Abstract
Australia has the highest prevalence of actinic keratoses (AK) worldwide. Because of the risk of transformation of AK to invasive squamous cell carcinomas, consensus guidelines recommend that AK are removed using appropriate therapies to prevent progression to invasive disease. Daylight photodynamic therapy (PDT) is emerging as an efficacious treatment for AK, particularly for patients who require treatment of large areas of chronic actinic damage that can be exposed easily to daylight. Daylight PDT with methyl aminolevulinate (MAL) cream is a simple treatment for AK, almost painless, well tolerated and convenient, requiring minimal time in the clinic. Randomised controlled studies from northern Europe and Australia support the use of daylight PDT as an effective therapy for grade I and II AK on the face and scalp. There is sufficient daylight to conduct daylight PDT in Australia at any time of the year and during most weather conditions. Hence, daylight PDT with MAL can be included as an effective and well‐tolerated new treatment option for the treatment of AK in Australia. These consensus recommendations provide guidelines for Australian clinicians on the use of daylight PDT in the treatment of diagnosed AK.
Keywords: actinic keratosis, daylight photodynamic therapy, metvix, solar keratosis
Abbreviations
- AK
actinic keratoses
- c‐PDT
conventional photodynamic therapy
- MAL
methyl aminolevulinate
- PDT
photodynamic therapy
- PpIX
protoporphyrin IX
- SCC
squamous cell carcinoma
Introduction
Actinic keratoses (AK), also known as solar keratoses, are pre‐cancerous lesions that commonly occur in sun‐exposed skin.1 In Australia, where rates are highest, AK affects 40 to 60% of adults aged over 40 years.1, 2 Using the Olsen three‐point scale,3 AK lesions are categorised as grade I (single or few lesions, better felt than seen), grade II (moderately thick lesions, easily felt and seen), or grade III (hyperkeratotic lesions). Significantly, AK have the potential to progress to invasive squamous cell carcinomas (SCC), however the actual risk of malignant transformation is poorly defined, with the reported risk varying from <0.025 to 16% per year.4, 5, 6 Because of the malignant potential and the unpredictability of the transformation from AK to SCC, consensus guidelines recommend that AK are removed using appropriate therapies to prevent progression to invasive disease.5, 7, 8
Conventional photodynamic therapy (c‐PDT) utilising photosensitising agents such as 5‐aminolaevulinic acid or methyl aminolevulinate (MAL) has emerged as a successful treatment for AK, particularly for the treatment of large areas of field change.5, 7, 9 During treatment, the topically applied agents are converted to photoactive porphyrins (e.g., protoporphyrin IX [PpIX]), through enzymes in the haem biosynthetic pathway10 and afterwards activated by dedicated light‐emitting equipment (e.g. red light‐emitting diode light sources). These photoactive metabolites are formed in greater concentrations by dysplastic and neoplastic cells in contrast to normal keratinocytes, resulting in the fast and specific destruction of lesions upon light exposure. However, while c‐PDT is efficacious, the common adverse effects of burning, stinging and associated pain are an impediment for patients with widespread lesions on the face and scalp.9, 10 In addition, c‐PDT requires relatively long incubation times (hours), which can be inconvenient for patients, and dedicated equipment, which can limit availability and increase costs.10
Daylight photodynamic therapy (daylight PDT) with MAL cream (Metvix, Galderma Laboratories, Paris, France) is a new therapy for AK that offers advantages over c‐PDT in cost, time and tolerability. Daylight PDT is a simple treatment that uses visible light as the light source for the photoactivation of PpIX, thereby eliminating the requirement for costly instrumentation and prolonged in‐clinic times. Significantly, daylight PDT is a tolerable, almost painless treatment, because of the continuous production and activation of active PpIX and the prolonged low intensity nature of photoactivation.11
International consensus recommendations for daylight PDT for treatment of AK have been developed primarily by and for dermatologists in Europe and Canada.12 Since this consensus, a phase III study has been performed in Australia confirming the efficacy and tolerability of daylight PDT.13 Local practice guidelines on the use of daylight PDT in Australia are needed because of the differences in weather conditions from those of Europe and the extent of the solar damage and patients' characteristics in the Australian population. The aim of this position paper is to provide practical, consensus‐driven recommendations on daylight PDT for Australian clinicians (Table 1).
Table 1.
Development of a position statement on the use of daylight photodynamic therapy (PDT) with methyl aminolevulinate (MAL) for patients with actinic keratoses (AK)
| Variable | Development of the position statement |
|---|---|
| Objective | To generate consensus on the patient population most suitable for daylight PDT and how and when to treat patients with AK in Australia |
| Expert panel | Nine dermatologists from around Australia convened during a face‐to‐face meeting held in Sydney on 12 April 2014 to assess the clinical evidence of published clinical trials on the use of daylight PDT. A draft version of the position statement was developed from the minutes of the meeting and circulated to all members of the expert panel (authors) for revision and approval. |
| Method | A search of MEDLINE via PubMed (1966 to April 2014) was conducted and all relevant articles were identified using the following search terms: ‘actinic keratosis’, ‘actinic keratoses’, ‘solar keratosis’, ‘solar keratoses’, ‘photodynamic therapy’, ‘daylight’. No limits were placed on the search. |
| Levels of evidence | All publications retrieved were assessed for eligibility. All levels of evidence as defined by the National Health & Medical Research Council † were considered. In the absence of evidence, consensus recommendations from the expert panel were obtained. |
†Level I = systematic review of randomised controlled trials, level II = randomised controlled trial, Level III‐1 = pseudorandomised controlled trial, level III‐2 = comparative studies with concurrent controls, level III‐3 = comparative study without concurrent controls, level IV = case series.
Literature Review
The efficacy and safety of daylight PDT with MAL in the treatment of AK has been assessed in four randomised trials conducted in Europe11, 14, 15, 16, 17 and one randomised controlled trial conducted in Australia.13 The European trials consisted of a small exploratory study comparing daylight PDT with c‐PDT,11 two comparative studies with different concentrations of MAL cream16 and daylight exposure times14, 15 and, most recently, a phase III randomised trial comparing daylight PDT with c‐PDT (only preliminary results reported).17 In all trials, daylight PDT resulted in high total lesion response rates (> 70% grade I) with participants experiencing little to no pain during daylight exposure (Table 2).
Table 2.
Characteristics of randomised studies assessing the efficacy and/or safety of daylight photodynamic therapy (PDT) with methyl aminolevulinate (MAL) in subjects with actinic keratoses (AK) of the face or scalp
| Publication country/region | Study design treatment arms | Number enrolled (completed) mean age, sex, AK severity | Mean daylight exposure time | Mean lesion response rate (reduction at 3 months from BL) | Mean maximal pain scores ± SD during daylight exposure † |
|---|---|---|---|---|---|
|
Wiegell et al. 200811
northern Europe |
RCT, SB, single centre, split‐face daylight PDT vs c‐PDT |
n = 30 (29) 78 years, 77% M AK (severity NR) |
150 min (defined in protocol) |
Daylight PDT vs c‐PDT: 79 vs 71% (P = 0.13) |
Daylight PDT vs c‐PDT: 2.0 ± 1.9 vs 6.7 ± 2.2; P < 0.001 |
|
Wiegell et al. 200916
northern Europe |
RCT, DB, single centre, split‐face 16 vs 8% MAL |
n = 30 (29) 71 years, 87% M Thin AK ‡ |
244 min |
16% MAL vs 8% MAL: 77 vs 80% (P = 0.37) |
16% vs 8% MAL: 3.7 ± 2.4 vs 3.6 ± 2.4; P = 0.74 |
|
Wiegell et al. 2011, 201214, 15
northern Europe |
RCT, multicentre 2 vs 3 h § daylight exposure |
n = 145 (142), including 120 (119) grade 1 grade 1: 72 years, 81% M Mild to thick AK ‡ |
2 h: 131 min 3 h: 187 min |
2 vs 3 h, grade 1: 77 vs 75% (P = 0.57) 2 and 3 h pooled: grade I: 76% (n = 120 patients) ‡ grade II: 61% (n = 127 patients) ‡ grade III: 49% (n = 67 patients) ‡ |
2 vs 3 h, grade 1: 1.3 ± 1.5 vs 1.3 ± 1.5; P = 0.94 All patients: min 0, max 3 |
|
Rubel et al. 201413
Australia |
RCT, SB, multicentre, noninferiority (efficacy), superiority (safety), split‐face daylight PDT vs c‐PDT |
n = 100 (92) 70 years, 75% M Mild AK with or without moderate AK ‡ |
120 min (defined in protocol) |
daylight PDT vs c‐PDT: 89 vs 93% (noninferior: 95% CI −6.8 to −0.3) 96% of mild lesions maintained complete response after 24 weeks |
daylight PDT vs c‐PDT: 0.8 ± 1.2 (min 0, max 5) vs 5.7 ± 2.3 (min 0, max 10), P < 0.001 |
†Mean maximal pain score based on a numerical rating scale from 0 (no pain) to 10 (extreme pain). ‡The thickness of each lesion was graded according to Olsen et al. 19913 where grade I (mild) = slightly palpable AK, more easily felt than seen, grade II (moderate) = easily felt, and grade III (thick) = very thick or obvious AK. §Total time of exposure to daylight following application of MAL cream. BL, baseline; c‐PDT, conventional photodynamic therapy with red light‐emitting diode light; CI, confidence interval; DB, double‐blind; M, male; max, maximum; min, minimum; NR, not reported; RCT, randomised controlled trial; SB, single‐blind.
The Australian study was a 24‐week, phase III, randomised intra‐individual controlled trial of efficacy (noninferiority: daylight PDT vs c‐PDT) and safety (superiority of daylight PDT vs c‐PDT for maximal pain) enrolling 100 participants with predominantly (97 participants) mild AK. Although most lesions were mild, participants were severely affected, with approximately 14 lesions on each side of the face or scalp.13 Treatment with daylight PDT (1379 lesions at baseline) or c‐PDT (1372 lesions at baseline) was randomly assigned to either side of participants' face or scalp, and findings showed that daylight PDT was noninferior to c‐PDT with regard to lesion response (primary end‐point: 89 vs 93%, respectively; 95% CI: −6.8 to −0.3; week 12). Most lesions that regressed after treatment did not recur by the end of the study (week 24, daylight PDT: 96%, c‐PDT: 97%), showing a high maintenance response at 6 months. Participant‐reported pain was significantly lower with daylight PDT than with c‐PDT (Table 2) and fewer participants experienced treatment‐related adverse events (39/100 vs 59/100 participants, respectively).13 The most frequently related adverse events were dermatologic in nature. No treatment‐related adverse events were considered to be serious or severe, or led to study discontinuation.
Most participants who were treated with daylight PDT or c‐PDT in the clinical trials experienced erythema and desquamation.11, 15, 16 Although one study showed more severe erythema and desquamation in participants who were treated with daylight PDT than with c‐PDT,11 these effects were most likely a result of sunburn, which can be avoided with the application of a chemical sunscreen to the treatment site and entire sun‐exposed area.13, 16
Preliminary results from the recent European phase III study (conducted in northern and southern European countries) confirmed the Australian results, with one daylight PDT session noninferior to c‐PDT at 12 weeks in terms of AK grade I and II lesion response rates, and a very low frequency of participant‐reported pain with daylight PDT.17
Whom to Treat with Daylight PDT with Methyl Aminolevulinate Cream
Based on available data and clinical experience of the experts, daylight PDT is recommended for treatment of grade I and II AK on the face and scalp. Daylight PDT is also recommended as a first‐line therapy for patients who have received multiple treatments (e.g., cryotherapy) that have led to postinflammatory hypopigmentation. Although patients enrolled in the Australian study had individual lesions treated,13 the expert panel agreed that daylight PDT is recommended for use in patients who require field therapy (i.e., large areas of actinic damage) to treat visible lesions and subclinical disease, depending on the clinical need. This is consistent with current guidelines for treatment of AK7 and is supported by findings from the studies of daylight PDT (including field treatment) in northern Europe.11, 14, 15, 16
As daylight PDT requires exposure of the affected skin to daylight, the treatment is most suitable for lesions and areas of actinic damage on the face and scalp.12 Findings from a subgroup analysis of AK severity showed that following daylight PDT, lesion response rates of thin AK (76% [1572 lesions at baseline]) were higher than with moderate or thick AK (61% (974 lesions at baseline), 49% (222 lesions at baseline), as is also the case with c‐PDT and other topical treatments.14 Hence, the expert panel agreed that daylight PDT is suitable for lesions that are grade I or grade II based on the Olsen three‐point scale.3 Daylight PDT is not recommended for grade III (hyperkeratotic lesions), although the expert panel agreed that pretreatment of these lesions to reduce hyperkeratosis (i.e., with a keratolytic agent or physical removal) may be considered.
How to Treat Patients with Daylight PDT with Methyl Aminolevulinate Cream
Treatment period and weather conditions
In the Australian study, 2 h of daylight exposure across seven centres from March to June, 2012 was found to be sufficient to demonstrate similar efficacy of daylight PDT with c‐PDT, irrespective of meteorological conditions13 (Table 3). This finding is consistent with a recent meteorological study showing that there is sufficient daylight to conduct daylight PDT in Australia all year round, from as far south as Hobart to as far north as Darwin, irrespective of weather conditions when it is not raining (Fig. 1).18 Therefore, the expert panel recommends that daylight PDT in Australia can be conducted in all weather conditions except rain.
Table 3.
Percent change from baseline in the total number of treated actinic keratosis (AK) lesions per side of face or scalp after one treatment of daylight photodynamic therapy (daylight PDT) and conventional‐photodynamic therapy (c‐PDT) with methyl aminolevulinate (MAL) at 12 weeks in Australian patients13 exposed to different meteorological conditions
| Metrological conditions | Number of patients | Treatment administered (%) | |
|---|---|---|---|
| Daylight PDT | c‐PDT | ||
| Sunny | 63 | 89 | 91 |
| Partially cloudy | 24 | 88 | 94 |
| Cloudy | 9 | 86 | 95 |
Figure 1.
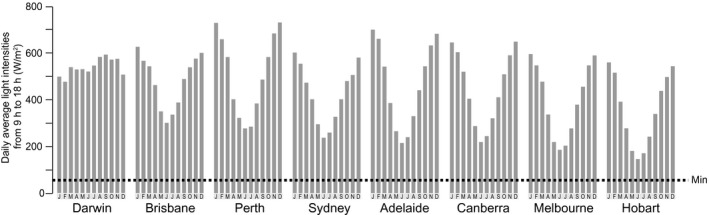
Daily average global radiation showing the monthly range of effective daylight exposure for daylight photodynamic therapy (PDT) across several Australian cities. The horizontal line indicates the minimum effective light intensity level (40 W/m2) for daylight PDT. Data from Spelman and colleagues. 18
The timing of daylight PDT should take into account patients' preferences for more extreme weather conditions (e.g. heat, cold, humidity) to encourage their compliance with the 2‐h exposure time. The treatment can be conducted within office hours, providing sufficient time is available for 2 h of daylight exposure following the application of MAL cream.
What to tell patients before treatment
Clinicians should explain the differences between visible light and UV light to ensure the patients understand the rationale for daylight exposure during treatment with daylight PDT, and why a chemical sunscreen is needed during and following treatment. Daylight PDT utilises visible light, which has a longer wavelength (380 to 780 nm) than UV light (100 to 380 nm) (Fig. 2). Therefore, patients can be protected from harmful UV rays during daylight PDT by applying a chemical sunscreen to the treatment area and by covering all non‐treatment areas. This sunscreen will not prevent visible light from penetrating and activating PpIX in the skin. In particular, clinicians should emphasise that physical sunscreens (e.g. zinc oxide, iron oxide, titanium dioxide or colour tints) prevent visible light from activating PpIX and may compromise its efficacy. The expert panel recommends that patients are asked to attend the clinic wearing long‐sleeved, loose clothing (including long trousers or a skirt) that covers all body areas except for the treatment sites.
Figure 2.
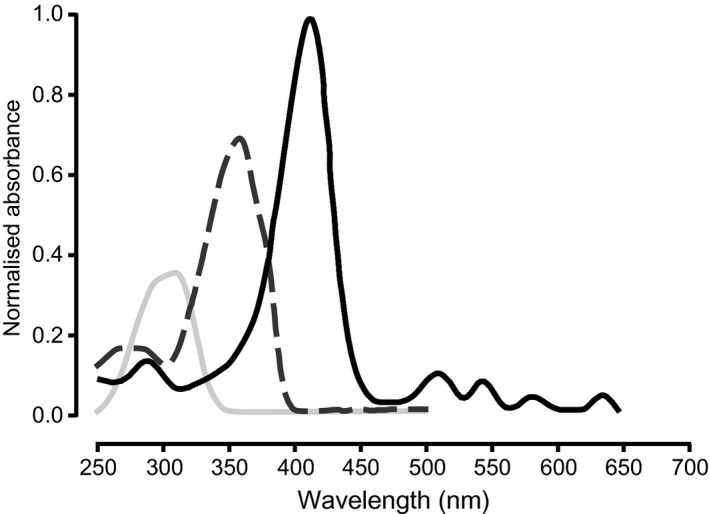
Comparison of  protoporphyrin IX (PpIX) absorption spectrum and spectrums for
protoporphyrin IX (PpIX) absorption spectrum and spectrums for  generic chemical UV‐A and
generic chemical UV‐A and  UV‐B sunscreens.
UV‐B sunscreens.
Because daylight PDT is almost painless,13 the expert panel recommends that patients are made aware of the potential for skin reactions (e.g. erythema, desquamation), which will peak in intensity at 2–3 days and rapidly resolve. Most skin reactions on the face and scalp resolve within 7 days of treatment and any residual erythema usually resolves within 3 months. Daylight PDT has a low risk of scarring or hypopigmentation following treatment13 and, similar to c‐PDT, daylight PDT results in improved cosmesis.19
Protocol before MAL cream application
Evidence from the clinical trials conducted to date suggest that the affected area should be prepared before the application of MAL cream13, 14, 15, 16 as follows (Table 4). Firstly, the affected area(s) should be washed (e.g. to remove make‐up or moisturiser). Secondly, a chemical sunscreen of sun protection factor 30 or higher should be applied to protect all sun‐exposed areas of the skin during the 2‐h exposure to daylight. As described above, physical sunscreens must be avoided. Third, the surface of the lesions or areas of actinic damage should be prepared to remove any scales or scabs. Skin preparation is important as it can assist with the penetration of MAL cream into the treatment area and contribute to improved cosmetic outcomes.19
Table 4.
Protocol for daylight photodynamic therapy (daylight PDT) with methyl aminolevulinate (MAL) in patients with actinic keratoses (AK) of the face or scalp
| Variable | Recommendation |
|---|---|
| Patient population | Patients with grades I and II AK on the face and scalp |
| Treatment period | Any time of the year within office hours, ensuring sufficient time is available for 2 h of daylight exposure following the application of MAL cream |
| Weather conditions | All weather conditions except rain; dependent on the toleration of patients to heat, cold, and humidity |
| Treatment modality |
|
| Follow up | According to usual clinical practice; treated lesions should be evaluated after 3 months and, if necessary, a second treatment session should be given. |
The expert panel agreed that, in clinical practice, clinicians may apply sunscreen before or after skin preparation. In one retrospective case series,20 sunscreen was applied to patients with thin to moderately thick AK after curettage. Sixteen of the 18 patients achieved a complete lesion response.
Curettage is the recommended method for skin preparation of the affected areas11, 13, 14, 15, 16 as it is effective, acceptable and relatively inexpensive.21 Other inexpensive methods like abrasive skin preparation pads, such as those used in cardiology before electrocardiogram or mechanical or chemical microdermabrasion may be considered, depending on the treatment area and availability of equipment.19 Some methods (e.g. microneedling ablative or nonablative fractional laser) may exacerbate localised side‐effects such as pain and local skin reactions.22
Application of MAL cream
Following skin preparation, a thin, even layer of MAL cream is applied to the lesion and surrounding area of normal skin or to the entire area of actinic damage. MAL cream can be applied by a clinician or other qualified staff by spatula or hand using disposable gloves, with no occlusion. The expert panel recognised that, in clinical practice, a thin layer of MAL cream is sufficient.17 In addition, preliminary evidence suggests that thinner applications of MAL cream (0.1 mm, 0.2 mm and 0.5 mm) result in similar levels of PpIX fluorescence compared with a 1‐mm application.17 Therefore, the expert panel recommends 1 to 2 g of MAL cream to treat a full face, based on their experience.
Daylight exposure
Daylight exposure should begin immediately or within a maximum of 30 min of applying MAL cream. Patients must then remain outdoors, comfortably, in full daylight so that they receive a total of 2‐h exposure to daylight after the application of MAL cream. In one clinical trial, a small but significant association (P = 0.02, r2 = 0.17) was found between the first sensation of pain and a delay greater than 30 min in exposure to daylight after the application of MAL cream.16 Together with anecdotal evidence from a retrospective case series,20 these findings suggest that patients who remain indoors for longer than 30 min after applying MAL cream may be at risk of increased pain, presumably arising from the greater accumulation of PpIX before sun exposure.23
There is no evidence to support that daylight PDT with daylight exposure times shorter than 2 h is efficacious or to suggest that longer exposure times may result in greater efficacy.15 The expert panel recommends that daylight PDT is conducted for 2 h, as this is the exposure time that is required to produce and activate PpIX. If the daylight exposure is reduced to 1 h, the production of PpIX might be insufficient.
Patients are to remain outdoors and avoid water‐based activities or activities that may result in excessive perspiration. The expert panel recommends that patients who are uncomfortable in full daylight take shelter, if necessary, from time to time in a shaded outdoor area. Patients should avoid darkly shaded areas (e.g. those close to a building or at the back of a balcony or deck) where there is a lack of diffuse light. Patients should ensure that the treatment area is continuously exposed to daylight (e.g. by not wearing a scarf or hat if the affected area is on the scalp). If it begins to rain before the MAL cream has been applied, the expert panel recommends that the treatment is postponed. If it begins to rain after the MAL cream is applied, the expert panel recommends that the patient takes shelter from the rain in an undercover outdoor area with diffuse light and completes the 2‐h exposure to daylight. Daylight exposure longer than 2 h is not recommended due to the increased risk of inflammation (e.g. the increased risk of sunburn).
Completion of treatment
After daylight exposure is complete, any residual MAL cream should be washed off, either at the clinic or the patient's home. The expert panel recommends that patients who may not be compliant with the exposure time can be asked to return to the clinic immediately after the 2‐h exposure to daylight. The expert panel also recommends that patients apply a sunscreen at the end of daylight exposure and continue to protect the treated areas from the sun until sundown of the treatment day. They should also apply a moisturiser at night to hydrate the skin for up to 1 week following treatment.
Follow up
In all randomised studies conducted to date, patients have been evaluated at 3 months after daylight PDT.11, 13, 14, 15, 16 The expert panel recommends that patients should be followed up, evaluated and treated for any residual keratotic lesions, according to usual clinical practice. Figure 3 shows a patient before treatment with daylight PDT, as well as at 5 days and 10 days after treatment.
Figure 3.
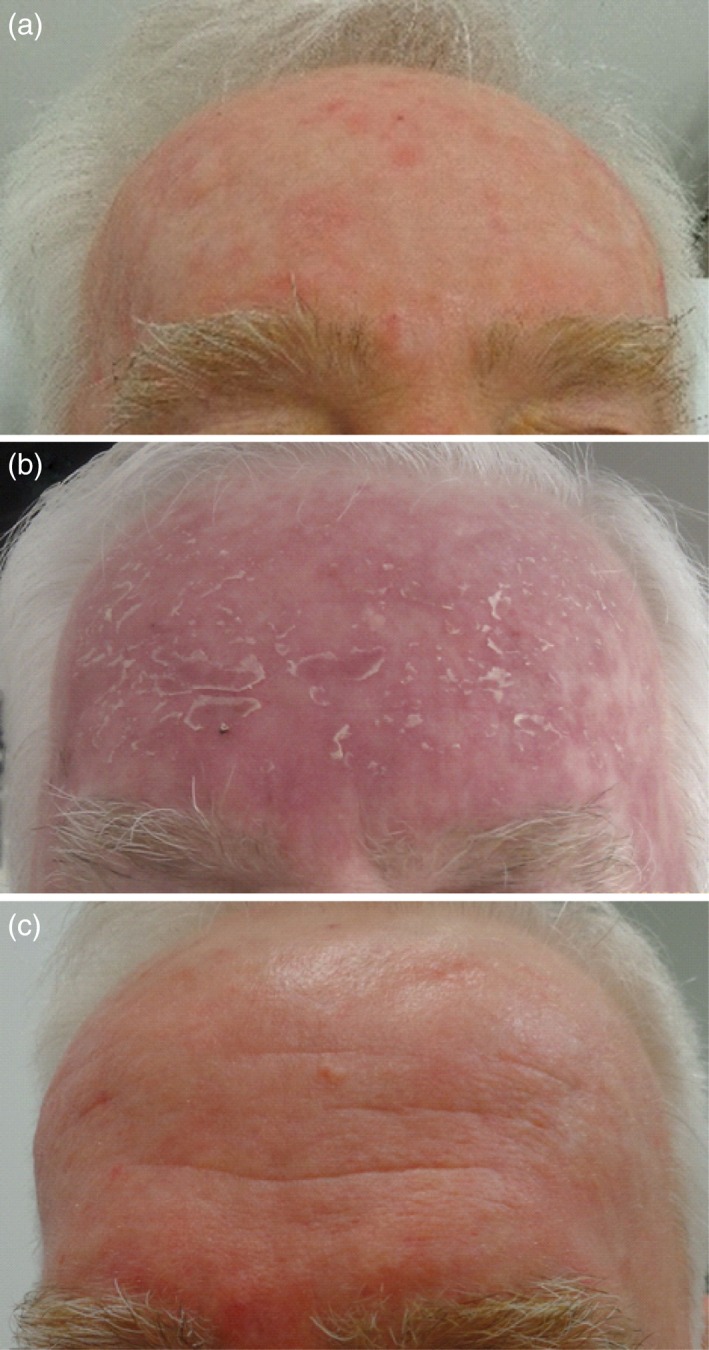
Photographs of a patient with actinic keratoses (a) before treatment with daylight photodynamic therapy, (b) 5 days after treatment, and (c) 10 days after treatment.
Conclusion
This position paper is based on a consensus assessment of published clinical trials by an expert panel of dermatologists and is the first guideline for Australian clinicians on the use of daylight PDT (Table 4). Although c‐PDT is a successful treatment for AK,5, 9, 24 the unpredictability of tolerance during treatment in some patients, extended treatment times, and costly instrumentation has limited its use. Daylight PDT is a simple and practical new treatment option for AK that allows large areas of actinic damage to be treated with reduced in‐clinic treatment times and little or no pain. Hence, daylight PDT with MAL can be included as a new and effective option for treatment of grade I and II AK on the face and scalp.
Acknowledgements
The Consensus Meeting was funded by an unrestricted grant from Galderma Australia Pty Ltd, Sydney, New South Wales, Australia. Medical writing assistance was provided by Serina Stretton, PhD, Certified Medical Publication Professional of ProScribe – Envision Pharma Group and was funded by Galderma Australia Pty Ltd. ProScribe's services complied with international guidelines for good publication practice.
Jo‐Ann See, FACD. Stephen Shumack, FACD. Dedee F Murrell, FACD. Diana M Rubel, FACD. Pablo Fernández‐Peñas, FACD. Robert Salmon, FACD. Daniel Hewitt, FACD. Peter Foley, FACD. Lynda Spelman, FACD.
Conflict of interest: Jo‐Ann See has received honoraria as an Advisory Board member from Galderma and has undertaken clinical trials with daylight PDT (Metvix, Galderma). Peter Foley has received honoraria as an Advisory Board member from Galderma, CSL Ltd, 3M/iNova Pharmaceuticals (Valeant Pharmaceuticals), LEO Pharma/Peplin, Clinuvel Pharmaceuticals, Roche, and Aspen, has undertaken clinical trials with daylight PDT (Metvix, Galderma) and is undertaking or has undertaken clinical trials with competitor products to Metvix for CSL, 3M/iNova Pharmaceuticals (Valeant Pharmaceuticals), LEO Pharma/Peplin, Clinuvel Pharmaceuticals, and Roche, receives consulting fees from LEO Pharma/Peplin, Clinuvel Pharmaceuticals, Roche, and Aspen, and has received travel grants from Galderma and LEO Pharma/Peplin. Daniel Hewitt has received honoraria as an Advisory Board member from Galderma and has undertaken clinical trials with daylight PDT (Metvix, Galderma). Dedee F. Murrell has received honoraria as an Advisory Board member from Galderma, has undertaken clinical trials with daylight PDT (Metvix, Galderma) and receives consulting fees from LEO Pharma A/S. Pablo Fernández‐Peñas has received honoraria as an Advisory Board member from Galderma, has undertaken clinical trials with daylight PDT (Metvix, Galderma) and is undertaking clinical trials with competitor products to Metvix for LEO Pharma. Diana M. Rubel has received honoraria as an Advisory Board member from Galderma, has undertaken clinical trials with daylight PDT (Metvix, Galderma) and is undertaking clinical trials with competitor products to Metvix for LEO Pharma. Robert Salmon has received honoraria as an Advisory Board member, from Galderma. Stephen Shumack has received honoraria as an Advisory Board member from Galderma and has undertaken clinical trials with daylight PDT (Metvix, Galderma). Lynda Spelman has received honoraria as an Advisory Board member from Galderma, has undertaken clinical trials with daylight PDT (Metvix, Galderma) and is undertaking clinical trials with competitor products to Metvix for LEO Pharma.
All authors participated in the development of the recommendations and in the drafting, critical revision and in the approval of the final version of the manuscript.
References
- 1. Marks R. Epidemiology of non‐melanoma skin cancer and solar keratoses in Australia: a tale of self‐immolation in Elysian fields. Australas. J. Dermatol. 1997; 38 (Suppl. 1): S26–29. [DOI] [PubMed] [Google Scholar]
- 2. Frost CA, Green AC. Epidemiology of solar keratoses. Br. J. Dermatol. 1994; 131: 455–464. [DOI] [PubMed] [Google Scholar]
- 3. Olsen EA, Abernethy L, Kulp‐Shorten C et al A double‐blind, vehicle‐controlled study evaluating masoprocol cream in the treatment of actinic keratoses of the head and neck. J. Am. Acad. Dermatol. 1991; 24: 738–743. [DOI] [PubMed] [Google Scholar]
- 4. Marks R, Rennie G, Selwood TS. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet 1988; 1: 795–797. [DOI] [PubMed] [Google Scholar]
- 5. Holmes C, Foley P, Freeman M et al Solar keratosis: epidemiology, pathogenesis, presentation and treatment. Australas. J. Dermatol. 2007; 48: 67–74, quiz 5–6. [DOI] [PubMed] [Google Scholar]
- 6. Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J. Am. Acad. Dermatol. 2000; 42: 4–7. [DOI] [PubMed] [Google Scholar]
- 7. Stockfleth E, Ferrandiz C, Grob JJ et al Development of a treatment algorithm for actinic keratoses: a European Consensus. Eur. J. Dermatol. 2008; 18: 651–659. [DOI] [PubMed] [Google Scholar]
- 8. Stockfleth E. The paradigm shift in treating actinic keratosis: a comprehensive strategy. J. Drugs Dermatol. 2012; 11: 1462–1467. [PubMed] [Google Scholar]
- 9. Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin. Cosmet. Investig. Dermatol. 2014; 7: 145–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kennedy JC, Pottier RH. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J. Photochem. Photobiol. B. 1992; 14: 275–292. [DOI] [PubMed] [Google Scholar]
- 11. Wiegell SR, Haedersdal M, Philipsen PA et al Continuous activation of PpIX by daylight is as effective as and less painful than conventional photodynamic therapy for actinic keratoses; a randomized, controlled, single‐blinded study. Br. J. Dermatol. 2008; 158: 740–746. [DOI] [PubMed] [Google Scholar]
- 12. Wiegell SR, Wulf HC, Szeimies RM et al Daylight photodynamic therapy for actinic keratosis: an international consensus: International Society for Photodynamic Therapy in Dermatology. J. Eur. Acad. Dermatol. Venereol. 2012; 26: 673–679. [DOI] [PubMed] [Google Scholar]
- 13. Rubel DM, Spelman L, Murrell DF et al Daylight PDT with methyl aminolevulinate cream as a convenient, similarly effective, nearly painless alternative to conventional PDT in actinic keratosis treatment: a randomised controlled trial. Br. J. Dermatol. 2014; 171: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 14. Wiegell SR, Fabricius S, Gniadecka M et al Daylight‐mediated photodynamic therapy of moderate to thick actinic keratoses of the face and scalp: a randomized multicentre study. Br. J. Dermatol. 2012; 166: 1327–1332. [DOI] [PubMed] [Google Scholar]
- 15. Wiegell SR, Fabricius S, Stender IM et al A randomized, multicentre study of directed daylight exposure times of 1(1/2) vs. 2(1/2) h in daylight‐mediated photodynamic therapy with methyl aminolaevulinate in patients with multiple thin actinic keratoses of the face and scalp. Br. J. Dermatol. 2011; 164: 1083–1090. [DOI] [PubMed] [Google Scholar]
- 16. Wiegell SR, Haedersdal M, Eriksen P et al Photodynamic therapy of actinic keratoses with 8% and 16% methyl aminolaevulinate and home‐based daylight exposure: a double‐blinded randomized clinical trial. Br. J. Dermatol. 2009; 160: 1308–1314. [DOI] [PubMed] [Google Scholar]
- 17. Wulf H. Optimizing the amount of Metvix in daylight PDT. Euro‐PDT 13th Annual Congress, 31 May–1 Jun 1; Madrid, Spain. 2013.
- 18. Spelman L, Rubel DM, Murrell DF et al Treatment of the face and scalp solar keratosis with daylight‐mediated photodynamic therapy is possible throughout the year in Australia: evidence from a clinical and meteorological study. Australas. J. Dermatol. 2015; doi: 10.1111/ajd.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Szeimies RM, Lischner S, Philipp‐Dormston W et al Photodynamic therapy for skin rejuvenation: treatment options – results of a consensus conference of an expert group for aesthetic photodynamic therapy. J. Dtsch Dermatol. Ges. 2013; 11: 632–636. [DOI] [PubMed] [Google Scholar]
- 20. Braathen LR. Daylight photodynamic therapy in private practice in Switzerland: gain without pain. Acta Derm. Venereol. 2012; 92: 652–653. [DOI] [PubMed] [Google Scholar]
- 21. Tran DT, Salmon R. Field treatment of facial and scalp actinic keratoses with photodynamic therapy: survey of patient perceptions of treatment satisfaction and outcomes. Australas. J. Dermatol. 2011; 52: 195–201. [DOI] [PubMed] [Google Scholar]
- 22. Torezan L, Chaves Y, Niwa A et al A pilot split‐face study comparing conventional methyl aminolevulinate‐photodynamic therapy (PDT) with microneedling‐assisted PDT on actinically damaged skin. Dermatol. Surg. 2013; 39: 1197–1201. [DOI] [PubMed] [Google Scholar]
- 23. Wiegell SR, Stender IM, Na R et al Pain associated with photodynamic therapy using 5‐aminolevulinic acid or 5‐aminolevulinic acid methylester on tape‐stripped normal skin. Arch. Dermatol. 2003; 139: 1173–1177. [DOI] [PubMed] [Google Scholar]
- 24. Morton CA, Szeimies RM, Sidoroff A et al European guidelines for topical photodynamic therapy part 1: treatment delivery and current indications – actinic keratoses, Bowen's disease, basal cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2013; 27: 536–544. [DOI] [PubMed] [Google Scholar]


