Abstract
OBJECTIVE
To explore whether electrochemiluminescence (ECL) assays can help improve prediction of time to type 1 diabetes in the TrialNet autoantibody-positive population.
RESEARCH DESIGN AND METHODS
TrialNet subjects who were positive for one or more autoantibodies (microinsulin autoantibody, GAD65 autoantibody [GADA], IA-2A, and ZnT8A) with available ECL-insulin autoantibody (IAA) and ECL-GADA data at their initial visit were analyzed; after a median follow-up of 24 months, 177 of these 1,287 subjects developed diabetes.
RESULTS
Univariate analyses showed that autoantibodies by radioimmunoassays (RIAs), ECL-IAA, ECL-GADA, age, sex, number of positive autoantibodies, presence of HLA DR3/4-DQ8 genotype, HbA1c, and oral glucose tolerance test (OGTT) measurements were all significantly associated with progression to diabetes. Subjects who were ECL positive had a risk of progression to diabetes within 6 years of 58% compared with 5% for the ECL-negative subjects (P < 0.0001). Multivariate Cox proportional hazards models were compared, with the base model including age, sex, OGTT measurements, and number of positive autoantibodies by RIAs. The model with positivity for ECL-GADA and/or ECL-IAA was the best, and factors that remained significantly associated with time to diabetes were area under the curve (AUC) C-peptide, fasting C-peptide, AUC glucose, number of positive autoantibodies by RIAs, and ECL positivity. Adding ECL to the Diabetes Prevention Trial risk score (DPTRS) improved the receiver operating characteristic curves with AUC of 0.83 (P < 0.0001).
CONCLUSIONS
ECL assays improved the ability to predict time to diabetes in these autoantibody-positive relatives at risk for developing diabetes. These findings might be helpful in the design and eligibility criteria for prevention trials in the future.
Introduction
Relatives of those with type 1 diabetes have an increased risk of developing type 1 diabetes, especially once they become positive for multiple pancreatic islet autoantibodies (1–3). These relatives are eligible to be screened for islet autoantibodies through the TrialNet Pathway to Prevention Study (4,5). TrialNet is an international consortium of investigators dedicated to studying the etiology of type 1 diabetes with the goal of preventing or reversing the progression of type 1 diabetes. TrialNet follows these subjects with serial longitudinal autoantibody testing for the development of islet autoantibodies to insulin (6), GAD65 (7), IA-2 (8), and ZnT8 (9) and type 1 diabetes and offers close monitoring for autoantibody-positive subjects through HbA1c testing and oral glucose tolerance test (OGTT) (10). Autoantibody-positive subjects may also be eligible for prevention trials within TrialNet. Factors involved in risk of progression to diabetes have been previously shown to include young age at seroconversion, positivity for multiple autoantibodies, high autoantibody levels, persistent positivity for insulin autoantibody (IAA), and high autoantibody affinity (1,11–13). Although multiple autoantibody–positive subjects have an 80% risk of developing diabetes within 15 years, the rate of progression of these high-risk individuals varies significantly, from a few months to >10 years (2,14), and factors involved in the rate of progression are not yet well understood.
Over the past couple of years, the Barbara Davis Center for Childhood Diabetes (BDC) has been developing new electrochemiluminescence (ECL) assays for islet autoantibodies. These new assays have been shown to be more disease specific and more sensitive than current gold standard radioassays, especially for IAA (15–17). The goal of this study was to explore whether ECL assays can help improve prediction of time to type 1 diabetes in the TrialNet autoantibody-positive population.
Research Design and Methods
Study Population
The TrialNet Pathway to Prevention Study screens relatives of patients with type 1 diabetes for the presence of islet autoantibodies and offers close monitoring and/or prevention trials. TrialNet subjects (n = 1,287) positive for one or more autoantibodies by radioimmunoassays (RIAs) (microinsulin autoantibody [mIAA], GAD65 autoantibody [GADA], IA-2A, and ZnT8A) with available ECL-IAA and ECL-GADA at their initial visit were analyzed. All TrialNet subjects are tested for RIA autoantibodies. However, only subjects with at least one positive islet autoantibody undergo HbA1c testing and OGTT. Subjects with negative islet autoantibodies are eligible for yearly rescreening of islet autoantibodies if <18 years of age; only a minority of these “control” subjects undergo yearly HbA1c and OGTT (n = 89). Because of the small number of autoantibody-negative subjects with available metabolic markers, this study only included subjects with at least one positive islet autoantibody by RIA. OGTT measurements (area under the curve [AUC] glucose, fasting glucose, fasting C-peptide, and AUC C-peptide) were available on a subset of these subjects (n = 759) who are included in multivariate analyses. All subjects provided written informed consent, and assent when applicable, and the study was approved by the ethical boards of all participating institutions.
Islet Autoantibodies
Measurement of islet autoantibodies to insulin, GAD65, IA-2, and ZnT8 was performed in the Clinical Immunology Laboratory at the BDC, the core immunology laboratory for antibody testing for the TrialNet study, using RIAs as previously described (9,18,19). In the 2015 Islet Autoantibody Standardization Program (IASP) Workshop, sensitivities and specificities were 52 and 100% respectively for mIAA, 82 and 99% respectively for GADA, 72 and 100% respectively for IA-2A, and 70 and 97% respectively for ZnT8A.
The ECL assays for both mIAA and GADA have been previously described (16,20). In brief, serum samples were mixed with both sulfo-tag and biotin-labeled antigen proteins (either proinsulin or GAD65) for overnight incubation at 4°C. The antigen-antibody complexes with biotin were captured by a streptavidin-coated plate and sulfo-tag gave the signals with ECL. The results were expressed as an index against internal standard–positive controls of either insulin or GAD65 monoclonal antibody. The ECL assay cutoff indexes of 0.006 for mIAA or 0.023 for GADA were set at the 99th percentile over 100 healthy control subjects and the ECL interassay coefficients of variation were 4.8% (n = 20) for mIAA and 8.8% (n = 10) for GADA, respectively. In the 2015 IASP Workshop, sensitivities and specificities for the ECL assays were 60 and 98% respectively for mIAA, and 78 and 96% respectively for GADA, among patients with newly diagnosed type 1 diabetes.
Statistical Analysis
Statistical analyses were performed using PRISM (GraphPad Software, Inc., La Jolla, CA) and SAS version 9.4 (SAS Institute, Cary, NC). Proportions were compared using χ2 or Fisher exact test. Follow-up time was defined as time from the initial visit to development of diabetes or most recent visit for those subjects who did not develop diabetes. Cox proportional hazards regression was used to obtain hazard ratios (HRs) with 95% CIs. The proportional hazards assumption was tested for all variables, with only HbA1c showing an interaction with time. Six multivariable Cox proportional hazards models predicting time to develop diabetes were compared using Akaike information criterion (AIC). The base model contained age, sex, AUC C-peptide, fasting C-peptide, AUC glucose, and number of positive autoantibodies by RIAs. Other models included the covariates in the base model and added autoantibodies by RIAs or ECL results: continuous RIA (titers), categorical RIA (pos/neg), continuous ECL (titers), categorical ECL (pos/neg for ECL-IAA; pos/neg for ECL-GADA), categorical combined ECL (positivity for ECL-GADA and/or ECL-IAA). These six models were repeated with the addition of HbA1c and HLA DR3/4-DQ8 genotype.
Survival analysis was performed for the development of diabetes according to ECL positivity using the log-rank test. Receiver operating characteristic (ROC) curves were generated to examine the addition of ECL positivity to the Diabetes Prevention Trial risk score (DPTRS) (21). The DPTRS, developed in order to predict risk for diabetes (22), includes age, log BMI, log fasting C-peptide, the glucose sums of 30-, 60-, 90-, and 120-min values, and the C-peptide sums of 30-, 60-, 90-, and 120-min values.
An antibody risk score (ABRS) was previously developed from a proportional hazards model that included both RIA positivity and autoantibody levels according to the following (23):
 |
In a subgroup of subjects who had complete DPTRS, ABRS, and ECL information (n = 606), Cox proportional hazards models predicting time to develop diabetes were performed to compare the three following models: DPTRS and ABRS; DPTRS and ECL; and DPTRS, ABRS, and ECL (ECL positivity defined as positivity for ECL-GADA and/or ECL-IAA).
Results
After a median follow-up of 24 months (IQR 8–57), 177 of the 1,287 subjects developed type 1 diabetes. Characteristics of TrialNet autoantibody-positive subjects are shown in Table 1. The autoantibody-positive subjects without diabetes had a median age of 16 years (IQR 9–37) at initial visit compared with 10 years (IQR 7–14) for those autoantibody-positive subjects who developed diabetes (P < 0.0001). The majority (n = 136 [77%]) of those who developed diabetes had multiple positive autoantibodies, whereas only 22% (n = 249) of the subjects without diabetes had multiple positive autoantibodies (P < 0.0001). BMI assessed by categories (underweight/normal/overweight/obese) was similar between groups at both the initial and most recent visits (P = 0.34 and 0.55, respectively).
Table 1.
Characteristics of TrialNet autoantibody-positive subjects (n = 1,287)
| Characteristics | Ab+ subjects without diabetes (n = 1,110) | Ab+ subjects with diabetes (n = 177) | P value |
|---|---|---|---|
| Age at initial visit (years) | 16 (9–37) | 10 (7–14) | <0.0001 |
| Age at last visit (years) | 19 (12–39) | 11 (8–17) | <0.0001 |
| Sex, female | 669 (60) | 88 (50) | 0.02 |
| Multiple (≥2) Ab+ at initial visit | 249 (22) | 136 (77) | <0.0001 |
| HLA DR3/4-DQ8 (yes) | 71 (15) | 33 (25) | 0.007 |
| BMI at initial visit (% underweight/normal/overweight/obese) | 1/57/22/20 | 2/62/18/18 | 0.35 |
Data are median (IQR) or n (%), unless specified otherwise. Boldface type indicates P < 0.05. Ab+, autoantibody positive.
Univariate analyses of TrialNet autoantibody-positive subjects are shown in Supplementary Table 1. Univariate analyses confirmed that age, HLA-DR3/4*0302 genotype, number of positive autoantibodies by RIAs, ECL autoantibodies, RIA autoantibodies (except GADA), and metabolic factors (HbA1c, AUC C-peptide, fasting C-peptide, and AUC glucose but not fasting glucose) are all associated with progression to diabetes in these autoantibody-positive subjects. Missing information in subjects who did not complete a monitoring visit includes OGTT measurements, HbA1c, and HLA testing, and therefore these subjects were not included in multivariate analyses. Univariate analyses limited to the 759 subjects included in multivariate analyses were similar except for sex, which was no longer a significant factor (P = 0.07), and GADA, which became significant (HR 1.6 [95% CI 1.1–2.5], P = 0.02; data not shown). A total of 150 out of the 759 subjects progressed to diabetes.
Characteristics of TrialNet autoantibody-positive subjects by ECL status are shown in Supplementary Table 2. Compared with ECL-negative subjects, subjects positive for both ECL-IAA and ECL-GADA are more likely to have a higher frequency of the HLA DR3/4-DQ8 genotype, lower fasting C-peptide, lower AUC C-peptide, and higher AUC glucose; they are also more likely to be younger at baseline visit and have multiple positive autoantibodies with a higher frequency of all RIA autoantibodies.
Six multivariable Cox proportional hazards models predicting time to develop diabetes were compared using AIC. The base model contained age, sex, AUC C-peptide, fasting C-peptide, AUC glucose, and number of positive autoantibodies by RIAs. Other models included the covariates in the base model and added autoantibodies by RIAs or ECL results (as continuous or categorical values). The model with positivity for ECL-GADA and/or ECL-IAA was best and had the lowest AIC score of 1,516 (Table 2). Factors that remained significantly associated with time to diabetes were AUC glucose, AUC C-peptide, fasting C-peptide, number of positive autoantibodies by RIAs, and ECL positivity. The base model had an AIC of 1,536, whereas the other four models had AIC values similar to the base model.
Table 2.
Multivariate Cox proportional hazards models in TrialNet subjects (n = 759)
| Covariate | HR (95% CI) | P value |
|---|---|---|
| Age | 0.99 (0.97–1.01) | 0.354 |
| Sex (female) | 0.94 (0.67–1.32) | 0.736 |
| AUC glucose (units = 100) | 1.02 (1.01–1.03) | <0.0001 |
| AUC C-peptide (units = 100) | 0.76 (0.69–0.82) | <0.0001 |
| Fasting C-peptide | 1.45 (1.12–1.87) | 0.004 |
| Number of positive antibodies by RIAs | 2 vs. 1: 2.80 (1.66–4.73) | <0.0001 |
| 3 vs. 1: 3.33 (2.04–5.46) | ||
| 4 vs. 1: 3.81 (2.15–6.74) | ||
| ECL (GADA or IAA): positive vs. negative | 6.90 (2.46–19.32) | 0.0002 |
Six multivariate Cox proportional hazards models were compared with base model. Association with progression to type 1 diabetes was analyzed. Only results of best multivariate model are shown. The base model contained age, sex, AUC C-peptide, fasting C-peptide, AUC glucose, and number of positive antibodies by RIAs. Other models included base model and adding antibodies by RIAs or ECL results (as continuous or categorical value). The model with ECL-IAA and ECL-GADA combined had the lowest AIC and thus was the best model. A total of 150 out of the 759 subjects developed diabetes. Boldface type indicates P < 0.05.
The six models were repeated with the addition of HbA1c and HLA DR3/4-DQ8 in all subjects with available information (n = 581); a total of 125 out of the 581 subjects developed diabetes. Again, the model with positivity for ECL-GADA and/or ECL-IAA had the lowest AIC (1,211) and thus was the best model (Supplementary Table 3). The base model had an AIC of 1,235, whereas the other four models had AIC values similar to the base model.
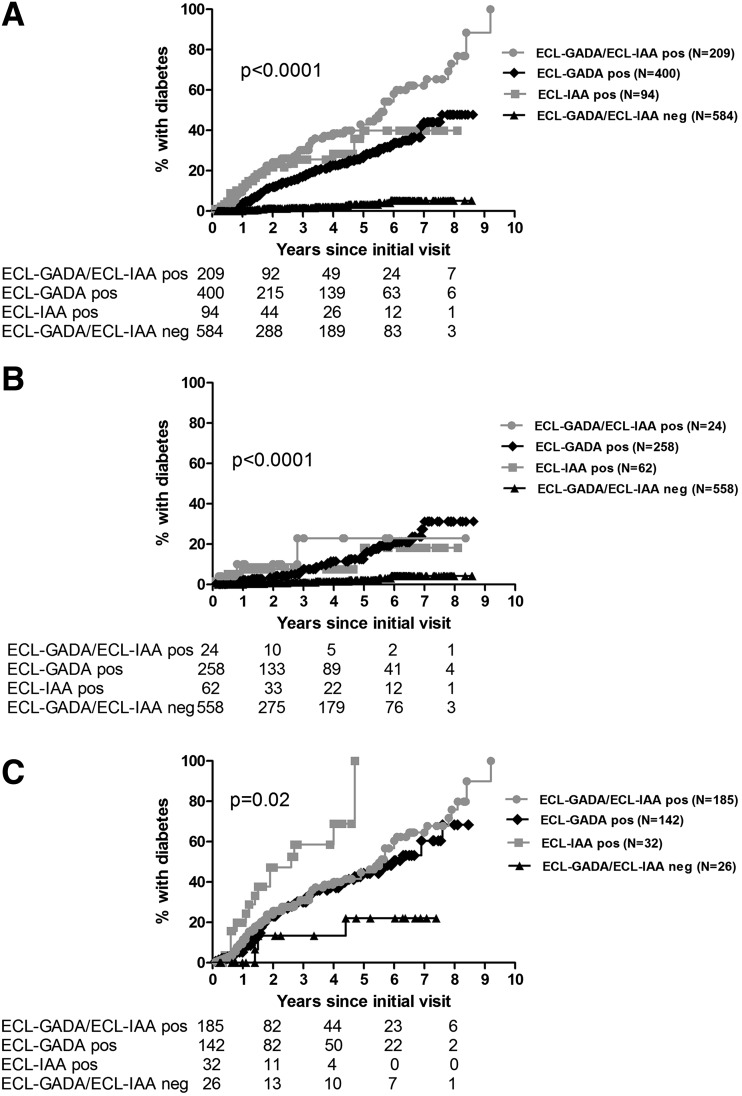
When survival curves were generated by ECL status, it was apparent that the combined ECL positivity (i.e., positivity for ECL-GADA and ECL-IAA) was associated with much higher risk of the development of diabetes overall (Fig. 1A) (P < 0.0001). By year 6 after the participant’s initial visit, 58% of those positive for both ECL-IAA and ECL-GADA developed diabetes. Risk for development of diabetes by 6 years was 40% for those positive for ECL-IAA only and 34% for those positive for ECL-GADA only. Only 5% of the subjects who were negative for either ECL assay developed diabetes by the 6-year follow-up after their initial visit.
Figure 1.
Development of diabetes by ECL status. A: All subjects (n = 1,287). B: Subjects positive for one autoantibody by RIAs (n = 902). C: Subjects positive for two or more autoantibodies by RIAs (n = 385). Survival analysis was performed for the development of diabetes since initial visit according to ECL positivity using the log-rank test. ECL-GADA/ECL-IAA pos, positive for both ECL-GADA and ECL-IAA; ECL-GADA pos, positive for ECL-GADA only; ECL-IAA pos, positive for ECL-IAA only; ECL-GADA/ECL-IAA neg, negative for both ECL-GADA and ECL-IAA.
Survival curves analyzing only subjects positive for one autoantibody by RIAs (n = 902) showed a much higher risk for development of diabetes by year 6 after the participant’s initial visit for subjects who were ECL positive (23% if both ECL-IAA and ECL-GADA positive, 18% if only ECL-IAA positive, and 21% if only ECL-GADA positive) compared with those subjects who were ECL negative (4%, P < 0.0001) (Fig. 1B). When looking at subjects positive for two or more autoantibodies by RIAs (n = 385), the risk for development of diabetes was again much higher for those subjects who were ECL positive: 61% by 6 years if both ECL-IAA and ECL-GADA positive, 69% by 4 years if only ECL-IAA positive, and 51% by 6 years if only ECL-GADA positive compared with 22% for those subjects who were ECL negative (Fig. 1C) (P = 0.02); follow-up for the only ECL-IAA–positive group was by 4 years as the number of subjects in that group over time was small.
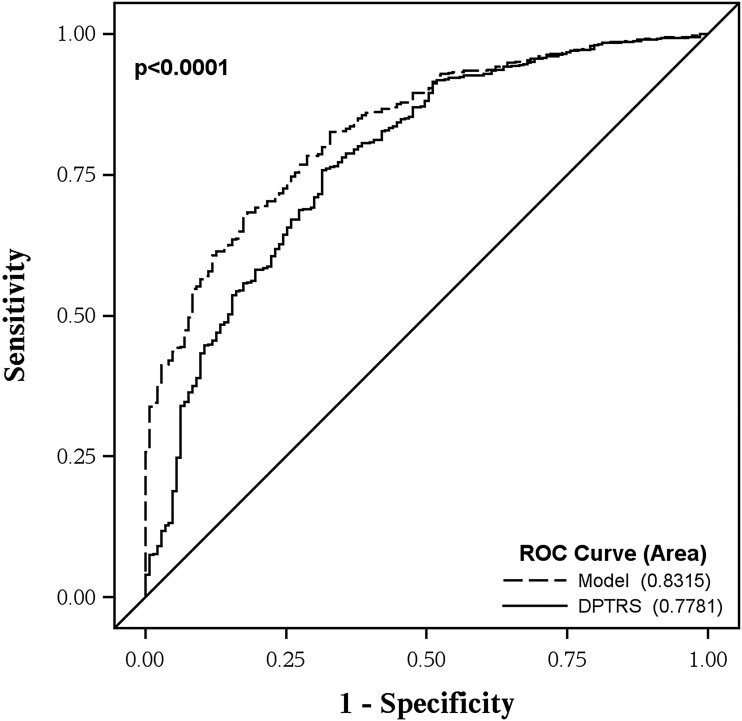
Adding the ECL categorical assay status (positive for one or more ECL assay) to the DPTRS helped improve the prediction accuracy of type 1 diabetes (Fig. 2). The AUC for DPTRS was 0.78, which improved to 0.83 when adding ECL to DPTRS (P < 0.0001).
Figure 2.
ROC curves comparing DPTRS alone vs. DPTRS and ECL. ROC curves were generated to examine the addition of ECL positivity to the DPTRS. DPTRS, DPTRS alone; model, includes ECL positivity in addition to DPTRS.
In a subgroup of subjects who had complete DPTRS, ABRS, and ECL information (n = 606), Cox proportional hazards models predicting time to develop diabetes with either DPTRS/ABRS or DPTRS/ECL had a similar AIC (1,248 and 1,252, respectively), while the best model was the one which included DPTRS, ABRS, and ECL (AIC 1,223). Type 1 diabetes was strongly related to all of the variables in the model (DPTRS: P < 0.0001; ABRS: P < 0.0001; ECL: P = 0.0004).
Conclusions
Data from prospective, longitudinal studies of individuals at risk for developing type 1 diabetes have contributed to the understanding of presymptomatic type 1 diabetes. Recently, a scientific statement from the JDRF, the Endocrine Society, and the American Diabetes Association was published with three defined stages (14): stage 1 is defined as the presence of β-cell autoimmunity (as evidenced by the presence of two or more islet autoantibodies) with normoglycemia, stage 2 as the presence of β-cell autoimmunity with dysglycemia (still presymptomatic), and stage 3 as onset of symptomatic disease. Although the risk for diabetes for these subjects is very high, the rate of progression from onset of β-cell autoimmunity to onset of diabetes is variable, lasting from months to decades (24). This study found that ECL assays improved the ability to predict time to diabetes in the TrialNet autoantibody-positive relatives at risk for developing type 1 diabetes. Furthermore, the addition of ECL assays to the DPTRS improved the accuracy of risk classification with a better AUC than DPTRS alone.
Overall, 167 of the 177 (94%) subjects who developed diabetes were positive for at least one ECL assay, i.e., the majority of subjects who developed diabetes were picked up by either ECL-IAA or ECL-GADA. Of note, all TrialNet subjects had RIA measurements for mIAA, GADA, IA-2A, and ZnT8A, whereas only IAA and GADA were measured by ECL assays. As all TrialNet subjects are tested for RIA autoantibodies, this study tested whether the addition of ECL autoantibody data can improve diabetes prediction. Previous studies have shown that ECL assays are more sensitive and more disease specific than RIA assays (15–17). Several risk scores for diabetes have been developed, including the DPTRS (22,25) and an ABRS (23); a total of 672 out of the current 1,287 (52%) subjects were included in the latter paper by Sosenko et al. (23). The ABRS described in that paper included both positivity and titers of all four RIA autoantibodies as in the current paper, but no ECL results. In this paper, six multivariable Cox proportional hazards models predicting time to develop diabetes were compared; the best model was the one including positivity for ECL-GADA and/or ECL-IAA.
In a subgroup of subjects who had complete DPTRS, ABRS, and ECL information (n = 606), the DPTRS/ECL model gave similar results to the DPTRS/ABRS model. The DPTRS/ECL model was based on only two autoantibodies (ECL-IAA and ECL-GADA), whereas the DPTRS/ABRS model included all four RIA autoantibodies. However, the best model was the one that included the DPTRS, the ABRS, and ECL positivity; type 1 diabetes was strongly associated with all of the variables in the model. Thus, it appears that autoantibody affinity, as indicated by ECL, contributes to the prediction of type 1 diabetes independently of metabolic status, autoantibody type, and autoantibody titer. As ECL assays are developed for other islet autoantibodies (ECL-IA2 and ECL-ZnT8), the prediction of time to diabetes may be further refined.
TrialNet is currently using the DPTRS as one of the criteria for more intense monitoring with OGTTs every 6 months instead of yearly. In this study, the DPTRS was improved by adding baseline ECL assays to the risk score. On the other hand, subjects negative for both ECL-IAA and ECL-GADA have a much lower risk for diabetes (5%) and could benefit from less intensive monitoring. A more accurate risk score for diabetes would help in further stratifying risk and surveillance for subjects in studies such as TrialNet. Among subjects positive for single GADA or single mIAA by RIA, those subjects who were also positive for ECL assay (ECL-IAA or ECL-GADA) showed higher autoantibody affinity (26). The higher affinity of these autoantibodies detected by ECL assay may be explained by the fact that the ECL assay is a bivalent assay designed to capture all immunoglobulins.
Factors involved in rate of progression to diabetes are only partially known. Other prospective studies, such as the Diabetes Autoimmunity Study in the Young (DAISY), the Type 1 Diabetes Prediction and Prevention Project (DIPP), and The Environmental Determinants of Diabetes in the Young (TEDDY) have shown that higher IAA and IA-2A levels (but not GADA levels) are associated with time to diabetes, after adjusting for first-degree relative status, number of autoantibodies, age at first persistent confirmed autoantibodies, and HLA genotypes (12,13,27). In TrialNet at-risk subjects, positivity for ECL-IAA or ECL-GADA was a significant predictor for time to diabetes (with HR 7), while adding ECL titers to the base model did not improve prediction of time to diabetes compared with the base model alone. Eligibility for current prevention trials typically requires the presence of at least two positive islet autoantibodies. In both single and multiple RIA autoantibody groups, the risk for development of diabetes was much higher for those subjects who were ECL positive compared with those who were ECL negative: 20 vs. 4% respectively for those subjects with single autoantibody by RIAs and 50–70 vs. 20% respectively for those subjects with multiple autoantibodies by RIAs. Adding ECL testing at baseline visit in studies such as TrialNet might allow for better stratification into eligibility trials, as autoantibody-positive subjects also positive for ECL have a much higher risk for diabetes than ECL-negative subjects (26).
The limitations of this study include that only ECL-IAA and ECL-GADA have been measured in a subset of subjects followed in TrialNet as ECL assays are not routinely performed in TrialNet subjects. In addition, ECL assays were only measured at baseline with no longitudinal results available for ECL assays.
In conclusion, ECL assays improved the ability to predict time to diabetes in these antibody-positive relatives at risk for developing diabetes. These findings might be helpful in both the monitoring of subjects at risk for diabetes and the design of prevention trials in the future.
Supplementary Material
Article Information
Acknowledgments. The authors thank David Boulware (University of South Florida, Tampa, FL) for statistical analyses.
Funding. This research was supported by National Institutes of Health (NIH) grant DK32083, JDRF 47-2013-581, and American Diabetes Association grant 1-14-CD-17. The Type 1 Diabetes TrialNet Pathway to Prevention Study Group is a clinical trials network funded by the NIH through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, through the cooperative agreements U01-DK-061010, U01-DK-061034, U01-DK-061042, U01-DK-061058, U01-DK-085465, U01-DK-085453, U01-DK-085461, U01-DK-085463, U01-DK-085466, U01-DK-085499, U01-DK-085504, U01-DK-085505, U01-DK-085509, U01-DK-103180, U01-DK-103153, U01-DK-085476, and U01-DK-103266, and the JDRF.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the JDRF.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.F. researched data and wrote the manuscript. L.P. and D.M. researched data. L.Y., A.M., J.K., J.S., and P.G. contributed to discussion and reviewed and edited the manuscript. A.K.S. designed the study, contributed to discussion, and reviewed and edited the manuscript. A.K.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-0302/-/DC1.
References
- 1.Orban T, Sosenko JM, Cuthbertson D, et al.; Diabetes Prevention Trial-Type 1 Study Group . Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2009;32:2269–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziegler AG, Rewers M, Simell O, et al. . Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chmiel R, Giannopoulou EZ, Winkler C, Achenbach P, Ziegler AG, Bonifacio E. Progression from single to multiple islet autoantibodies often occurs soon after seroconversion: implications for early screening. Diabetologia 2015;58:411–413 [DOI] [PubMed] [Google Scholar]
- 4.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al.; TrialNet Natural History Committee; Type 1 Diabetes TrialNet Study Group . The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes 2009;10:97–104 [DOI] [PubMed] [Google Scholar]
- 5.Skyler JS, Greenbaum CJ, Lachin JM, et al. . Type 1 Diabetes TrialNet–an international collaborative clinical trials network. Ann N Y Acad Sci 2008;1150:14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenbaum CJ, Palmer JP, Kuglin B, Kolb H. Insulin autoantibodies measured by radioimmunoassay methodology are more related to insulin-dependent diabetes mellitus than those measured by enzyme-linked immunosorbent assay: results of the Fourth International Workshop on the Standardization of Insulin Autoantibody Measurement. J Clin Endocrinol Metab 1992;74:1040–1044 [DOI] [PubMed] [Google Scholar]
- 7.Baekkeskov S, Aanstoot H-J, Christgau S, et al. . Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase [published correction appears in Nature 1990;347:782]. Nature 1990;347:151–156 [DOI] [PubMed] [Google Scholar]
- 8.Gianani R, Rabin DU, Verge CF, et al. . ICA512 autoantibody radioassay. Diabetes 1995;44:1340–1344 [DOI] [PubMed] [Google Scholar]
- 9.Wenzlau JM, Moua O, Sarkar SA, et al. . SlC30A8 is a major target of humoral autoimmunity in type 1 diabetes and a predictive marker in prediabetes. Ann N Y Acad Sci 2008;1150:256–259 [DOI] [PubMed] [Google Scholar]
- 10.Vehik K, Beam CA, Mahon JL, et al.; TrialNet Natural History Study Group . Development of autoantibodies in the TrialNet Natural History Study. Diabetes Care 2011;34:1897–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siljander HT, Simell S, Hekkala A, et al. . Predictive characteristics of diabetes-associated autoantibodies among children with HLA-conferred disease susceptibility in the general population. Diabetes 2009;58:2835–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steck AK, Johnson K, Barriga KJ, et al. . Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes: diabetes autoimmunity study in the young. Diabetes Care 2011;34:1397–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steck AK, Vehik K, Bonifacio E, et al.; TEDDY Study Group . Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY). Diabetes Care 2015;38:808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Insel RA, Dunne JL, Atkinson MA, et al. . Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015;38:1964–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu L, Dong F, Miao D, Fouts AR, Wenzlau JM, Steck AK. Proinsulin/insulin autoantibodies measured with electrochemiluminescent assay are the earliest indicator of prediabetic islet autoimmunity. Diabetes Care 2013;36:2266–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miao D, Guyer KM, Dong F, et al. . GAD65 autoantibodies detected by electrochemiluminescence assay identify high risk for type 1 diabetes. Diabetes 2013;62:4174–4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miao D, Steck AK, Zhang L, et al.; Type 1 Diabetes TrialNet Study Group . Electrochemiluminescence assays for insulin and glutamic acid decarboxylase autoantibodies improve prediction of type 1 diabetes risk. Diabetes Technol Ther 2015;17:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu L, Rewers M, Gianani R, et al. . Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab 1996;81:4264–4267 [DOI] [PubMed] [Google Scholar]
- 19.Bonifacio E, Yu L, Williams AK, et al. . Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for National Institute of Diabetes and Digestive and Kidney Diseases Consortia. J Clin Endocrinol Metab 2010;95:3360–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu L, Miao D, Scrimgeour L, Johnson K, Rewers M, Eisenbarth GS. Distinguishing persistent insulin autoantibodies with differential risk: nonradioactive bivalent proinsulin/insulin autoantibody assay. Diabetes 2012;61:179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sosenko JM, Krischer JP, Palmer JP, et al.; Diabetes Prevention Trial-Type 1 Study Group . A risk score for type 1 diabetes derived from autoantibody-positive participants in the Diabetes Prevention Trial-Type 1. Diabetes Care 2008;31:528–533 [DOI] [PubMed] [Google Scholar]
- 22.Sosenko JM, Skyler JS, Mahon J, et al.; Type 1 Diabetes TrialNet Study Group; Diabetes Prevention Trial-Type 1 Study Group . The application of the Diabetes Prevention Trial-Type 1 risk score for identifying a preclinical state of type 1 diabetes. Diabetes Care 2012;35:1552–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sosenko JM, Skyler JS, Palmer JP, et al.; Type 1 Diabetes TrialNet Study Group; Diabetes Prevention Trial-Type 1 Study Group . The prediction of type 1 diabetes by multiple autoantibody levels and their incorporation into an autoantibody risk score in relatives of type 1 diabetic patients. Diabetes Care 2013;36:2615–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014;383:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sosenko JM, Skyler JS, Palmer JP; Diabetes Type 1 TrialNet and Diabetes Prevention Trial-Type 1 Study Groups . The development, validation, and utility of the Diabetes Prevention Trial-Type 1 Risk Score (DPTRS). Curr Diab Rep 2015;15:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steck AK, Fouts A, Miao D, et al.; TrialNet Study Group . ECL-IAA and ECL-GADA can identify high-risk single autoantibody-positive relatives in the TrialNet Pathway to Prevention Study. Diabetes Technol Ther 2016;18:410–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parikka V, Näntö-Salonen K, Saarinen M, et al. . Early seroconversion and rapidly increasing autoantibody concentrations predict prepubertal manifestation of type 1 diabetes in children at genetic risk. Diabetologia 2012;55:1926–1936 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.