered orally to rats for 28 days. These results are in contrast to the study of Wolf and Weisbrode (2003) who observed small decreases in liver and spleen weights. The reason for these differences is not known but may relate to the method of extract preparation or the animal species used.
A toxicological evaluation of an ethanol extract of S. chinensis roots was conducted in rats and shown to be devoid of toxicity up to a dose of 2000 mg/kg (Kannan et al., 2011). An increase in liver weight was observed at this dose, which may be as much as 50 times a typical human dose, and may relate to enzyme induction that has been reported at very high, non‐therapeutic doses (Yokotani et al., 2013).
The blood levels of alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase, markers of liver damage associated with the streptozotocin‐induced diabetes, were significantly decreased following the administration of 40 mg mangiferin per kilogram per day for 30 days (Sellamuthu et al., 2014). In addition, the levels of red blood cells and white blood cells were also improved, demonstrating the non‐toxic nature of mangiferin and as well as its beneficial effects with respect to diabetes.
Finally, it should be noted that several authors reported no evidence of adverse effects in conjunction with previously discussed biochemical studies involving administration of Salacia extracts to animals (Ruvin Kumara et al., 2005; Yoshino et al., 2009; Yokotani et al., 2013).
Pharmacologically Active Constituents
A wide range of chemical constituents have been isolated from Salacia species with the composition depending on the geographic origin of the plants, the plant parts examined, and the species involved (Akaki et al., 2014). Isolated constituents from various Salacia species are summarized in Table 2. For example, a study compared the salacinol and kotalanol content of roots, stems, leaves, and fruits of S. reticulata, S. oblonga, and S. chinensis (Muraoka et al., 2010). Roots of S. reticulata contained the highest content of these compounds relative to the other plant parts and other two species. Neokotalanol was found to be the major constituent in samples from Thailand while salacinol was the primary constituent in roots and stems from Sri Lanka and India (Akaki et al., 2014).
Table 2.
Isolated constituents from Salacia species
| Plant species | Active ingredients |
|---|---|
| S. chinensis | Lignans schisandrin, deoxyschisandrin, gomisins, and pregomisin |
| S. oblonga | Salacinol, Kotalanol, and Kotalagenin‐16‐acetate (a triterpene) |
| T. cordifolia | syringin, cordifolioside A, magnoflorine, and tinocordiside |
| E. offinalis | Carnosol, Carnosic acid, rosmadial and rosmanol, epirosmanol, and methyl carnosate |
| C. longa | Curcumin (diferuloylmethane), demethoxycurcumin, bisdemethoxycurcumin, turmerone, atlantone, and zingiberene |
| G. sylvestre | Oleanane‐type triterpenoid saponins known as gymnemic acids (in the form of tigloyl, methylbutyroyl etc.), derivatives of deacylgymnemic acid (DAGA) that is the 3‐O‐>glucuronide of gymnemagenin (3,16,21,22,23,28‐hexahydroxy‐olean‐12‐ene) |
| S. reticulata | 1,3,8‐Trihydroxyanthraquinone, chrysophanol(1,8‐dihydroxy‐3‐methylanthraquinone), physcion (1,8‐dihydroxy‐3‐methyl‐6‐methoxyanthraquinone), aloe‐emodin (3‐carbinol‐1,8‐dihydroxyanthraquinone), lunatin (3‐methoxy‐1,6,8‐trihydroxyanthraquinone), emodin (6‐methyl‐1,3,8‐trihydroxyanthraquinone), and chrysophanol‐10,10'‐bianthrone |
| S. macrosperma | Norbellidifolin, 1‐hydroxy‐3,7, 8‐trimethoxy‐xanthone, norswertianolin, swertianolin, 1,3,7,8‐tetrahydroxyxanthone‐8‐O‐beta‐ d‐glucopyranoside, swertiamatin, decentapicrin, coniferl aldehyde, sinapaldehyde, balanophonin, together with beta‐sitosterol, daucosterol, and oleanolic acid . |
| Swertia macrosperma |
The sugar‐based sulfonium sulfates salicinol, ponkoranol, kotalanol, and salaprinol are believed to be major contributors to the anti‐diabetogenic effects of Salacia species (Akaki et al., 2014; Mohan et al., 2014). The corresponding de‐0‐sulfonated compounds (Akaki et al., 2014; Mohan et al., 2014) as well as neoponkoranol and neosalaprinol (Xie et al., 2011) have also been isolated. Mangiferin (a xanthone) is also present in S. reticulata and other Salacia species and has been shown to be an inhibitor of sucrase, isomaltase (α‐glucosidases), and aldose reductase activities (Yoshikawa et al., 2001). More pharmacological and mechanistic studies have been conducted with mangiferin than other Salacia constituents.
Various polyphenolic proanthocyanidin oligomers have been isolated from S. reticulata leaves (Koga et al., 2013). The main constituents were shown to be epigallocatechin, epicatechin, and epiafzelechin with flavanol polymerization estimated to be about five units in length. These polyphenols were believed to be responsible for the pancreatic lipase inhibitory activity of hydroalcoholic leaf extracts and thus lipid lowering activity.
A number of other minor constituents also have been identified and characterized from S. reticulata and other Salacia species including various triterpenes and sesquiterpenes (Arunakumara and Subasinghe, 2010; Oe and Ozaki, 2008; Gao et al., 2008; Morikawa et al., 2003). The roles of these minor constituents with respect to the observed pharmacological properties of extracts are not known. Based on various safety studies, they also do not appear to exhibit significant toxicological properties because of the amounts present in the extracts.
In summary, mangiferin, salicinol, ponkoranol, kotalanol, and salaprinol appear to be the primary compounds responsible for the anti‐diabetic effects of Salacia. The flavanols epigallocatechin, epicatechin, and epiafzelechin appear to be primarily responsible for the lipid‐lowering effects of Salacia preparations. Various triterpenes and sesquiterpenes are present as minor constituents of Salacia and may not be present in sufficient concentrations to exhibit significant pharmacological effects.
Discussion
Various animal studies have examined the effects of extracts of S. reticulata as well as S. oblonga and S. chinensis on a number biochemical, metabolic, and histopathological parameters. The extracts have been shown to modulate blood glucose and lipid levels via a number of credible mechanisms, provide cardiac and hepatic protection, exhibit antiinflammatory and antioxidant properties, and modulate increases in body weight gain. Extracts used have involved various solvents, with aqueous extracts primarily being used.
Plant parts used have included leaves, roots, stems, and root bark. When comparing various species and plant parts, the roots of S. reticulata were shown to exhibit greatest activity with respect to inhibiting postprandial blood glucose levels.
A number of clinical investigations have been conducted with Salacia extracts. These studies have indicated that aqueous extracts when administered to type II diabetic subjects effectively control blood glucose levels and do so without adverse effects. Furthermore, blood lipids are also modulated. In addition, tissue damaging glycosylation reactions involving elevated glucose levels are also decreased as indicated by lowered levels of HbA1c. Lack of adequate detail with respect to plant part used and methods of extract preparation constitutes the major problems associated with a number of the clinical studies. As a consequence, these studies cannot be reproduced by other investigators.
Various toxicity studies have been conducted in rats and mice. The results indicate that doses of extracts as high as 2000–2500 mg/kg per day are without adverse effect. Several studies indicate that liver hypertrophy may occur, although one study indicated that lower liver weights were observed. However, in general, the doses at which hepatic hypertrophy have occurred are many‐fold higher than a typical human dose.
The hypoglycemic and hypolipidemic mechanisms of action of Salacia extracts have been examined by various investigators, and it is evident that multiple mechanisms involving multiple constituents exist. For example, Salacia root extracts modulate multiple targets including α‐glucosidase, aldose reductase, and pancreatic lipase, PPAR‐α‐mediated transcription of lipogenic genes, GLUT4 transporter, and angiotensin II type 1 receptor.
The relative role of these various mechanisms may depend upon the relative concentrations of the various active agents in a given extract. The relative proportion of the active constituents will depend upon the extraction solvent and process, Salacia species involved, plant part used, growing and harvesting conditions, soil, and genetic variations. Lack of standardization of extracts and the general lack of knowledge of the composition of the extracts used represent a major problem and point of concern.
The most prominent active agents in Salacia include salacinol, kotalanol, mangiferin, and kotalagenin 16‐acetate. Mangiferin has been the most extensively studied individual constituent. Numerous minor constituents also exhibit pharmacological activity. A major advantage of using an herbal extract is the existence of multiple mechanisms involving multiple ingredients. A disadvantage is the fact that standardization is difficult because multiple active constituents are present.
Finally, no human clinical study to date has assessed the ability of Salacia extracts to serve as a weight loss agent, although this has been clearly established in animal studies. It is assumed that Salacia extracts can support weight loss and weight management because they are able to modulate glucose and lipid metabolism. Long‐term human studies assessing weight loss as well as safety are needed. The most useful would be studies conducted with defined chemical compositions.
Conflict of Interest
The authors have no conflicts of interest to report.
Stohs, S. J. , and Ray, S. (2015) Anti‐diabetic and Anti‐hyperlipidemic Effects and Safety of Salacia reticulata and Related Species. Phytother. Res., 29: 986–995. doi: 10.1002/ptr.5382.
References
- Akaki J, Morikawa T, Miyaka S, et al 2014. Evaluation of Salacia species as anti‐diabetic natural resources based on quantitative analysis of eight sulphonium constituents: a new class of α‐glucosidase inhibitors. Phytochem Anal 25: 544–550. [DOI] [PubMed] [Google Scholar]
- Akase T, Shimada T, Harasawa Y, et al 2011. Preventive effects of Salacia reticulata on obesity and metabolic disorders in TSOD mice. Evid Based Compl Alt Med . DOI:10.1093/ecam/nep052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunakumara KKIU, Subasinghe S. 2010. Salacia reticulata WIGHT: A review of botany, phytochemistry and pharmacology. Trop Agr Res Extens 13: 41–47. [Google Scholar]
- Bhat BM, Raghuveer CV, D'Souza V, Manjrekaar PA. 2012. Antidiabetic and hypolipidemic effect of S. oblonga in streptozotocin‐induced diabetic rats. J Clin Diagn Res 6: 1685–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar CN, Madhyastha S, Benjamin S, et al 2009. Free radical scavenging activities and anti‐diabetic properties of various extracts of Salacia reticulata . J Physiol Sci 21: 48–57. [Google Scholar]
- Collene AL, Hertzler SR, Williams JA, et al 2005. Effects of a nutritional supplement containing Salacia oblonga extract and insulinogenic amino acids on postprandial glycemia, insulinemia, and breath hydrogen responses in healthy adults. Nutrition 21: 848–854. [DOI] [PubMed] [Google Scholar]
- Flammang AM, Erexson GL, Mirwald JM, et al 2007. Toxicological and cytogenetic assessment of a Salacia oblonga extract in a rat subchronic study. Food Chem Toxicol 45: 1954–1962. [DOI] [PubMed] [Google Scholar]
- Gao XH, Xie N, Feng F. 2008. Studies on chemical constituents of Salacia prinoides . Zhong Yao Cai 31: 1348–1351. [PubMed] [Google Scholar]
- Giron MD, Sevillano N, Salto R, et al 2009. Salacia oblonga extract increases glucose transporter 4‐mediated glucose uptake in L6 rat myotubes: role of mangiferin. Clin Nutr 28: 565–574. [DOI] [PubMed] [Google Scholar]
- He L, Rong X, Jiang J, et al 2011. The ayurvedic medicine Salacia oblonga attenuates diabetic renal fibrosis in rats: suppression of angiotensin II AT1 signalling. Evid Based Compl Alt Med . DOI:10.1093/ecam/nepo95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcock PM, Hertzler SR, Williams JA, et al 2005. Effects of a medical food containing an herbal alpha‐glucosidase inhibitor on postprandial glycemia and insulinemia in healthy adults. J Amer Diet Assoc 105: 65–71. [DOI] [PubMed] [Google Scholar]
- Huang TH, Yang Q, Harada M, et al 2006. Salacia oblonga root improves cardiac lipid metabolism in Zucker diabetic fatty rats: modulation of cardiac PPAR‐alpha‐mediated transcription of fatty acid metabolic genes. Toxicol Appl Pharmacol 210: 78–85. [DOI] [PubMed] [Google Scholar]
- Huang TH, He L, Qin Q, et al 2008. Salacia oblonga root decreases cardiac hypertrophy in Zucker diabetic fatty rats: inhibition of cardiac expression of angiotensin II type 1 receptor. Diabetes Obes Metab 10: 574–585. [DOI] [PubMed] [Google Scholar]
- Im R, Mano HJ, Nakatani S, et al 2008. Safety evaluation of the aqueous extract Kothala himbutu (Salacia reticulata) stem in the hepatic gene expression profile of normal mice using DNA microarrays. Biosci Biotechnol Biochem 72: 3075–3083. [DOI] [PubMed] [Google Scholar]
- Im R, Mano H, Matsuura T, et al 2009. Mechanisms of blood glucose‐lowering effect of aqueous extract from stems of Kotala himbutu (Salacia reticulata) in the mouse. J Ethnopharmacol 121: 234–240. [DOI] [PubMed] [Google Scholar]
- Ismail TS, Gopalakrishnan S, Begum VH, et al 1997. Anti‐inflammatory activity of Salacia oblonga Wall. and Azima tetracantha Lam. J Ethnopharmacol 56: 145–152. [DOI] [PubMed] [Google Scholar]
- Jayawardena MH, de Alwis NM, Hettigoda V, et al 2005. A double blind randomized placebo controlled cross over study of a herbal preparation containing Salacia reticulata in the treatment of type 2 diabetes. J Ethnopharmacol 97: 215–218. [DOI] [PubMed] [Google Scholar]
- Kajimoto O, Kawamuri S, Shimoda H, et al 2000. Effects of diet containing Salacia reticulata on mild type 2 diabetes in humans – a placebo controlled cross over trail. J Jap Soc Nutr Food Sci 53: 199–205. [Google Scholar]
- Kannan M, Saravanan S, Sagayam S, et al 2011. Toxicological evaluation of ethanolic extract of Salacia chinensis roots in Wistar albino rats. Int J Univ Pharm Life Sci 1: 145–153. [Google Scholar]
- Karunanayake EH, Welihinda J, Sirimanne SR, et al 1984. Oral hypoglycemic actrivity of some medicinal plants of Sri Lanka. J Ethnopharmacol 11: 223–231. [DOI] [PubMed] [Google Scholar]
- Kataoka K. 2006. The hypoglycemic potential and safety profile of Salacia reticulate Wight extract powder in healthy and diabetic subjects. New Diet Ther 20: 47–53. [Google Scholar]
- Kishino E, Ito T, Fujita K, et al 2006. A mixture of Salacia reticulata (Kotala himbutu) aqueous extract and cyclodextrin reduces the accumulation of visceral fat mass in mice and rats with high‐fat diet‐induced obesity. J Nutr 136: 433–439. [DOI] [PubMed] [Google Scholar]
- Kishino E, Ito T, Fujita K, et al 2009. A mixture of Salacia reticulata (Kotala himbutu) aqueous extract and cyclodextrin reduces body weight gain, visceral fat accumulation, and total cholesterol and insulin increases in male Wistar fatty rats. Nutr Res 29: 55–63. [DOI] [PubMed] [Google Scholar]
- Koga K, Hisamura M, Kanetaka T, et al 2013. Proanthocyanidin oligomers isolated from Salacia reticulate leaves potently inhibit pancreatic lipase activity. J Food Sci 78: 105–111. [DOI] [PubMed] [Google Scholar]
- Koteshwar P, Raveeendra KR, Allan JJ, et al 2013. Effect of NR‐Salacia on post‐prandial hyperglyceria: a randomized double‐blind, placebo‐controlled, cross‐over study in healthy vounteers. Phamracogn Mag 9: 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian GA, Manjushi V, Nair SS, et al 2014. Short‐term effect of G‐400, a polyherbal formulation in the management of hyperglycemia and hyperlipidemia conditions in patients with type 2 diabetes. Nutrition 30: 1156–1164. [DOI] [PubMed] [Google Scholar]
- Krishnakumar K, Augusti KT, Vijayammal PL. 1999. Hypoglycaemic and anti-oxidant activity of Salacia oblonga Wall. extract in streptozotocin-induced rats. Indian J. Physiol. Pharmacol 43: 510–514. [PubMed] [Google Scholar]
- Li Y, Peng G, Wen S, et al 2004. Salacia oblonga improves cardiac fibrosis and inhibits postprandial hyperglycemia in obese Zucker rats. Life Sci 75: 1735–1746. [DOI] [PubMed] [Google Scholar]
- Li Y, Huang TH, Yamahara J. 2008. Salacia root, a unique ayurvedic medicine, meets multiple targets in diabetes and obesity. Life Sci 82: 1045–1049. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Murakami T, Yashiro K, et al 1999. Anti‐diabetic principles of natural medicines. IV. Aldose reductase and α‐glucosidase inhibitors from the roots of Salacia oblonga WALL. (Celastraceae): structure of a new friedalane‐type triterpene, kotalagenin 16‐acetate. Chem Pharm Bull 47: 1725–1729. [DOI] [PubMed] [Google Scholar]
- Mohan S, Eskandari R, Pinto BM. 2014. Naturally occurring sulfonium ion glucosidase inhibitors and their derivatives: a promising class of potential antri‐diabetic agents. Acc Chem Res 47: 211–225. [DOI] [PubMed] [Google Scholar]
- Morikawa T, Kishi A, Pongpiriyadacha Y, Matsuda H, Yoshikawa M. 2003. Structures of new friedelane‐type triterpenes and eudesmane‐type sesquiterpenes and aldose reductase inhibitors form Salacia reticulata . J Nat Prod 55: 1191–1196. [DOI] [PubMed] [Google Scholar]
- Morikawa T, Akaki J, Ninomiya K et al. 2015. Salacinol and related analogs: new leads for type 2 diabetes therapeutic candidates from the Thai traditional natural medicine Salacia chinensis. Nutrients 7: 1480–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka O, Morikawa T, Miyake S, et al 2010. Quantitative determination of potent alpha‐glucosidase inhibitors, salacinol and kotalanol, in Salacia species using liquid chromatograph‐mass spectrometry. J Pharm Biomed Anal 52: 770–773. [DOI] [PubMed] [Google Scholar]
- Muruganandan S, Gupta S, Kataria M, et al 2002. Mangiferin protects the streptozotocin‐induced oxidative damage to cardiac and renal tissues in rats. Toxicology 176: 165–173. [DOI] [PubMed] [Google Scholar]
- Muruganandan S, Srinivasan K, Gupta S, et al 2005a. Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. J Ethnopharmacol 97: 497–501. [DOI] [PubMed] [Google Scholar]
- Muruganandan S, Lal J, Gupta PK. 2005b. Immunotherapeutic effects of mangiferin mediated by inhibition of oxidative stress to activated lymphocytes, neutrophils and macrophages. Toxicology 215: 57–68. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Zhang Y, Matsuda H, et al 2011. Chemical structures and hepatoprotective effects of constituents from the leaves of Salacia chinensis . Chem Pharm Bull 59: 1020–1028. [DOI] [PubMed] [Google Scholar]
- Nataka K, Taniguchi Y, Yoshioka N, et al 2011. A mixture of Salacia oblonga extract and IP‐PA1 reduces fasting plasma glucose (FPG) and low‐density lipoprotein (LDL) cholesterol levels. Nutr Res Pract 5: 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oe H, Ozaki S. 2008. Hypoglycemic effect of 13‐membered ring thiocyclitol, a novel alpha‐glucosidase inhibitor from Kothala‐himbutu (Salacia reticulata). Biosci Biothechnol Biochem 72: 1962–1964. [DOI] [PubMed] [Google Scholar]
- Radha R, Amrithaveni M. 2009. Role of medicinal plant Salacia reticulata in the management of type II diabetic subjects. Anc Life Sci 29: 14–16. [PMC free article] [PubMed] [Google Scholar]
- Rajashree R, Parineetha P, Bjhat P, et al 2011. Effects of a mixture of Salacia reticulata W. and Catharanthus roseus L. extracts in streptozotocin‐induced juvenile diabetic rats. J Physiol Biomed Sci 24: 5–8. [Google Scholar]
- Rong X, Kim MS, Su N, et al 2008. An aqueous extract of Salacia oblonga root, a herb‐derived peroxisome proliferator‐activated receptor‐alpha activator, by oral gavage for 28 days induces gender‐dependent hepatic hypertrophy in rats. Food Chem Toxicol 46: 2165–2172. [DOI] [PubMed] [Google Scholar]
- Ruvin Kumara NKVM, Pathirana RN, Pathirana C. 2005. Hyperglycemic activity of the root and stem of Salacia reticulata var. β‐diandra in alloxan diabetic rats. Pharmaceut Bull 43: 219–225. [Google Scholar]
- Sekiguchi Y, Mano H, Nakatani S, Shimizu J, Kobata K, Wada M. 2012. Anti‐proliferative effects of Salacia reticulata leaves hot‐water extract on interleukin‐1β‐activated cells derived from the synovium of rheumatoid arthritis model mice. BMC Res Notes 5: 198 DOI:10.1186/1756-0500-5-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellamuthu PS, Arulselvan P, Muniappan BP, Fakurazi S, Kandasamy M. 2013. Mangiferin from Salacia chinensis prevents oxidative stress and protects pancreatic β‐cells in streptozotocin‐induced diabetic rats. J Med Food 16: 719–727. [DOI] [PubMed] [Google Scholar]
- Sellamuthu PS, Arulselvan P, Fakurazi S, Kandasamy M. 2014. Beneficial effects of mangiferin isolated from Salacia chinensis on biochemical and hematological parameters in rats with streptozotocin‐induced diabetes. Pak J Pharm Sci 27: 161–167. [PubMed] [Google Scholar]
- Shimada T, Nagai E, Harasawa Y, et al 2010. Metabolic disease prevention and suppression of fat accumulation by Salacia reticulata . J Nat Med 64: 266–274. [DOI] [PubMed] [Google Scholar]
- Shimada T, Nagai E, Harasawa Y, et al 2011. Salacia reticulata inhibits differentiation of 3 T3‐L1 adipocytes. J Ethnopharmacol 136: 67–74. [DOI] [PubMed] [Google Scholar]
- Shimada T, Nakayama Y, Harasawa Y, et al 2014. Salacia reticulata has therapeutic effects on obesity. J Nat Med 68: 668–676. [DOI] [PubMed] [Google Scholar]
- Shimoda H, Kawamori S, Kawahara Y. 1998. Effects of an aqueous extract of Salacia reticulata, a useful plant in Sri Lanka, on postprandial hyperglycemia in rats and humans. Nippon Eiyo Shokuryo Gakkaishi 151: 279–287. [Google Scholar]
- Shimoda H, Fujimura T, Makino K, et al 1999. Safety profile of extractive from trunk of Salacia reticulata (Colastraceae). J Food Hyg Soc Jap 40: 198–205. [Google Scholar]
- Shimoda H, Asano I, Yamada Y. 2001. Antigenicity and phototoxicity of water‐soluble extract from Salacia reticulata (Celastraceae). Shokuhin Eiseigaku Zasshi 42: 144–147. [DOI] [PubMed] [Google Scholar]
- Shivaprasad HN, Bhanumathy M, Sushma T, et al 2013. Salacia reticulata improves serum lipid profiles and glycemic control in patients with pre‐diabetes and mild to moderate hyperlipidemia: a double‐blind, placebo‐controlled, randomized trial. J Med Food 16: 564–568. [DOI] [PubMed] [Google Scholar]
- Sim L, Jayakanthan K, Mohan S, et al 2010. New glucosidase inhibitors for an ayurvedic herbal treatment for type 2 diabetes: structures and inhibition of human intestinal maltase‐glucoamylase with compounds from Salacia reticulata . Biochemistry 49: 443–451. [DOI] [PubMed] [Google Scholar]
- Singh RG, Rathore SS, Wani IA, et al 2015. Effects of Salacia oblonga on cardiovascular risk factors in chronic kidney disease patients: a prospective study. Saudi J Kidney Dis Transpl 26: 61–66. [DOI] [PubMed] [Google Scholar]
- Suwannalert P, Kariya R, Suzu I, Okada S. 2014. The effects of Salacia reticulata on anti‐cellular oxidants and melanogenesis inhibition in alpha‐MSHstimulated and UV irradiated B16 melanoma cells. Nat Prod Commun 9: 551–554. [PubMed] [Google Scholar]
- Tanimura C, Terada I, Hiramatu K, et al 2005. Effect of a mixture of aqueous extract from Salacia reticulata (Kotala himbutu) and cyclodextrin on the serum glucose and the insulin levels in sucrose tolerance test and on serum glucose level changes and gastrointestinal disorder by massive ingestion. Yonago Igaku ZAsshi 56: 85–93. [Google Scholar]
- Venkateswarlu V, Kokate CK, Rambhau D, Veeresham C. 1993. Antidiabetic activity of roots of Salacia macrosperma . Planta Med 59: 391–393. [DOI] [PubMed] [Google Scholar]
- Williams JA, Choe YS, Noss MJ, et al 2007. Extract of Salacia oblonga lowers acute glycemia in patients with type 2 diabetes. Amer J Clin Nutr 86: 124–130. [DOI] [PubMed] [Google Scholar]
- Wolf BW, Weisbrode SE. 2003. Safety evaluation of an extract form Salacia oblonga . Food Chem Toxicol 41: 867–874. [DOI] [PubMed] [Google Scholar]
- Xie W, Tanabe G, Akaki J, et al 2011. Isolation, structure identification and SAR studies on the thiosugar sulfonium salts, neosalaprinol and neoponkoranol, as potent α‐glucosidase inhibitors. Bioorg Med Chem 19: 2015–2022. [DOI] [PubMed] [Google Scholar]
- Yokotani K, Chiba T, Sato Y, et al 2013. Effect of three herbal extracts on cytochrome P450 and possibility of interactions with drugs. Shakuhin Eiseiguku Zasshi 54: 56–64. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Nishida N, Shimoda H, et al 2001. Polyphenol constituents from Salacia species: quantitative analysis of mangiferin with alpha‐glucosidase and aldose reductase inhibitory activities. Yakugaku Zasshi 121: 371–378. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Shimoda H, Nisida N, et al 2002a. Salacia reticulata and its polyphenolic constituents with lipase inhibitory and lipolytic activities have mild anti‐obesity effects in rats. J Nutr 132: 1819–1824. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Ninomiya K, Shimoda H, Nishida N, Matsuda H. 2002b. Hepatoprotective and antioxidant properties of Salacia reticulata: preventive effects of phenolic constituents on CCl4‐induced liver injury in mice. Biol Pharm Bull 25: 72–76. [DOI] [PubMed] [Google Scholar]
- Yoshino K, Miyauchi Y, Kanetaka T, et al 2009. Anti‐diabetic activity of a leaf extract prepared from Salacia reticulata in mice. Biosci Biotechnol Biochem 73: 1096–1104. [DOI] [PubMed] [Google Scholar]


