Abstract
Background
The comparative effectiveness of the two treatment options (surgical clipping and endovascular coiling) for ruptured cerebral aneurysms has not been studied in real-world practice in the United States. We investigated the association of treatment method for ruptured cerebral aneurysms and outcomes.
Methods
We performed a retrospective cohort study of elderly patients who underwent treatment for ruptured cerebral aneurysms from 2007 to 2012, using a 100% sample of Medicare fee-for-service claims data. An instrumental variable analysis was used to control for unmeasured confounding and create pseudo-randomization on treatment method. In sensitivity analysis, controlling only for measured confounding, we used propensity score conditioning and inverse probability weighting, with mixed effects to account for clustering at the HRR level.
Results
During the study period, there were 3,210 patients, who underwent treatment for ruptured cerebral aneurysms, and met the inclusion criteria. Of these, 1,206 (37.6%) had surgical clipping, and 2,004 (62.4%) had endovascular coiling. Instrumental variable analysis demonstrated no difference of coiling in comparison to clipping for 1-year postoperative mortality (OR, 1.04; 95%CI, 0.70-1.54), likelihood of discharge to rehabilitation (OR, 1.07; 95%CI, 0.72-1.58), or 30-day readmission rate (OR, 1.14; 95%CI, 0.70-1.87). Clipping however was associated with 2.7 days longer length of stay (LOS) (95%CI, 0.45-4.99). The same associations were present in propensity score adjusted and inverse probability weighted models.
Conclusions
In a cohort of Medicare patients, we did not demonstrate a difference in mortality, rate of discharge to rehabilitation, and readmissions between clipping and coiling of ruptured cerebral aneurysms. Clipping was associated with slightly longer LOS.
Keywords: cerebral aneurysms, subarachnoid hemorrhage, clipping, coiling, instrumental variable, Medicare
INTRODUCTION
Cerebral aneurysm rupture causes subarachnoid hemorrhage (SAH), and is associated with major morbidity and mortality for as many as 65% of the cases.1,2 Securing the aneurysm after rupture and preventing further bleeding can be achieved with two treatment options.1,3 Surgical clipping involves a craniotomy and placement of a clip on the blood vessel to exclude the weakened area, whereas endovascular coiling is a minimally invasive angiographic technique achieving aneurysm obliteration from within the blood vessel.1,2 The International Study for Aneurysm Treatment (ISAT)3 demonstrated that clipping was associated with higher 1 year morbidity and mortality in comparison to coiling for patients with ruptured cerebral aneurysms. This data fueled an explosive growth in the number of endovascular procedures performed in patients with SAH making coiling the predominant treatment modality in this patient population.1,4
However, concerns have been raised for the application of these results in real-world practice. Many argue that the expansion of endovascular options has encouraged treatment of ruptured cerebral aneurysms by less experienced, low-volume proceduralists.5 Efficacy demonstrated in the context of carefully controlled clinical trials3,6,7 often does not translate into real-world effectiveness. Lessons learned from trials of carotid endarterectomy and stenting have supported this notion, with complication rates higher among non-trial participants.8,9 In addition, extrapolation of the results of ISAT3,7 and other trials6 on the elderly can be limited, given the different vascular profile (more calcified, tortuous arterial anatomy), and comorbidity burden of this population.
We performed a cohort study of Medicare patients presenting with SAH, investigating the comparative effectiveness of clipping and coiling. The association of treatment used with 1-year mortality, 30-day readmission, length of stay, and discharge to rehabilitation was examined. In order to control for unmeasured confounding, we used an instrumental variable (IV) approach, creating pseudo-randomization on the treatment method. Lastly, to investigate the robustness of our findings, we utilized a battery of approaches (controlling only for measured confounding) including regression adjustment, propensity score adjustment, and inverse probability weighting (IPW), whereas mixed effects methods were employed to control for clustering at the HRR level.
METHODS
Data and cohort creation
This study was approved by the Dartmouth Committee for Protection of Human Subjects. We used 100% of Medicare Denominator file and corresponding Medicare inpatient and outpatient claims, Parts A (Inpatient) and B (Outpatient), 2007-2012 (MedPAR, Carrier and Outpatient files) to select patients presenting with aneurysmal SAH. All patients older than 65 years old and disabled individuals are enrolled in Medicare. We obtain identifiable data that allow us to follow patients longitudinally through episodes of care and hospitalizations. Data is provided by the Centers for Medicare and Medicaid Services and is used under strict security provisions protected by multiple firewalls. SAH patients were identified based on one or more inpatient diagnostic codes from the International Classification of Diseases, Ninth Revision (ICD-9 430, excluding 094.87 for ruptured syphilitic aneurysm, 437.4 for cerebral arteritis, 747.81, 39.53, or 92.30 for arteriovenous malformation, 800.0-801.9, 803.0-804.9, 850.0-854.1, and 873.0-873.9 for traumatic hemorrhage) between 2007 and 2012. For cohort inclusion, patients were required to be (1) continuously enrolled in fee-for-service (FFS) Medicare Parts A, and B for 12 months before index diagnosis, and (2) be age 65 or older at the time of index diagnosis.
Intervention
We used ICD-9-CM codes to identify patients with aneurysmal SAH, who underwent clipping (ICD-9-CM code 39.51) or coiling (ICD-9-CM code 39.52 (should also have a code 88.41 and no 39.51 during the same hospitalization), 39.72, 39.75, 39.76 39.79) between 2007 and 2012. For patients with multiple interventions, only the first one was included in the final cohort.
Outcome variables
The primary outcome was 1-year post-procedure mortality, in order to mirror the time point used by prior randomized trials.3,6 Secondary outcomes were: length of stay (LOS) during the initial hospitalization, rate of discharge to rehabilitation, and rate of 30-day post-discharge readmission.
Covariates
Sex-age categories (65-69, 70-74, 75-79, 80-84, 85-99) were created, as well as five ethnicity and race categories (Asian, Black, Hispanic, Native American, and other, with white being the excluded variable). The enrollee's ZIP code was used to match to 2010 Census data on income and poverty. We included the ZIP-level poverty rate separately, from the income variable, to reflect the differing distribution of income within the ZIP code.
Comorbidities, diagnosed (in more than 2 outpatient and/or 1 inpatient encounters) at any time in the 12-month look-back (before the intervention), for which outcomes were adjusted (Supplemental Table I), included: hypertension, myocardial infarction, cardiac arrhythmia, congestive heart failure, hyperlipidemia, coagulopathy, hypertension, ischemic stroke, peripheral vascular disease, chronic obstructive pulmonary disease (COPD), other pulmonary disease, diabetes, obesity, alcohol abuse, malignancy, and dementia.
Each facility was identified with one of the 306 Hospital Referral Region (HRR) in the United States as used by The Dartmouth Atlas of Health Care. An HRR is a region served by a hospital or group of hospitals that offer cardiovascular and neurosurgical procedures, so that each HRR includes at least one tertiary care hospital. All ZIP codes in the United Sates were assigned to HRR on the basis of the migration patterns of hospital use among the elderly population. The coiling rate in each HRR was calculated by dividing the number of coiling procedures in an HRR by the number of total interventions for ruptured cerebral aneurysms in the same location and time period.
Statistical analysis
To overcome confounding (the non-random selection of patients for either treatment) due to covariates not captured in our data (such as the clinical condition of the patients upon presentation, aneurysm size and location), which cannot be addressed with traditional methods, we employed an instrumental variable analysis.10 This analysis uses the differences in practice patterns across regions to simulate the structure of a randomized trial, in an observational setting. It attempts to create balance of unmeasured covariates among treatment groups. This advanced observational technique has been used before by clinical researchers, to answer comparative effectiveness questions for different interventions. The goal is to simulate randomization, especially when the baseline functional characteristics of the patients are unknown (similar to our application).11-13 We provide more details on using an instrumental variable analysis in our Supplemental Methods.
Use of coiling varies widely across HRR. Patients tend to seek care for ruptured aneurysms, a medical emergency, close to their residence. Someone who lives in an HRR where coiling is primarily offered, is more likely to receive this treatment. The IV approach depends on the assumption that HRR coiling rates affect the outcomes only by promoting the use of coiling (exclusion restriction criterion), while they are otherwise unrelated to unmeasured risk factors affecting the outcome. HRR coiling rates were not correlated with average predicted mortality within an HRR, based on known confounders (r=0.03, P>0.10) suggesting case-mix balance between HRRs. A practical rule14 for employing an instrument is that the F-statistic (or chi-square for a binary exposure) in the first stage regression exceeds 10. This value was 390 in our study, when using HRR coiling rates as an instrument for coiling. In sensitivity analysis, we used the differential distance of the patient's residence to facilities preferentially offering clipping versus coiling. Although the results were qualitatively the same, this second IV approach had minimal ability to discriminate between treatments, and resulted in high variance. Therefore, this was not used further.
We subsequently calculated the odds ratio for the association of clipping with the outcomes, using a logistic regression model with an IV analysis, in a moments-based approach, as previously described in the literature.15-17 HRR coiling rate was used as an instrument for coiling, and we additionally adjusted for all other covariates listed above. For linear outcomes, we employed a multiple linear regression model with an IV analysis, sometimes referred to as 2-stage least squares.
In sensitivity analysis, to assess the robustness of our results we used different analytical methods to control for measured confounding, two of which are based on propensities. To derive the propensity of clipping versus coiling we developed a prediction model using logistic regression, based on the covariates described above. To compare death at 1 year, 30-day readmission and discharge to rehabilitation between coiling and clipping, we employed multivariable logistic regression, logistic regression with adjustment (stratification) by quantiles (we chose the number of quantiles to be 15, the cube root of the sample size) of the propensity score, and IPW logistic regression. These models included the patient's HRR as a random effects variable to control for clustering. For length of stay we employed the corresponding versions of multiple linear regression models. Logarithmic transformation of LOS did not change the results, and is therefore not reported further. In further sensitivity analysis we used “discharge to home” as an outcome. The observed associations did not change (as compared to discharge to rehabilitation) and therefore these results are not reported further. Lastly, we repeated all analyses after stratification for teaching status of the hospital, and for high-volume institutions. The magnitude and the direction of the associations did not change, and are not reported further.
Finally, we plotted the survival of our cohort, using a Kaplan-Meier estimator, stratified for treatment technique, as well as IPW-adjusted Kaplan-Meier18. In further analysis, for the mortality outcomes, we modeled the dependence of time to death on the treatment of ruptured cerebral aneurysms, using a Cox proportional hazards ratio analysis. Patients were censored on death, and disenrollment from FFS Medicare. This model included all the covariates listed above. We additionally utilized propensity score stratification, and IPW to improve adjustment for known confounders, and IV analysis to adjust for unknown confounders19
Given that we had 2,004 patients undergoing coiling and 1,206 clipping, we had an 80% power to detect a difference in mortality as small as 5.0% (such as 35% vs. 40%), at an α-level of 0.05. Patients with missing data (3% of poverty and income) were excluded from further analysis. All probability values were the result of two sided tests. SAS version 9.4 (SAS Institute, Cary, NC), and the 64-bit version of R.2.12.2 (R Foundation for Statistical Computing) were used for statistical analysis.
RESULTS
Patient characteristics
From 2007-2012, there were 3,210 Medicare patients who underwent treatment for ruptured cerebral aneurysms, and met the inclusion criteria for the study. Of these, 1,206 (37.6%) underwent surgical clipping, and 2,004 (62.4%) endovascular coiling. The respective distribution of exposure variables between the two methods of treatment can be found in Table 1. Figure 1 demonstrates the distribution of coiling rates per HRR for patients with ruptured aneurysms.
Table 1.
Patient characteristics
| Clipping | Coiling | Z-value | |
|---|---|---|---|
| Age, mean (SD) | 73.5 (6.2) | 75.3 (6.8) | 7.4 |
| Male gender | 275 (22.8%) | 533 (26.6%) | −2.4 |
| African-Americans | 135 (11.2%) | 208 (10.4%) | 0.7 |
| Income* | $44,800 (17,900) | $45,700(17,700) | −1.4 |
| Poverty* | 137 (11.4%) | 210 (10.5%) | 3.1 |
| Comorbidities¶ | |||
| Hypertension | 421 (34.9%) | 859 (42.9%) | −4.5 |
| Hyperlipidemia | 166 (13.7%) | 294 (14.7%) | −0.7 |
| Chronic obstructive pulmonary disease | 20 (1.7%) | 33 (1.6%) | 0.02 |
| Myocardial infarction | 97 (8.0%) | 223 (11.1%) | −2.8 |
| Cardiac arrhythmia | 51 (4.2%) | 135 (6.7%) | −3.0 |
| Coagulopathy | ∅ | 17 (0.8%) | −1.2 |
| Renal insufficiency | 42 (3.5%) | 67 (3.3%) | 0.2 |
| Congestive heart failure | 27 (2.2%) | 82 (4.1%) | −2.8 |
| Pulmonary disease§ | 25 (2.1%) | 47 (2.3%) | −0.5 |
| Obesity | ∅ | ∅ | 0.5 |
| Alcohol abuse | ∅ | ∅ | −0.8 |
| Dementia | ∅ | 31 (1.5%) | −2.0 |
| Ischemic stroke | 39 (3.2%) | 89 (4.4%) | −1.7 |
| Diabetes | 123 (10.2%) | 241 (12.0%) | −1.6 |
| Peripheral vascular disease | 51 (4.2%) | 142 (7.1%) | −3.3 |
| Malignancy | 58 (4.8%) | 132 (6.6%) | −2.1 |
SD: Standard Deviation
Output represents crude numbers and percentages in parentheses
The enrollee's ZIP code was used to match to 2010 Census data on income and poverty.
Based on 12-month look-back before the date of the procedure
Non COPD
Output suppressed to comply with the reporting rules of Medicare, which do not allow printing of output involving less than 11 patients
Figure 1.
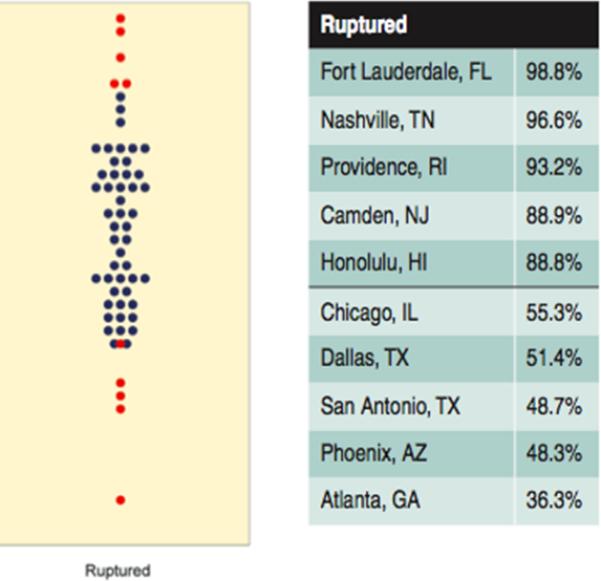
Percent of Medicare beneficiaries treated for ruptured cerebral aneurysms using coiling (2007-2012). Each blue dot represents the percent of Medicare beneficiaries who were treated for cerebral aneurysms with coiling in one of 306 hospital referral regions in the U.S. Red dots indicate the regions with the 5 lowest and 5 highest rates. The names of the latter can be found on the right. (Bekelis K, Goodney RP, Dzebisashvili N, Goodman DC, Bronner KK. Variation in the Care of Surgical Conditions: Cerebral Aneurysms. Lebanon, NH, 2014, reproduced with permission)
Mortality
Overall, 438 (36.3%) deaths were recorded (Table 2) in the first year after clipping, and 821 (41.0%) after coiling. As demonstrated in Table 3, clipping was associated with decreased 1-year mortality (OR, 0.82; 95% CI, 0.71-0.95) in the unadjusted analysis. However, there was no association of treatment with mortality when using an instrumental variable analysis (OR, 1.04; 95% CI, 0.70-1.54). Adjusting for measured confounders with a multivariable logistic regression model (Table 3) confirmed the lack of association of clipping with 1-year mortality (OR, 0.97; 95% CI, 0.83-1.14), which persisted after propensity score adjustment (OR, 0.98; 95% CI, 0.83-1.15), and IPW (OR, 0.94; 95% CI, 0.84-1.06).
Table 2.
Outcomes
| Clipping | Coiling | P-value* | |
|---|---|---|---|
| 1-year mortality | 438 (36.3%) | 821 (41.0%) | 0.008 |
| 30-day readmission | 214 (17.7%) | 308 (15.4%) | 0.09 |
| Discharge to rehabilitation | 369 (30.5%) | 526 (26.3%) | 0.009 |
| Length-of-stay (SD) | 19.9 (12.4) | 17.6 (12.5) | <0.0001 |
SD: Standard Deviation
Output represents crude numbers and percentages in parentheses unless otherwise indicated. Length-of-stay is measured in days
Based on Chi Square or t-test as appropriate
Table 3.
Association of clipping with primary outcome measures
| Models | ||||
|---|---|---|---|---|
| 1-year mortality⌘ | Mortality (time-to-event)§ | |||
| OR (95% CI) | P-value | HR (95% CI) | P-value | |
| Crude | 0.82 (0.71-0.95) | 0.008 | 0.83 (0.74-0.93) | 0.002 |
| Instrumental variable analysis¶ | 1.04 (0.70-1.54) | 0.837 | 0.97 (0.70-1.36) | 0.880 |
| Multivariable regression* | 0.97 (0.83-1.14) | 0.726 | 0.94 (0.83-1.06) | 0.299 |
| Propensity score adjustment* | 0.98 (0.83-1.15) | 0.782 | 0.94 (0.84-1.06) | 0.325 |
| Inverse probability weighting* | 0.94 (0.84-1.06) | 0.345 | 0.92 (0.85-1.01) | 0.338 |
OR: Odds Ratio; 95% CI: 95% Confidence Interval; HR: Hazards Ratio
HRR coiling rate (fraction of coiling of total procedures performed) was used as an instrument of choice of treatment
Mixed effects; Includes patient's HRR as a random effect variable
Analyses based on logistic regression
Analyzed based on a Cox proportional hazard model (limited to 1-year follow-up)
Additionally, we did not demonstrate an association of treatment technique with mortality in further time-to-event analyses, using a Cox model with IV analysis (HR, 0.97; 95% CI, 0.70-1.36). Similar results were seen with multivariable Cox proportional hazard method (Table 3) (HR, 0.94; 95% CI, 0.83-1.06), propensity score adjusted Cox model (HR, 0.94; 95% CI, 0.84-1.06), and IPW Cox model (HR, 0.92; 95% CI, 0.85-1.01). Figure 2 demonstrates a Kaplan-Meier plot of the survival during follow up after clipping or coiling for the treatment of ruptured cerebral aneurysms.
Figure 2.
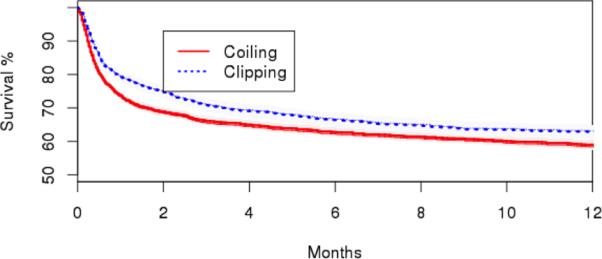
Kaplan-Meier estimates of survival for patients with ruptured aneurysms after surgical clipping or endovascular coiling. Adjusted estimates are presented. Shaded areas represent 95% Confidence Intervals. Adjustment was performed with an inverse probability weighted (IPW) logistic regression model.
Length-of-stay (LOS)
The average LOS was 19.9 days (SD 12.4) for patients undergoing clipping, and 17.6 days (SD 12.5) days for patients undergoing coiling (Table 2). As demonstrated in Table 4, clipping was associated with longer LOS in comparison to coiling (2.31; 95% CI, 1.41-3.20), in the crude analysis. Instrumental variable analysis also demonstrated that clipping was associated with 2.72 days longer LOS (95% CI, 0.45-4.99). This persisted (Table 4) after mixed effects multivariable linear regression modeling (1.78; 95% CI, 0.86-2.71), propensity score stratification (1.86; 95% CI, 0.94-2.79), and IPW (1.90; 95% CI, 0.92-2.87).
Table 4.
Association of clipping with secondary outcome measures
| Models | ||||||
|---|---|---|---|---|---|---|
| Discharge to rehabilitation⌘ | Length-of-stay (days)○ | 30-day readmissions⌘ | ||||
| OR (95% CI) | P-value | Beta (95% CI) | P-value | OR (95% CI) | P-value | |
| Crude | 1.25 (1.06-1.47) | 0.009 | 2.31 (1.41-3.20) | <0.0001 | 1.18 (0.98-1.44) | 0.085 |
| Instrumental variable analysis¶ | 1.07 (0.72-1.58) | 0.754 | 2.72 (0.45-4.99) | <0.0001 | 1.44 (0.70-1.87) | 0.591 |
| Multivariable regression* | 1.13 (0.95-1.34) | 0.161 | 1.78 (0.86-2.71) | <0.0001 | 1.20 (0.98-1.47) | 0.075 |
| Propensity score adjustment* | 1.13 (0.95-1.34) | 0.172 | 1.86 (0.94-2.79) | <0.0001 | 1.21 (0.98-1.48) | 0.071 |
| Inverse probability weighting* | 1.16 (0.95-1.33) | 0.165 | 1.90 (0.92-2.87) | <0.0001 | 1.19 (0.98-1.38) | 0.072 |
OR: Odds Ratio; 95% CI: 95% Confidence Interval
HRR coiling rate (fraction of coiling of total procedures performed) was used as an instrument of coiling
Mixed effects; Includes patient's HRR as a random effect variable
Analyses based on logistic regression
Analyses based on linear regression; point estimates are beta coefficients
Discharge to rehabilitation
369 (30.5%) patients were discharged to rehabilitation after clipping, and 526 (26.3%) after coiling (Table 2). As demonstrated in Table 4, clipping was associated with higher rates of discharge to rehabilitation in comparison to coiling (OR, 1.25; 95% CI, 1.06-1.47), in the unadjusted analysis. However, instrumental variable analysis (OR, 1.07; 95% CI, 0.72-1.58) did not demonstrate an association of treatment technique with discharge to rehabilitation. This persisted (Table 4) after mixed effects multivariable logistic regression modeling (OR, 1.13; 95% CI, 0.95-1.34), propensity score stratification (OR, 1.13; 95% CI, 0.95-1.34), and IPW (OR, 1.16; 95% CI, 0.95-1.33).
30-day readmission
214 (17.7%) readmissions were recorded in the immediate 30-day post-discharge period after clipping, and 308 (15.4%) after coiling (Table 2). As demonstrated in Table 4 clipping was not associated with a lower rate of 30-day readmission in comparison to coiling (OR, 1.18; 95% CI, 0.98-1.44), in the crude analysis. Instrumental variable analysis (OR, 1.44; 95% CI, 0.70-1.87) confirmed the lack of association. This persisted (Table 4) after mixed effects multivariable logistic regression modeling (OR, 1.20; 95% CI, 0.98-1.47), propensity score stratification (OR, 1.21; 95% CI, 0.98-1.48), and IPW (OR, 1.19; 95% CI, 0.98-1.38).
DISCUSSION
In the Medicare population of patients presenting with aneurysmal SAH, we did not identify an association of surgical clipping or endovascular coiling with 1-year mortality, discharge to rehabilitation, or 30-day readmission. Clipping, a more invasive procedure, was associated with slightly longer LOS. These results were consistent across techniques to control for measured and unmeasured confounders. In recent years, the pendulum has swung dramatically in favor of coiling for ruptured aneurysms.1 However, significant regional variation persists in the United States,1 with coiling rates ranging from 36.6% in Atlanta, GA, to 98.9% in Fort Lauderdale, FL.1
Prior randomized clinical trials have shown a clear benefit of endovascular options over surgical clipping in the first year after intervention.1,3,6,7 Molyneux et al3 in their landmark ISAT study demonstrated that 23.7% of SAH patients undergoing coiling were dead or dependent 1 year postoperatively compared to 30.6% of patients undergoing clipping. Further long-term results from this cohort confirmed a persistent survival benefit for coiled patients, although its magnitude was less pronounced.7 The publication of ISAT was met with significant criticism,20 mainly focusing on the execution of the trial only in Europe, and the selection criteria applied. Aneurysms from the anterior circulation were over-represented, raising concerns about the generalization of these findings. In order to address some of these drawbacks, the BRAT trial6 was performed in a single US institution, including a wider selection of aneurysm locations. The results of this study were almost identical to the ISAT trial one year postoperatively.6
Although the impact of these trials has been fundamental in the treatment of cerebral aneurysms, there is limited data on the real-world comparative effectiveness of clipping and coiling. Concerns have been raised that the expansile use of endovascular techniques has been driven by lower volume centers with inferior outcomes.5,21 In addition the application of these techniques in patients with age characteristics, comorbidity profile, and aneurysm locations outside of the strict criteria set by randomized trials might yield suboptimal or otherwise unexpected results. An inherent limitation of an observational study investigating the performance of these treatments would be the bias introduced by the pre-selection of patients for either intervention. In order to overcome this, we used an instrumental variable technique to simulate the effects of randomization on treatment. In addition, we created a cohort of almost all elderly patients in the United States, giving a true picture of national practice in this population, which is not represented in prior clinical trials.3,6
Contrary to these studies,3,6 our analysis could not identify a difference in 1-year mortality, discharge to rehabilitation, and 30-day readmissions between clipping and coiling for elderly patients with SAH. This observation might reflect the favorable risk-profile of the population included in randomized trials. Another possibility is that coiling might be associated with a higher rate of complications in the elderly, given their tortuous vascular anatomy and calcified vessels, making endovascular access and navigation challenging. Lastly the widespread availability of coiling options, performed by multiple specialties without strict certification criteria and standardization of training,1 might lead to suboptimal results in the community.
Going forward, answering these questions and monitoring the performance of treatment options for ruptured cerebral aneurysms in the community can be achieved by the creation of large, long-term registries, with such efforts currently being underway.22 Another consideration is that using mortality as an endpoint might not be the most suitable given the very small apparent difference between clipping and coiling in our cohort. Quality of life outcome measures (such as the modified Rankin scale), or patient satisfaction metrics could be used instead in future registry investigations.
Our study has limitations common to administrative databases. First, this is an observational study, and there is still a possibility of residual confounding. We used multiple techniques (propensity score stratification, IPW, HRR random effects, IV analysis), yielding consistent results to account for known and unknown confounders. Our first stage F-statistic was consistent with a strong instrument,14 and it is unlikely that the regional rate of coiling will be associated with procedural mortality in any other way, than the choice of treatment. Second, coding inaccuracies can affect our estimates. However, coding for procedures is rarely inaccurate, given that it is a revenue generator, and is under scrutiny by payers.
Third, claims data do not provide metrics on the postoperative neurologic status of the patients (i.e. modified Rankin score), re-treatment rates, chronic pain, or quality of life. Therefore we cannot analyze the difference of clipping and coiling, in regards to these measures. Fourth, findings among this older, American population may not be generalizable to younger or otherwise dissimilar populations. Fifth, we have no information on aneurysm size and location, baseline functional status, or surgeon experience. In order to control for these unobservable variables we used an instrumental variable technique, which simulates the effect of randomization, and has been commonly used in similar setting in the past to address such bias.11-13 Lastly, causal inference is hard to establish based on observational data, even when using an IV analysis.10
Conclusions
Treatment options for ruptured cerebral aneurysms and their impact on outcomes in real-world practice remain an issue of debate. Our study found little difference in 1-year survival and discharge to rehabilitation between patients undergoing elective coiling or clipping of ruptured cerebral aneurysms. Future comparative effectiveness studies will likely need to be based on prospective registries, using quality outcome metrics, when determining how treatments compare in the community.
Supplementary Material
Acknowledgments
Funding Statement: “Supported by grants from the National Institute on Aging (PO1-AG19783), and the National Institutes of Health Common Fund (U01-AG046830). The funders had no role in the design, execution, or interpretation of the study, or the manuscript preparation”
Footnotes
Competing Interests Statement: “There are no competing interests”
Contributorship Statement:
“KB-concept, design, manuscript preparation, data interpretation
DG- data analysis, statistical analysis, data interpretation, critical review of manuscript
YS- data analysis, statistical analysis, data interpretation, critical review of manuscript
AO- data analysis, statistical analysis, data interpretation, critical review of manuscript
NL- data interpretation, critical review of manuscript
PG- data interpretation, critical review of manuscript
TM- data analysis, statistical analysis, data interpretation, critical review of manuscript”
Data sharing: “All data are included in the study”
REFERENCES
- 1.Bekelis K, Goodney RP, Dzebisashvili N, et al. Variation in the Care of Surgical Conditions: Cerebral Aneurysms. In: Practice TDIfHPaC, editor. A Dartmouth Atlas of Health Care Series. Lebanon, NH: 2014. [PubMed] [Google Scholar]
- 2.Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med. 2006;355(9):928–39. doi: 10.1056/NEJMra052760. [DOI] [PubMed] [Google Scholar]
- 3.Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267–74. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 4.Qureshi AI, Vazquez G, Tariq N, et al. Impact of International Subarachnoid Aneurysm Trial results on treatment of ruptured intracranial aneurysms in the United States. Clinical article. J Neurosurg. 2011;114(3):834–41. doi: 10.3171/2010.6.JNS091486. [DOI] [PubMed] [Google Scholar]
- 5.Zacharia BE, Ducruet AF, Hickman ZL, et al. Technological advances in the management of unruptured intracranial aneurysms fail to improve outcome in New York state. Stroke. 2011;42(10):2844–49. doi: 10.1161/STROKEAHA.111.619767. [DOI] [PubMed] [Google Scholar]
- 6.McDougall CG, Spetzler RF, Zabramski JM, et al. The Barrow Ruptured Aneurysm Trial. J Neurosurg. 2012;116(1):135–44. doi: 10.3171/2011.8.JNS101767. [DOI] [PubMed] [Google Scholar]
- 7.Molyneux AJ, Kerr RS, Birks J, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol. 2009;8(5):427–33. doi: 10.1016/S1474-4422(09)70080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jalbert JJ, Nguyen LL, Gerhard-Herman MD, et al. Outcomes After Carotid Artery Stenting in Medicare Beneficiaries, 2005 to 2009. JAMA Neurol. 2015 Jan 12; doi: 10.1001/jamaneurol.2014.3638. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Feasby TE, Kennedy J, Quan H, et al. Real-world replication of randomized controlled trial results for carotid endarterectomy. Arch Neurol. 2007;64(10):1496–500. doi: 10.1001/archneur.64.10.1496. [DOI] [PubMed] [Google Scholar]
- 10.Garabedian LF, Chu P, Toh S, et al. Potential bias of instrumental variable analyses for observational comparative effectiveness research. Ann Intern Med. 2014;161(2):131–38. doi: 10.7326/M13-1887. [DOI] [PubMed] [Google Scholar]
- 11.Neuman MD, Rosenbaum PR, Ludwig JM, et al. Anesthesia technique, mortality, and length of stay after hip fracture surgery. JAMA. 2014;311(24):2508–17. doi: 10.1001/jama.2014.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan HJ, Norton EC, Ye Z, et al. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA. 2012;307(15):1629–35. doi: 10.1001/jama.2012.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xian Y, Holloway RG, Chan PS, et al. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305(4):373–80. doi: 10.1001/jama.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staiger D, Stock JH. Instrumental Variables Regression with Weak Instruments. Econometrica. 1997;65(3):557–86. [Google Scholar]
- 15.Foster EM. Instrumental Variables for logistic regression: an illustration. Soc Sci Res. 1997;26:287–504. [Google Scholar]
- 16.Johnston K, Gustafson P, Levy AR, et al. Use of IVs in the analysis of generalized linear models in the presence of omitted confounding with applications to epidemiological research. Stat Med. 2008;27:1539–56. doi: 10.1002/sim.3036. [DOI] [PubMed] [Google Scholar]
- 17.Rassen JA, Schneeweiss S, Glynn RJ, et al. IV analysis for estimation of treatment effects with dichotomous outcomes. Am J Epidemiol. 2009;169(3):273–84. doi: 10.1093/aje/kwn299. [DOI] [PubMed] [Google Scholar]
- 18.MacKenzie T, Brown JR, Likosky DS, et al. Review of Case-Mix Adjusted Survival Curves. Ann Thorac Surg. 2012;93(5):1416–25. doi: 10.1016/j.athoracsur.2011.12.094. [DOI] [PubMed] [Google Scholar]
- 19.MacKenzie TA, Tosteson T, Morden NE, et al. Using Instrumental Variables to Estimate a Cox's Proportional Hazards Regression Subject to Additive Confounding. Health Serv Outcomes Res Methodol. 2014;14:54–68. doi: 10.1007/s10742-014-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harbaugh RE, Heros RC, Hadley MN. More on ISAT. Lancet. 2003;361(9359):783–84. doi: 10.1016/S0140-6736(03)12641-4. [DOI] [PubMed] [Google Scholar]
- 21.Zacharia BE, Bruce SS, Carpenter AM, et al. Variability in outcome after elective cerebral aneurysm repair in high-volume academic medical centers. Stroke. 2014;45(5):1447–52. doi: 10.1161/STROKEAHA.113.004412. [DOI] [PubMed] [Google Scholar]
- 22.NeuroPoint Alliance The National Neurosurgery Quality and Outcomes Database (N2QOD). Secondary The National Neurosurgery Quality and Outcomes Database (N2QOD) 2015 http://www.neuropoint.org/NPA N2QOD.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


