Abstract
IMPORTANCE
Salivary duct carcinoma is a rare, aggressive malignancy of the salivary glands. Owing to its rare nature, clinical data are limited, and only a few clinical studies comprise more than 50 patients.
OBJECTIVE
To review the University of Pittsburgh Medical Center’s experience with salivary duct carcinoma over a 20-year period, focusing on demographics, presentation, treatment, and outcome.
DESIGN, SETTING, AND PARTICIPANTS
This investigation was a retrospective cohort study in a multihospital institution with tertiary referral. A pathology database was reviewed for all cases of histopathologically diagnosed salivary duct carcinoma from January 1, 1995, to October 20, 2014. Patients who were referrals for pathology review only and were never seen at the institution were excluded. In total, 75 study patients were identified. The electronic medical record was reviewed for details regarding demographics, presentation, treatment, and outcome, including overall survival (OS) and disease-free survival (DFS). This study was supplemented with a review of the institution’s Head and Neck Oncology Database for further clinical details.
MAIN OUTCOMES AND MEASURES
Primary outcome measures consisted of OS and DFS.
RESULTS
The study sample comprised 75 participants with a mean age at diagnosis of 66.0 years (age range, 33–93 years), and 29% (n = 22) were female. Most primary tumors were from the parotid gland (83%), with the next most frequent site being the submandibular gland (12%). Overall, 41% of the cases were carcinoma ex pleomorphic adenoma. Rates of other histologic features included the following: perineural invasion (69%), extracapsular spread (58%), ERBB2 (formerly HER2) positivity (31%) (62% of those who were tested), and vascular invasion (61%). The median OS was 3.1 years, and the median DFS was 2.7 years. Univariate Kaplan-Meier survival analyses demonstrated that facial nerve sacrifice and extracapsular spread were associated with lower OS (2.38 vs 5.11 years and 2.29 vs 6.56 years, respectively) and DFS (2.4 vs 3.88 years and 1.44 vs 4.5 years, respectively). Although underpowered, multivariable analysis demonstrated significantly worse OS in patients with N2 and N3 disease (hazard ratio [HR] 8.42, 95% CI, 1.84–38.5) but did not show significantly worse DFS or OS for facial nerve sacrifice or extracapsular spread. There was no association between ERBB2 positivity and survival and no difference in survival between patients receiving radiation therapy vs radiation therapy plus chemotherapy. No patients had recurrence or distant metastasis after 5 disease-free years.
CONCLUSIONS AND RELEVANCE
Salivary duct carcinoma is an aggressive disease. A large number of cases in this review were carcinoma ex pleomorphic adenoma and had classic negative prognostic indicators, such as perineural invasion, vascular invasion, and extracapsular spread. ERBB2 positivity was not associated with any difference in survival. Facial nerve involvement appears to indicate worse prognosis, as does nodal stage higher than N1. Recurrence and metastasis after 5 years are rare.
Salivary duct carcinoma (SDC) is a highly aggressive but very rare malignancy, estimated to represent approximately 1% to 3% of all salivary malignancies1–3 (upto 6% in one Finnish study). It was first described by Kleinsasser et al4 in 1968 owing to its histologic similarity to ductal carcinoma of the breast. It occurs predominately in the parotid gland, and most patients die within 3 years, with overall survival (OS) reported as low as 42% for stage I disease and 23% for stage IV disease.5 Similar to its breast cancer counterpart, many of these tumors overexpress erb-B2, which has been reported to be a negative prognostic indicator.6–8 The mainstay of therapy is surgery and radiation therapy, but good control remains challenging.9,10
Salivary duct carcinoma is a malignancy that will be rarely seen by most physicians, and providing education and information to patients about prognosis is challenging. Indeed, there are only a few single-institution studies5,10,11 so far that include more than 50 patients. We examined the clinical features and outcomes of the University of Pittsburgh Medical Center’s experience with SDC over the past 20 years to provide more literature regarding factors that affect the course of this disease.
Methods
The study was reviewed and approved by the University of Pittsburgh Institutional Review Board. An electronic medical record search was conducted for all pathologic diagnoses of SDC or salivary ductal carcinoma at one institution from January 1, 1995, to October20, 2014. Patients who were outside referrals for pathology review only and cases for which clinical information could not be obtained through the electronic medical record (eg, operative reports) were excluded. Clinical information was obtained from the University of Pittsburgh Medical Center’s Head and Neck Oncology Database and from the electronic medical record. For our study, tumor grading was rarely available in the electronic medical record and was not included in our analysis. ERBB2 (formerly HER2) (OMIM 164870) status was considered positive if moderately or strongly positive by erbB-2 staining or if positive (more than equivocal) by ERBB2 gene amplification studies.
Overall survival and disease-free survival (DFS) were calculated and compared graphically with Kaplan-Meier survival curves using a software program (GraphPad Prism, version 6.05 for Windows; GraphPad Software). The log-rank test (and where appropriate the log-rank test for trend) was used to compare survival statistically. Cox proportional hazards models were fit to perform multivariable survival analysis. These models included sex, age, primary tumor subsite, T stage and N stage, adjuvant therapy, diagnosis of carcinoma ex pleomorphic adenoma, perineural invasion (PNI), extracapsular spread (ECS), vascular invasion, ERBB2 positivity, and facial nerve sacrifice. Univariate analysis only included data points that were reported (ie, positive or negative), but “unknown” values were not included. All P values were calculated using 2-sided hypotheses. The threshold for statistical significance was set at P < .05. However, given the small sample size and associated possibility of type II error, we also reported P < .10 as demonstrating a trend. Statistical analyses of survival were conducted using a software program (Stata 11.1 Intercooled; StataCorp LP).
Results
We identified 75 total cases of histopathologically diagnosed SDC from January 1, 1995, to October 20, 2014. The median follow-up was 4.6 years (55 months). Demographics and treatment characteristics are summarized in Table 1. Most patients were male, and the mean age at diagnosis was 66.0 years. There was no significant difference between the sexes in the mean age at presentation. Most primary tumors were from the parotid gland (83% [n = 62]), with the next most frequent site being the submandibular gland (12% [n = 9]). The most common presenting symptom was a painless parotid or neck mass, although some patients with advanced disease were initially seen with facial weakness or paralysis. Almost all patients (95% [n = 71]) underwent resection of their tumors. A large number of patients were seen with T4 disease (39% [n = 29]), and most patients had more than 2 positive lymph nodes (stage N2b), although 12% (n = 9) had no lymph nodes removed (stage Nx). Three patients were initially seen with distant metastasis. Most patients (81% [n = 61]) had adjuvant radiation therapy, with about half also receiving chemotherapy. No patients received chemotherapy alone. Thirty-one patients (41%) had pathologic features suggestive of SDC arising out of a pleomorphic adenoma (ie, carcinoma ex pleomorphic adenoma). Additional rates of pertinent histopathologic features included the following: PNI (69% [n = 52]), ECS (58% [38 of 66]), ERBB2 positivity (31% [n = 23]) (62% [23 of 37] of those who were tested), and vascular invasion (61% [n = 46]) (Table 2). The preoperative level of facial nerve function was not available for a large majority of patients, so facial nerve sacrifice at the time of operation was used as a surrogate for preoperative facial nerve involvement. More than half of the patients (55% [33 of 60]) who underwent parotidectomy required facial nerve sacrifice during the procedure. Recurrence, when noted, initially occurred most often at the primary site or neck, although distant metastases often followed. Distant metastases were most commonly seen in the lungs, but brain and bone metastases were also noted.
Table 1.
Demographics and Presentation or Treatment
| Variable | Value (N = 75)a |
|---|---|
| Sex, No. (%) | |
| Male | 53 (71) |
| Female | 22 (29) |
| Age at diagnosis, mean (range), y | |
| Overall | 66.0 (33–93) |
| Male | 65.3 (33–93) |
| Female | 67.8 (37–84) |
| Smoking history, No. (%) | |
| Positive | 43 (57) |
| Negative | 25 (33) |
| Unknown | 7 (9) |
| Alcohol use, No. (%) | |
| Positive | 41 (55) |
| Negative | 24 (32) |
| Unknown | 10 (13) |
| Primary tumor subsite, No. (%) | |
| Parotid gland | 62 (83) |
| Submandibular gland | 9 (12) |
| Oral cavity | 1 (1) |
| Parapharyngeal space | 1 (1) |
| Hard palate | 1 (1) |
| Unknown | 1 (1) |
| Adjuvant therapy, No. (%) | |
| Chemotherapy and radiation therapy | 30 (40) |
| Radiation therapy only | 31 (41) |
| None | 13 (17) |
| Unknown | 1 (1) |
| Pathologic T stage, No. (%) | |
| T1 | 14 (19) |
| T2 | 14 (19) |
| T3 | 13 (17) |
| T4 | 29 (39) |
| Tx | 5 (7) |
| Pathologic N stage, No. (%) | |
| N0 | 12 (16) |
| N1 | 5 (7) |
| N2a | 2 (3) |
| N2b | 46 (61) |
| N2c | 1 (1) |
| Nx | 9 (12) |
| M stage, No. (%) | |
| M0 | 72 (96) |
| M1 | 3 (4) |
Unless otherwise indicated, data are presented as number (percentage).
Table 2.
Surgical and Pathologic Features
| Variable | No./Total No. (%)(N = 75) |
|---|---|
| Procedure | |
| Tumor resection | 71 (95) |
| Biopsy only | 4 (5) |
| Facial nerve sacrifice during parotidectomy (main trunk or branches)a | |
| Yes | 33/60 (55) |
| No | 27/60 (45) |
| Perineural invasion | |
| Yes | 52 (69) |
| No | 14 (19) |
| Unknown | 9 (12) |
| Carcinoma ex pleomorphic adenoma | |
| Yes | 31 (41) |
| No | 42 (56) |
| Unknown | 2 (3) |
| Extracapsular spread | |
| Yes | 38/66 (58) |
| No | 26/66 (39) |
| Unknown | 2/66 (3) |
| ERBB2 status | |
| Positive | 23 (31) |
| Negative | 14 (19) |
| Unknown | 38 (51) |
| Vascular invasion | |
| Yes | 46 (61) |
| No | 12 (16) |
| Unknown | 17 (23) |
Sixty-two patients had a parotid mass, and only 60 patients underwent parotidectomy.
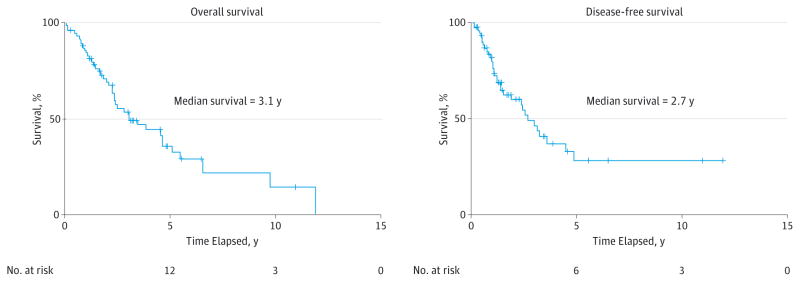
The median OS was 3.1 years, and the median DFS was 2.7 years (Figure). No patients had recurrence or distant metastasis after 5 disease-free years. The results of univariate Kaplan-Meier survival analyses are summarized in Table 3. Facial nerve sacrifice and ECS were significant negative prognostic indicators for OS and DFS. Both PNI and vascular invasion demonstrated a trend for decreasing OS and were significant for decreasing DFS. Increased T stage and N stage were associated with significantly worse OS but not DFS. A diagnosis of carcinoma ex pleomorphic adenoma demonstrated a trend for predicting worse DFS but was not associated with OS. Contrary to prior findings,5 there was no association between ERBB2 positivity and survival. No difference in OS or DFS was observed between patients receiving radiation therapy vs radiation therapy plus chemotherapy, although regimens were not standardized.
Figure.
Overall and Disease-Free Survival
The number at risk at the time of median survival is 25 for overall survival and 19 for disease-free survival.
Table 3.
Univariate Analysis of Overall and Disease-Free Survival, Including the Median Survival
| Variable | Overall Survival
|
Disease-Free Survival
|
||
|---|---|---|---|---|
| Median, y | P Value | Median, y | P Value | |
| Pathologic T stage | ||||
|
| ||||
| T1 | 4.57 | .009a | 2.68 | .17 |
|
|
|
|||
| T2 | 6.57 | ND | ||
|
|
|
|||
| T3 | 2.51 | 1.53 | ||
|
|
|
|||
| T4 | 2.12 | 2.40 | ||
|
| ||||
| Pathologic N stage | ||||
|
| ||||
| N0 | ND | .06 | ND | .11 |
|
|
|
|||
| N1 | 6.56 | 2.68 | ||
|
|
|
|||
| N2 | 2.44 | 2.37 | ||
|
| ||||
| Carcinoma ex pleomorphic adenoma | ||||
|
| ||||
| Yes | 3.48 | .48 | ND | .08a |
|
|
|
|||
| No | 2.44 | 2.55 | ||
|
| ||||
| Perineural invasion | ||||
|
| ||||
| Yes | 2.51 | .06 | 2.55 | .006a |
|
|
|
|||
| No | ND | ND | ||
|
| ||||
| Extracapsular spread | ||||
|
| ||||
| Yes | 2.29 | .003a | 1.44 | .005a |
|
|
|
|||
| No | 6.56 | 4.50 | ||
|
| ||||
| Vascular invasion | ||||
|
| ||||
| Yes | 2.40 | .10a | 1.9 | .03a |
|
|
|
|||
| No | 4.57 | ND | ||
|
| ||||
| ERBB2 positivity | ||||
|
| ||||
| Yes | 2.44 | .95 | 2.40 | .33 |
|
|
|
|||
| No | 2.82 | 3.59 | ||
|
| ||||
| Facial nerve sacrifice | ||||
|
| ||||
| Yes | 2.38 | .008a | 2.40 | .02a |
|
|
|
|||
| No | 5.11 | 3.88 | ||
|
| ||||
| Adjuvant therapy | ||||
|
| ||||
| Radiation therapy only | 4.65 | .88 | 3.15 | .87 |
|
|
|
|||
| Chemotherapy and radiation therapy | 2.82 | 2.37 | ||
Abbreviation: ND, not defined (because of survival >50%).
Significant.
Multivariable survival models were fit (Table 4). Male sex was associated with significantly worse DFS. Parotid gland subsite (vs submandibular gland), vascular invasion, and facial nerve sacrifice were associated with worse DFS: the submandibular gland hazard ratio (HR) was 0.08 (95% CI, 0.01–1.12; P = .06), the vascular invasion HR was 5.30 (95% CI, 0.95–29.60; P = .06), and the facial nerve sacrifice HR was 3.60 (95% CI, 0.91–14.25; P = .07). However, the effect did not achieve statistical significance. Advanced age and N stage higher than N1 were significantly associated with worse OS: the age HR was 1.08 (95% CI, 1.04–1.13; P < .001), and the N2 or N3 HR was 8.42 (95% CI, 1.84–38.50; P = .006).
Table 4.
Multivariable Analysis of Overall and Disease-Free Survival
| Variable | Overall Survival
|
Disease-Free Survival
|
||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age | 1.08 (1.04–1.13)a | <.001a | 1.02 (0.97–1.06) | .44 |
|
| ||||
| Sex | ||||
|
| ||||
| Male | 1 [Reference] | NA | 1 [Reference] | NA |
|
| ||||
| Female | 0.81 (0.32–2.03) | .65 | 0.33 (0.11–0.96)a | .04a |
|
| ||||
| Primary tumor subsite | ||||
|
| ||||
| Parotid gland | 1 [Reference] | NA | 1 [Reference] | NA |
|
| ||||
| Submandibular gland | 0.26 (0.04–1.64) | .15 | 0.08 (0.01–1.12)a | .06a |
|
| ||||
| Pathologic T stage | ||||
|
| ||||
| T1 or T2 | 1 [Reference] | NA | 1 [Reference] | NA |
|
| ||||
| T3 or T4 | 0.95 (0.37–2.43) | .91 | 0.52 (0.17–1.62) | .26 |
|
| ||||
| Unknown | 2.45 (0.10–58.75) | .58 | 0.00 (0.00–0.00)a | <.001a |
|
| ||||
| Pathologic N stage | ||||
|
| ||||
| N0 or N1 | 1 [Reference] | NA | 1 [Reference] | NA |
|
| ||||
| N2 or N3 | 8.42 (1.84–38.50)a | .006a | 0.71 (0.18–2.77) | .62 |
|
| ||||
| Unknown | 2.64 (0.08–87.45) | .59 | 0.12 (0.01–1.90) | .13 |
|
| ||||
| Carcinoma ex pleomorphic adenoma | ||||
|
| ||||
| No | 1 [Reference] | NA | 1 [Reference] | NA |
|
| ||||
| Yes | 1.39 (0.64–3.03) | .41 | 1.18 (0.41–3.45) | .76 |
|
| ||||
| Perineural invasion | ||||
|
| ||||
| No | 1 [Reference] | NA | 1 [Reference] | NA |
|
| ||||
| Yes | 0.87 (0.12–6.01) | .88 | 2.16 (0.30–15.53) | .44 |
|
| ||||
| Unknown | 1.22 (0.07–21.84) | .89 | 11.73 (0.78–175.89)a | .07a |
|
| ||||
| Extracapsular spread | ||||
|
| ||||
| No | 1 [Reference] | NA | 1 [Reference] | NA |
|
| ||||
| Yes | 1.66 (0.69–3.96) | .25 | 2.24 (0.83–6.02) | .11 |
|
| ||||
| Unknown | 2.15 (0.07–64.84) | .66 | 5.55 (0.51–59.95) | .16 |
|
| ||||
| Vascular invasion | ||||
|
| ||||
| No | 1 [Reference] | NA | 1 [Reference] | NA |
|
| ||||
| Yes | 0.68 (0.17–2.75) | .58 | 5.30 (0.95–29.60)a | .06 |
|
| ||||
| Unknown | 0.31 (0.05–2.06) | .23 | 2.18 (0.30–15.77) | .44 |
|
| ||||
| ERBB2 positivity | ||||
|
| ||||
| No | 1 [Reference] | NA | 1 [Reference] | NA |
|
| ||||
| Yes | 2.48 (0.73–8.47) | .15 | 1.49 (0.37–6.01) | .57 |
|
| ||||
| Unknown | 4.03 (1.24–13.10)a | .02a | 0.58 (0.14–2.36) | .44 |
|
| ||||
| Facial nerve sacrifice | ||||
|
| ||||
| No | 1 [Reference] | NA | 1 [Reference] | NA |
|
| ||||
| Yes | 2.70 (0.81–8.96) | .10 | 3.60 (0.91–14.25)a | .07 |
|
| ||||
| Unknown | 1.62 (0.40–6.52) | .50 | 4.53 (0.83–24.63)a | .08 |
Abbreviations: HR, hazard ratio; NA, not applicable; ND, not defined.
Significant.
Discussion
Salivary duct carcinoma is a rare entity, and the literature is consequently limited. To date, the largest SDC study is a review of the Surveillance, Epidemiology, and End Results database from 1973 to 2008 by Jayaprakash et al.3 They reviewed 228 cases, almost two-thirds of which were diagnosed in the last 8 years of the study. Negative prognostic indicators for OS and DFS were age 50 years or older, tumor size, and lymph node involvement, with no apparent survival benefit of radiation therapy. Poorly or undifferentiated tumors were also associated with worse prognosis, as was advanced stage. However, one of the drawbacks with pathologic features in such a study is in the confounding bias of a large variety of physicians diagnosing and treating a rare disease that is still being characterized. For some time, many low-grade cribriform cystadenocarcinomas were previously characterized as low-grade SDC,12 and some authors have even suggested calling these intraductal carcinomas instead.13 Reevaluation of previous diagnoses among experienced pathologists often reveals that changes are made on repeat phenotyping.14 As such, single-institution series at large tertiary referral centers, although small, are the most homogeneous and therefore may be the most reliable studies.
However, there are few large single-institution series of SDC, with only 3 series that examine more than 50 patients.5,10,11 Jaehne et al5 performed the first large study in 2005, examining 50 patients with SDC with a mean follow-up of 96 months. Consistent with prior literature,15,16 most patients were male, the mean age at diagnosis was 62.5 years, the primary site was the parotid gland, and two-thirds of the patients were initially seen with advanced disease, in this case stage T3/4. Most patients (56%) had cervical lymph node metastasis at diagnosis, and local recurrence was seen in 48% of patients only 17 months after treatment.
Two other large studies were published in the past 2 years. Roh et al10 reported on 56 patients seen for a median follow-up of 71 months. In their study, there were a large number of submandibular SDCs (38%), but 75% of patients still were initially seen with stage IV disease. Advanced disease stage (stage T3 or T4, N+ disease, or stages III or IV) and PNIs were associated with worse overall, disease-specific, and progression-free survival. On multivariable analysis, N+ disease was associated with worse OS, disease-specific survival, progression-free survival, and distant metastasis–free survival. There was no association between ECS and survival.
In 2015, Johnston et al11 looked at 54 cases over 11 years, with a median follow-up of 5.7 years and a 5-year OS of 43%. Univariate analysis showed significantly decreased OS with N2b/c stage, ECS, and lymphovascular invasion, and multivariable analysis confirmed poor OS with N2b or N2c stage and demonstrated a trend for ECS. Johnston et al also reported a large comprehensive review of the major series with their study. The number of total cases previously reported in institutional series was approximately 442 in 17 studies. From these data and other literature searches, our study of 75 patients over a 20-year span appears to be the largest single-institution study to date.
The median OS and DFS were 3.1 years and 2.7 years, respectively, for the cohort (Figure), which is poor and consistent with other literature. Walvekar et al17 previously reported that most high-grade parotid malignancies recur in the first 18 months, although only 9%(10 patients) in their study had SDC. In contrast, our cohort of SDC in all sites had more than half of the recurrences occur after 2 years (Figure). However, there was no disease recurrence after 5 disease-free years in our study, which is helpful information both in terms of knowing how long to follow up patients and in helping counsel patients about their prognosis after that period. Forty-one percent (n = 31) of our cases were carcinoma ex pleomorphic adenoma, which is a rate double that usually seen.9 Salivary duct carcinoma ex pleomorphic adenoma may portend a better prognosis, with our data showing a trend for better DFS but not OS.
In addition, we examined multiple variables associated with worse prognosis in previous studies, including facial nerve involvement (by the surrogate of facial nerve sacrifice), PNI, vascular invasion, T stage, and N stage. We also looked at nodal ECS, which is known to be a negative prognostic factor in other head and neck cancers and was found to be related to survival by Johnston et al11 and Maxwell et al18 but not by Roh et al.10 All these variables trended toward or showed significantly worse survival, as summarized in Table 3, with the most significance seen in ECS and facial nerve sacrifice. We also examined erb-B2 status. In the study by Jaehne et al,5 moderate and strong staining for erb-B2 was seen in 17.7% and 20.6% of patients, respectively, with worse OS and distant metastasis seen in patients with erb-B2 overexpression. Other authors have also found that erb-B2 overexpression is associated with poor prognosis.6,8 We found no such association in the present study, and other recent large studies11,19 have found no relationship either. However, erb-B2 testing was performed only for approximately half of the patients in our study. Because the data are mixed, it remains to be seen how ERBB2 positivity affects prognosis. However, because it is present in some cohorts in up to 64% of patients19 (and in 62% [23 of 37] of patients tested in our cohort), ERBB2 positivity may be an avenue for specific targeting agents, such as the anti–erb-B2 monoclonal antibody trastuzumab.7,20
The multivariable analysis was performed using the same variables as the univariate analysis, with additional variables of age, sex, and subsite (although these variables decreased the power of the analysis). Age was borne out in multivariable testing as a negative prognostic indicator for OS, which is expected, but so was N stage above N1. This difference in OS, with worse OS for N2 and above, was also reported by Johnston et al.11 It is unclear why DFS was not also worse in this group in our cohort. It may be that treatment-related toxicity due to radiation therapy, chemotherapy, or both contributed to poorer survival in this population, while the cancer itself has been successfully treated. T stage was not associated with survival, reflecting the overall aggressiveness of the disease. Facial nerve sacrifice, parotid gland subsite, and vascular invasion demonstrated a trend for worse DFS in multivariable testing. Female sex was associated with significantly better DFS, although with the large difference in the numbers of male vs female patients, this finding may be a sampling error. There was no significant difference in outcome between radiation therapy and radiation therapy plus chemotherapy. However, this comparison is difficult in a heterogeneous population who received different regimens, in regard to both radiation therapy and chemotherapy, over the course of 20 years. A larger study or meta-analysis may be able to better ascertain if the addition of chemotherapy actually affects OS or DFS.
Our study has several limitations. It is retrospective and has frequent gaps in the data due to the length of time and change in tools available over the time course of the review (eg, lack of erb-B2 testing and occasionally no comment on perineural or vascular invasion). Although this investigation is the largest study of SDC to date, the sample size is still small, and the multivariable analyses were underpowered. In addition, facial nerve sacrifice, although a surrogate for preoperative facial nerve paresis or paralysis, may not be as reliable for facial nerve weakness as preoperative clinical examination information, which was not available.
Conclusions
Salivary duct carcinoma is an aggressive and rare disease with poor prognosis. A surprisingly large number of our cases were carcinoma ex pleomorphic adenoma. Advanced stage at presentation was common. Negative prognostic indicators associated with worse survival included PNI, vascular invasion, ECS, higher N stage, and facial nerve involvement. erb-B2, previously reported as a negative prognostic indicator,5,6,8 was not associated with survival. Multivariable analysis was performed, revealing that male sex was significantly associated with worse DFS and that age and advanced nodal stage (≥N2) were significantly associated with worse OS. Facial nerve sacrifice, parotid gland subsite, and vascular invasion trended toward an association with DFS and OS, but these differences did not achieve statistical significance. Recurrence or metastasis was common, involving locoregional and distant sites. However, recurrence after 5 disease-free years did not occur in our study and is likely rare after this period, helping to guide follow-up and patient counseling.
Acknowledgments
Funding/Support: Dr Duvvuri was supported in part by the Department of Veterans Affairs Biomedical Laboratory Research and Development.
Footnotes
Conflict of Interest Disclosures: None reported.
Disclaimer: This work does not reflect the views of the US government or the Department of Veterans Affairs.
Previous Presentation: This study was presented as a poster at the Combined Otolaryngology Spring Meetings; April 22–23, 2015; Boston, Massachusetts.
Author Contributions: Drs Gilbert and Sharma had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: All authors.
Acquisition, analysis, or interpretation of data: Gilbert, Sharma, Schmitt.
Drafting of the manuscript: Gilbert, Sharma.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Gilbert, Sharma.
Administrative, technical, or material support: Gilbert, Sharma.
Study supervision: Schmitt, Johnson, Ferris, Duvvuri, Kim.
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Salovaara E, Hakala O, Bäck L, et al. Management and outcome of salivary duct carcinoma in major salivary glands. Eur Arch Otorhinolaryngol. 2013;270(1):281–285. doi: 10.1007/s00405-012-1997-4. [DOI] [PubMed] [Google Scholar]
- 2.Luukkaa H, Klemi P, Leivo I, et al. Salivary gland cancer in Finland 1991–96: an evaluation of 237 cases. Acta Otolaryngol. 2005;125(2):207–214. doi: 10.1080/00016480510003174. [DOI] [PubMed] [Google Scholar]
- 3.Jayaprakash V, Merzianu M, Warren GW, et al. Survival rates and prognostic factors for infiltrating salivary duct carcinoma: analysis of 228 cases from the Surveillance, Epidemiology, and End Results database. Head Neck. 2014;36(5):694–701. doi: 10.1002/hed.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleinsasser O, Klein HJ, Hübner G. Salivary duct carcinoma: a group of salivary gland tumors analogous to mammary duct carcinoma [in German] Arch Klin Exp Ohren Nasen Kehlkopfheilkd. 1968;192(1):100–105. [PubMed] [Google Scholar]
- 5.Jaehne M, Roeser K, Jaekel T, Schepers JD, Albert N, Löning T. Clinical and immunohistologic typing of salivary duct carcinoma: a report of 50 cases. Cancer. 2005;103(12):2526–2533. doi: 10.1002/cncr.21116. [DOI] [PubMed] [Google Scholar]
- 6.Sugano S, Mukai K, Tsuda H, et al. Immunohistochemical study of c-erbB-2 oncoprotein overexpression in human major salivary gland carcinoma: an indicator of aggressiveness. Laryngoscope. 1992;102(8):923–927. doi: 10.1288/00005537-199208000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Nabili V, Tan JW, Bhuta S, Sercarz JA, Head CS. Salivary duct carcinoma: a clinical and histologic review with implications for trastuzumab therapy. Head Neck. 2007;29(10):907–912. doi: 10.1002/hed.20614. [DOI] [PubMed] [Google Scholar]
- 8.Felix A, El-Naggar AK, Press MF, et al. Prognostic significance of biomarkers (c-erbB-2, p53, proliferating cell nuclear antigen, and DNA content) in salivary duct carcinoma. Hum Pathol. 1996;27(6):561–566. doi: 10.1016/s0046-8177(96)90162-8. [DOI] [PubMed] [Google Scholar]
- 9.Wee DT, Thomas AA, Bradley PJ. Salivary duct carcinoma: what is already known, and can we improve survival? J Laryngol Otol. 2012;126(suppl 2):S2–S7. doi: 10.1017/S0022215112000412. [DOI] [PubMed] [Google Scholar]
- 10.Roh JL, Lee JI, Choi SH, et al. Prognostic factors and oncologic outcomes of 56 salivary duct carcinoma patients in a single institution: high rate of systemic failure warrants targeted therapy. Oral Oncol. 2014;50(11):e64–e66. doi: 10.1016/j.oraloncology.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Johnston ML, Huang SH, Waldron JN, et al. Salivary duct carcinoma: treatment, outcomes, and patterns of failure. Head Neck. doi: 10.1002/hed.24107. [published online April 27, 2015] [DOI] [PubMed] [Google Scholar]
- 12.Laco J, Podhola M, Dolezalova H. Low-grade cribriform cystadenocarcinoma of the parotid gland: a neoplasm with favorable prognosis, distinct from salivary duct carcinoma. Int J Surg Pathol. 2010;18(5):369–373. doi: 10.1177/1066896910367649. [DOI] [PubMed] [Google Scholar]
- 13.Kuo YJ, Weinreb I, Perez-Ordonez B. Low-grade salivary duct carcinoma or low-grade intraductal carcinoma? review of the literature. Head Neck Pathol. 2013;7(suppl 1):S59–S67. doi: 10.1007/s12105-013-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams L, Thompson LD, Seethala RR, et al. Salivary duct carcinoma: the predominance of apocrine morphology, prevalence of histologic variants, and androgen receptor expression. Am J Surg Pathol. 2015;39(5):705–713. doi: 10.1097/PAS.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 15.Hosal AS, Fan C, Barnes L, Myers EN. Salivary duct carcinoma. Otolaryngol Head Neck Surg. 2003;129(6):720–725. doi: 10.1016/S0194-59980301386-X. [DOI] [PubMed] [Google Scholar]
- 16.Guzzo M, Di Palma S, Grandi C, Molinari R. Salivary duct carcinoma: clinical characteristics and treatment strategies. Head Neck. 1997;19(2):126–133. doi: 10.1002/(sici)1097-0347(199703)19:2<126::aid-hed7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Walvekar RR, Andrade Filho PA, Seethala RR, et al. Clinicopathologic features as stronger prognostic factors than histology or grade in risk stratification of primary parotid malignancies. Head Neck. 2011;33(2):225–231. doi: 10.1002/hed.21433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maxwell JH, Ferris RL, Gooding W, et al. Extracapsular spread in head and neck carcinoma: impact of site and human papillomavirus status. Cancer. 2013;119(18):3302–3308. doi: 10.1002/cncr.28169. [DOI] [PubMed] [Google Scholar]
- 19.Han MW, Roh JL, Choi SH, et al. Prognostic factors and outcome analysis of salivary duct carcinoma. Auris Nasus Larynx. 2015;42(6):472–477. doi: 10.1016/j.anl.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Skálová A, Stárek I, Kucerová V, Szépe P, Plank L. Salivary duct carcinoma: a highly aggressive salivary gland tumor with HER-2/neu oncoprotein overexpression. Pathol Res Pract. 2001;197(9):621–626. doi: 10.1078/0344-0338-00136. [DOI] [PubMed] [Google Scholar]