Abstract
OBJECTIVE
This study tests the effectiveness of expert guidelines for diabetes prevention: lifestyle intervention with addition of metformin, when required, among people with prediabetes.
RESEARCH DESIGN AND METHODS
The Diabetes Community Lifestyle Improvement Program (D-CLIP) is a randomized, controlled, translation trial of 578 overweight/obese Asian Indian adults with isolated impaired glucose tolerance (iIGT), isolated impaired fasting glucose (iIFG), or IFG+IGT in Chennai, India. Eligible individuals were identified through community-based recruitment and randomized to standard lifestyle advice (control) or a 6-month, culturally tailored, U.S. Diabetes Prevention Program–based lifestyle curriculum plus stepwise addition of metformin (500 mg, twice daily) for participants at highest risk of conversion to diabetes at ≥4 months of follow-up. The primary outcome, diabetes incidence, was assessed biannually and compared across study arms using an intention-to-treat analysis.
RESULTS
During 3 years of follow-up, 34.9% of control and 25.7% of intervention participants developed diabetes (P = 0.014); the relative risk reduction (RRR) was 32% (95% CI 7–50), and the number needed to treat to prevent one case of diabetes was 9.8. The RRR varied by prediabetes type (IFG+IGT, 36%; iIGT, 31%; iIFG, 12%; P = 0.77) and was stronger in participants 50 years or older, male, or obese. Most participants (72.0%) required metformin in addition to lifestyle, although there was variability by prediabetes type (iIFG, 76.5%; IFG+IGT, 83.0%; iIGT, 51.3%).
CONCLUSIONS
Stepwise diabetes prevention in people with prediabetes can effectively reduce diabetes incidence by a third in community settings; however, people with iIFG may require different interventions.
Introduction
Randomized controlled trials have shown the efficacy of lifestyle interventions or metformin for reducing diabetes conversion among individuals with impaired glucose tolerance (IGT) (1–5). The American Diabetes Association (ADA) and the International Diabetes Federation recommend stepwise diabetes prevention (lifestyle modification plus metformin when risk remains elevated) for individuals with any form of prediabetes, defined as isolated IGT (iIGT), isolated impaired fasting glucose (iIFG), or IFG+IGT (6,7). However, no large diabetes prevention trial has compared the effects of diabetes prevention across the prediabetes spectrum, and no study has tested the effectiveness of the stepwise diabetes prevention recommendations.
The incredible diabetes burden reflects our failure to translate proven evidence for prevention into action on a wider scale. Worldwide, 415 million people have diabetes, and this number will reach 642 million by 2040 (8). Most individuals with diabetes, 75%, live in low- and middle-income countries (LMICs) (8), where the condition has especially marked effects on health and economic prosperity.
The Diabetes Community Lifestyle Improvement Program (D-CLIP) (9) is a randomized controlled, diabetes prevention trial in adults with iIGT, iIFG, or IFG+IGT in which standard of care is compared with a culturally tailored lifestyle education curriculum based on the U.S. Diabetes Prevention Program (DPP) plus stepwise addition of metformin when needed. D-CLIP was conducted in Asian Indians, a population at elevated risk for developing diabetes even at younger ages and lower BMIs (10–13) and possibly with dual susceptibility to insulin resistance and early β-cell dysfunction (14,15). In this study we tested the effectiveness of guideline-based, stepwise diabetes prevention by comparing the incidence of diabetes between control and intervention participants and by determining whether intervention effects differ across baseline prediabetes type, HbA1c level, age, sex, BMI level, or family history of diabetes.
Research Design and Methods
D-CLIP is a randomized, controlled translational research study. Detailed study methods are described elsewhere (9), and details pertinent to this analysis are discussed below. The Emory University Institutional Review Board (IRB-00016503) and the Madras Diabetes Research Foundation Ethics Committee approved the study procedures and materials.
Participants
D-CLIP included overweight or obese (World Health Organization Asian-specific cut points: BMI 23 to <27.5 kg/m2 for overweight, BMI ≥27.5 kg/m2 for obese and/or waist circumference ≥90 cm for men or ≥80 cm for women) (16) adults aged 20–65 years with prediabetes (IFG: fasting plasma glucose [FPG] 5.6–6.9 mmol/L and/or IGT: 2-h, postload glucose of 7.8–11.0 mmol/L) (17). Individuals with diabetes, major health conditions impeding participation in an unsupervised lifestyle change program, or current pregnancy or breastfeeding were excluded.
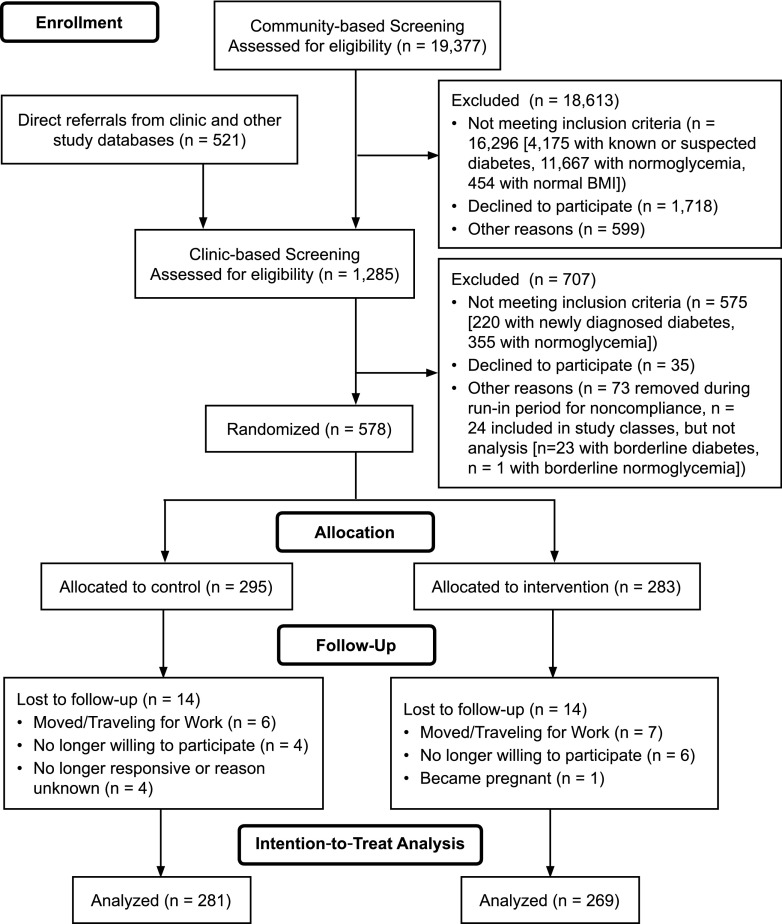
Individuals provided written informed consent before screening and randomization. A detailed discussion of recruitment and enrollment is available elsewhere (18). Briefly, two-step screening included:1) community-based screening camps at housing/apartment blocks, worksites, schools, and churches and during community health events (e.g., World Diabetes Day health screening) with a short demographic questionnaire and anthropometry and finger-stick, random capillary glucose measurements; and 2) clinic-based screening with questionnaires, anthropometric measurements, blood pressure measurement, a fasting blood draw, and a 2-h oral glucose tolerance test (OGTT) for individuals found in step 1 to be at risk for having prediabetes (initially, random capillary glucose ≥6.1 mmol/L, although this was later changed to ≥5.6 mmol/L to improve the pace of recruitment). A smaller sample of individuals with probable or known prediabetes, identified from clinic referrals, attended only clinic-based screening (step 2 above). Figure 1 details inclusion and exclusion at each step of enrollment.
Figure 1.
D-CLIP trial profile.
A brief run-in period followed screening, whereby noncompliant individuals (never attended the first intervention class or the control group education class) were removed from the study. The baseline characteristics between noncompliant individuals and participants remaining in the study were not significantly different. The study enrolled 24 individuals, 12 per study arm, with borderline glucose levels (0.06–0.28 mmol/L outside the ADA cutoffs for diabetes [n = 23] or normoglycemia [n = 1]) who were not included in the analysis. The study physicians requested that these individuals be allowed to participate in the study because they felt that lifestyle education would be beneficial. A sensitivity analysis was conducted comparing the primary end point with and without these individuals.
Randomization and Masking
The study site coordinator (R.H.) provided a list of eligible study identifiers to the U.S.-based coordinator (M.B.W., who had no interaction with study participants) weekly for randomization using a random-number list created in SAS software (SAS Institute, Inc., Cary, NC). The study site coordinator then informed participants of their allocation group. Because of the nature of the trial and the inclusion of group-specific questions in the study questionnaire, study participants, staff, and investigators could not be blinded to group allocation.
Interventions
Control and intervention activities were conducted at the study site, a diabetes care and research institution in Chennai, India, with extensive experience in diabetes treatment and prevention. Classes and data collection visits were designed to fit within participants’ schedules, with most activities occurring on weekends. Control arm participants received the study site’s standard of care for prediabetes: a single day with one-on-one visits with a physician, a dietitian, and a fitness trainer and one group class on diabetes prevention (e.g., following a low-fat diet rich in complex carbohydrates and fresh fruits and vegetables, increasing physical activity). Metformin prescription for diabetes prevention is not standard of care at the study site, so no control arm participants received metformin. Aside from follow-up data collection visits, control participants had no additional contacts with study staff.
The stepwise intervention included lifestyle classes plus metformin when needed. The lifestyle curriculum was based on the DPP, with lessons modified to be group-based and culturally appropriate. Weekly classes included 16 core intervention classes in months 0–4 on active lifestyle changes, followed by 8 maintenance classes in months 5–6. Like the DPP, participants had two study goals: ≥7% weight loss and ≥150 min weekly of moderate-intensity exercise. Participants were trained on improving diet quality and reducing dietary intake through keeping weekly food diaries, adhering to individual goals for total fat intake, reducing portion sizes, and increasing intake of fiber-rich foods. A trained lifestyle modification team, including a health coach, a fitness instructor, and a community volunteer peer leader taught each cohort of 8–24 participants (18 total cohorts; median cohort size, 16). At 4 months or later (after the core lifestyle curriculum was completed), intervention participants were prescribed metformin at a dose of 500 mg twice daily if they were considered at high risk of converting to diabetes, defined as having IFG+IGT or IFG+ HbA1c ≥5.7% (39 mmol/mol). After the lifestyle classes ended, intervention participants had minimal contacts with study staff (phone calls every 6 months to schedule study testing visits and LISTSERV postings for holidays).
Study Testing and Outcome Measures
Testing visits occurred at the study site at baseline, postcore intervention (month 4), postmaintenance (month 6), and every 6 months until study closeout and included study questionnaires, anthropometric measurements, blood pressure testing, and fasting blood draws. Three-sample (0, 30, and 120 min) 75-g OGTTs were performed annually.
The primary outcome, diabetes incidence, was diagnosed on the basis of a single, annual OGTT or the semiannual FPG test. Diagnostic cut points for diabetes were based on ADA criteria, FPG ≥7.0 mmol/L or 2-h glucose ≥11.1 mmol/L (17). Secondary outcomes included weight, waist circumference, FPG, 2-h glucose, HbA1c, physical activity, diet, and metformin adherence. Covariates included age, sex, BMI, prediabetes category, HbA1c, and self-reported family history of diabetes (having a first-degree relative with diabetes). Mean minutes of weekly, self-reported physical activity were estimated based on the following questions: 1) “how many days per week do you exercise;” and 2) “on average how long does each exercise session last” (possible values: 0–15, 16–30, 31–45, 46–60, or >60 min). Weekly physical activity was categorized as reaching study goals (≥150 min/week) or not. Dietary intake was measured using a Food Frequency Questionnaire developed for South Indian populations (19). Adherence to metformin was assessed by pill counts at each study visit.
Study physicians reviewed study records and an adverse event questionnaire to determine whether adverse events occurred and whether they were study related. If needed, the participant met with the study physician to review the event and make changes. Participants were also in frequent contact with study staff, who referred participants to the study physician if an adverse event was suspected.
Statistical Analysis
A data safety officer monitored the study. The analysis was conducted in SAS 9.4 software and followed an intention-to-treat principle. The study was designed to provide 80% power to detect a 35% difference in diabetes incidence between groups assuming a 10% loss to follow-up, an α of 0.05, and an annual conversion rate to diabetes of 9%.
Intervention adherence was assessed by evaluating 1) class attendance; 2) changes in dietary intake (using repeated-measures models); the percentage of participants reaching 3) physical activity and 4) weight loss goals at 6 and 12 months; and 5) metformin adherence. Changes over time in intermediate outcomes of weight, waist circumference, HbA1c, FPG, and 120-min glucose were modeled using repeated-measures analysis.
Time to diabetes, survival probabilities, and associated SEs were quantified through life-table methods. Cumulative incidences of diabetes in intervention and control participants were compared through product-limit curves and the log-rank test. Risk reduction and tests for heterogeneity across baseline covariates were assessed by proportional hazards regression at a significance level of P < 0.1. Baseline age, BMI, waist circumference, and HbA1c values were examined categorically and continuously. The number needed to treat to prevent one case of diabetes and the 95% CI were calculated using survival probabilities at 3 years and the Greenwood estimate of the SE.
Results
Study Enrollment and Follow-up
Recruitment (September 2009-February 2012) included community-based screening of 19,377 individuals, of which 18,613 were ineligible for or declined additional screening (Fig. 1). The remaining 764 individuals plus 521 referrals from clinic databases attended clinic-based screening (n = 1,285). From these, 707 people were excluded (575 ineligible, 35 unwilling, and 97 other reasons), and 578 individuals were randomized to intervention (n = 283) or control (n = 295). Most of excluded individuals at each stage of screening were ineligible for the trial. Participants (63.2% male; mean age, 44.4 [SD 9.3] years) had a mean BMI of 27.9 (SD 3.7) kg/m2, and 30.2% had iIFG, 29.7% had iIGT, and 40.1% had IFG+IGT (Table 1). Mean follow-up time was 2.54 years (range 4–48 months). Including individuals with borderline baseline glucose levels in the analysis resulted in a small, insignificant strengthening of the intervention effect, so these individuals were excluded from the reported results. Loss to follow-up (individuals lacking all follow-up data) was 4.7% (intervention, 4.7%; control, 4.6%), leaving 281 control subjects and 269 intervention participants for the primary outcome assessment.
Table 1.
Baseline characteristics by groups
| Characteristics* | Overall (N = 576) | Control (n = 293) | Intervention (n = 283) |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 364 (63.2) | 183 (62.5) | 181 (64.0) |
| Female | 212 (36.8) | 110 (37.5) | 102 (36.0) |
| Education, n (%) | |||
| High school or less | 222 (38.7) | 110 (37.8) | 112 (39.6) |
| Undergraduate/technical degree or greater | 352 (61.3) | 181 (62.2) | 171 (60.4) |
| Monthly income, n (%) | |||
| <10,000 rupees | 149 (28.4) | 71 (26.4) | 78 (30.6) |
| 10,000–25,000 rupees | 210 (40.1) | 111 (41.3) | 99 (38.8) |
| >25,000 rupees | 165 (31.5) | 87 (32.3) | 78 (30.6) |
| Family history of diabetes, n (%) | 330 (57.1) | 169 (57.3) | 161 (56.9) |
| Age, years, mean (SD) | 44.4 (9.3) | 44.0 (9.5) | 44.8 (9.0) |
| Weight, kg, mean (SD) | 74.6 (11.4) | 74.7 (11.4) | 74.6 (11.3) |
| BMI, kg/m2, mean (SD) | 27.9 (3.7) | 27.8 (3.7) | 27.9 (3.7) |
| BMI categories, n (%) | |||
| Normal | 33 (5.7) | 18 (6.1) | 15 (5.3) |
| Overweight | 262 (45.3) | 134 (45.4) | 128 (45.2) |
| Obese | 283 (49.0) | 143 (48.5) | 140 (49.5) |
| Waist circumference, cm, mean (SD) | 94.8 (9.1) | 94.8 (8.8) | 94.7 (9.4) |
| Plasma glucose, mmol/L, mean (SD) | |||
| Fasting | 5.7 (0.5) | 5.7 (0.5) | 5.7 (0.5) |
| 30-min postload | 9.7 (1.4) | 9.6 (1.5) | 9.8 (1.4) |
| 120-min postload | 8.3 (1.5) | 8.4 (1.4) | 8.2 (1.5) |
| HbA1c, %, mean (SD) | 6.0 (0.5) | 6.0 (0.5) | 6.0 (0.5) |
| HbA1c, mmol/mol, mean (SD) | 42 (5.5) | 42 (5.5) | 42 (5.5) |
| Glucose intolerance level, n (%) | |||
| iIFG | 174 (30.1) | 84 (28.5) | 90 (31.8) |
| iIGT | 172 (29.8) | 89 (30.2) | 83 (29.3) |
| IGT+IFG | 232 (40.1) | 122 (41.4) | 110 (38.9) |
| Reported any exercise, n (%) | 331 (57.6) | 167 (57.2) | 164 (58.0) |
| Average minutes of exercise, n (%) | |||
| <150 min | 431 (75.2) | 221 (75.7) | 210 (74.7) |
| ≥150 min | 142 (24.8) | 71 (24.3) | 71 (25.3) |
| Daily dietary intake, kcal, mean (SD) | |||
| Total calories | 2,970.6 (865.0) | 2,999.7 (839.6) | 2,942.0 (889.9) |
| Calories from fat | 825.8 (287.0) | 839.5 (276.7) | 812.2 (296.7) |
| Calories from carbohydrates | 1,803.2 (523.9) | 1,815.8 (513.0) | 1,791.0 (535.1) |
*Family history of diabetes defined as one or more first-degree relatives (parent, sibling, or child) with diabetes.
Changes in Diabetes Incidence
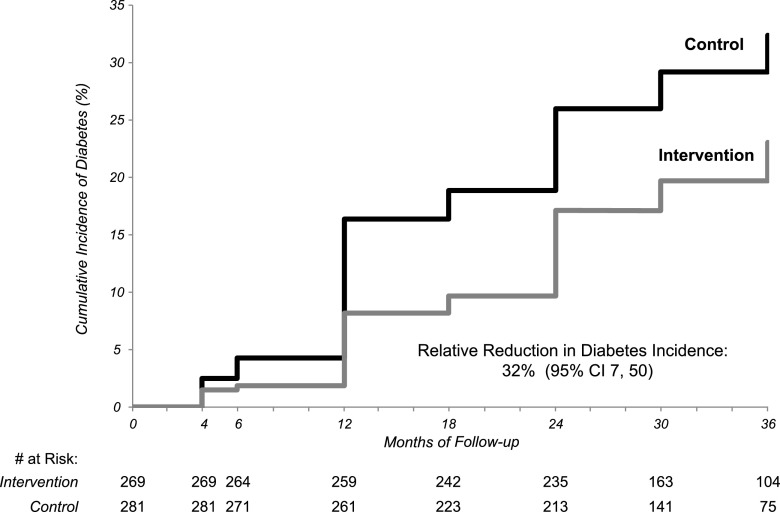
During the 3 years of follow-up, there was a 32% (95% CI 7–50) relative reduction in diabetes incidence in intervention participants compared with control subjects (Table 2). The incidence remained lower in the lifestyle participants throughout the follow-up (Fig. 2). At 3 years, 34.9% of control subjects (n = 98) and 25.7% of intervention participants (n = 69) had developed diabetes for an average annual incidence of diabetes of 11.1% and 7.8%, respectively (P = 0.014), and the number needed to treat to prevent one case of diabetes with the D-CLIP intervention was 9.8 (95% CI 5.4–53.9).
Table 2.
Incidence of diabetes incidence and risk reduction and numbers needed to treat in D-CLIP intervention and control participants by population subgroup
| N | Incidence density (cases/100 person-years) |
Reduction in incidence* |
Number needed to treat |
||||
|---|---|---|---|---|---|---|---|
| Control | Intervention | % | 95% CI | n | 95% CI | ||
| Overall | 550 | 14.2 | 9.8 | 32 | 7, 50 | 9.8 | 5.4, 53.9 |
| Age, years† | |||||||
| ≤35 | 96 | 14.5 | 10.4 | 34 | −46, 70 | 4.0 | 2.1, 46.0 |
| 36–50 | 292 | 13.7 | 10.0 | 21 | −21, 49 | 13.9 | 5.4, −25.0 |
| ≥51 | 162 | 15.1 | 9.0 | 47 | 6, 70 | 10.1 | 4.0, −20.6 |
| Sex† | |||||||
| Male | 344 | 14.5 | 9.6 | 37 | 7, 58 | 9.2 | 4.7, 245.6 |
| Female | 206 | 13.9 | 10.1 | 24 | −27, 54 | 12.0 | 4.6, −19.0 |
| BMI, kg/m2† | |||||||
| 23 to <27.5 | 246 | 10.5 | 8.7 | 14 | −43, 48 | 46.3 | 10.9, −20.5 |
| ≥27.5 | 273 | 19.1 | 10.5 | 49 | 23, 66 | 6.8 | 4.3, 16.6 |
| Prediabetes type† | |||||||
| iIFG | 166 | 7.2 | 6.5 | 12 | −80, 57 | 15.5 | 7.8, 854.4 |
| iIGT | 162 | 10.7 | 7.4 | 31 | −31, 64 | 11.0 | 5.4, −188.3 |
| IFG+IGT | 222 | 22.2 | 14.5 | 36 | 3, 57 | 12.3 | 5.4, −43.6 |
| HbA1c, % (mmol/mol)† | |||||||
| <5.7 (<39) | 117 | 7.7 | 6.1 | 13 | −106, 64 | 19.3 | 7.9, −43.1 |
| 5.7–6.4 (39–46) | 340 | 15.3 | 8.1 | 50 | 25, 67 | 8.2 | 5.2, 19.6 |
| ≥6.5 (≥48) | 93 | 19.4 | 20.3 | −18 | −114, 35 | 256.4 | 5.9, −6.2 |
| Family history† | |||||||
| No | 200 | 12.8 | 7.3 | 46 | 5, 70 | 8.3 | 3.8, −55.5 |
| Yes | 349 | 15.1 | 11.3 | 23 | −12, 47 | 10.8 | 5.1, −80.1 |
*Based on the hazard ratio.
†Tests for heterogeneity across strata were not statistically significant except for HbA1c (P = 0.05).
Figure 2.
Cumulative incidence of diabetes by study arm in the D-CLIP trial from baseline to year 3.
The RRRs were stronger in some subgroups (Table 2). When comparing across prediabetes type, the intervention effects were only significant among participants with IFG+IGT (36% [95% CI 3–57]), although the relative reduction in incidence was similar in magnitude among iIGT participants (31%). For individuals with iIFG, the RRR was 12% (95% CI −80 to 57). The reduction in incidence was strongest among older participants and men. Reduction in diabetes incidence was more than two-times higher in obese participants than in overweight participants. The differences in RRR across glycemic status group, BMI category, age, and sex did not reach statistical significance.
Changes in Intermediate Outcomes
Change in weight and waist circumference differed significantly between intervention and control participants during the 3 years of follow-up (P < 0.001 for each) (Supplementary Fig. 1). The intervention group lost weight throughout the 6-month intervention period, with a maximum weight loss at 6 months (weight loss: −2.4 [SD 2.7] kg/–3.2% [SD 3.4] at 4 months and −2.9 [SD 2.9] kg/–4.0% [SD 3.8] at 6 months). The control arm only lost weight in months 0–4 (−0.8 [SD 2.2] kg/–1.0% [SD 2.9]). Similarly, waist circumference decreased in the intervention arm by month 4 (−3.6 [SD 4.6] cm) and month 6 (−3.9 [SD 4.3] cm), but only in months 0–4 in the control group (−1.5 [SD 3.6] cm). Glucose measures (Supplementary Fig. 2) were significantly lower in the intervention arm at most (HbA1c, FPG) or all (2-h glucose) follow-up visits. FPG and HbA1c both decreased more steeply in the intervention group until month 6, when lifestyle education classes ended. The 2-h glucose decreased slightly in the intervention group between baseline and 1 year and increased thereafter.
Intervention Adherence
Participants attended an average of 12 (SD 3.9) core intervention classes: 22% of participants attended all 16 (n = 63), 70% attended 12 or more (n = 196), and 90% attended at least half (n = 253). Class attendance did not vary by sex; however, significantly fewer participants in the youngest age group (≤35 years) attended 75% or more of the study classes (46.9% [n = 23 of 49] compared with 73.0% of those aged 36–50 [n = 116 of 159] and 76.0% [n = 57 of 75] of those aged 51 or older, P = 0.0009).
Calories, carbohydrates, and fat intake all improved (P < 0.001 for all) during the 6-month intervention period (6-month intakes were 2,586.4 [SD 820.0] kcal, 1,558.7 [SD 506.0] kcal, and 719.0 [SD 273.4] kcal, respectively). In the control arm, there were slight, nonsignificant decreases in calorie, carbohydrate, and fat intake.
About half of intervention participants reported reaching the physical activity goal (≥150 min/week) at 6 months (51.1% [n = 121 of 237]) or 1 year (52.1% [n = 124 of 238]). Men were more likely than women to reach physical activity goals at 6 months (43.8% [n = 130 of 297] vs. 23.8% [n = 43 of 181], respectively, P < 0.0001) and at 1 year (50.5% [n = 149 of 295] vs. 35.0% [n = 62 of 177], respectively, P = 0.0011). At 1 year, 54.1% (n = 79 of 146) of participants in the oldest age category (≥51 years) reached the exercise goal compared with 42.4% (n = 111 of 262) of those aged 36–50 years, and 32.8% (n = 21 of 64) of those aged ≤35 years (P = 0.0088). The percentage of control individuals reaching this level of physical activity was lower (25.9% [n = 52 of 201] at 6 months and 37.7% [n = 87 of 231] at 1 year). Conversely, very few intervention participants reached the 7% weight loss goal (0 at 6 months, 2 at 1 year).
During the trial, 188 of the intervention participants (72%) presented with both IFG and either IGT or elevated HbA1c and were eligible for metformin. Of those, only 20 individuals (11%) refused the metformin prescription, citing a desire to continue with lifestyle intervention alone, and 13 (7%) accepted the prescription but never took any of the metformin tablets. Of the metformin users, 97 (52%) initiated metformin after the core intervention classes (month 4). Most individuals with baseline iIFG or IFG+IGT required metformin during the trial (76.5% [n = 65 of 85] and 83.0% [n = 83 of 100], respectively), whereas 51.3% (n = 40 of 78) of individuals with iIGT at baseline were prescribed metformin. Mean adherence to metformin was 69.6% (SD 37.9).
Adverse Effects
There were no severe adverse events (e.g., hospitalization, severe injury or illness) related to participation in the study, no injuries related to the exercise program, and no adverse events from diet changes made. All participants survived to the end of follow-up. Some participants reported mild or moderate gastritis related to taking metformin, but none of these cases were severe enough to stop taking the medication. One participant developed a rash after taking metformin, which resolved after metformin was discontinued.
Conclusions
A stepwise diabetes prevention program reduced the 3-year diabetes risk by 32% (95% CI 7–50) in overweight or obese Asian Indian adults with any form of prediabetes. There was evidence, however, indicating heterogeneity of effect across prediabetes type, with the strongest benefit in people with combined IFG+IGT (36%), followed by iIGT (31%) and then iIFG (12%).
The overall RRR shown here is similar to that reported at 3 years among the lifestyle (28.5%) and lifestyle plus metformin (28.2%) arms in the Indian Diabetes Prevention Program (IDPP) (3), but less than that shown at 2.8 years in the DPP (58%) (1), 4 years in the Finnish Diabetes Prevention Study (DPS) (58%) (20), 6 years in the diet and physical activity education group in the Da Qing IGT and Diabetes Study (42%) (2), and 4 years in the Japanese lifestyle intervention trial (67.4%) (5). This lower reported effect is likely, at least in part, because D-CLIP recruited people across the prediabetes spectrum, including those with iIFG, whereas the other studies only included individuals with IGT (2,3,5,20) or IGT+elevated FPG (1). In a multicenter diabetes prevention study of lifestyle modification among adults with iIFG or IFG+IGT, lifestyle intervention, although highly effective among individuals with IFG+IGT (hazard ratio 0.41, 95% CI 0.24–0.69), did not reduce diabetes risk among individuals with iIFG (hazard ratio 1.17, 95% CI 0.50–2.74) (21).
In addition, baseline risk among all D-CLIP participants was 14.2% per annum, considerably higher than the 11.0% annual risk shown for individuals with IGT+elevated FPG in DPP (1); among comparable people in D-CLIP, namely those with IFG+IGT, the risk was 22.2% per annum, supporting data showing that Asian Indians have a higher rate of prediabetes-to-diabetes conversion (22). The high percentage of intervention participants requiring metformin, 72%, and the fact that half required metformin within 4 months of trial enrollment further supports this and raises questions about whether factors other than insulin resistance are involved in the pathogenesis of type 2 diabetes in Asian Indians.
The program appeared to be less effective in people with iIFG; there was only a 12% (not significant) RRR, and a higher proportion of individuals with baseline iIFG required metformin (IFG+IGT or IFG+ HbA1c ≥5.7% [39 mmol/mol] at 4 months or later), indicating a failure of lifestyle to curtail disease progression. iIFG may be a phenotype more related to poor insulin secretion and gluconeogenesis than to insulin resistance, and if so, lifestyle interventions may be insufficient because they target the wrong pathophysiological mechanism. D-CLIP was also more effective in participants with no family history of diabetes (46%), a group likely affected by weight or lifestyle-related insulin resistance, than in those with a family history of diabetes (23%) and genetic susceptibility to insulin resistance, β-cell dysfunction, or both. Furthermore, the D-CLIP intervention might not have been ideal for those with iIFG. The Mediterranean diet, which has been inversely associated with IFG (23), might be more appropriate than the low-fat diet used in D-CLIP. On-going lifestyle intervention trials for individuals with IFG (24,25) will be important for determining the best course of action for this group. In addition, other drug classes that act more directly on β-cell function (e.g., gliptins) might be better candidate drugs for this group than metformin, which increases insulin sensitivity and inhibits gluconeogenesis (26).
There were differences in RRR by age, BMI group, and sex that were not statistically significant but might indicate a trend. Like the DPP (1), the strongest intervention effect was among the oldest participants, perhaps a result of the beneficial effects of weight loss and increased physical activity on age-related peripheral insulin resistance (27). Older D-CLIP participants also met exercise goals more frequently at 1 year and were more likely to attend 75% of the study classes than the younger participants. However, pooled data from the IDPP studies found no difference in RRR when comparing individuals younger than age 45 or 45 years of age or older (28). The difference between D-CLIP and these studies might be due to differences in age cutoffs or intervention methods. Regardless, the data reported from the D-CLIP trial add further support for targeting diabetes prevention efforts in all age groups, including older adults.
This study differed from the DPP (1) and a meta-analysis of the IDPP trials (28) in that individuals with obese-level BMIs showed markedly higher diabetes risk reduction than individuals with lower, but still overweight, BMIs (a 49% reduction vs. a nonsignificant 14% reduction). Participation in the intervention reduced diabetes incidence among obese participants to the incidence rate found among overweight control subjects (10.5 cases/100 person-years). Further analyses are needed to clarify the factors associated with this increased intervention success, but it is possible that obese individuals were more motivated to make the necessary lifestyle changes or that improved lifestyle, particularly increased physical activity, resulted in greater improvements in peripheral insulin sensitivity in the obese group. Alternatively, people with lower BMIs and high diabetes risk may be more β-cell deficient and thus less amenable to interventions targeting insulin resistance.
Also, unlike the DPP (1) and a recent meta-analysis of diabetes prevention studies (29), we found that the intervention effect was stronger in men than in women. Women reported more barriers to joining the study initially (30), which influenced recruitment outcomes and might have influenced lifestyle changes. Although class attendance did not differ by sex, men were significantly more likely to reach exercise goals at 6 months and 1 year.
D-CLIP intervention arm participants reached maximum weight loss (2.9 kg/4.0%) at 6 months, although only two participants reached the 7% weight loss goal. This weight loss was less than that seen in the DPP (7 kg/7%) or the DPS (4.2 kg/4.7%) (1,20); however, mean baseline weight and BMI were lower in D-CLIP, so a smaller weight loss may be more attainable. A meta-analysis of U.S.-based DPP translation studies also showed a mean weight loss of 4.0%, indicating that the weight loss seen in D-CLIP aligns with other DPP translation research (31). In diabetes prevention studies in Asian populations in India, China, and Japan (2,3,5), weight loss was similar to or less than that seen in D-CLIP. Even with no weight loss, studies in Asia report significant reductions in diabetes incidence (2,3), which might indicate that in populations with a lower average BMI, physiological changes other than weight loss may be more influential in reducing diabetes risk. Waist circumference loss at 6 months in D-CLIP (3.9 cm) exceeded the 1-year loss in the DPP (2.7 cm) (32). Abdominal adiposity is common in Asian Indians and can present at lower BMIs than in other ethnicities (33), so a waist circumference decrease may better represent adiposity loss than weight change.
Although lifestyle intervention participants showed short-term improvements in adiposity and glucose markers, all measures increased over longer follow-up. The inability of interventions to sustain improvements in anthropometry or glycemic control has previously been shown (1,34,35). The consistency of these patterns in multiple studies indicates a need for further research on maintenance of weight loss and other lifestyle changes associated with glucose control.
D-CLIP is a large, well-randomized trial with good follow-up, attendance, and adherence. This is the first large diabetes prevention translation trial to include individuals with all three types of prediabetes and the first study to test expert group recommendations for stepwise diabetes prevention. This study was conducted in a region at high-risk for diabetes and can provide important data for understanding diabetes prevention in LMIC settings. This study also has a large population of men, an underrepresented group in diabetes prevention trials (31).
The major weakness of this study is the lack of power for subgroup comparisons; however, several results do indicate interesting patterns that warrant further investigation. Also, the simplistic assessment of physical activity may not accurately reflect true activity. Finally, the D-CLIP study population was ethnically homogenous, which might affect generalizability; however, the inclusion of individuals across the prediabetes spectrum makes these results more broadly applicable to community-level diabetes prevention.
In conclusion, the D-CLIP trial shows that expert recommendations of adding metformin in a stepwise manner to lifestyle education is an effective method for preventing or delaying diabetes in adults with prediabetes, even in a resource-challenged setting like an LMIC. However, further research is needed to better understand diabetes prevention among people with iIFG. This is especially important because iIFG is the more common form of prediabetes in many racial/ethnic groups like Asian Indians (36–38). The possible need for specialized interventions for diabetes prevention among different categories of prediabetes has important public health and clinical significance.
Supplementary Material
Article Information
Funding. This project is supported by a BRiDGES grant from the International Diabetes Federation (LT07-115). BRiDGES, an International Diabetes Federation project, is supported by an educational grant from Lilly Diabetes. Additional support was provided by the Global Health Institute at Emory University. M.B.W. received funding from two National Institute of Diabetes and Digestive and Kidney Diseases T32 grants (5T3-2DK-007298-33 and T32-DK-007734-16). L.R.S. received funding from the Human Health Molecules to Humankind program funded by the Burroughs Wellcome Fund grant BWF 1008188.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.B.W. was primary author of the manuscript and conducted some analysis. H.R. oversaw data collection and participant management at the study site. L.R.S. conducted the primary analysis. M.B.W., H.R., K.M.V.N., and V.M. designed the trial. M.B.W., H.R., and R.M.A. developed the lifestyle intervention curriculum. All authors contributed to the manuscript discussion, provided edits to the text, and reviewed and approved the manuscript. K.M.V.N. and V.M. are co-primary investigators of the study and provided senior leadership and direction to the team. M.B.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5–9 June 2015 (39).
Footnotes
Clinical trial reg. no. NCT01283308, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-1241/-/DC1.
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan XR, Li GW, Hu YH, et al. . Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 3.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V; Indian Diabetes Prevention Programme (IDPP) . The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–297 [DOI] [PubMed] [Google Scholar]
- 4.Lindström J, Louheranta A, Mannelin M, et al.; Finnish Diabetes Prevention Study Group . The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003;26:3230–3236 [DOI] [PubMed] [Google Scholar]
- 5.Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract 2005;67:152–162 [DOI] [PubMed] [Google Scholar]
- 6.Alberti KG, Zimmet P, Shaw J. International Diabetes Federation: a consensus on type 2 diabetes prevention. Diabet Med 2007;24:451–463 [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association Prevention or delay of type 2 diabetes. Sec. 5. In Standards of Medical Care in Diabetes—2015. Diabetes Care 2015;38(Suppl.):S31–S32 [DOI] [PubMed] [Google Scholar]
- 8.International Diabetes Federation IDF Diabetes Atlas. 7th ed. Brussels, Belgium, International Diabetes Federation, 2015 [Google Scholar]
- 9.Weber MB, Ranjani H, Meyers GC, Mohan V, Narayan KM. A model of translational research for diabetes prevention in low and middle-income countries: the Diabetes Community Lifestyle Improvement Program (D-CLIP) trial. Prim Care Diabetes 2012;6:3–9 [DOI] [PubMed] [Google Scholar]
- 10.Gujral UP, Pradeepa R, Weber MB, Narayan KM, Mohan V. Type 2 diabetes in South Asians: similarities and differences with white Caucasian and other populations. Ann N Y Acad Sci 2013;1281:51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber MB, Oza-Frank R, Staimez LR, Ali MK, Narayan KM. Type 2 diabetes in Asians: prevalence, risk factors, and effectiveness of behavioral intervention at individual and population levels. Annu Rev Nutr 2012;32:417–439 [DOI] [PubMed] [Google Scholar]
- 12.Nakagami T, Qiao Q, Carstensen B, et al.; DECODE-DECODA Study Group . Age, body mass index and type 2 diabetes-associations modified by ethnicity. Diabetologia 2003;46:1063–1070 [DOI] [PubMed] [Google Scholar]
- 13.Ramachandran A, Snehalatha C, Vijay V. Low risk threshold for acquired diabetogenic factors in Asian Indians. Diabetes Res Clin Pract 2004;65:189–195 [DOI] [PubMed] [Google Scholar]
- 14.Mohan V, Amutha A, Ranjani H, et al. . Associations of β-cell function and insulin resistance with youth-onset type 2 diabetes and prediabetes among Asian Indians. Diabetes Technol Ther 2013;15:315–322 [DOI] [PubMed] [Google Scholar]
- 15.Staimez LR, Weber MB, Ranjani H, et al. . Evidence of reduced β-cell function in Asian Indians with mild dysglycemia. Diabetes Care 2013;36:2772–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163 [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association Classification and diagnosis of diabetes. Sec. 2. In Standards of Medical Care in Diabetes—2015. Diabetes Care 2015;38(Suppl.):S8–S16 [DOI] [PubMed] [Google Scholar]
- 18.Ranjani H, Weber MB, Anjana RM, Lakshmi N, Narayan KM, Mohan V. Recruitment challenges in a diabetes prevention trial in a low- and middle-income setting. Diabetes Res Clin Pract 2015;110:51–59 [DOI] [PubMed] [Google Scholar]
- 19.Sudha V, Radhika G, Sathya RM, Ganesan A, Mohan V. Reproducibility and validity of an interviewer-administered semi-quantitative food frequency questionnaire to assess dietary intake of urban adults in southern India. Int J Food Sci Nutr 2006;57:481–493 [DOI] [PubMed] [Google Scholar]
- 20.Tuomilehto J, Lindström J, Eriksson JG, et al.; Finnish Diabetes Prevention Study Group . Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 21.Saito T, Watanabe M, Nishida J, et al.; Zensharen Study for Prevention of Lifestyle Diseases Group . Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med 2011;171:1352–1360 [DOI] [PubMed] [Google Scholar]
- 22.Anjana RM, Shanthi Rani CS, Deepa M, et al. . Incidence of diabetes and prediabetes and predictors of progression among Asian Indians: 10-year follow-up of the Chennai Urban Rural Epidemiology Study (CURES). Diabetes Care 2015;38:1441–1448 [DOI] [PubMed] [Google Scholar]
- 23.Viscogliosi G, Cipriani E, Liguori ML, et al. . Mediterranean dietary pattern adherence: associations with prediabetes, metabolic syndrome, and related microinflammation. Metab Syndr Relat Disord 2013;11:210–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duijzer G, Haveman-Nies A, Jansen SC, ter Beek J, Hiddink GJ, Feskens EJ. SLIMMER: a randomised controlled trial of diabetes prevention in Dutch primary health care: design and methods for process, effect, and economic evaluation. BMC Public Health 2014;14:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marinik EL, Kelleher S, Savla J, Winett RA, Davy BM. The Resist Diabetes trial: rationale, design, and methods of a hybrid efficacy/effectiveness intervention trial for resistance training maintenance to improve glucose homeostasis in older prediabetic adults. Contemp Clin Trials 2014;37:19–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Utzschneider KM, Tong J, Montgomery B, et al. . The dipeptidyl peptidase-4 inhibitor vildagliptin improves beta-cell function and insulin sensitivity in subjects with impaired fasting glucose. Diabetes Care 2008;31:108–113 [DOI] [PubMed] [Google Scholar]
- 27.Amati F, Dubé JJ, Coen PM, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care 2009;32:1547–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nanditha A, Snehalatha C, Ram J, et al. . Impact of lifestyle intervention in primary prevention of Type 2 diabetes did not differ by baseline age and BMI among Asian-Indian people with impaired glucose tolerance. Diabet Med. 16 January 2016. [Epub ahead of print]. DOI: 10.1111/dme.13071 [DOI] [PubMed] [Google Scholar]
- 29.Glechner A, Harreiter J, Gartlehner G, et al. . Sex-specific differences in diabetes prevention: a systematic review and meta-analysis. Diabetologia 2015;58:242–254 [DOI] [PubMed] [Google Scholar]
- 30.Ranjani H, Weber MB, Anjana RM, Lakshmi N, Narayan KM, Mohan V. Recruitment challenges in a diabetes prevention trial in a low- and middle-income setting. Diabetes Res Clin Pract 2015;110:51–59 [DOI] [PubMed]
- 31.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood) 2012;31:67–75 [DOI] [PubMed] [Google Scholar]
- 32.Fujimoto WY, Jablonski KA, Bray GA, et al.; Diabetes Prevention Program Research Group . Body size and shape changes and the risk of diabetes in the diabetes prevention program. Diabetes 2007;56:1680–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerji MA, Faridi N, Atluri R, Chaiken RL, Lebovitz HE. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab 1999;84:137–144 [DOI] [PubMed] [Google Scholar]
- 34.Kouvelioti R, Vagenas G, Langley-Evans S. Effects of exercise and diet on weight loss maintenance in overweight and obese adults: a systematic review. J Sports Med Phys Fitness 2014;54:456–474 [PubMed] [Google Scholar]
- 35.Lindström J, Peltonen M, Eriksson JG, et al.; Finnish Diabetes Prevention Study (DPS) . Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia 2013;56:284–293 [DOI] [PubMed] [Google Scholar]
- 36.Anjana RM, Pradeepa R, Deepa M, et al.; ICMR–INDIAB Collaborative Study Group . Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia 2011;54:3022–3027 [DOI] [PubMed] [Google Scholar]
- 37.Sentell TL, He G, Gregg EW, Schillinger D. Racial/ethnic variation in prevalence estimates for United States prediabetes under alternative 2010 American Diabetes Association criteria: 1988-2008. Ethn Dis 2012;22:451–458 [PMC free article] [PubMed] [Google Scholar]
- 38.Cowie CC, Rust KF, Byrd-Holt DD, et al. . Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health and Nutrition Examination Survey 1999-2002. Diabetes Care 2006;29:1263–1268 [DOI] [PubMed] [Google Scholar]
- 39.Weber MB, Harish R, Staimez LR, et al. . Reduction in diabetes incidence differs by prediabetes type in a randomized translational trial of prevention (Abstract). Diabetes 2015;64(Suppl. 1):LB46 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.