Abstract
Aims
Hyperkalaemia in heart failure patients limits use of cardioprotective renin–angiotensin–aldosterone system inhibitors (RAASi). Sodium zirconium cyclosilicate (ZS‐9) is a selective potassium ion trap, whose mechanism of action may allow for potassium binding in the upper gastrointestinal tract as early as the duodenum following oral administration. ZS‐9 previously demonstrated the ability to reduce elevated potassium levels into the normal range, with a median time of normalization of 2.2 h and sustain normal potassium levels for 28 days in HARMONIZE—a Phase 3, double‐blind, randomized, placebo‐controlled trial. In the present study we evaluated management of serum potassium with daily ZS‐9 over 28 days in heart failure patients from HARMONIZE, including those receiving RAASi therapies.
Methods and results
Heart failure patients with evidence of hyperkalaemia (serum potassium ≥5.1 mmol/L, n = 94) were treated with open‐label ZS‐9 for 48 h. Patients (n = 87; 60 receiving RAASi) who achieved normokalaemia (potassium 3.5–5.0 mmol/L) were randomized to daily ZS‐9 (5, 10, or 15 g) or placebo for 28 days. Mean potassium and proportion of patients maintaining normokalaemia during days 8–29 post‐randomization were evaluated. Despite RAASi doses being kept constant, patients on 5 g, 10 g, and 15 g ZS‐9 maintained a lower potassium level (4.7 mmol/L, 4.5 mmol/L, and 4.4 mmol/L, respectively) than the placebo group (5.2 mmol/L; P<0.01 vs. each ZS‐9 group); greater proportions of ZS‐9 patients (83%, 89%, and 92%, respectively) maintained normokalaemia than placebo (40%; P < 0.01 vs. each ZS‐9 group). The safety profile was consistent with previously reported overall study population.
Conclusion
Compared with placebo, all three ZS‐9 doses lowered potassium and effectively maintained normokalaemia for 28 days in heart failure patients without adjusting concomitant RAASi, while maintaining a safety profile consistent with the overall study population.
Keywords: hyperkalaemia, ZS‐9, heart failure, RAAS
Introduction
Hyperkalaemia is a life‐threatening condition associated with serious cardiac arrhythmias, conduction system abnormalities, and increased mortality.1, 2 The prevalence of hyperkalaemia has been increasing because of higher rates of patients with chronic kidney disease (CKD), heart failure (HF), and diabetes, as well as the broader use of renin–angiotensin–aldosterone system inhibitors (RAASi).3, 4, 5 Specifically, the growing use of RAASi and mineralocorticoid receptor antagonists (MRAs)—as a result of proven clinical benefit in at‐risk populations (particularly those with HF)—has led to higher rates of hyperkalaemia and related hospitalizations and deaths as these agents increase serum potassium levels as a result of their mechanism of action.6 Commonly used treatments for hyperkalaemia, such as polymer exchange resins [i.e. sodium polystyrene sulphonate (SPS)] have significant limitations, including not having been evaluated in randomized trials.7, 8, 9 Furthermore, SPS is poorly tolerated, associated with severe and even fatal gastrointestinal (GI) complications, calling into question its suitability for chronic use.10, 11, 12, 13 Other management strategies for hyperkalaemia, including restricted dietary intake of potassium and treatments such as insulin, sodium bicarbonate, and inhaled beta‐2‐adrenergic agonists, have shown limited and temporary efficacy, and are not always feasible.14, 15, 16
RAASi, including angiotensin‐converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), and MRAs have been shown to decrease morbidity and mortality in patients with HF,17, 18, 19 but are also known to impair potassium excretion, particularly when given at full dose.20, 21, 22 Discontinuation or dose reduction of such therapies decreases hyperkalaemia but is associated with substantial long‐term risk because of the significant cardiorenal benefit associated with their use. Consequently, although clinical trials have indicated that optimal ACEi, ARB, and MRA dosing may reduce cardiovascular‐related hospitalizations and mortality,21 utilization studies have demonstrated that RAASi dosing is often not optimized in HF patients,23, 24, 25 with renal dysfunction and hyperkalaemia frequently cited as barriers to optimal application of RAASi in daily clinical practice.26, 27, 28
Several studies have documented that only approximately one‐third of HF patients eligible for RAASi therapy are receiving the target dose.29, 30 Optimal dosing of RAASi may be increased in patients with HF, if hyperkalaemia is addressed and properly managed in these patients. However, current standard‐of‐care therapies for hyperkalaemia, including agents such as SPS, have uncertain efficacy, poor tolerability, and are associated with severe GI toxicity.10, 31 There is a clinical need for agents that can reliably and safely reduce serum potassium in patients with HF, and effectively maintain normal potassium in patients who are receiving RAASi.
Sodium zirconium cyclosilicate (ZS‐9) is an orally administered, non‐absorbed, highly selective K+ ion trap, which binds potassium even in the presence of competing ions throughout the intestinal tract. Preclinical studies have suggested that ZS‐9 exerts its greatest effects in the duodenum.32 The results of two recently published phase 3 randomized trials in over 1000 patients demonstrated that ZS‐9 rapidly normalized serum potassium levels and maintained potassium in the normal range in a broad cross‐section of hyperkalaemic patients.33, 34 In the phase 3 Hyperkalaemia Randomized Intervention Multidose ZS‐9 Maintenance (HARMONIZE) study of 258 patients with hyperkalaemia, treatment with ZS‐9 resulted in rapid and significant reductions in potassium from 5.6 mmol/L at baseline to 4.5 mmol/L at 48 h, with a median time to normalization of 2.2 h. After achieving normal potassium levels, patients were randomized to receive placebo (treatment withdrawal) or ZS‐9 (5, 10, or 15 g) for 28 days; during this randomized phase, all three doses of ZS‐9 resulted in significantly lower potassium levels and greater proportion of patients maintaining normokalaemia vs. placebo, while demonstrating a safety profile similar to placebo, with no ZS‐9 treatment‐related serious adverse events and no impact on magnesium.34
In this study, we specifically focused on the efficacy of ZS‐9 in maintaining normal potassium levels in HF patients who participated in the HARMONIZE study.
Methods
Study population
The details of the HARMONIZE study are reported elswhere.34 The study recruited adult ambulatory patients with hyperkalaemia (serum potassium ≥5.1 mmol/L) from 44 cardiology, nephrology and general research sites in the USA, Australia, and South Africa (March–August 2014). Key exclusion criteria were renal dialysis, active and significant cardiac arrhythmias and treatment with SPS. Patients initially received open‐label ZS‐9 10 g TID for 48 h; those achieving normokalaemia (serum potassium 3.5–5.0 mmol/L) were randomized (4 : 4 : 4 : 7) to ZS‐9 (5, 10 or 15 g) or placebo once daily for 28 days (Figure 1). Concomitant medications could not be adjusted per protocol. Patients completing the randomized phase were eligible for entry in an 11‐month open‐label extension phase. For this analysis, we specifically focused on patients with a history of HF who participated in the HARMONIZE trial. Patients who developed significant hypokalaemia (serum potassium <3.0 mmol/L) at any time, severe hyperkalaemia (serum potassium >6.2 mmol/L) or significant arrhythmias (ventricular tachycardia/fibrillation, new atrial fibrillation/flutter, paroxysmal supraventricular tachycardia, second‐ or third‐degree atrioventricular block or significant bradycardia [heart rate <40 bpm]) during the maintenance phase were withdrawn from the study and referred to their treating physician.
Figure 1.
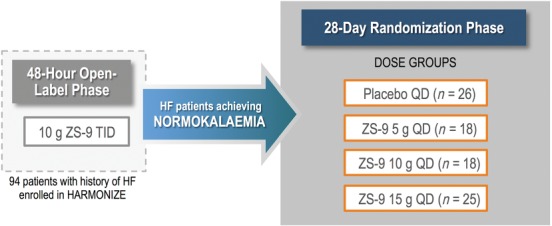
Study design overview. Patients with hyperkalaemia and a history of heart failure (HF) in the Hyperkalaemia Randomized Intervention Multidose ZS‐9 Maintenance (HARMONIZE) study received ZS‐9 10 g during the 48‐h open‐label phase. Those who achieved normokalaemia were randomized to placebo (randomized withdrawal) or ZS‐9 (5 g, 10 g, or 15 g) for 28 days.
The study was conducted in accordance with the ICH E6 (R1) guidelines of good clinical practice and the declaration of Helsinki. The study was approved by national regulatory authorities in each country and the institutional review board or local ethics committee for each site.
Potassium measurements
All potassium levels were measured (after an 8‐h fast) in whole blood with a point‐of‐care device (i‐STAT; Abbott Laboratories, Princeton, NJ). All samples were then sent to a central laboratory for analysis (AU680 chemistry analyzer; Beckman‐Coulter, Brea, CA). Patient eligibility for randomization was based on i‐STAT potassium values in the morning of study day 3 (following 48 h of treatment with ZS‐9 at 10 g, this times daily, TID, to lower potassium). Following randomization, potassium was measured pre‐dose on select days throughout the 28‐day randomized phase of the study. Statistical analyses were based on central laboratory values. No protocol‐directed advice on dietary potassium was provided to patients.
The primary outcome for this analysis was mean serum potassium, determined from days 8 to 29 after randomization. Other outcomes included the proportion of patients that were normokalaemic (mean potassium <5.1 mmol/L) during days 8–29 following randomization and adverse events. Per protocol, secondary objectives of HARMONIZE included evaluation of safety and efficacy of ZS‐9 in patients with HF. Patients in HARMONIZE who met eligibility criteria could enrol in an open‐label extension phase of once‐daily ZS‐9 for 11 months (NCT02107092).
Statistical analysis
All statistical analyses were conducted as previously described.34 Briefly, a longitudinal generalized estimating equation (GEE) model was used to simultaneously compare each active dose (highest to lowest) vs. placebo for the mean day 8–29 values. The GEE model is a repeated measure, mixed effect model. Fisher Exact test was used to measure proportion of normokalaemic patients at measured time‐intervals and patients with mean day 8–29 potassium <5.1 mmol/L. For the open‐label phase efficacy endpoints, change from baseline in potassium at all time‐points were assessed using one‐sample t‐test to test the null hypotheses that the mean changes from baseline were 0. A two‐sided P‐value of <0.05 was considered statistically significant for all endpoints. All analyses were conducted using SAS v9.1 (SAS Institute, Inc., Cary, NC, USA).
Results
Study population
Of 94 patients who had a history of HF at baseline, 87 (93%) achieved normokalaemia after 48 h of ZS‐9 treatment and were randomized to either once‐daily ZS‐9 at the 5 g (n = 18), 10 g (n = 18), or 15 g (n = 25) dose, or placebo (n = 26). Of the seven patients who were not randomized, two withdrew consent before completing the open‐label phase, and five completed the 48‐h open‐label phase but withdrew because they did not meet the serum potassium target (3.5–5.0 mmol/L; n = 4), as determined by a local i‐STAT device, or did meet electrocardiogram (ECG) withdrawal criteria (n = 1).
Baseline characteristics are listed in Table 1. Across patients, the median age was 69 years, 64% were male, and 86% were white; 71% of patients had a history of diabetes mellitus, 76% had a history of chronic kidney disease, and 81% had estimated glomerular filtration rate (eGFR) of <60 mL/min.1.73 m2. At baseline, 69% of patients were treated with RAASi (44% on ACEi, 23% on ARB, and 11% on MRA), which were continued for the duration of the study in all patients receiving these agents. Of these patients, 43% had mild hyperkalaemia (serum potassium 5.0 to <5.5 mmol/L), 45% had moderate hyperkalaemia (serum potassium 5.5 to <6.0 mmol/L), and 13% had severe hyperkalaemia (potassium ≥6.0 mmol/L) at baseline.
Table 1.
Baseline characteristics of heart failure patients by treatment arm
| Open‐label phase | Randomized phase | ||||
|---|---|---|---|---|---|
| ZS‐9 10 g (n = 94) | Placebo (n = 26) | ZS‐9 dose | |||
| 5 g (n = 18) | 10 g (n = 18) | 15 g (n = 25) | |||
| Median age, years (range) | 69.0 (36–89) | 69.0 (45–82) | 70.5 (48–89) | 69.0 (46–85) | 68.0 (36–82) |
| Sex, n (%) | |||||
| Male | 60 (63.8) | 16 (61.5) | 12 (66.7) | 9 (50.0) | 20 (80.0) |
| Female | 34 (36.2) | 10 (38.5) | 6 (33.3) | 9 (50.0) | 5 (20.0) |
| Race, n (%) | |||||
| White | 81 (86.2) | 22 (84.6) | 16 (88.9) | 15 (83.3) | 21 (84.0) |
| Black/African American | 12 (12.8) | 3 (11.5) | 2 (11.1) | 3 (16.7) | 4 (16.0) |
| Serum potassium, n (%) | |||||
| <5.5 mmol/L | 40 (42.6) | 12 (46.2) | 6 (33.3) | 6 (33.3) | 11 (44.0) |
| 5.5 to <6.0 mmol/L | 42 (44.7) | 10 (38.5) | 11 (61.1) | 9 (50.0) | 11 (44.0) |
| ≥6.0 mmol/L | 12 (12.8) | 4 (15.4) | 1 (5.6) | 3 (16.7) | 3 (12.0) |
| eGFR, n (%) | |||||
| <30 mL/min.1.73 m2 | 39 (41.4) | 8 (30.8) | 8 (44.4) | 9 (50.0) | 10 (40) |
| <60 mL/min.1.73 m2 | 76 (80.9) | 22 (84.6) | 13 (72.2) | 16 (88.9) | 20 (80.0) |
| Comorbidities, n (%)a | |||||
| Chronic kidney disease | 71 (75.5) | 19 (73.1) | 12 (66.7) | 15 (83.3) | 18 (72.0) |
| Diabetes mellitus | 67 (71.3) | 18 (69.2) | 13 (72.2) | 16 (88.9) | 17 (68.0) |
| Heart failure | 94 (100) | 26 (100) | 18 (100) | 18 (100) | 25 (100) |
| RAASi medication, n (%) | 65 (69.1) | 21 (80.8) | 11 (61.1) | 14 (77.8) | 14 (56.0) |
| ACEi | 41 (43.6) | 18 (69.2) | 5 (27.8) | 7 (38.9) | 11 (44.0) |
| ARB | 22 (23.4) | 3 (11.5) | 5 (27.8) | 6 (33.3) | 3 (12.0) |
| MRA | 10 (10.6) | 4 (15.4) | 2 (11.1) | 2 (11.1) | 1 (4.0) |
ACE, angiotensin‐converting enzyme; ARB, angiotensin‐receptor blockers; eGFR, estimated glomerular filtration rate; MRA, mineralocorticoid receptor antagonists; RAASi, renin‐angiotensin‐aldosterone system inhibitor.
History of chronic kidney disease or diabetes mellitus collected at study entry.
Efficacy
Before randomization, the mean baseline serum potassium in 94 patients with HF was 5.6 mmol/L [95% confidence interval (CI) 5.5–5.7], which was normalized to 4.4 mmol/L (95% CI 4.3–4.5) after 48 h of ZS‐9 treatment (Figure 2). The median time to normalization was 2.0 h. Within 24 h, 90% of patients had serum potassium <5.1 mmol/L; within 48 h, 99% of patients had serum potassium <5.1 mmol/L.
Figure 2.
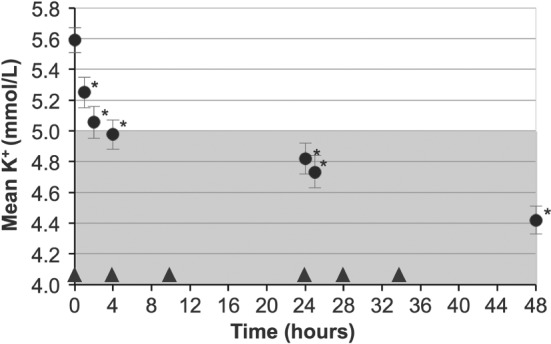
Mean serum potassium over time in patients treated with ZS‐9 10 g three times daily (circles) for 48 h during the open‐label phase. The shaded portion represents normal potassium levels. Bars indicate 95% confidence intervals. Triangles indicate administration of ZS‐9 dose. *P < 0.001 for comparisons against placebo.
During the 28‐day randomized withdrawal phase, patients in the 5 g, 10 g, and 15 g dose groups maintained lower serum potassium levels at 4.7 mmol/L (95% CI 4.5–4.9), 4.5 mmol/L (95% CI 4.3–4.6), and 4.4 mmol/L (95% CI 4.2–4.5), respectively, compared with patients on placebo who returned to hyperkalaemia [5.2 mmol/L; 95% CI 5.0–5.4; P < 0.001, ZS‐9 (all doses) vs. placebo; Figures 3 and 4]. Greater proportions of HF patients receiving ZS‐9 maintained normokalaemia (mean potassium <5.1 mmol/L, days 8–29) during the randomized phase (83%, 89%, and 92% of patients in the 5 g, 10 g, and 15 g dose groups, respectively) compared with the placebo arm [40%; P < 0.01 for ZS‐9 (all doses) vs. placebo]. Efficacy findings were consistent among HF patients regardless of continued concomitant RAASi medication.
Figure 3.
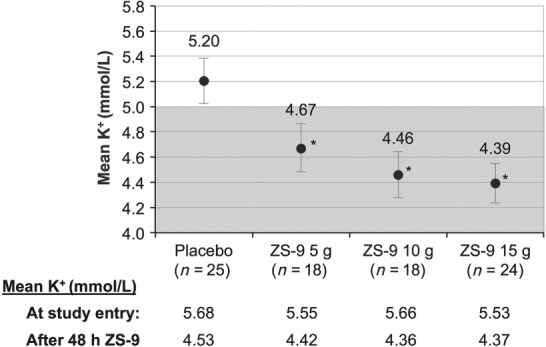
Mean serum potassium, days 8–29 after randomization, placebo vs. ZS‐9 5 g, 10 g, and 15 g dose groups. Mean baseline serum potassium values before and after 48 h of ZS‐9 treatment are shown below the graph for each dose group. The shaded portion represents normal potassium levels. Bars indicate 95% confidence interval. *P < 0.001 for comparisons against placebo.
Figure 4.
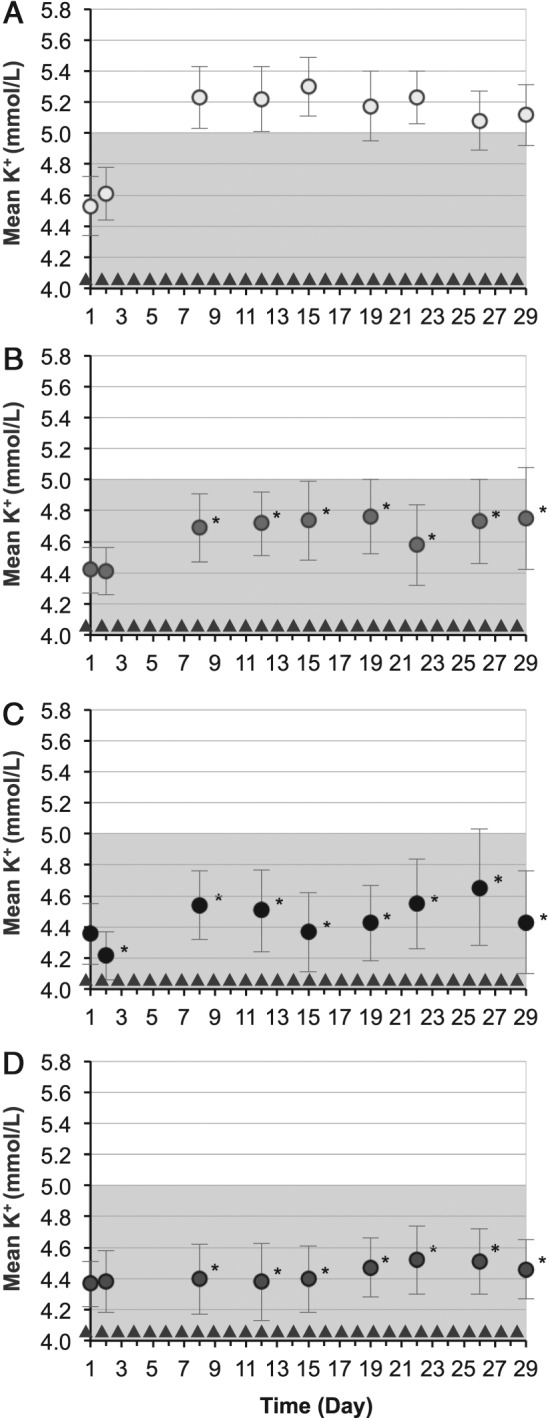
Mean serum potassium over time for the duration of the study (circles): (A) placebo (n = 25), (B) ZS‐9 5 g dose group (n = 18), (C) ZS‐9 10 g dose group (n = 18), and (D) ZS‐9 15 g dose group (n = 24). Triangles indicate administration of ZS‐9 dose or placebo. The shaded portion represents normal potassium levels. Bars indicate 95% confidence intervals. *P < 0.05 for comparisons against placebo.
Safety
Adverse events were reported in 10 HF patients (10.6%) in the 48‐h open‐label phase; nausea and dizziness were the most common, occurring in two patients (2.1%) each. Adverse events occurring in two or more HF patients after randomization are presented in Table 2. The most commonly reported adverse event during the maintenance phase was oedema, occurring in one, two, and five patients in the 5 g, 10 g, and 15 g dose groups, respectively, and one patient in the placebo arm. Eight of the nine cases were peripheral oedema, four of which did not require treatment despite continued ZS‐9 dosing, and no patient discontinued the study because of oedema. Generalized oedema occurred in one patient with severe HF and a history of oedema requiring diuretic treatment. This occurrence of oedema was attributed to discontinuation of diuretics by the patient's family physician before initiation of the study. Six of the nine oedema patients entered the extension study, continuing once‐daily ZS‐9, and none have experienced new oedema (with 149 total exposure weeks). No patient receiving 15 g doses of ZS‐9 in the extension study has experienced oedema (with 278 total exposure weeks). Notably, the oedema rate in HF patients in the extension study is numerically lower than the oedema rate in HF patients on placebo during the randomized phase (0.00119 vs. 0.00132 oedema per day of exposure).
Table 2.
Summary of all adverse events occurring in two or more patients in any treatment arm
| Adverse event, n | Placebo (n = 26) | ZS‐9 dose | ||
|---|---|---|---|---|
| 5 g (n = 18) | 10 g (n = 18) | 15 g (n = 25) | ||
| Any event | 9 | 10 | 7 | 15 |
| Oedemaa | 1 | 1 | 2 | 5b |
| Fatigue | 0 | 0 | 1 | 2 |
| Anaemia | 0 | 0 | 0 | 2 |
| Nasopharyngitis | 1 | 0 | 0 | 2 |
| Upper respiratory tract infection | 0 | 2 | 0 | 0 |
| Hypertension | 1 | 1 | 1 | 2 |
Eight of the nine cases were peripheral oedema, four of which did not require treatment despite continued ZS‐9 treatment, and no patient discontinued the study because of oedema. Six of nine patients entered the extension study and none have experienced new oedema (149 total exposure weeks).
Generalized oedema occurred in one patient with severe heart failure and a history of oedema requiring diuretic treatment. This occurrence of oedema was attributed to discontinuation of diuretics by the patient's family physician before initiation of the study.
Gastrointestinal events were reported in five patients (5.3%) during the open‐label phase. After randomization, GI events occurred in one patient (5.6%) in the 5 g dose group, none in the 10 g dose group, and three (12%) in the 15 g dose group, compared with five (19.2%) in the placebo group. No clinically significant cases of hypokalaemia (serum potassium <3.0 mmol/L) or cardiac arrhythmias occurred. Laboratory analyses showed mild hypokalaemia (3.0 to <3.5 mmol/L) occurring in one patient in the 10 g dose group and three patients in the 15 g dose group; each case resolved with protocol‐directed dose adjustments. None of the cases of hypokalaemia were reported as adverse events. There were no treatment‐related serious adverse events in any ZS‐9 dose groups.
Discussion
Angiotensin‐converting enzyme inhibitor, ARB, and MRA therapy are cornerstones of modern HF therapy, decreasing morbidity and mortality in patients with HF. Unfortunately, these RAASi therapies impair potassium excretion, thereby causing or exacerbating hyperkalaemia. The development of hyperkalaemia in HF patients often results in the reduction RAASi dosage to a level that is suboptimal for the treatment of their cardiovascular disease. Our current options for hyperkalaemia are not ideal given that they are transient, require active management, and are invasive and expensive. For example, treatments such as insulin, sodium bicarbonate, and inhaled beta‐2‐adrenergic agonists cause a temporary intracellular shift of potassium, dialysis is costly and invasive, and organic polymer resins have not been evaluated in randomized trials and are associated with severe GI complications.
In the present paper we describe the safety and efficacy of once‐daily ZS‐9 to achieve and maintain normokalaemia in HF patients with hyperkalaemia. Our findings in HF patients with hyperkalaemia demonstrate that, compared with placebo, once‐daily ZS‐9 resulted in lower potassium levels and greater proportion of patients who maintained normokalaemia for up to 4 weeks. The majority of patients (69%) received RAASi during the study and achieved and maintained normokalaemia without adjustment of their RAASi dose. The data described here in HF patients are consistent with results from two phase 3 randomized controlled trials where ZS‐9 rapidly normalized serum potassium within hours and continued to maintain normal potassium for up to 28 days in a high proportion of patients on all ZS‐9 doses, all ZS‐9 doses reduced serum aldosterone, and efficacy was similar across patients on and not on RAASi. These results suggest that in HF patients, including those on ACEi, ARB, and/or MRA, ZS‐9 demonstrated a safety profile similar to the overall HARMONIZE population, was fast‐acting with a median time to serum potassium normalization of 2 h, and maintained normokalaemia in patients with HF without the need to reduce or cease life‐saving RAASi therapies.
The results of this study should be interpreted in the context of several potential limitations. First, our analysis focused on the subgroup of patients with HF from the HARMONIZE study population, the numbers of patients within each treatment subgroup were small, and interpretability of the results must take into consideration the limitations inherent to treatment in a clinical trial setting. A diagnosis of HF was established based on patient history; no data were collected on New York Heart Association (NYHA) class, echocardiogram, or ejection fraction of enrolled patients. Second, hospitalized patients, those receiving dialysis, and those with life‐threatening arrhythmias were excluded from the HARMONIZE trial. Third, although the findings of this study support the ability of ZS‐9 to enable continuation of RAASi use in HF patients, additional studies will be valuable in confirming whether ZS‐9 use can enable up‐titration or optimal dosing of RAASi in these patients. Finally, the HARMONIZE trial followed patients for up to 4 weeks, therefore the long‐term evaluation of ZS‐9 efficacy and safety would be valuable and these studies are currently ongoing.
In conclusion, compared with placebo, all three doses of ZS‐9 resulted in lower potassium levels and effectively maintained normokalaemia for 28 days in hyperkalaemic patients with HF, including those on RAASi. These data suggest that ZS‐9 may enable continuation of important RAASi use in patients with HF and represents a promising addition to the future of HF therapy.
Funding
This work was supported by ZS Pharma Inc.
Conflict of interest
S.D.A. has received fees for speaking and consultancy from ZS Pharma, Inc., the maker of ZS‐9, and Bayer Pharmaceuticals, Inc. M.K. is a consultant for ZS Pharma, Inc., the maker of ZS‐9, and is currently an investigator and conducting research sponsored by this company. F.Z. declares that he receives steering committee fees from Bayer, Boston Scientific, Janssen, Novartis, Pfizer, Resmed, and Takeda, and consultant/scientific advisory board fees from: Air Liquide, Amgen, CVRx, Relypsa, Servier, St. Jude, Stealth peptide, ZS Pharma, Inc., Quantuum Genomics. He is co‐founder of CRS. His institution receives research grants from Roche Diagnostics. P.vdM. received consultancy fees from ZS Pharma, Inc., the maker of ZS‐9. G.F. participates in committees of trials sponsored by Novartis, Bayer, Cardiorentis, European Union. H.S.R. and B.S. are employees of and hold stock or stock options in ZS Pharma, Inc., the maker of ZS‐9. P.T.L. holds stock and options in ZS Pharma, Inc., the maker of ZS‐9, and is currently conducting research sponsored by this company. A.Y. is a paid consultant for and holds stock or stock options in ZS Pharma, Inc., the maker of ZS‐9. P.A.M., I.L.P. and P.P. report no conflicts of interest. P.D. does not own any stocks nor conducts research, nor is a member of a speakers bureau of any company that makes the same or similar products.
Acknowledgment
Medical writing support was provided by Xelay Acumen, Inc.
The copyright line for this article was changed on 13 November 2015 after original online publication.
References
- 1. An JN, Lee JP, Jeon HJ, Kim DH, Oh YK, Kim YS, Lim CS. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care 2012;16:R225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khanagavi J, Gupta T, Aronow WS, Shah T, Garg J, Ahn C, Sule S, Peterson S. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci 2014;10:251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jain N, Kotla S, Little BB, Weideman RA, Brilakis ES, Reilly RF, Banerjee S. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol 2012;109:1510–1513. [DOI] [PubMed] [Google Scholar]
- 4. Navaneethan SD, Nigwekar SU, Sehgal AR, Strippoli GFM. Aldosterone antagonists for preventing the progression of chronic kidney disease: a systematic review and meta‐analysis. Clin J Am Soc Nephrol 2009;4:542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Svensson M, Gustafsson F, Galatius S, Hildebrandt PR, Atar D. How prevalent is hyperkalemia and renal dysfunction during treatment with spironolactone in patients with congestive heart failure? J Card Fail 2004;10:297–303. [DOI] [PubMed] [Google Scholar]
- 6. Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. New Engl J Med 2004;351:543–551. [DOI] [PubMed] [Google Scholar]
- 7. Kessler C, Ng J, Valdez K, Xie H, Geiger B. The use of sodium polystyrene sulfonate in the inpatient management of hyperkalemia. J Hosp Med 2011;6:136–140. [DOI] [PubMed] [Google Scholar]
- 8. Scherr L, Ogden DA, Mead AW, Spritz N, Rubin AL. Management of hyperkalemia with a cation‐exchange resin. New Engl J Med 1961;264:115–119. [DOI] [PubMed] [Google Scholar]
- 9. Flinn RB, Merrill JP, Welzant WR. Treatment of the oliguric patient with a new sodium‐exchange resin and sorbitol; a preliminary report. New Engl J Med 1961;264:111–115. [DOI] [PubMed] [Google Scholar]
- 10. Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med 2013;126:264.e9–e24. [DOI] [PubMed] [Google Scholar]
- 11. Yuan CM, Nee R, Little DJ, Abbott KC. Incidence of sodium polystyrene sulfonate‐associated colonic necrosis. Am J Med 2013;126:e13. [DOI] [PubMed] [Google Scholar]
- 12. Watson MA, Baker TP, Nguyen A, Sebastianelli ME, Stewart HL, Oliver DK, Abbott KC, Yuan CM. Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Am J Kidney Dis 2012;60:409–416. [DOI] [PubMed] [Google Scholar]
- 13. Castillo‐Cejas MD, De‐Torres‐Ramírez I, Alonso‐Cotoner C. Colonic necrosis due to calcium polystyrene sulfonate (Kalimate) not suspended in sorbitol. Rev Esp Enferm Dig 2013;105:232–234. [DOI] [PubMed] [Google Scholar]
- 14. Fordjour KN, Walton T, Doran JJ. Management of hyperkalemia in hospitalized patients. Am J Med Sci 2014;347:93–100. [DOI] [PubMed] [Google Scholar]
- 15. Goraya N, Wesson DE. Dietary management of chronic kidney disease: protein restriction and beyond. Curr Opin Nephrol Hypertens 2012;21:635–640. [DOI] [PubMed] [Google Scholar]
- 16. Łoniewski I, Wesson DE. Bicarbonate therapy for prevention of chronic kidney disease progression. Kidney Int 2014;85:529–535. [DOI] [PubMed] [Google Scholar]
- 17. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. New Engl J Med 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 18. The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. New Engl J Med 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 19. Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. New Engl J Med. 1992;327:669–677. [DOI] [PubMed] [Google Scholar]
- 20. Konstam MA, Neaton JD, Dickstein K, Drexler H, Komajda M, Martinez FA, Riegger GAJ, Malbecq W, Smith RD, Guptha S, Poole‐Wilson PA. Effects of high‐dose versus low‐dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double‐blind trial. Lancet 2009;374:1840–1848. [DOI] [PubMed] [Google Scholar]
- 21. Packer M, Poole‐Wilson PA, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Rydén L, Thygesen K, Uretsky BF. Comparative effects of low and high doses of the angiotensin‐converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group . Circulation 1999;100:2312–2318. [DOI] [PubMed] [Google Scholar]
- 22. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. New Engl J Med 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 23. Lenzen MJ, Boersma E, Reimer WJMSO, Balk AHMM, Komajda M, Swedberg K, Follath F, Jimenez‐Navarro M, Simoons ML, Cleland JGF. Under‐utilization of evidence‐based drug treatment in patients with heart failure is only partially explained by dissimilarity to patients enrolled in landmark trials: a report from the Euro Heart Survey on Heart Failure. Eur Heart J 2005;26:2706–2713. [DOI] [PubMed] [Google Scholar]
- 24. Peters‐Klimm F, Laux G, Campbell S, Müller‐Tasch T, Lossnitzer N, Schultz J‐H, Remppis A, Jünger J, Nikendei C. Physician and patient predictors of evidence‐based prescribing in heart failure: a multilevel study. PloS One 2012;7:e31082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bungard TJ, McAlister FA, Johnson JA, Tsuyuki RT. Underutilisation of ACE inhibitors in patients with congestive heart failure. Drugs 2001;61:2021–2033. [DOI] [PubMed] [Google Scholar]
- 26. Edep ME, Shah NB, Tateo IM, Massie BM. Differences between primary care physicians and cardiologists in management of congestive heart failure: relation to practice guidelines. J Am Coll Cardiol 1997;30:518–526. [DOI] [PubMed] [Google Scholar]
- 27. Echemann M, Zannad F, Briançon S, Juillière Y, Mertès PM, Virion JM, Villemot JP. Determinants of angiotensin‐converting enzyme inhibitor prescription in severe heart failure with left ventricular systolic dysfunction: the EPICAL study. Am Heart J 2000;139:624–631. [DOI] [PubMed] [Google Scholar]
- 28. Houghton AR, Cowley AJ. Why are angiotensin converting enzyme inhibitors underutilised in the treatment of heart failure by general practitioners? Int J Card 1997;59:7–10. [DOI] [PubMed] [Google Scholar]
- 29. Albert NM, Yancy CW, Liang L, Zhao X, Hernandez AF, Peterson ED, Cannon CP, Fonarow GC. Use of aldosterone antagonists in heart failure. JAMA 2009;302:1658–1665. [DOI] [PubMed] [Google Scholar]
- 30. Maggioni AP, Anker SD, Dahlström U, Filippatos G, Ponikowski P, Zannad F, Amir O, Chioncel O, Leiro MC, Drozdz J, Erglis A, Fazlibegovic E, Fonseca C, Fruhwald F, Gatzov P, Goncalvesova E, Hassanein M, Hradec J, Kavoliuniene A, Lainscak M, Logeart D, Merkely B, Metra M, Persson H, Seferovic P, Temizhan A, Tousoulis D, Tavazzi L. Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2013;15:1173–1184. [DOI] [PubMed] [Google Scholar]
- 31. Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion‐exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol 2010;21:733–735. [DOI] [PubMed] [Google Scholar]
- 32. Stavros F, Yang A, Leon A, Nuttall M, Rasmussen HS. Characterization of structure and function of ZS‐9, a K+ selective ion trap. PloS One 2014;9:e114686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Packham DK, Rasmussen HS, Lavin PT, El‐Shahawy MA, Roger SD, Block G, Qunibi W, Pergola P, Singh B. Sodium zirconium cyclosilicate in hyperkalemia. New Engl J Med 2014;372:222–231. [DOI] [PubMed] [Google Scholar]
- 34. Kosiborod M, Rasmussen HS, Lavin P, Qunibi WY, Spinowitz B, Packham D, Roger SD, Yang A, Lerma E, Singh B. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia. JAMA 2014;312:2223–2233. [DOI] [PubMed] [Google Scholar]


